Abstract
Objective
Computed tomography (CT)-based treatment planning for cervical cancer has allowed investigation into the volumetric radiation dose delivered to the rectum. The goal of intracavitary brachytherapy is to maximize the tumor dose while decreasing the dose to normal tissue like the rectum. We investigated the effects of tandem angle and maximum rectal distention on rectal dose delivered in HDR brachytherapy for locally advanced cervical cancer.
Methods and Materials
Between July 2007 and January 2010, 97 brachytherapy treatment planning CT scans from the first and last implant of 51 patients with locally advanced cervical cancer were reviewed. The rectum was manually contoured from the ischial tuberosity to the bottom of the sacroiliac joint. The maximum rectal distention was determined by measuring the largest anterior-posterior diameter of the rectum superior to the tandem ring and inferior to the end of the applicator. A volumetric measurement of the maximum and mean rectal dose, dose to 2cc (D2cc), dose to 1cc (D1cc) of the rectum was calculated. The tandem angle and the ICRU rectal point were recorded, and a dose volume histogram was referenced.
Results
The mean maximum rectal distention was 3.01cm. The mean D1cc, D2cc, mean rectal dose, maximum rectal dose, and ICRU rectal dose were 3.03 Gy, 2.78 Gy, 4.19 cGy, 1.40 cGy, and 2.99 Gy per treatment, respectively. In a multivariate analysis controlling for surface area, tandem angle, and body mass index (BMI), there was a significant increase in D2cc with increasing rectal distention (P=.016). There were no significant findings when observing the effects of tandem angle on D2cc.
Conclusion
Rectal distention significantly impacts D2cc delivered in HDR brachytherapy. In contrast, tandem angle does not. Concerted efforts to decrease rectal distention should be considered during treatment planning and delivery.
Keywords: Tandem angle, Brachytherapy, Rectal Distention, CT, Cervical Cancer
Introduction
Intracavitary brachytherapy in conjunction with pelvic external beam radiotherapy (EBRT) remains the standard of care in the treatment of patients with locally advanced cervical cancer (1). The efficacy of any such treatment is defined by maximizing the dose to the tumor while minimizing the dose to the surrounding normal tissues. Accurate calculation of radiation doses to tissues therefore remains an integral part of the treatment process. This is especially critical in high-dose-rate (HDR) brachytherapy due to the increased risk of complication from the high dosage of radiation used in treatment (2).
Traditionally, doses to the target and surrounding organs have been calculated by two-dimensional (2D) methods using orthogonal plain films in conjunction with the Internal Commission on Radiation Units and Measurement (ICRU) points. However, three-dimensional (3D) treatment planning has been gaining popularity with improvements in the quality of computed tomography (CT) and magnetic resonance imaging (MRI) scans. This is in large part due to the increased accuracy of 3D over the 2D method (3-5). According to a recent survey by the American Brachytheraphy Society (ABS), over 57% of clinicians currently implementing brachytherapy use CT or MRI based planning over 2D plain films (6).
With the help of treatment planning software, it is now possible to directly contour the 3D images and generate a dose volume histogram (DVH). The improved accuracy of DVH over ICRU rectal points has been investigated in several studies (3, 7). This has allowed us to accurately calculate the tumor doses relative to doses delivered to surrounding normal structures, such as the rectum. The rectum is a common site of side effects during brachytherapy due to its proximity to cervix and potential subsequent high dose region in brachytherapy. Figures 1 and 2 demonstrate the close proximity of the rectum in a patient with locally advanced cervical cancer seen on T2 weighted MRI images. Late rectal side effects during brachytherapy include bleeding, stenosis, and fistula formation. Given the correlation between minimizing radiation to the rectum and decreased gastrointestinal (GI) toxicity (8), it is of interest to determine the effects of these variables on the rectal dose.
Figure 1.
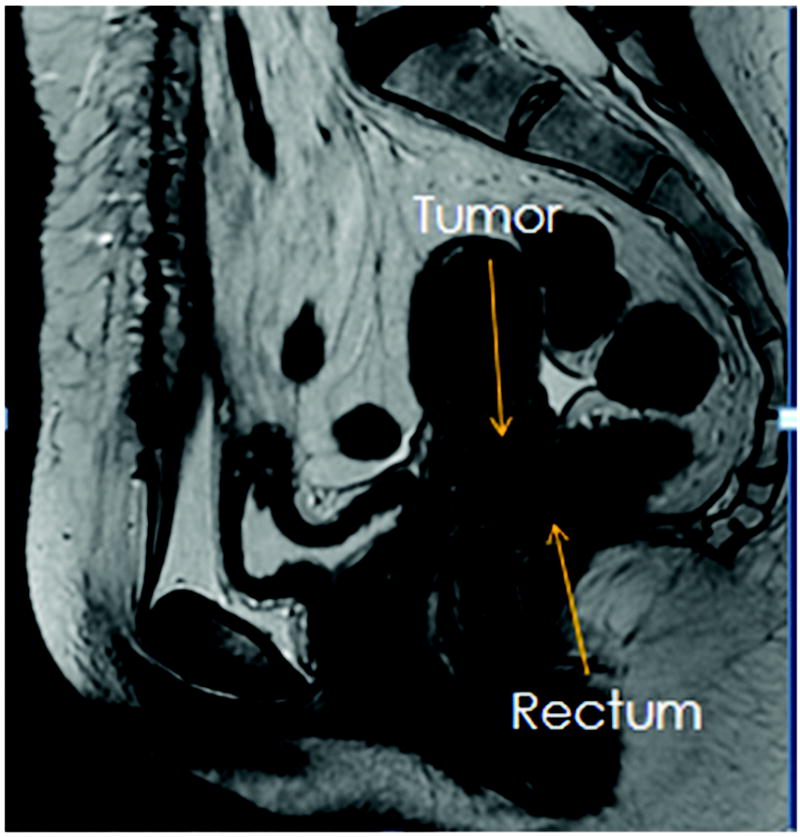
Sagittal T2 weighted MRI image to demonstrate the close proximity of the anterior rectal wall to the cervix and gross tumor
Figure 2.
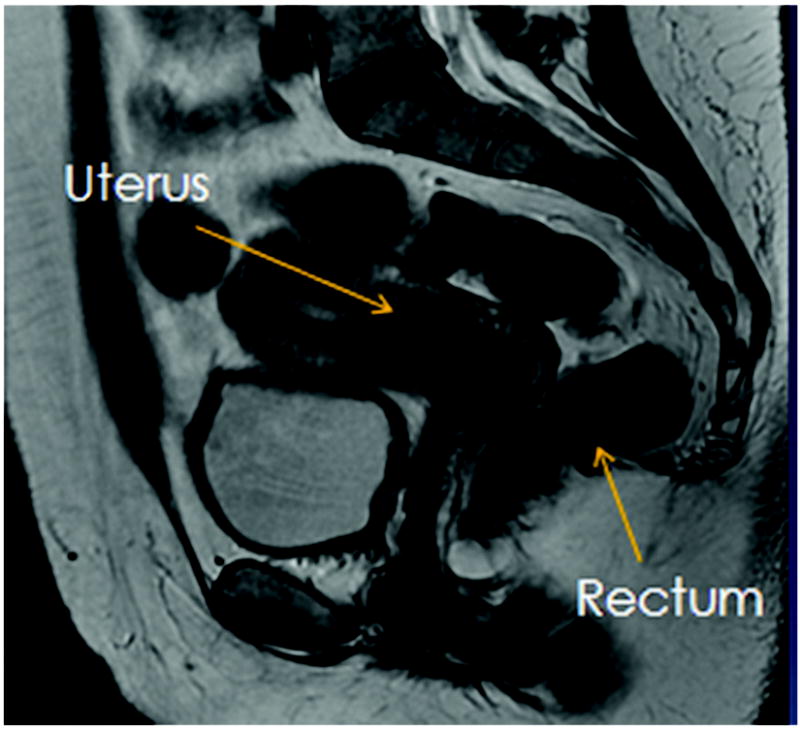
Sagittal T2 weighted MRI image to demonstrate the close proximity of the anterior rectal wall to the cervix and gross tumor
Intracavitary brachytherapy is more likely than EBRT to produce an inhomogenous dose distribution and cause rectal complications from points of high rectal dose (9). The possibility of developing rectal complications is best quantified from measuring the dose to 2cc (D2cc) of the rectum. To our knowledge, there has not been any reported literature on the effects of rectal distention and tandem angle on the rectal dose during brachytherapy for cervical cancer. Hence, to assess the impact of rectal distention and tandem angle on D2cc during brachytherapy, we have designed an investigation based on a clinical study of 51 women diagnosed with locally advanced cervical cancer.
Methods and Materials
Patient Characteristics
Between July 2007 and January 2010, 51 patients with locally advanced cervical cancer were treated with definitive radiation therapy and HDR brachytherapy. Ninety-seven brachytherapy treatment CT scans were retrospectively reviewed. For each patient, the CT scans from the first and last implant were examined in our study. Patients were staged based on the International Federation of Gynecology and Obstetrics (FIGO) classification.
Treatment Characteristics
Patients were initially treated with concurrent cisplatin chemotherapy and EBRT to the whole pelvis. The EBRT dose was given at five fractions per week for a total of 45 Gy in twenty-five fractions. If the patient needed a parametrial or lymph node boost, this was given after whole pelvic radiation therapy. This was followed by intracavitary HDR brachytherapy with the use of a fixed and rigid tandem ring applicator. Tandem angle of the applicator was preset at 30, 45, or 60 and was selected based on the patient’s anatomy and physician’s discretion. A rectal retractor was placed and vaginal packing with gauze was used to minimize applicator movement. Both orthogonal radiographs and pelvic CT images with 3mm slice thickness were obtained after the applicator was placed.
3D Treatment Planning
On the orthogonal radiograph, the following reference points were identified: ICRU rectal points and Manchester’s A and B points. The ICRU rectal point was defined as being located on the lateral plain film 5mm behind the packing at the level of the tandem ring. The HDR prescription dose was 6 Gy for five fractions or 8 Gy for three fractions to Point A. The tandem and ring were weighted in a standard loading pattern.
Varian Eclipse Brachyvision (v 8.1) was used for 3D treatment planning. CT images were imported and a 3D image was generated. The rectum was manually contoured from the ischial tuberosity to the bottom of the sacroiliac joint. Maximum rectal distention was defined as the largest anterior-posterior diameter of the rectum in the region superior to the location where the tandem bisects tandem ring, and inferior to the proximal and superior portion of the applicator.
The dose to 2cc (D2cc) and dose to 1cc (D1cc) were determined based on the dose volume histogram generated from the contoured rectum. Maximum rectal dose was calculated by determining the maximum absolute dose received by a point on the contoured rectum. The mean rectal dose was calculated by determining the mean of all rectal point doses on the contoured rectum.
Statistical Analysis
The effects of rectal distention, tandem angle, and other factors on D2cc were analyzed using linear mixed effects models incorporating a random effect for patient. Linear mixed effects models yield P-values and confidence intervals that account for the correlation between multiple observations from the same patient (10). All statistical analyses were performed using the statistical software environment R. Linear mixed effects modeling was conducted using the R package nlme (11).
Results
Patient and Tumor Characteristics
51 patients were included in the study. The average age was 54.6 years (ranging from 33 to 88 years). Patients were treated with brachytherapy over an elapsed average of 18 days (ranging from 6-36 days). The majority of the patient population had stage IIB cervical cancer (40.4% n=21), with the others ranging from IB1 to IIIB. The histopathology distribution showed a large prevalence of squamous cell carcinoma (78.8% n=41). Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Patient Characteristics | |
|---|---|
| Patients (n) | 51 |
| Average age (years) | 54.6 (33-88) |
| Average Time of Brachytherapy Duration (days) | 18 (6-36) |
| Cervical Cancer Stage (IB1 - IVA) | |
| IB1 | 5 (9.6%) |
| IB2 | 9 (17.3%) |
| IIA | 2 (3.85%) |
| IIB | 21 (40.4%) |
| IIIA | 1 (1.9%) |
| IIIB | 12 (23.1%) |
| IVA | 1 (1.9%) |
| IVB | 1 (1.9%) |
| Pathology (n) | |
| Squamous cell carcinoma | 41 (78.8%) |
| Adenocarcinoma | 8 (15.4%) |
| Adenosquamous carcinoma | 3 (5.77%) |
Rectal Dose Characteristics
D1cc was 3.03 Gy (range 1.00-5.85 Gy) and mean D2cc was 2.78 Gy (range 0.96-5.41 Gy). The mean of the ICRU point dose was 2.99 Gy (range 1.02-6.37 Gy). The mean of maximum rectal dose was 4.19 cGy (range 2.71-5.35 cGy) and for the mean rectal dose was 1.40 cGy (range 1.13-1.61 cGy). The maximum rectal distention mean was 3.01 cm (range 1.50-4.90 cm). Rectal distension is seen in Figure 3. The key rectal dose measurements are summarized in Table 2.
Figure 3.
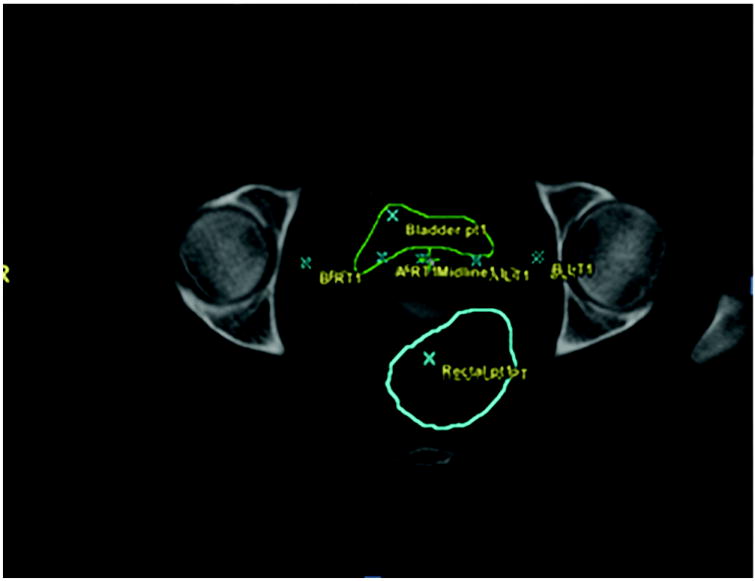
Axial view of the HDR planning CT depicting rectal distention. The rectal wall is contoured in blue
Table 2.
Summary of dose to ICRU rectal point measured in 2D plans and dose to 1cc and 2cc of rectum using 3D DVH parameters
| Variable | Mean | Range |
|---|---|---|
| D1cc (Gy) | 3.03 ± 1.08 | 1.00-5.85 |
| D2cc (Gy) | 2.78 ± 0.96 | 0.96—5.41 |
| ICRU (Gy) | 2.99 ± 1.04 | 1.02-6.37 |
| Mean Dose (cGy) | 1.40± 0.21 | 1.13-1.61 |
| Maximum Dose (cGy) | 4.20 ± 1.08 | 2.71-5.35 |
| Maximum Distention (cm) | 3.01 ± 0.70 | 1.50-4.90 |
2D = two-dimensional. 3D = three-dimensional. DVH = dose volume histogram. D1cc and D2cc = radiation dose to 1cc and 2cc of rectum respectively. ICRU = Internal Commission on Radiation Units and Measurement.
Rectal Dose Statistics
Univariate analysis using linear mixed effects models showed that, D2cc increased significantly with increasing rectal distension with an estimated increase of 6.58 Gy for each additional centimeter of distention (Figure 4). D2cc also increased significantly in multivariate analysis using linear mixed effect models (P < 0.016) when adjusting for the effects of tandem angle, body mass index (BMI), and surface area.
Figure 4.
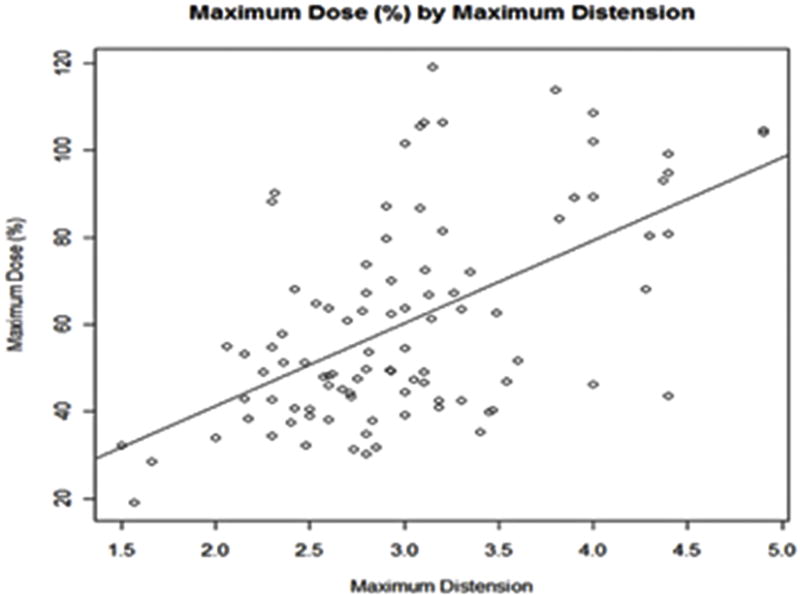
(Plot Chart) Maximum rectal dose percentage (%) as a function of maximum rectal distention
Tandem Angle Characteristics
Of the 97 implantations examined in this paper, 52 (54%) had a tandem angle of 60 degrees. Thirty-six (37%) implantations had a tandem angle of 45 degrees, and 7 (9%) implantations had a tandem angle of 30 degrees.
Tandem Angle Statistics
D2cc was compared between tandem angle groups using linear mixed effects models. D2cc did not differ significantly between tandem angle groups in univariate analysis (P = 0.796), or in multivariate analysis adjusting for maximum rectal distention, BMI, and surface area (P = 0.744). Figure 5 shows boxplots of each outcome by tandem angle.
Figure 5.
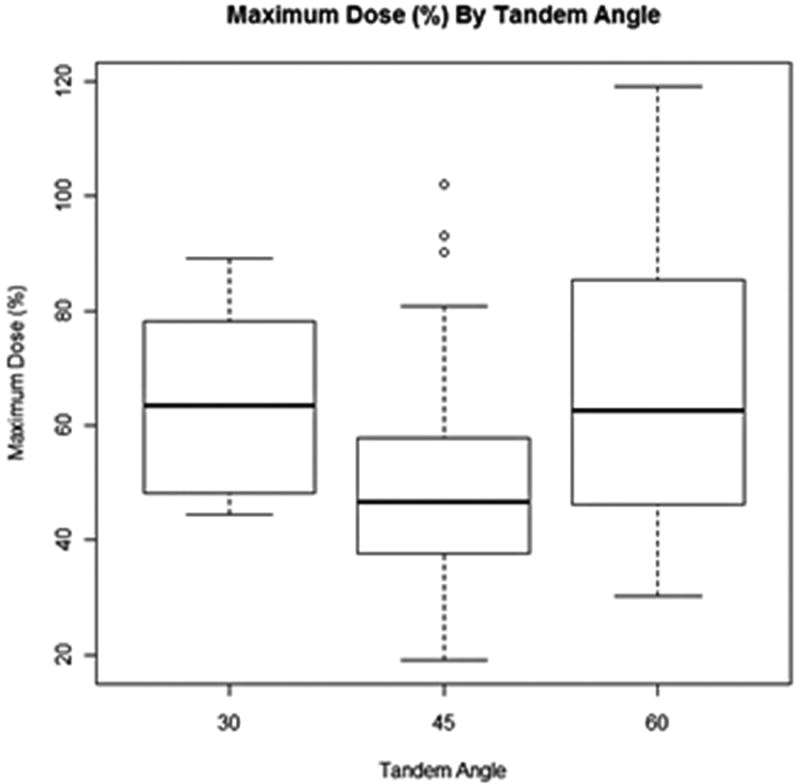
Maximum rectal dose percentage (%) as a function of maximum tandem angle distention
Discussion
Our investigation shows that rectal distention has a statistically significant impact on D2cc during HDR intracavitary brachytherapy for locally advanced cervical cancer. Based on our results, we would expect patients with more rectal distention to have greater rectal side effects during brachytherapy. In a study by Lee et al., prostate cancer patients exposed to higher doses of radiation had an increased likelihood of developing Grade 2-3 rectal toxicity (12). No study to date has found any connection between rectal dose and rectal toxicity in cervical cancer patients. Future studies may look into this further; however, it is important to control for confounders such as age, comorbidities, and surgical history (13-15).
Similarly, our results are consistent with a study by Merrick et al. that showed prostate cancer patients with a more distended rectum were subjected to higher doses of radiation. This was true for both D1cc and D2cc. The authors of the study concluded that minimizing the rectal distention before prostate brachytherapy is likely to minimize potential rectal toxicity (16). Both the Lee and Merrick studies involved patients undergoing treatment for prostate cancer. Differences in patient anatomy and treatment procedure may bring the applicability of these findings to cervical cancer treatment into question.
Even though our research results have been consistent with those of other studies, our study of rectal distention has limitations. Given that CT scans are obtained at a fixed point in time prior to the brachytherapy procedure, the rectal distention seen on the CT may not be indicative of the rectal distention during brachytherapy. The time between imaging and procedure can take hours, especially if imaging is done outside the procedure room. Also, the moving of the patient from imaging to the procedure room may impact the position of the applicator (17). For our study, in order to decrease this variability, we minimized applicator movement by using interior vaginal packing as well as a rigid external immobilization device that was secured to the distal aspect of the applicator and fixed to a rigid treatment board. However, vaginal packing has the potential to displace the distal portion of the rectum during brachytherapy and decrease the dose received by the rectum (18). The rectal rectractor has also been shown to decrease the dose received by the rectum (19).
Additionally, we did not measure sigmoid distention, which may impact the D2cc of the rectum given its proximity to the uterine body. A concern is that the sigmoid may have been contoured as the rectum given the definition of the rectum as being in the region superior to the ischial tuberosity and inferior to the sacroiliac joint. However, in our study we did take into consideration situations when the rectum angled to the left or the right. Once the rectosigmoid flexure was reached, we stopped the contouring such that the sigmoid was not included as part of the rectum.
In our investigation, we found no statistically significant relationship between tandem angle and D2cc. As a result, we expect the tandem angle to have minimal impact on rectal morbidity. There are a few issues that may have influenced the results of our research. We did not take into consideration differences in the length of tandem angle, amount of radiopaque gauze used in vaginal packing, and asymmetric radiation dose generated from the applicator. These variables may have had an impact on the relationship between tandem angle and D2cc. A significant source of variation also stems from changes in the position of the applicator during brachytherapy (17). Controlling for all these variables remains difficult, as they are not as easily measured.
While tandem angle does not affect D2cc, it is still an important factor to consider during brachytherapy. As noted earlier, it has the possibility of impacting the dose distribution generated and hence the total dose of radiation received by both the surrounding tissues and tumor. A suboptimal tandem angle has the potential to lead to inadequate target dose coverage. However, there have not been any studies to date definitively showing the impact of tandem angle on the radiation dose distribution. Further studies may determine how the tandem angle influences the dose distribution.
Further investigation is also needed to clarify the clinical benefits of minimizing rectal distention and hence rectal dose when treating cervical cancer. Efforts should nonetheless be taken by the clinician to minimize the extent of rectal distention during treatment with HDR brachytherapy given the potential to benefit the patient. From our observation, the presence of stool and gas correlated most with rectal distention (21). Therefore, physicians should seek to limit the amount of stool and gas within the patient before starting brachytherapy. This should benefit the patient by decreasing the dose of radiation received by the rectum.
The patient also benefits further from the more accurate imaging to further define the rectum relative to the target volume. A study performed in prostate cancer patients showed that tumor control was significantly decreased in patients with distended rectum as the CT scans were not representative of the patient’s anatomy (22). Hence, the potential benefit of decreasing rectal distention not only includes fewer rectal side effects but also improved accuracy in targeting the volume of interest in radiation oncology. It is important to note that the study involved prostate cancer patients and therefore its applicability to cervical cancer patients is of interest and needs further evaluation.
There are currently no recommendations regarding bowel preparation before beginning HDR brachytherapy for locally advanced cervical cancer. A few methods to reduce bowel distention include modification of diet before the procedure, having the patient defecate before undergoing radiotherapy, administration of an enema prior to the procedure, or the use of laxatives. Furthermore, vaginal packing may cause proximal distention of the rectum. Hence, placing the rectal retractor past the level of vaginal packing may reduce rectal distention. Adapting such guidelines in regular practice has the potential to benefit the patient.
Rectal distention impacts the rectal dose delivered in HDR intracavitary brachytherapy for cervical cancer. Decreasing the amount of stool and gas preoperatively should minimize the radiation to the rectum and therefore potentially minimize the rectal side effects. Some methods include controlling the diet before undergoing radiotherapy, having the patient defecate preceding treatment, using enema or mild laxatives.
Acknowledgments
Funding support:
National Center for Research Resources, National Institutes of Health, grant number UL1 RR024146 (Recipient B. Durbin-Johnson)
Morton Levitt Research Grant (Recipient J.Lim)
Footnotes
Financial Disclosures: None
References
- 1.Haie-Meder C, Morice P, Castiglione M. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v37–40. doi: 10.1093/annonc/mdq162. [DOI] [PubMed] [Google Scholar]
- 2.Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:201–211. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 3.Gao M, Albuquerque K, Chi A, et al. 3D CT-based volumetric dose assessment of 2D plans using GEC-ESTRO guidelines for cervical cancer brachytherapy. Brachytherapy. 2010;9:55–60. doi: 10.1016/j.brachy.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Haie-Meder C, Chargari C, Rey A, et al. DVH parameters and outcome for patients with early-stage cervical cancer treated with preoperative MRI-based low dose rate brachytherapy followed by surgery. Radiother Oncol. 2009;93:316–321. doi: 10.1016/j.radonc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Pelloski CE, Palmer M, Chronowski GM, et al. Comparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62:131–137. doi: 10.1016/j.ijrobp.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104–109. doi: 10.1016/j.ijrobp.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Van de Kamer JB, De Leeuw AA, Moerland MA, et al. Determining DVH parameters for combined external beam and brachytherapy treatment: 3D biological dose adding for patients with cervical cancer. Radiother Oncol. 2010;94:248–253. doi: 10.1016/j.radonc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Montana GS, Fowler WC. Carcinoma of the cervix: analysis of bladder and rectal radiation dose and complications. Int J Radiat Oncol Biol Phys. 1989;16:95–100. doi: 10.1016/0360-3016(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 9.Kim RY, Shen S, Duan J. Image-based three-dimensional treatment planning of intracavitary brachytherapy for cancer of the cervix: dose-volume histograms of the bladder, rectum, sigmoid colon, and small bowel. Brachytherapy. 2007;6:187–194. doi: 10.1016/j.brachy.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 11.Team RDC. R: A language and environment for statistical computin. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 12.Lee WR, Hanks GE, Hanlon AL, et al. Lateral rectal shielding reduces late rectal morbidity following high dose three-dimensional conformal radiation therapy for clinically localized prostate cancer: further evidence for a significant dose effect. Int J Radiat Oncol Biol Phys. 1996;35:251–257. doi: 10.1016/0360-3016(96)00064-8. [DOI] [PubMed] [Google Scholar]
- 13.Kapp DS, Fischer D, Gutierrez E, et al. Pretreatment prognostic factors in carcinoma of the uterine cervix: a multivariable analysis of the effect of age, stage, histology and blood counts on survival. Int J Radiat Oncol Biol Phys. 1983;9:445–455. doi: 10.1016/0360-3016(83)90060-3. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama Y, Van Nagell JR, Jr, Utley J, et al. Radiation and small bowel complications in cervical carcinoma therapy. Radiology. 1974;112:699–703. doi: 10.1148/112.3.699. [DOI] [PubMed] [Google Scholar]
- 15.Unal A, Hamberger AD, Seski JC, et al. An analysis of the severe complications of irradiation of carcinoma of the uterine cervix: treatment with intracavitary radium and parametrial irradiation. Int J Radiat Oncol Biol Phys. 1981;7:999–1004. doi: 10.1016/0360-3016(81)90150-4. [DOI] [PubMed] [Google Scholar]
- 16.Merrick GS, Butler WM, Dorsey AT, et al. The effect of constipation on rectal dosimetry following prostate brachytherapy. Med Dosim. 2000;25:237–241. doi: 10.1016/s0958-3947(00)00047-9. [DOI] [PubMed] [Google Scholar]
- 17.Ebruli C, Demiral AN, Cetingoz R, et al. The variability of applicator position among high dose rate intracavitary brachytherapy applications in cervical cancer patients treated with ring & tandem applicators. Tumori. 2007;93:432–438. doi: 10.1177/030089160709300505. [DOI] [PubMed] [Google Scholar]
- 18.Saini AS, Zhang GG, Finkelstein SE, et al. Dose reduction study in vaginal balloon packing filled with contrast for HDR brachytherapy treatment. Int J Radiat Oncol Biol Phys. 2011;80:1263–1267. doi: 10.1016/j.ijrobp.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Lee KC, Kim TH, Choi JH, et al. Use of the rectal retractor to reduce the rectal dose in high dose rate intracavitary brachytherapy for a carcinoma of the uterine cervix. Yonsei Med J. 2004;45:113–122. doi: 10.3349/ymj.2004.45.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Thomadsen B. Achieving Quality in Brachytherapy. Madison, Wis: Institute of Physics Publishing; 2000. [Google Scholar]
- 21.Stillie AL, Kron T, Fox C, et al. Rectal filling at planning does not predict stability of the prostate gland during a course of radical radiotherapy if patients with large rectal filling are re-imaged. Clin Oncol (R Coll Radiol) 2009;21:760–767. doi: 10.1016/j.clon.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Heemsbergen WD, Hoogeman MS, Witte MG, et al. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 GY versus 78 Gy. Int J Radiat Oncol Biol Phys. 2007;67:1418–1424. doi: 10.1016/j.ijrobp.2006.11.014. [DOI] [PubMed] [Google Scholar]


