Abstract
Purpose
A number of studies have previously assessed the role of teaching interventions to improve organ-at-risk (OAR) delineation. We present a preliminary study demonstrating the benefit of a combined atlas and real time software based-feedback intervention to aid in contouring of OARs in the head and neck.
Methods and Materials
The study consisted of a baseline evaluation, a real-time feedback intervention, atlas presentation, and a follow-up evaluation. At baseline evaluation, 8 resident observers contoured 26 organs-at-risk on a computed tomography scan without intervention or aid. They then received feedback comparing their contours both statistically and graphically to a set of atlas-based expert contours. Additionally, they received access to an atlas to contour these structures. The resident observers were then asked to contour the same 26 organs-at-risk on a separate computed tomography scan with atlas access. In addition, 6 experts (5 radiation oncologists specializing in the head and neck, and 1 neuroradiologist) contoured the 26 organs-at-risk on both scans. A STAPLE composite of the expert contours was used as a gold-standard set for analysis of organs-at-risk contouring.
Results
Of the 8 resident observers who initially participated in the study, 7 completed both phases of the study. Dice Similarity Coefficients (DSCs) were calculated for each user-drawn structure relative to the expert STAPLE composite for each structure. Mean DSC across all structures increased between Phase 1 and Phase 2 for each resident observer demonstrating a statistically significant improvement in overall OAR-contouring ability (p < 0.01). Additionally, intervention improved contouring in 16/26 delineated organs-at-risk across resident observers at a statistically significant level (p ≤ 0.05), including all otic structures and suprahyoid lymph node levels of the head and neck.
Conclusions
Our data suggest that a combined atlas and real-time feedback-based educational intervention detectably improves contouring of OARs in the head and neck.
Introduction
In order to plan for IMRT, manual segmentation (contouring) of regions of interest (ROIs), either tumors or organs-at-risk (OARs), is performed by physician observers. As these ROIs serve as the input functions for all subsequent planning steps, accurate segmentation, leading to the proper voxel assignment of both tumors and organs-at-risk, is crucial to optimize therapeutic ratio. However, data shows there is a great degree of inter-observer variability in manual ROI segmentation.1 Both under- and over-contouring of tumors and OARs can have deleterious consequences, leading to local failure and normal tissue sequelae respectively. The importance of accurate manual segmentation and the high demonstrated inter-observer operator-dependence of this process indicate a specific and substantial impediment to execution of multi-institutional clinical trials involving conformal radiotherapy.2 Despite the requirement for accurate ROI delineation for radiation therapy treatment planning, instruction in target definition is often based on ad hoc instruction, with limited educational resources provided to many residents.3 Previous cooperative group studies involving practicing physicians suggest that reference to a simple anatomic atlas can substantially standardize and improve conformality of target volumes to an expert reference.4 Likewise, Bekelman5 and Tai6 have demonstrated educational interventions may improve trainee target definition.
Consequently, we sought to investigate the potential gain of a standardized atlas-based, software feedback-assisted intervention to improve head and neck OAR/ROI segmentation, having developed an open source on-line segmentation analysis software.7-9 The specific aims of the current study were:
Estimate potential improvement in OAR/ROI manual segmentation conformance with a multi-expert composite ROI attributable to a combined atlas/visual software-feedback educational intervention.
Validate utility of an open-source software solution for execution of said educational study.
Hypothesis-generation and sample size estimation for future prospective series.
Materials and Methods
Approval and Compliance
Institutional Review Board approval as an exempt, 45 CFR 46.101(b)(4)-compliant study was obtained, allowing collection of anonymized DICOM files. Clinical datasets were anonymized and stripped of identifiers, and fictionalized case histories were constructed for all cases.
Study Design
This single-arm pilot, prospective feasibility analysis was designed to determine the requisite sample and effect size required for a planned larger atlas-based software-feedback assisted effort. The study was designed as a test-retest sequence, with comparison to a “gold-standard” multi-expert composite ROI (Figure 1).
Figure 1.
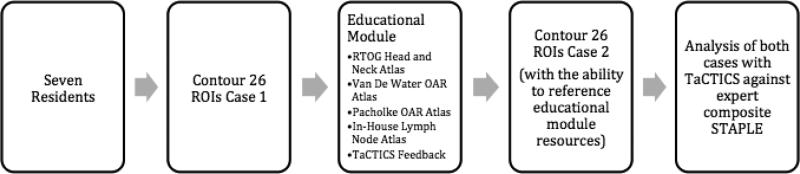
Study Design/workflow.
Software Utilization
For this study we utilized TaCTICS (Target Contour Testing/Instructional Computer Software, https://github.com/kalpathy/tacticsRT), which has been presented in detail previously.7-9 TaCTICS provides a data collection and analysis platform for manual or automated segmentation ROIs. TaCTICS is capable of collecting, displaying, and analyzing ROIs with multiple distinct metrics, and can generate multi-observer probabilistic composite ROIs using Warfield's STAPLE (Simultaneous Truth And Performance Level Estimation)10 methodology. This feature was used for the current study to create multi-expert estimation of a ground truth “gold-standard” ROIs. TaCTICS was also used to calculate Dice Similarity Coefficients (vide infra) for analysis of individual resident ROIs.
Observer Manual Segmentation/Educational Intervention
Eight resident observers were asked to contour all structures listed in Table 1 on axial CT images obtained from a patient with a head and neck malignancy, using their normal clinical practice (but without referring to any atlas). TaCTICS software7-9 was used for ROI submission.
Table 1.
List of 26 ROIs/OARs to contour grouped by category.
| Lymph Node Levels | Ear Structures |
| Left Level 1 | Left Cochlea |
| Left Level 2 | Right Cochlea |
| Left Level 3 | Left Middle Ear |
| Left Level 4 | Right Middle Ear |
| Left Level 5 | Left Vestibular Apparatus |
| Right Level 1 | Right Vestibular Apparatus |
| Right Level 2 | Salivary Glands |
| Right Level 3 | Left Parotid Gland |
| Right Level 4 | Right Parotid Gland |
| Right Level 5 | Left Sublingual Gland |
| Retropharyngeal Level | Right Sublingual Gland |
| Velar/Palatal Structures | Left Submandibular Gland |
| Lower Lip | Right Submandibular Gland |
| Upper Lip | |
| Soft Palate |
After contouring this case, the resident observers were asked to contour the same structures on a new case after an educational intervention. This intervention consisted of real-time feedback (within 5 minutes), as both numerical and visual DSC scoring of submitted ROIs (Supplemental Figure A). Feedback included axial slice-by-slice ROI comparisons with other submitted user ROIs (Supplemental Figure B), as well as two expert sets of contours defined as a “reference caution” (Supplemental Figure C), and a “reference flag” (Supplemental Figure D). Segmentations outside the “reference caution” indicated an ROI > 0.25 cm outside an atlas-based ROI contour for said OAR or > 0.5 cm outside an atlas-based ROI contour for atlas-based lymph node levels (conceptually equivalent to a clinical trial “minor deviation”). Segmentations outside the “reference flag” indicated ROIs > 0.5 cm outside an atlas-based ROI contour for an OAR, or > 1.0 cm outside atlas-based lymph node levels ROIs (conceptually a “major deviation”).
Simultaneously, observers were provided immediate access to relevant peer-reviewed reference atlases11-13 as well an in-house reference atlas (courtesy XXXXXXXXX, MD). Upon online submission of the second case ROIs, TaCTICS again provided feedback and metrics as described above.
Five expert head and neck radiation oncology attendings (XXX, XXX, XXX, XX, XXX) and one neuroradiologist (XX) were asked to manually segment the same OAR ROIs for both cases. Using Warfield's STAPLE10 methodology, a probabilistic estimate of ground truth segmentation of these contours was generated to create idealized “gold-standard” ROIs using TaCTICS. This multi-expert probabilistic composite ROI set was then used for all subsequent comparisons.
TaCTICS was used to calculate the Dice similarity coefficient (DSC) for all resident observer ROIs for all residents prior to the atlas/feedback intervention and after the intervention. The DSC is defined as:
where A represents each resident observer OAR ROI and G is the “gold-standard” multi-expert STAPLE contour. The DSC characterizes the intersection of the user with the reference STAPLE while penalizing observers for excessively large contours.14
Statistics
Statistical analysis was performed using the JMP software package. The Wilcoxon Signed-Rank test was used as a non-parametric measure to determine two outcomes. The first outcome that was assessed was whether the intervention improved a user's ability to contour a particular OAR ROI as measured by DSC as compared to a expert composite STAPLE of the same OAR ROI. The second outcome that was assessed was whether the intervention improved a user's ability to contour the set of all OAR ROIs. A non-Bonferroni-corrected confidence level of a = 0.05 was considered statistically significant for this hypothesis generating pilot study. Pre-study power and sample size calculations using G*Power15 were performed using a minimum asymptotic relative efficiency16 of the Wilcoxon Signed Rank test of 0.864 relative to its parametric equivalent, the paired t-test, to ensure an equivalent 1 - b = 0.80 using a large effect size (≥ 0.7) for seven users. Post-hoc evaluation of detected effect sizes for all OAR ROIs, again performed using G*Power15, demonstrated an evident mean±SD effect size (calculated as Cohen's D) of 0.77±0.32, broadly consistent with pre-study estimates.
Results
Feasibility
Seven resident observers completed both phases (assigned henceforth as Users A-G). All six experts contoured all 26 structures on both cases with two exceptions. One expert omitted contouring the right cochlea while another omitted right Level 2 on the second case. STAPLE composites were generated from all 6 expert contours excepting the two aforementioned OARs for which composites were generated from 5 expert contours. TaCTICS was used to analyze overlap of the structures and obtain DSCs for each resident observer for each structure relative to the STAPLE multi-expert composite ROIs. A summary of the DSCs by structure is seen in Table 2. Notable is the improvement in mean DSC for all resident observers for all OAR ROIs. Of note, in the initial phase, at least one resident observer was unable to contour the majority of the structures with any overlapping voxels relative to the expert composite.
Table 2.
Summary of DSC Across Structures for All Resident Observers
| Mean Resident DSC | Median Resident DSC | Range of Resident DSC | Wilcoxon Signed Rank | ||||
|---|---|---|---|---|---|---|---|
| Organ-at-Risk | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | (*p < 0.05) |
| Lymph Node Levels | |||||||
| Left Level 1 | 0.38±0.36 | 0.66±0.22 | 0.16 | 0.79 | 0.00-0.78 | 0.31-0.82 | 0.008* |
| Left Level 2 | 0.42±0.30 | 0.64±0.19 | 0.54 | 0.69 | 0.00-0.75 | 0.28-0.82 | 0.008* |
| Left Level 3 | 0.46±0.31 | 0.68±0.13 | 0.36 | 0.75 | 0.00-0.78 | 0.43-0.78 | 0.039* |
| Left Level 4 | 0.39±0.36 | 0.55±0.17 | 0.52 | 0.63 | 0.00-0.75 | 0.31-0.75 | 0.148 |
| Left Level 5 | 0.42±0.26 | 0.57±0.14 | 0.51 | 0.57 | 0.00-0.70 | 0.30-0.72 | 0.109 |
| Right Level 1 | 0.40±0.37 | 0.67±0.21 | 0.18 | 0.79 | 0.00-0.82 | 0.33-0.83 | 0.016* |
| Right Level 2 | 0.42±0.28 | 0.63±0.19 | 0.53 | 0.68 | 0.00-0.70 | 0.26-0.81 | 0.008* |
| Right Level 3 | 0.47±0.31 | 0.68±0.10 | 0.38 | 0.74 | 0.00-0.80 | 0.50-0.77 | 0.109 |
| Right Level 4 | 0.42±0.37 | 0.58±0.13 | 0.63 | 0.62 | 0.00-0.77 | 0.33-0.69 | 0.289 |
| Right Level 5 | 0.40±0.27 | 0.54±0.15 | 0.53 | 0.54 | 0.00-0.66 | 0.30-0.76 | 0.055 |
| RP Level | 0.27±0.20 | 0.40±0.17 | 0.33 | 0.48 | 0.00-0.45 | 0.04-0.52 | 0.016* |
| Ear Structures | |||||||
| Left Cochlea | 0.18±0.14 | 0.48±0.36 | 0.18 | 0.63 | 0.00-0.35 | 0.00-0.82 | 0.031* |
| Right Cochlea | 0.15±0.12 | 0.50±0.32 | 0.16 | 0.64 | 0.00-0.30 | 0.00-0.75 | 0.031* |
| Left Middle Ear | 0.28±0.28 | 0.52±0.22 | 0.15 | 0.54 | 0.00-0.67 | 0.07-0.72 | 0.039* |
| Right Middle Ear | 0.26±0.26 | 0.52±0.22 | 0.11 | 0.58 | 0.00-0.64 | 0.08-0.75 | 0.039* |
| Left Vestibular Apparatus | 0.02±0.03 | 0.42±0.32 | 0.00 | 0.45 | 0.00-0.07 | 0.00-0.81 | 0.031* |
| Right Vestibular Apparatus | 0.01±0.02 | 0.50±0.25 | 0.00 | 0.58 | 0.00-0.05 | 0.00-0.69 | 0.031* |
| Velar/Palatal Structures | |||||||
| Lower Lip | 0.30±0.10 | 0.31±0.13 | 0.26 | 0.31 | 0.20-0.50 | 0.12-0.52 | 0.469 |
| Upper Lip | 0.25±0.28 | 0.27±0.15 | 0.10 | 0.26 | 0.00-0.70 | 0.00-0.51 | 0.422 |
| Soft Palate | 0.47±0.29 | 0.49±0.25 | 0.63 | 0.53 | 0.00-0.76 | 0.00-0.70 | 0.344 |
| Salivary Glands | |||||||
| Left Parotid Gland | 0.76±0.17 | 0.85±0.05 | 0.81 | 0.84 | 0.39-0.87 | 0.77-0.91 | 0.023* |
| Right Parotid Gland | 0.77±0.09 | 0.85±0.06 | 0.81 | 0.87 | 0.58-0.84 | 0.74-0.91 | 0.016* |
| Left Sublingual Gland | 0.06±0.15 | 0.28±0.21 | 0.00 | 0.38 | 0.00-0.40 | 0.00-0.49 | 0.047* |
| Right Sublingual Gland | 0.07±0.18 | 0.29±0.17 | 0.00 | 0.33 | 0.00-0.49 | 0.10-0.57 | 0.039* |
| Left Submandibular Gland | 0.59±0.40 | 0.82±0.10 | 0.82 | 0.84 | 0.00-0.86 | 0.61-0.89 | 0.055 |
| Right Submandibular Gland | 0.64±0.40 | 0.84±0.08 | 0.86 | 0.86 | 0.00-0.89 | 0.66-0.91 | 0.234 |
Atlas Intervention with Real-Time Feedback Improves Contouring of Multiple Head and Neck OARs
Atlas-introduction with real-time software-based visual feedback demonstrated p < 0.05 for multiple candidate ROIs by Wilcoxon Signed-Rank Analysis (Table 2). Improved resident DSC conformance with expert ROIs was seen for otic structures (bilateral cochleae, bilateral middle ears, bilateral vestibular apparatuses), lymph node levels above the hyoid (bilateral levels 1 and 2 and the retropharyngeal space) as well as bilateral parotid and sublingual glands. Figure 2 illustrates the alteration in an individual resident observer's conformance with expert STAPLE ROIs for lymph node Level 1 after the atlas/software intervention. There was no significant improvement in DSC of the ROIs for the velar and palatal structures.
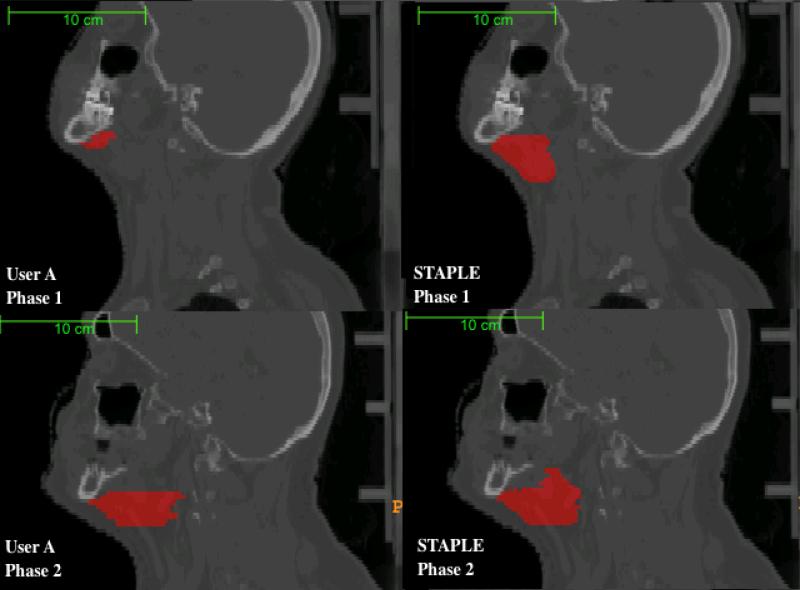
Figure 2.
Segmentation of Lymph Node Level I by User A relative to Expert STAPLE Composites. Phase 1 represents the initial segmentation, while Phase 2 represents the segmentation after intervention. Cranio-caudal contouring of Lymph Node Level I in Phase 2 is improved after atlas/feedback intervention.
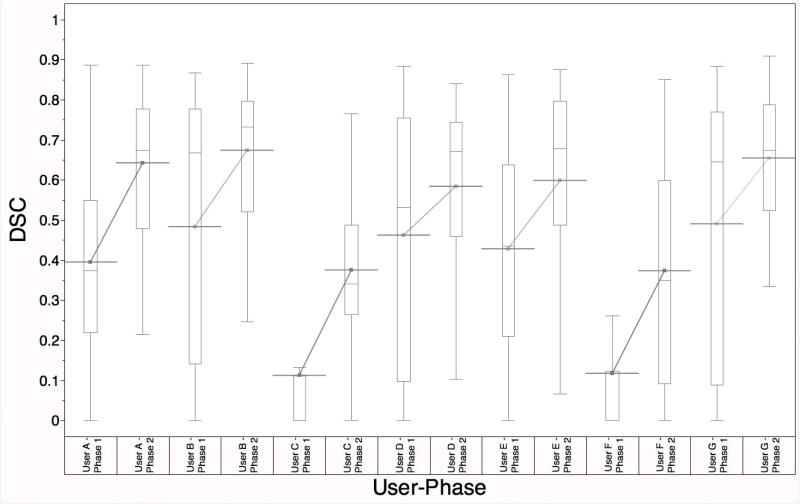
Additionally, the average user conformance with expert STAPLE ROIs across all ROIs improved after the intervention (p = 0.0078). Figure 3 shows the improvement in the mean DSC for each individual user across all 26 contoured structures.
Figure 3.
DSC distribution for Users A through G before and after the intervention, with improvement in mean DSC for each resident observer. (p = 0.0078)
The Benefit of Atlas Intervention May Relate to Level of Training and OAR Size
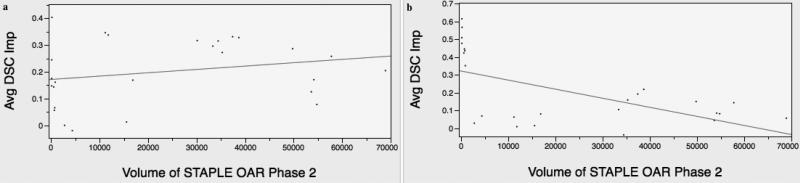
Post-hoc secondary analysis was performed to assess potential trends in resident observer OAR contouring experience and ROI DSC interval improvement post-intervention. Users A, B and G were “Upper level” third or fourth year radiation oncology residents. Users C and F were incoming PGY-1 residents matched to radiation oncology programs, and Users D and E were in their first two years of residency in radiation oncology, grouped as “Junior Level”. For both junior (p < 0.0001) and upper (p < 0.0001) level resident observers, there was a statistically significant mean DSC improvement in subgroup analysis. (Supplemental Figure E) Figure 4 demonstrates a plot of average change in DSC versus STAPLE composite OAR size from Phase 2 for Junior (Figure 4a, R2 = 0.06) and Upper (Figure 4b, R2 = 0.33) Level resident observers. This shows that for experienced trainee observers, there is demonstrable gain primarily in the contouring of small-volume OARs while for novice users the gain is seen across all OARs.
Figure 4.
Volume-dependent correlation was seen among upper level resident observers (4b), with significant improvement in low-volume OAR segmentation, while no correlation was noted for junior level observers (4a).
Discussion
The utility of atlas-based and other teaching interventions in improving contouring of ROIs has been addressed by a number of authors showing mixed effects. Our previous work demonstrated improvement in OAR segmentation when using an atlas-based intervention4. Here, we perform a prospective feasibility study to evaluate a combination of an atlas-based educational component with a real-time software feedback and visualization assessment, and also to estimate effect size and perform sample size calculation for a future larger scale directed atlas-based, software-assisted longitudinal effort planned with the goal of improving segmentation of difficult to contour organs-at-risk in the head and neck.
Our study demonstrated detectable improvement in overall contouring of OAR ROIs through the use of the aforementioned intervention. Cumulatively, resident observers improved their average DSC across all OAR ROIs demonstrating that through the use of our intervention, an individual's overall contouring of normal OAR ROIs more closely approximated expert observers. In a previous technical paper, we reported such interventions lead to more homogenous contours among trainees.8In toto, our intervention appears to improve both contouring uniformity, and accuracy as compared to an expert-derived “gold-standard”.
Our study also demonstrated OAR-specific differentials in contouring improvement with detectable improvement in suprahyoid lymph node level, otic, parotid and sublingual gland ROIs after the intervention. We suspect improvement in contouring of the suprahyoid and retropharyngeal lymph node levels is related to the complex anatomy of the upper neck. Thus, a more precise visual/atlas-based anatomical definition, as obtained from our intervention, is more readily characterized. A similar rationale applies to sub-centimeter otic structures and sublingual glands. Regarding the parotids, improved contouring was likely due to a novice error in neglecting to contour the deep lobe of the parotid gland. There was no improvement in contouring of the ROIs for the velar and palatal structures selected for this study.
Segmentation of head and neck ROIs is notoriously difficult as OARs are particularly small and confined to an anatomically complex region. Bekelman et al.5 examined the utility of a teaching intervention in contouring tumor ROIs in the head and neck. 14 residents segmented three CTVs on 6 CT slices of a single base-of-tongue case. The residents then underwent a series of oncology and anatomy seminars, including didactics and a hands-on sessions before recontouring. There was improvement in the node-negative neck ROIs, but difficulty remained in coverage of subclinical disease. These data, in concert with our findings, show training interventions have potential to improve head and neck segmentation.
Our web-based intervention provides an ideal mechanism for low-cost educational and clinical trial implementation. As an open-source online training system, radiation oncology departments need not invest additional educational funding, and clinical trialists may easily implement web-based training/credentialing programs for ROI quality-assurance. During this study, observers downloaded DICOM images into their treatment planning system, exported contours as an RTSTRUCT file from their treatment planning system, and uploaded these files for real-time feedback and analysis. We have since developed an entirely web-based ROI segmentation system to streamline this process. As this entire intervention was web-based, it provides a blueprint for a low-cost mechanism to train and credential future radiation oncologists in the segmentation of head and neck OAR ROIs.
Conclusions
The results of our study demonstrate that a combined atlas-based and real-time feedback intervention was associated with improved contouring of OAR ROIs in the head and neck, as defined by ROI conformance with a multi-expert gold-standard.
Supplementary Material
Acknowledgements
CDF received support from the American Society for Clinical Oncology Conquer Cancer Foundation Young Investigator Award, and the National Institutes of Health Clinician Scientist Loan Repayment Program (L30 CA136381). JKC received funding support from a National Institute of Health/National Library of Medicine grant (4R00LM009889). TaCTICS software was developed by JKC and CDF with support from the Society of Imaging Informatics in Medicine (SIIM) Product Development Grant. These funders played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication. The authors wish to gratefully thank Daniel Baseman, MD and Anna Harris, MD for their efforts and participation in the aforementioned study, and Prasanna Vibhute, MD for permission to use his in-house nodal atlas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests Notifications: CDF has served as a consultant to GE Medical Systems.
Preliminary clinical portions of this data were selected for a presentation at the ASTRO 2011 Annual Meeting, Miami Beach, FL, October 5, 2011 and early technical findings presented at the American Medical Informatics Association 2011 Annual Symposium, Washington, October 22, 2011.
References
- 1.Hong TS, Tomé WA, Harari PM. Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol. 1032012:92–98. doi: 10.1016/j.radonc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okunieff P, Kachnic LA, Constine LS, et al. Report from the Radiation Therapy Committee of the Southwest Oncology Group (SWOG): Research Objectives Workshop 2008. Clin. Cancer Res. 152009:5663–5670. doi: 10.1158/1078-0432.CCR-09-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik R, Oh JL, Roeske JC, Mundt AJ. Survey of resident education in intensity-modulated radiation therapy. Technology in cancer research & treatment. 2005 Jun;4(3):303–309. doi: 10.1177/153303460500400310. [DOI] [PubMed] [Google Scholar]
- 4.Fuller CD, Nijkamp J, Duppen JC, et al. Prospective randomized double-blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. International journal of radiation oncology, biology, physics. 2011 Mar 01;79(2):481–489. doi: 10.1016/j.ijrobp.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekelman JE, Wolden S, Lee N. Head-and-neck target delineation among radiation oncology residents after a teaching intervention: a prospective, blinded pilot study. International journal of radiation oncology, biology, physics. 2009 Mar 01;73(2):416–423. doi: 10.1016/j.ijrobp.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Li XA, Tai A, Arthur DW, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG Multi-Institutional and Multiobserver Study. International journal of radiation oncology, biology, physics. 2009 Apr 01;73(3):944–951. doi: 10.1016/j.ijrobp.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalpathy-Cramer J, Fuller CD. Target Contour Testing/Instructional Computer Software (TaCTICS): A Novel Training and Evaluation Platform for Radiotherapy Target Delineation. AMIA Annual Symposium ... 2010. [PMC free article] [PubMed]
- 8.Kalpathy-Cramer J, Bedrick SD, Boccia K. A pilot prospective feasibility study of organ-at-risk definition using Target Contour Testing/Instructional Computer Software (TaCTICS), a training and .... AMIA Annual ... 2011. [PMC free article] [PubMed]
- 9.Kalpathy-Cramer J, Awan M, Bedrick SD, Rasch CRN, Rosenthal DI, Fuller CD. Development of a Software for Quantitative Evaluation Radiotherapy Target and Organ-at-Risk Segmentation Comparison. Journal of Digital Imaging. 2012 doi: 10.1007/s10278-013-9633-4. In Submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE transactions on medical imaging. 2004 Jul;23(7):903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacholke HD, Amdur RJ, Schmalfuss IM, Louis D, Mendenhall WM. Contouring the middle and inner ear on radiotherapy planning scans. American journal of clinical oncology. 2005 May;28(2):143–147. doi: 10.1097/01.coc.0000143847.57027.16. [DOI] [PubMed] [Google Scholar]
- 12.van de Water TA, Bijl HP, Westerlaan HE, Langendijk JA. Delineation guidelines for organs at risk involved in radiation-induced salivary dysfunction and xerostomia. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009 Dec;93(3):545–552. doi: 10.1016/j.radonc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2003 Dec;69(3):227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Fotina I, Lütgendorf-Caucig C, Stock M, Pötter R, Georg D. Critical discussion of evaluation parameters for inter-observer variability in target definition for radiation therapy. Strahlenther Onkol. 1882012:160–167. doi: 10.1007/s00066-011-0027-6. [DOI] [PubMed] [Google Scholar]
- 15.Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007 doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 16.Feustal E, Davisson L. The asymptotic relative efficiency of mixed statistical tests. Information Theory. 1967 [Google Scholar]
- 17.Vorwerk H, Hess CF. Guidelines for delineation of lymphatic clinical target volumes for high conformal radiotherapy: head and neck region. Radiation oncology (London, England) 2011;6:97. doi: 10.1186/1748-717X-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasch C, Steenbakkers R, van Herk M. Target definition in prostate, head, and neck. Seminars in Radiation Oncology. 2005 doi: 10.1016/j.semradonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.