Abstract
The abuse of synthetic cathinones, widely known as bath salts, has been increasing since the mid-2000s. These substances are derivatives of the naturally occurring compound cathinone, which is the primary psychoactive component of khat. The toxicity of synthetic cathinones includes significant sympathomimetic effects, as well as psychosis, agitation, aggression, and sometimes violent and bizarre behavior. Mephedrone and methylenedioxypyrovalerone are currently the predominantly abused synthetic cathinones.
Keywords: designer drugs/chemistry, street drugs/pharmacology, substance-related disorders/epidemiology, alkaloids/poisoning
Introduction
A recent manifestation of the interaction between substance abusers, those seeking to profit by providing substances of abuse, and the desire of both parties to avoid legal censure, is the emergence of bath-salt abuse. Bath salts in this context refer to several synthetic cathinones sold as either bath salts or plant food, but not intended for any horticultural or personal hygiene use. Despite the ubiquitous packaging disclaimer “not intended for human consumption,” these substances are sold as “legal highs” and drugs of abuse.1
History
Cathinone is a naturally occurring phenylalkylamine which is found in the leaves of khat (Catha edulis), a shrub or small tree indigenous to East Africa and the Arabian Peninsula.2 Historical references to the chewing of khat leaves for their euphoric and stimulant effects dates back many centuries, and today this practice is prevalent in such countries as Somalia, Yemen, Kenya, and Ethiopia.3 Khat leaves contain multiple compounds, notably phenylalkylamine alkaloids that include norpseudoephedrine, cathinone, and cathine.4 Studies in the 1930s to determine the psychoactive components of khat initially erroneously identified norpseudoephedrine as the main psychoactive compound.4 In 1975, cathinone was isolated from khat leaves and determined to be its principal psychoactive component.5 Although cathinone is a chiral molecule, khat leaves contain only the S (−) enantiomer,6 and synthesis of the racemate was not achieved until 1982.7 Cathinone begins to decompose shortly after leaves are harvested, and thus fresh leaves are sought for chewing. This may explain why khat chewing has largely remained limited to geographic regions where Catha edulis grows.3 In distinction to synthetic cathinone abuse, habitual khat chewing reportedly results in only mild psychological dependence and mild withdrawal symptoms.8,9
As reviewed by Kelly, the earliest report of synthetic cathinone synthesis is in 1928 with the synthesis of methcathinone in Germany, followed by the synthesis of mephedrone in 1929.10 Today, there are approximately 30 known synthetic cathinones.10
Although many synthetic cathinones have been investigated as anorectics, central nervous system stimulants, and antidepressants, clinical utility has been hindered by problems with abuse and dependence. For instance, methcathinone was initially used as an antidepressant in the USSR but removed from clinical use due to abuse.11 Currently, the synthetic cathinone diethylpropion is available for use as an anorectic, but is infrequently prescribed due to abuse and dependence. In addition, diethylpropion has been shown to be neurotoxic in animal studies.12 Pyrovalerone has been used in the past for chronic fatigue,13 while the most clinically successful cathinone is bupropion, which is widely prescribed for depression and smoking cessation.14
Patterns of abuse
Large-scale abuse of synthetic cathinones began with the use of methcathinone in the USSR in the 1970s and 1980s.11 Clandestine methcathinone manufacture first appeared in the US in Michigan in 1991, followed by significant problems of abuse in the early 1990s.11
Since 2004, abuse of various synthetic cathinones has been reported in Asia, Israel, the EU, and the US, possibly fueled by a decrease in purity and availability of other stimulant drugs of abuse, including MDMA (3,4-methylenedioxy-N-methylamphetamine) and cocaine.15 Data regarding synthetic cathinones seized in the EU since 2006 reveals ten different substances, including mephedrone, methylone, and MDPV (3,4-methylenedioxypyrovalerone), with mephedrone involved in 89% of seizures in the UK.14 US Customs and Border Protection drug-seizure data report the seizure of multiple different synthetic cathinones between July 2009 and April 2011, including MDPV and mephedrone.1 User surveys, poison center reports, and case series in the US and Europe indicate that current synthetic cathinone abuse involves primarily mephedrone and MDPV, with methylone, naphyrone, and flephedrone being less often implicated.9,16–19
Law-enforcement data indicate that synthetic cathinone supply frequently originates in the People’s Republic of China, Pakistan, and India.1 Substances are then sold to the public via the Internet and in retail establishments, including “head shops,” gas stations, convenience stores, and skateboard shops. Products are labeled as bath salts, plant food/fertilizer, vacuum freshener, pond cleaner, and insect repellent, and are typically sold as tablets or white powders.1 While oral ingestion and nasal insufflation have been reported as the most common means of use,9,16 parenteral exposure has also been described, with a recent case series reporting injection as the most common means of use.18
There is little epidemiologic data regarding synthetic cathinone use. Survey data imply that at least among certain demographic groups, use may be widespread. In a 2010 online survey sponsored by a magazine popular with UK clubbers, 41.7% of 2200 respondents reported having used mephedrone.15,20 A 2010 survey of 1006 high school and university students in Scotland reported a 20.3% prevalence of mephedrone use.9 Evidence from poison center calls and drug seizures in the US support the concept that synthetic cathinone abuse is a recent and increasing phenomenon. Synthetic cathinone-related calls to US poison centers increased from zero in 2009 to 304 in 2010 and 6138 in 2011.16,21 Drug samples seized in the US and analyzed by state and local forensic laboratories reveal 34 reports of synthetic cathinones in 2009 and 628 in 2010.17,22
Coingestion of other drugs of abuse and alcohol frequently accompanies synthetic cathinone use.19,23,24 While there are no data regarding people seeking treatment for synthetic cathinone dependence/addiction, users have reported a strong compulsion to redose, as well as addiction/dependence.9
The legal status of cathinone analogues continues to evolve as new substances are produced in order to evade existing laws.1 Mephedrone and several other substituted cathinones were banned in the EU in 2010,23 and as of July 2012, mephedrone, MDPV, and methylone have been added to Schedule I of the Controlled Substances Act in the US.25
Chemistry
As seen in Figure 1, cathinones are structurally related to amphetamines.26 Both are substituted phenethylamines with cathinones possessing a ketone group at the β-carbon position. Figure 2 demonstrates the general structure of substituted cathinones. Various R-group substitutions give rise to the approximately 30 known cathinones, many of which have amphetamine analogues to which they are identical, save for the β-carbon ketone group.26 Thus, replacing a hydrogen with a ketone converts amphetamine to cathinone, methamphetamine to methcathinone, and MDMA to methylone.10 The structure of several cathinones recently implicated in recreational abuse can be seen in Figure 3.
Figure 1.
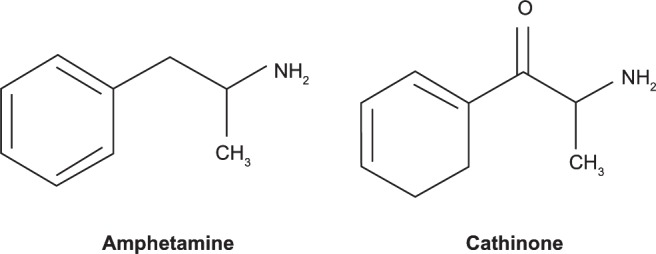
Amphetamine and cathinone structures.
Figure 2.
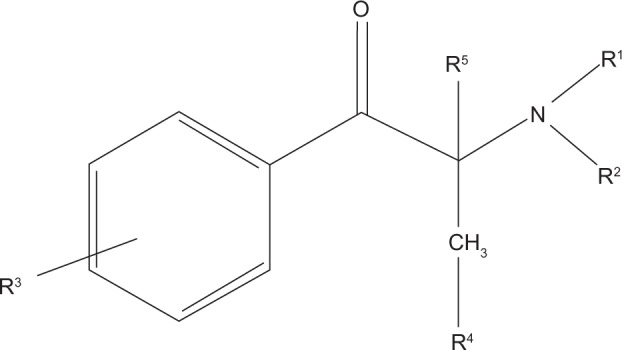
General cathinone derivative structure.
Figure 3.
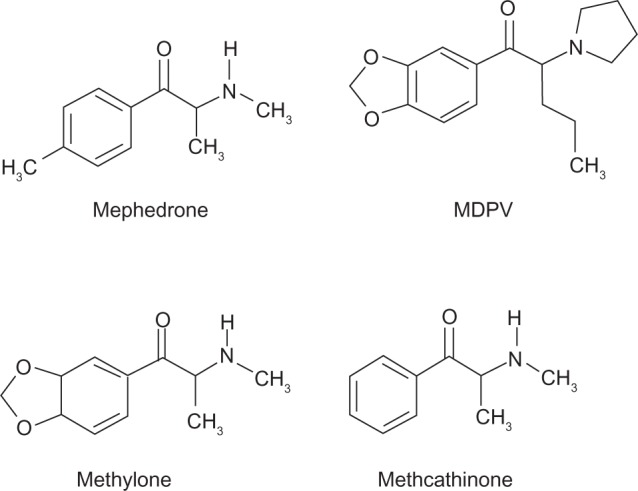
Selected substituted cathinone structures.
Abbreviation: MDPV, methylenedioxypyrovalerone.
Pharmacokinetics
At present, there is a scarcity of data concerning the pharmacokinetics of synthetic cathinones in humans. However, insights can be gleaned from animal data as well as inferred from studies of naturally occurring cathinones.
The pharmacokinetics of cathinone have been studied in humans by multiple investigators since the initial isolation of this compound from khat leaves in 1975.4 After chewing khat leaves, absorption takes place primarily in the oral mucosa, with a secondary contribution from absorption in the stomach and small intestine.27 Extraction of khat alkaloids by mastication has been reported to be very efficient;27 however, chewing results in delayed peak plasma concentrations when compared to administering oral cathinone. Following chewing khat leaves, time to peak plasma concentrations of 138 ± 39 minutes27 and 127 ± 30 minutes28 have been reported, while administration of gelatin cathinone capsules produced peak plasma concentrations in 72 minutes.29 Orally ingested cathinone undergoes extensive first-pass hepatic metabolism primarily to norpseudoephedrine with a smaller fraction converted to norephedrine.2,27,29,30 Very little cathinone is excreted unchanged, with studies in humans reporting urinary excretion of unchanged cathinone to be between 2%2 and 7%.31 The half-life of cathine is reported to be 5.2 ± 3.4 hours, and cathinone 1.5 ± 0.8 hours in humans.32
Research regarding synthetic cathinone pharmacokinetics in humans is lacking. There are no controlled studies of human in vivo synthetic cathinone pharmacokinetics; however, several studies examining urinary metabolites in people who claim to have ingested synthetic cathinones exist.33–36 In vivo animal studies have been published,33,35–37 as have in vitro investigations utilizing animal hepatocytes38,39 and human liver microsomes.36,37,40 Generalizing the findings of these studies is complicated not only by varying experimental models but also by the multiple different synthetic cathinones studied. However, within the framework of these limitations, several preliminary conclusions may be drawn. Similar to naturally occurring cathinone, synthetic cathinones appear to undergo extensive phase I and II metabolism,33–38,40 with little of the drug excreted unchanged in urine.35,37 Commonly identified phase I reactions are demethylation and oxidation, as well as reduction of the β-keto moiety.35,36,38 Glucuronidation of metabolites has been described by several investigators.37,38 Indirect evidence of sulfate conjugation has been reported;33 however, other investigations have been unable to confirm this.37,38 Human liver microsomes have been used by Meyer et al in two separate studies, with the finding that human cytochrome P450 (CYP) enzymes CYP2B6, CYP2C19, CYP2D6, and CYP1A2 are involved in synthetic cathinone metabolism.36,37 While there are no studies examining synthetic cathinone half-lives in humans, surveys of users suggest that the duration of effects of mephedrone and MDPV are “short,” with users reporting frequent redosing at 1- to 2-hour intervals and a duration of effects of approximately 2–4 hours.41,42 This is not inconsistent with findings in rat hepatocytes of a half-life of approximately 1 hour for mephedrone.38
Compared to amphetamines, the ketone group of cathinones confers a greater polarity and a predicted increased lipophilicity.43 Thus, diffusion across the blood–brain barrier may be decreased.43 However several pyrrolidine derivatives, including MDPV and MDPPP (3,4-methylenedioxy-α-pyrrolidinopropiophenone), have lower polarity and have shown high solubility in organic solvents.15 In addition, recent in vitro studies of mephedrone, MDPV, methylone, ethylone, butylone, and naphyrone demonstrated high blood–brain barrier permeability of all these synthetic cathinones.44
Mechanism of action
In vitro studies utilizing human and animal cell preparations suggest that cathinones acutely increase extracellular dopamine, norepinephrine, and serotonin levels. Similar to amphetamines, cathinones inhibit dopamine, norepinephrine, and serotonin plasma membrane and vesicular monoamine transporters, resulting in increased neurotransmitter synaptic concentration.45,46 Different cathinones display varying ability to cause increases in extracellular dopamine, norepinephrine, and serotonin levels, which may account for the different mood-altering effects, toxicity, and potential for addiction related to these compounds. Mephedrone, for instance, has been shown to significantly inhibit norepinephrine reuptake.46 While the expected sympathomimetic effects have not been specifically demonstrated in human studies, case reports do support a strong sympathomimetic effect of some synthetic cathinones, including mephedrone.18,24,47 The reported addictive potential of several synthetic cathinones is likely related to effects on increasing extracellular dopamine, as has been demonstrated in animal models.48
Toxicology
The current body of knowledge regarding the adverse effects of synthetic cathinones is based largely on case reports, data from poison centers, and surveys of users. Inherent flaws in these data are likely, as users may not be accurate regarding substances ingested, the amounts ingested, and the presence of coingestions. Compounding this is the limited ability to test accurately for synthetic cathinones,15 and purchased products that do not contain the substances advertised or contain unlisted compounds in addition to the advertised drug. Inaccurate labeling of products has been reported, with products containing synthetic cathinones other than the listed compound, other substances of abuse, including MDMA and ketamine, and pharmaceuticals, including acetaminophen, caffeine, benzocaine, and lidocaine.49,50 Additionally, products sold as cocaine and MDMA have been found to contain synthetic cathinones.51,52 Finally, a review of 15 products sold as “legal highs” in the USA found no ingredients were listed on any of the packages, thus making it very difficult for patients to report exposures accurately.19
Toxicity of naturally occurring cathinones
The reported toxicity of the natural substances cathinone and cathine appears less severe than many of their synthetic counterparts. This may be due to chewing khat representing the primary means of ingestion and the bulk of leaves that must be chewed in order to produce significant toxic effects.29 Described toxic effects of chewing khat include depression, irritability, insomnia, anorexia, and paranoid psychosis. Adverse cardiovascular effects include hypertension, tachycardia, and an increased incidence of acute myocardial infarction and cerebral vascular accidents.53 Chewing khat is also associated with an increased incidence of oral cancer.54
Toxicity of synthetic cathinones
Toxic effects of synthetic cathinones include sympathomimetic effects, as well as psychological effects, including aggression, agitation, paranoia, and delusions.16,19,55 Seizures, hyponatremia, hyperthermia, rhabdomyolysis, disseminated intravascular coagulation, renal failure, and hepatic failure have also been reported, as have several deaths.19,47,56,57 Several reports, primarily from Eastern Europe, document parkinsonism in patients following long-term parenteral use of methcathinone. Manganese contamination of homemade methcathinone has been identified as the cause.58,59
Chronic amphetamine use is known to be neurotoxic to dopaminergic neurons, resulting in long-term reductions in brain dopamine concentrations in chronic users.60 Serotonergic neurotoxicity as a result of MDMA use occurs in animals, and possibly humans.61 Whether synthetic cathinone abuse is related to dopaminergic or serotonergic neurotoxicity in humans is unknown; however, serotonin neurotoxicity has been described with methylone and mephedrone in rats.62
User-reported adverse effects
User-reported adverse effects of synthetic cathinone use commonly include agitation, paranoia, bruxism, palpitations, headache, and depression.9,63,64 A 2010 survey of 1006 students in Scotland with 205 mephedrone users found 56% reported at least one adverse effect, most commonly bruxism (28.3%) and paranoia (24.9%).9 Mixmag is a publication popular with UK clubbers and host of the large Mixmag Drugs Survey, which in 2012 included online responses from over 15,500 respondents. Commonly reported adverse mephedrone effects included depression (41%), agitation (23%), “overheating” (26%), severe headache (12%), and chest pain (10%).63 In a smaller study from Ireland utilizing privileged-access interviewing of eleven intravenous users of mephedrone, all users reported intense paranoia, and two reported extreme aggression and violence.64
Adverse effects reported to poison centers
Calls by physicians to the National Poisons Information Service in the UK from March 2009 to February 2010 included 188 calls regarding cathinones, with 131 of these concerning mephedrone. Reported adverse effects included agitation or aggression (24%), tachycardia (22%), confusion or psychosis (14%), chest pain (13%), and palpitations (11%).16 A report documenting synthetic cathinone-related calls to Texas poison centers in 2010 and 2011 found 362 calls, with common adverse effects being tachycardia (45.9%), agitation (39.2%), hypertension (21.0%), and hallucinations (17.7%).55 During an 8-month period in 2010 and 2011, 236 calls were received by poison centers in Kentucky and Louisiana regarding synthetic cathinone intoxication. Commonly reported toxicities included agitation (82%), combative behavior (57%), tachycardia (56%), hallucinations (40%), and paranoia (36%). This study also included descriptions of severe delusional behavior, including leaving a 2-year-old child in a highway “because she had demons,” firing guns at nonexistent people and “demons,” and destroying all the windows in a home and walking barefoot through the resulting broken glass. One patient died as a result of a self-inflicted gunshot wound while delusional.19
Adverse effects reported in case series
Multiple case reports from emergency departments concerning patients presenting with synthetic cathinone toxicity, without analytical confirmation of substances ingested, have been published. Findings are summarized in Table 1.
Table 1.
Synthetic cathinone toxicity: case reports with ≥15 patients without analytical confirmation of synthetic cathinone exposure
| Study | Patients | Reported exposure | Percent reporting
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Agitation | Tachycardia | HTN | Seizure | Chest pain | Hyperpyrexia | Elevated CK | Low sodium | |||
| CDC1 | 35 | MDPV, “bath salts” | 66 | 63 | 23 | 29 | a | a | 2.9 | a |
| Dargan et al23 | 72 | Mephedrone | 38.9 | 36.1 | 13.9 | 6.9 | 12.5 | 0 | 13.8b | 1.4 |
| Regan et al24 | 57 | Mephedrone | 40.4 | 79 | 74 | 3.5 | 24.6 | d | 33.3c | a |
| Wood et al47 | 15 | Mephedrone | 53.3 | 40 | 20 | 20 | b | a | a | a |
Notes: aNot reported; bCK measured in 18 patients, elevated in ten; cCK measured in 20 patients, elevated in 19; dindividual data not given. Temperature range of all patients 34.8°C–38.8°C.
Abbreviations: CK, creatine kinase; HTN, hypertension; MDPV, methylenedioxypyrovalerone; CDC, Centers for Disease Control and Prevention.
Case reports in which the presence of synthetic cathinones was confirmed by body-fluid or tissue analysis include multiple single-patient case reports, a series of three deaths involving methylone, and a series of seven patients with mephedrone toxicity.17,51,56,57,65–70 A series of 13 patients with confirmed MDPV intoxication has been published, but clinical findings in patients with confirmed and unconfirmed exposures are grouped together.19 In the report of seven patients with confirmed mephedrone ingestion, five of the patients had only synthetic cathinones detected on drug screening, while the other two patients also tested positive for cocaine. Adverse effects included heart rate >100 (five patients), systolic blood pressure >160 (three), agitation (four), and seizure (one). One patient had hyponatremia with a serum sodium concentration of 125 mmol/L, and one patient had rhabdomyolysis. No patients had significant hyperpyrexia.65 In the three reported deaths from methylone intoxication, all patients had hyperpyrexia and seizures, with metabolic acidosis, disseminated intravascular coagulation, and acute renal failure also reported.66 Single-patient case reports have also reported hyperpyrexia, seizures, hyponatremia, rhabdomyolysis, and metabolic acidosis.51,56,57,69
Conclusion
The recreational use of synthetic cathinones, widely known as bath salts, has been the subject of much recent interest. Synthetic cathinones are derivatives of the naturally occurring compound cathinone, which is the primary psychoactive component of khat. Cathinones are structurally related to amphetamines, and their mechanisms of action are thought to be similar. Although the first synthetic cathinones were synthesized in the 1920s, and recreational abuse can be traced back many decades, rapid increase in use began in the mid-2000s, likely fueled by the legality of these substances. Mephedrone and MDPV have been the dominant synthetic cathinones of abuse in recent years, and are implicated in an increasing number of emergency department visits due to adverse effects. Case series and poison center data indicate that toxicity includes significant sympathomimetic effects, as well as psychosis, agitation, aggression, and sometimes violent and bizarre behavior. Multiple deaths attributed to synthetic cathinone use have been reported. There is a paucity of data concerning the pharmacodynamics and pharmacokinetics of synthetic cathinones in humans, with current understanding based primarily on in vitro and animal studies. Long-term effects of synthetic cathinone use, including the potential for addiction/dependence, are largely unknown. Although mephedrone and MVPV are now illegal in many countries, the current landscape of synthetic cathinone abuse will likely continue to shift as new substances are developed and marketed, and as legal pressures change.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.US Department of Justice National Drug Intelligence Center Situation report: Synthetic cathinones (bath salts) – an emerging domestic threat 2011Available from: http://www.justice.gov/archive/ndic/pubs44/44571/44571p.pdfAccessed December 10, 2012
- 2.Kalix P, Braenden O. Pharmacological aspects of the chewing of khat leaves. Pharmacol Rev. 1985;37(2):149–164. [PubMed] [Google Scholar]
- 3.Review of the pharmacology of khat. Report of a WHO advisory group. Bull Narc. 1980;32(3):83–93. [No authors listed] [PubMed] [Google Scholar]
- 4.Szendrei K. The chemistry of khat. Bull Narc. 1980;32(3):5–35. [PubMed] [Google Scholar]
- 5.United Nations . Studies on the chemical composition of khat. III. Investigations on the phenylalkylamine fraction. UN document MNAR/11/1975. [Google Scholar]
- 6.Peterson DW, Maitai CK, Sparber SB. Relative potencies of two phenylalkylamines found in the abused plant Catha edulis, khat. Life Sci. 1980;27(22):2143–2147. doi: 10.1016/0024-3205(80)90496-8. [DOI] [PubMed] [Google Scholar]
- 7.Berrang BD, Lewin AH, Carroll FI. Enantiomeric alpha-aminopropiophenones (cathinone): preparation and investigation. J Org Chem. 1982;47(13):2643–2647. [Google Scholar]
- 8.Luqman W, Danowski TS. The use of khat (Catha edulis) in Yemen. Social and medical observations. Ann Intern Med. 1976;85(2):246–249. doi: 10.7326/0003-4819-85-2-246. [DOI] [PubMed] [Google Scholar]
- 9.Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM. 2010;103(11):875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- 10.Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3(7–8):439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- 11.Glennon RA, Young R, Martin BR, Dal Cason TA. Methcathione (“cat”): an enantiomeric potency comparison. Pharmacol Biochem Behav. 1995;50(4):601–606. doi: 10.1016/0091-3057(94)00348-3. [DOI] [PubMed] [Google Scholar]
- 12.Galvan-Arzate S, Santamaria A. Neurotoxicity of diethylpropion: neurochemical and behavioral findings in rats. Ann N Y Acad Sci. 2002;965:214–224. [PubMed] [Google Scholar]
- 13.Gardos G, Cole JO. Evaluation of pyrovalerone in chronically fatigued volunteers. Curr Ther Res Clin Exp. 1971;13(10):631–635. [PubMed] [Google Scholar]
- 14.Advisory Council on the Misuse of Drugs ACMD report on the consideration of the cathinones 2010Available from: http://www.homeoffice.gov.uk/acmd1/acmd-cathinodes-report-2010?view=BinaryAccessed November 1, 2012
- 15.Coppola M, Mondola R. Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food.”. Toxicol Lett. 2012;211(2):144–149. doi: 10.1016/j.toxlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 16.James D, Adams RD, Spears R, et al. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J. 2011;28(8):686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310–313. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Emergency department visits after use of a drug sold as “bath salts” – Michigan, November 13, 2010–March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624–627. [PubMed] [Google Scholar]
- 19.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 20.Dick D, Torrance C. Mixmag drugs survey. Mixmag. 2010;225:44–53. [Google Scholar]
- 21.American Association of Poison Control Centers (AAPCC) Bath salts data 2012Available from: https://aapcc.s3.amazonaws.com/files/library/Bath_Salts_Data_for_Website_1.09.2013.pdfAccessed December 15, 2012
- 22.Drug Enforcement Administration Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2009–2010 Springfield (VA)DEA; 2011Available from: http://www.deadiversion.usdoj.gov/nflis/2010rx_synth.pdfAccessed December 10, 2012 [Google Scholar]
- 23.Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test Anal. 2011;3(7–8):454–463. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- 24.Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J. 2011;28(12):1055–1058. doi: 10.1136/emj.2010.103093. [DOI] [PubMed] [Google Scholar]
- 25.Drug Enforcement Administration Schedules of controlled substances: placement of ezogabine into Schedule V. Final rule. Fed Regist. 2011;76(241):77895–77899. [PubMed] [Google Scholar]
- 26.Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol. 1992;70(2):77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 27.Toennes SW, Harder S, Schramm M, Niess C, Kauert GF. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br J Clin Pharmacol. 2003;56(1):125–130. doi: 10.1046/j.1365-2125.2003.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widler P, Mathys K, Brenneisen R, Kalix P, Fisch HU. Pharmacodynamics and pharmacokinetics of khat: a controlled study. Clin Pharmacol Ther. 1994;55(5):556–562. doi: 10.1038/clpt.1994.69. [DOI] [PubMed] [Google Scholar]
- 29.Brenneisen R, Fisch HU, Koelbing U, Geisshusler S, Kalix P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol. 1990;30(6):825–828. doi: 10.1111/j.1365-2125.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guantai AN, Maitai CK. Metabolism of cathinone to d-norpseudoephedrine in humans. J Pharm Sci. 1983;72(10):1217–1218. doi: 10.1002/jps.2600721029. [DOI] [PubMed] [Google Scholar]
- 31.Toennes SW, Kauert GF. Excretion and detection of cathinone, cathine, and phenylpropanolamine in urine after kath chewing. Clin Chem. 2002;48(10):1715–1719. [PubMed] [Google Scholar]
- 32.Kalix P. Pharmacological properties of the stimulant khat. Pharmacol Ther. 1990;48(3):397–416. doi: 10.1016/0163-7258(90)90057-9. [DOI] [PubMed] [Google Scholar]
- 33.Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioana Chem. 2010;397(3):1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- 34.Zaitsu K, Katagi M, Kamata HT, et al. Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine. Forensic Sci Int. 2009;188(1–3):131–139. doi: 10.1016/j.forsciint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Kamata HT, Shima N, Zaitsu K, et al. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica. 2006;36(8):709–723. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- 36.Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. J Mass Spectrom. 2010;45(12):1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- 37.Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. J Mass Spectrom. 2012;47(2):253–262. doi: 10.1002/jms.2960. [DOI] [PubMed] [Google Scholar]
- 38.Khreit OI, Grant MH, Zhang T, Henderson C, Watson DG, Sutcliffe OB. Elucidation of the phase I and phase II metabolic pathways of (+/−)-4′-methylmethcathinone (4-MMC) and (+/−)-4′-(trifluoromethyl) methcathinone (4-TFMMC) in rat liver hepatocytes using LC-MS and LC-MS(2) J Pharm Biomed Anal. 2013;72:177–185. doi: 10.1016/j.jpba.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Pawlik E, Plässer G, Mahler H, Daldrup T. Studies on the phase I metabolism of the new designer drug 3-fluoromethcathinone using rabbit liver slices. Int J Legal Med. 2012;126(2):231–240. doi: 10.1007/s00414-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 40.Mueller DM, Rentsch KM. Generation of metabolites by an automated online metabolism method using human liver microsomes with subsequent identification by LC-MS(n), and metabolism of 11 cathinones. Anal Bioanal Chem. 2012;402(6):2141–2151. doi: 10.1007/s00216-011-5678-8. [DOI] [PubMed] [Google Scholar]
- 41.Psychonaut WebMapping Research Group . Mephedrone Report. London: Institute of Psychiatry, King’s College London; 2009. [Google Scholar]
- 42.Newcombe R. Mephedrone: The Use of Mephedrone (M-cat, Meow) in Middlesbrough. Manchester: Lifeline Publications and Research; 2009. [Google Scholar]
- 43.Gibbons S, Zloh M. An analysis of the ‘legal high’ mephedrone. Bioorg Med Chem Lett. 2010;20(14):4135–4139. doi: 10.1016/j.bmcl.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 44.Simmler LD, Buser TA, Donzelli M, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cozzi NV, Sievert MK, Shulgin AT, Jacob P, 3rd, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381(1):63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167(2):407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28(4):280–282. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]
- 48.Kehr J, Ichinose F, Yoshitake S, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164(8):1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet – is there consistency in what you get? QJM. 2010;103(7):489–493. doi: 10.1093/qjmed/hcq056. [DOI] [PubMed] [Google Scholar]
- 50.European Monitoring Centre for Drugs and Drug Addiction . Report on the Risk Assessment of Mephedrone in the framework of the Council Decision on New Psychoactive Substances. Lisbon: EMCDDA; 2011. [Google Scholar]
- 51.Sauer C, Hoffmann K, Schimmel U, Peters FT. Acute poisoning involving the pyrrolidinophenone-type designer drug 4′-methyl-alpha-pyrrolidinohexanophenone (MPHP) Forensic Sci Int. 2011;208(1–3):e20–e25. doi: 10.1016/j.forsciint.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25(11):1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- 53.Al-Motarreb A, Briancon S, Al-Jaber N, et al. Khat chewing is a risk factor for acute myocardial infarction: a case-control study. Br J Clin Pharmacol. 2005;59(5):574–581. doi: 10.1111/j.1365-2125.2005.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balint EE, Falkay G, Balint GA. Khat – a controversial plant. Wien Klin Wochenschr. 2009;121(19–20):604–614. doi: 10.1007/s00508-009-1259-7. [DOI] [PubMed] [Google Scholar]
- 55.Forrester MB. Synthetic cathinone exposures reported to Texas poison centers. Am J Drug Alcohol Abuse. 2012;38(6):609–615. doi: 10.3109/00952990.2012.677890. [DOI] [PubMed] [Google Scholar]
- 56.Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Medicine. 2012;60(1):103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742. doi: 10.1016/S0140-6736(10)60891-4. [DOI] [PubMed] [Google Scholar]
- 58.Iqbal M, Monaghan T, Redmond J. Manganese toxicity with ephedrone abuse manifesting as parkinsonism: a case report. J Med Case Rep. 2012;6(1):52. doi: 10.1186/1752-1947-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yildirim EA, Esşsizoğlu A, Köksal A, Doğu B, Baybasş S, Gökalp P. [Chronic manganese intoxication due to methcathinone (ephedron) abuse: a case report] Turk Psikiyatri Derg. 2009;20(3):294–298. Turkish. [PubMed] [Google Scholar]
- 60.Ellison G. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur Neuropsychopharmacol. 2002;12(4):287–297. doi: 10.1016/s0924-977x(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 61.Puerta E, Hervias I, Aguirre N. On the mechanisms underlying 3,4-methylenedioxymethamphetamine toxicity: the dilemma of the chicken and the egg. Neuropsychobiology. 2009;60(3–4):119–129. doi: 10.1159/000253548. [DOI] [PubMed] [Google Scholar]
- 62.den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2012;103(3):501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Mixmag Mixmag’s drug survey: the results 2012Available from: http://www.mixmag.net/drugssurveyAccessed December 1, 2012
- 64.Van Hout MC, Bingham T. “A costly turn on”: patterns of use and perceived consequences of mephedrone based head shop products amongst Irish injectors. Int J Drug Policy. 2012;23(3):188–197. doi: 10.1016/j.drugpo.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Wood DM, Davies S, Greene SL, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol. 2010;48(9):924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- 66.Pearson JM, Hargraves TL, Hair LS, et al. Three fatal intoxications due to methylone. J Anal Toxicol. 2012;36(6):444–451. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- 67.Adamowicz P, Tokarczyk B, Stanaszek R, Slopianka M. Fatal mephedrone intoxication – a case report. J Anal Toxicol. 2013;37(1):37–42. doi: 10.1093/jat/bks085. [DOI] [PubMed] [Google Scholar]
- 68.Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol. 2010;6(3):327–330. doi: 10.1007/s13181-010-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8(1):69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derungs A, Schietzel S, Meyer MR, Maurer HH, Krahenbuhl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone) Clin Toxicol. 2011;49(7):691–693. doi: 10.3109/15563650.2011.592838. [DOI] [PubMed] [Google Scholar]


