Abstract
Stroke, the number four cause of death in the United States, is a greatly debilitating event resulting from insufficient blood supply to the brain (cerebral ischemia). Endothelial dysfunction, primarily characterized by dampened endothelial- dependent vasodilation, is a major contributor to the development and outcome of stroke. This review discusses the role of soluble epoxide hydrolase (sEH), an enzyme responsible for the degradation of vasoprotective eicosatrienoic acids (EETs), in the context of the cerebral vasculature and its contribution to the sexual dimorphic nature of stroke.
Keywords: sEH, ischemia, endothelia, EET, brain, sexual dimorphism
Stroke is a sexually dimorphic disease
Stroke is a major cause of mortality and morbidity worldwide. According to the American Stroke Association, stroke is the number four cause of death with approximately 795 000 individuals suffering a stroke each year in the United States alone [1]. Studies have shown that premenopausal women are at a lower risk of stroke compared to men of the same age. However, the incidence of stroke in women increases rapidly following the onset menopause [2,3]. While the mechanism of this apparent sexual dimorphism and these changes in stroke risk in women following menopause is not fully understood, sex hormones are thought to play an important role. Estrogen, in particular, has been shown to have vasoprotective properties, promoting vasodilation, and as such is thought to be a major mechanism contributing to the protection from stroke enjoyed by premenopausal women [4, 5]. The effects of estrogen on cerebral infarct have been summarized in Table 1.
Table 1.
The effects of sex and estrogen on cerebral infarct in rodent models of stroke.
| Ischemic Model | Species | Sexes Examined | Intervention/ Genetic Modification | Outcome | Mechanism/Important Findings | References |
|---|---|---|---|---|---|---|
| 2 h MCAO + 22 h reperfusion | Rat | Female, male, OVX | WT & SHR-SP | Female rats (SHR-SP & WT) had ↓ infarct size & ↑ laser-doppler flow during ischemia compared to male & OVX. | Endogenous estrogen improves stroke outcome, likely via neuroprotection and CBF preservation. | Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, & Hurn PD (1998) |
| Permanent MCAO | Rat | Female | SHR-SP & Wistar-Kyoto females in different parts of estrous cycle: proestrus (high estradiol levels) & metestrus (low estradiol levels); | Females in proestrus had smaller infarct sizes in SHR-SP rats, but infarct volumes were similar at all estradiol levels in Wistar-Kyoto rats | Proestrus part of cycle is protective relative to metestrus in stroke prone animals | Carswell HV, Dominiczak AF, & Macrae IM (2000) |
| 2 h MCAO + 22 h reperfusion | Mouse | Female, male, OVX | WT & bcl-2 overexpressing mice | WT female had ↓ infarct size compared with WT male, bcl-2 overexpression was protective in male, but provided no added benefit in female; OVX ↑ infarct size in WT female, but not in bcl-2 overexpressors. | Estrogen ↑ expression of bcl-2, which is involved in ↓ neuronal injury after ischemia | Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, et al. (2001) |
| 90 min MCAO + 22 h reperfusion | Mouse | Female, OVX | WT & ArKO | ArKO mice had greatest amount of total ischemic damage; OVX WT had less injury than ArKO; WT treated with aromatase inhibitor had ↑ damage. | P450 aromatase is important to female neuroprotection. | McCullough LD, Blizzard K, Simpson ER, Oz OK, & Hurn PD (2003) |
| 2 h MCAO + 22 h reperfusion | Rat | OVX | E2 vs placebo in ERKO and WT rats | E2 ↑ Ang-1 mRNA in WT, but not ERKO mice | Estrogen ↑ angiogenesis and enhances capillary density in the brain | Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, & Hurn PD (2005) |
| 2 h MCAO + 22 h reperfusion | Mouse | Female, male, OVX | WT & nNOS KO mice; pharmacological inhibition of nNOS | ↑ infarct size in nNOS KO intact females consistent with WT female; ↓ infarct size in nNOS KO males. Similar results seen with pharmacological inhibition in WT animals. | NO toxicity in the brain is specific to females and eradicate estrogen protection | McCullough LD, Zeng Z, Blizzard KK., Debchoudhury I, & Hurn PD (2005) |
| 2 h MCAO + 6 or 24 h reperfusion | Rat | OVX | OVX rats with & without E2 | E2 ↑ CART expression & secretion in neuron & CART decreases ischemic area | CART is highly induced in cerebral cortex by estradiol under ischemic conditions & is protective | Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, Hurn PD, et al. (2006) |
| Permanent MCAO | Rat | Female, OVX | E2 vs vehicle pretreatment | Decreased edema and infarct volume in E2 treated rats | E2 attenuates stimulation of BBB Na-K-Cl cotransporter activity, thereby decreasing edema & infarct volume | O'Donnell M, Lam T, Tran L, & Foroutan S (2006) |
| Permanent MCAO | Mouse | OVX | OVX implanted with vehicle vs. E2, MCAO 1 wk later | E2 treatment ↑ number of newborn neurons in dorsal SVZ after ischemia | Estradiol stimulates neurogenesis | Suzuki S, Gerhold LM, Böttner M, Rau SW, Cruz C, Yang E, Zhu H, et al. (2007) |
| 2 h MCAO + 22 h reperfusion | Rat | OVX | Infarct size measured in E2-treated OVX female with & without STAT3 inhibitor cucurbitacin | E2 ↑ P-STAT3; inhibiting STAT3 ↑ infarct size after MCAO in E2 treated OVX rats | P-STAT3 is involved in estradiol-mediated neuroprotection | Dziennis S, Jia T, Rønnekleiv OK, Hurn PD, & Alkayed NJ (2007) |
| Permanent MCAO | Mouse | OVX | WT & iNOS-null OVX females +/- exogenous E2 | E2 ↓ infarct size in WT OVX; iNOS KO OVX female had smaller infarct size than WT, but E2 did not further protect | Estradiol ↓, NOS, iNOS deletion is neuroprotective in OVX & estrogen-replaced females | Brown CM, Cruz CD, Yang E, & Wise PM (2008) |
| 2 h MCAO + 24 h reperfusion | Mouse | Female, male, OVX | WT & sEHKO mice | Infarct size ↓, in WT female compared with WT male; protection abolished after OVX; no sex difference in sEHKO mice. | sEH is important mechanism underlying sex differences in ischemia; estradiol decreases sEH. | Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, & Alkayed NJ (2009) |
| 90 min MCAO + 22 h reperfusion | Mouse | Female, male | Aged mice were given acute vs. chronic ERT prior to MCAO | Female mice on chronic ERT demonstrated improved outcomes after MCAO; females that had acute ERT did not; males benefitted from ERT regardless of the timing. | Chronic ERT after reproductive senescence may be protective, and this may be related to downregulation of NF-Kß via estrogen. | Liu F, Benashski SE, Xu Y, Siegel M, & McCullough LD (2011). |
MCAO, middle cerebral artery occlusion; min, minute; ArKO, aromatase knockout; WT, wild-type; OVX, ovariectomized; KO, knockout; SHR-SP, stroke prone spontaneously hypertensive; CBF, cerebral blood flow; E2, 17ß-estradiol; NOS, nitric oxide synthase; iNOS, inducible nitric oxide synthase; SVZ, subventricular zone; Ang-1, angiopoietin-1; ERKO, estrogen receptor alpha knockout; STAT, signal transducer & activator of transcription; PSTAT3, phosphorylated STAT3; CART, cocaine- & amphetamine-regulated transcript; sEHKO, soluble epoxide hydrolase knockout; sEH, soluble epoxide hydrolase; ERT, estrogen replacement therapy; NF-Kß, nuclear factor-Kß
Utilizing rodent models, multiple groups have been able to demonstrate that sex differences exist in the response to experimentally- induced ischemic injury in the brain. These sex differences manifest both in terms of infarct volume, and also in more subtle readouts such as differences in cerebral blood flow. Early experiments using gerbils showed that females are less vulnerable to severe neurological sequelae following unilateral common carotid artery ligation than males [6]. Later studies, also carried out carried out in gerbils, showed that female survival 24 hours following unilateral carotid artery occlusion was significantly higher than in males, with males displaying more histological damage than females [7]. This was verified in a rat model which demonstrated that female rats sustain smaller cortical and striatal infarct volumes following middle cerebral artery occlusion (MCAO) compared to their age-matched male counterparts. This sex difference was attributed to endogenous estrogen as female ovariectomy, which equalized plasma estrogen levels between males and females, abolished this observed sex difference [8]. Differences in cerebral blood flow were also noted in this study. Following the ischemic insult, cerebral blood flow as measured by laser-Doppler flowmetry, was higher in females than males. This phenomenon was also abolished by ovariectomy. There was a strong correlation between blood flow and ischemic damage, however the sex difference in stroke outcome was attributed to the ability of estrogen to act both as a neuroprotectant and maintain increased blood flow.
Although many factors have been shown to contribute to this sex difference downstream of estrogen [4], this review will focus on the enzyme soluble epoxide hydrolase (sEH). Its broad role in stroke will be discussed as well as its sexually dimorphic effects on cell survival and blood flow regulation with particular emphasis on the cerebral vasculature.
Soluble epoxide hydrolase and eicosatrienoic acids
Soluble epoxide hydrolase (sEH), the product of the EPHX2 gene, is a cytosolic enzyme responsible for the metabolism, and thus termination of action, of epoxyeicosatrienoic acids (EETs) [9, 10]. EETs are epoxide derivates of arachidonic acid, formed by cytochrome P450 epoxygenases; there are four regioisomers of EETs, which correspond to the double bond positions in their parent molecule: 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET [11]. While the hydrolase activity of sEH is mainly responsible for the hydration of EETs into their corresponding dihydroxyeicosatrienoic acids (DHETs), in vitro studies have shown that beta- oxidation and chain elongation also contribute to the metabolism of EETs [12].
EETs have multiple cellular actions and are involved in many signaling pathways, and have been the subject of many reviews [13, 14, 15, 16]. Among the important biological functions that EETs perform, they have been shown to be potent vasodilators, and as such have been suggested to be an endothelium- derived hyperpolarizing factor (EDHF) [17, 18, 19]. Importantly for the study of stroke, they have also been shown to provide protection from ischemic injury, both in the heart and in the brain [20, 21, 22, 23]. In cell culture models, they have been shown to be protective to endothelial cells following both serum deprivation- and staurosporine- induced apoptosis in pulmonary and coronary derived cell lines [24], as well as being involved in the protective effect of hypoxic preconditioning in brain astrocytes [25].
sEH is widely distributed throughout the body; it is present in the liver, kidney, blood vessels and intestinal tissues. Of relevance to the study of the role of sEH in cerebral ischemia, it has been shown to be expressed in multiple cell types in the brain, where is it localized to both vascular and non-vascular compartments. Within the vasculature, it is present in both endothelial cells and in the vascular smooth muscle [26,27], consistent with its role in regulating the availability of vasodilatory EETs levels in the cerebral vasculature. In the cortical surface vasculature, sEH is highly expressed in the perivascular vasodilator nerve fibers which innervate the pial arteries [28]; it is thought that the function of sEH expression in this region is the neurogenic control of cerebral vasculature. We have also previously shown sEH to be highly expressed in neuronal cell bodies and processes; its expression in these cells may play a role in protection of the brain via non- vascular methods as demonstrated by studies using cultured neurons exposed to ischemic conditions [29,30]. sEH is also expressed in the glial cells of the brain [31].
Role of soluble epoxide hydrolase in blood flow and tissue injury
As already mentioned, EETs are potent vasodilators and potentiate endothelium-dependent dilation; it therefore follows that hydrolysis of EETs to DHET by sEH will diminish the vasodilator potential of EETs [32, 33]. This has been demonstrated in spontaneously hypertensive rats (SHR). Consistent with higher sEH expression in the SHRs, they exhibit increased EET hydrolysis compared to normotensive rats. Treatment of the rats with a selective sEH inhibitor (N,N'-dicyclohexylurea) decreases blood pressure of the SHR [34], demonstrating that manipulating sEH levels is successful in regulating blood pressure.
There have been numerous studies in the heart demonstrating a beneficial role of decreased sEH expression or activity following ischemia. Using isolated hearts from sEH-null mice, Seubert et al., [35] showed that functional recovery was improved following global ischemia and that infarct volumes were smaller compared to wild-type mice. Furthermore, perfusion with the 14,15-EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (EEZE), prior to ischemia abolished this cardioprotective phenotype observed in the sEH-null mice, indicating that sEH-null mice are protected due to increased EET availability as a result of lack of their metabolism by sEH. Similar results were reported by Motoki et al. [36] following transient left coronary artery occlusion using a pharmacological inhibitor of sEH, 12-(3-Adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA-BE). Wild type mice treated with AUDA-BE sustained milder injury, an effect that was abolished on administration of EEZE.
In the brain, we have also shown protection following ischemia after inhibition of sEH. Infusion of the sEH inhibitor AUDA-BE resulted in a reduced infarct volume following transient middle cerebral artery occlusion (MCAO) in mice [29]. This protection was prevented by a P450 epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH), which would therefore inhibit the formation of EETs. The protection observed by sEH inhibition was therefore, as in the heart, linked to increased EET levels. Further studies on the brain using sEH- null mice confirmed the effect seen with pharmacological inhibition of sEH. sEH- null mice displayed reduced infarct size compared to wild- type. These mice have been shown to have higher 14,15-EET levels in the blood; this protection was therefore attributed to the reduced metabolism of circulating EETs [37, 38].
While EETs are established vasodilators, it is difficult to determine the mechanism of the protective effect of sEH inhibition in vivo. The above study using AUDA-BE in the brain found that cerebral blood flow (CBF) during occlusion was not altered between the inhibitor and control groups [29]. This may indicate that the reduced infarct volume observed may be due to a non-vascular mechanism. However, this does not rule out that sEH inhibition may have improved blood flow during the reperfusion period. In fact the protection enjoyed by the sEH- null mice was attributed to an increased vasodilator capacity in response to MCAO [37]. It is likely therefore that both vascular and non-vascular mechanisms contribute to the deleterious effect of sEH on outcome following ischemia.
In vitro studies have allowed us to demonstrate that sEH inhibition is also protective via flow-independent cytoprotective modes. Cultured cortical neurons transduced with an sEH variant, carrying the Arg287Gln mutation resulting in decreased hydrolase activity, was found to be protective against ischemia- induced cell death [30]. As this was carried out in cultured neurons, this protection against ischemia is independent of any vascular mechanisms and therefore demonstrates a cytoprotective role. This neuroprotective role of impaired sEH activity has been further verified by in vivo studies using spontaneously hypertensive stroke- prone (SHRSP) and normotensive Wistar Kyoto (WKY) rats chronically treated with the sEH inhibitor AUDA and subjected to MCAO. Simpkins et al. [39] demonstrated, by microarray, that sEH inhibition modulates gene expression of mediators of apoptosis, with up-regulation of anti-apoptotic mediators and down-regulation of pro-apoptotic mediators in WKY and SHRSP rats respectively. They did however also observe a vascular mechanism of protection in SHRSP rats. These demonstrated vascular remodeling, including increased microvessel density, which likely contributed to the reduced infarct volume following MCAO despite a lack in blood pressure reduction.
All of the above studies demonstrate that sEH inhibition or knock-down in animal models is protective against experimental cerebral ischemia, both by vascular and non-vascular mechanisms. Interestingly, polymorphisms identified in the human population have been shown to lead to altered, either increased or decreased, risk of stroke depending on the nature of the variation in the sEH gene [40, 41, 42]. Unsurprisingly therefore, sEH modulation has been identified as a therapeutic target for the treatment of ischemic stroke [39, 43, 44].
Vascular actions of soluble epoxide hydrolase
As already mentioned, EETs are potent vasodilators and are involved in endothelium-dependent responses of the vasculature; those produced by the vascular endothelium function as an EDHF [13, 19]. Much work on the vasoactive properties of sEH and EETs has been carried out in the coronary vasculature. Early studies showed that EETs and DHETs are incorporated into the phospholipids of aortic endothelial cells [45]. Treatment of coronary arteries with the epoxide hydrolase inhibitor 4-phenylchalcone oxide (4-PCO) has been shown to enhance 14,15-EET potentiation of bradykinin- induced relaxation; this was linked to EET incorporation into cell lipids [32]. It was therefore thought that epoxide hydrolases play a role in regulating EET incorporation into phospholipids, thus modulating endothelial function. In fact, 4-PCO was shown to block EET conversion to their corresponding DHETs and enhanced EET incorporation into cell lipids [33]. This was confirmed using a selective inhibitor of sEH, N,N’-dicyclohexylurea (DCU), which also decreased EET metabolism to DHET and enhanced 14,15-EET incorporation into endothelial lipids in culture [12].
Endothelial dysfunction (ED) manifests as impaired endothelium- dependent vasorelaxation to stimuli such as acetylcholine (ACh) or shear stress and plays a role in the pathogenesis of diseases such as hypertension and type II diabetes. The effect of sEH inhibition on endothelial function has been investigated in diabetic db/db mice, diet induced obese mice and angiotensin II- induced hypertensive rats, animal models of cardiometabolic diseases in which ED is present [46]. Zhang et al. [46] found that inhibition of sEH, using 1-(1-acetyl-piperidine-4-yl)-3-adamantan-1-yl-urea (AR9281), improved the impaired response of mesenteric arteries to application of ACh in diabetic mice. No effect was observed on the endothelium- independent, sodium nitroprusside- induced, vasorelaxation. A similar phenomenon was observed in the obese mice in which impaired endothelium- dependent vasorelaxation in mesenteric arteries and in the aorta was restored. In the hypertensive rats, inhibition of sEH also restored aortic endothelium-dependent relaxation to naive levels. EETs have been shown to modulate vascular function in diabetic and obese rodents, and as such, these beneficial effects of sEH inhibition were attributed to increasing the concentration of EETs [47, 48, 49]. This study also demonstrated that, in the diabetic mice, this effect of sEH inhibition was evident even in the presence of nitric oxide and prostacyclin inhibitors (L-NAME and indomethacin), therefore suggesting that this an augmentation of an EDHF response in the mesentery.
Soluble epoxide hydrolase is a sexually dimorphic enzyme
sEH has been shown to be a sexually dimorphic enzyme, as summarized in Table 2, with higher expression levels observed in the liver and kidneys of male than female mice [50, 51]. The phenotype for male mice lacking the sEH gene, EPHX2, is therefore more pronounced than in females. The null mutation results in decreased blood pressure in males compared to their wild-type counterparts. Whereas in females, disruption of the gene exerts a minimal effect on blood pressure, consistent with lower basal levels of sEH in females than males [50]. The beneficial effects of sEH inhibition in rodent models of vascular disease discussed in the previous paragraph were all observed in male mice [46].
Table 2.
Sexual dimorphism of sEH expression and effect on outcome following infarct.
| Cell Type/ Model | Species | Sex Difference Observed | Reference |
|---|---|---|---|
| Cortical Neurons | Mouse, rat | ↑ sEH expression and EPHX2 mRNA in WT male | Fairbanks SL, Young JM, Nelson JW, Davis CM, Koerner IP, & Alkayed NJ (2012) |
| Brain Endothelial Cells | Mouse | ↑ EPHX2 mRNA in WT male, ↓ EETs levels in WT male compared with WT female; males with ↑ cell death after in vitro ischemia; sex differences abolished with pharmacological inhibition of sEH | Gupta NC, Davis CM, Nelson JW, Young JM, & Alkayed NJ (2012) |
| Cerebral Vessels | Mouse | ↑ sEH expression and EPHX2 mRNA in WT male | Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, & Alkayed NJ (2009) |
| Whole Kidney | Mouse | ↑ sEH expression in WT male | Chanas B, Wang H, & Ghanayem BI (2003) Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, & Gonzalez FJ (2000) |
| Whole Liver | Mouse | ↑ sEH expression in WT male | Chanas B, Wang H, & Ghanayem BI (2003) |
| Whole Brain | Mouse | ↑ sEH expression in WT male, ↑ EETs quantity in WT Female | Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, & Alkayed NJ (2009) |
| Whole Brain | Rat | Estradiol reduces basal and post-ischemic levels of sEH | Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, & Alkayed NJ (2008) |
| Whole Brain/ MCAO | Mouse | sEH-null males protected after MCAO (with infarct size similar to WT females), no additional protection observed in sEH-null females | Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, & Alkayed NJ (2009) |
| Whole Brain/ MCAO | Mouse | Tie2-sEH overexpression leads to ↑ infarct size in females, but no infarct size difference in males | Zhang, et al. (in review) |
Abbreviations: sEH: soluble epoxide hydrolase; EPHX2: gene encoding sEH; WT: wild-type; EETs: epoxyeicosatrienoic acids; MCAO: middle cerebral artery occlusion.
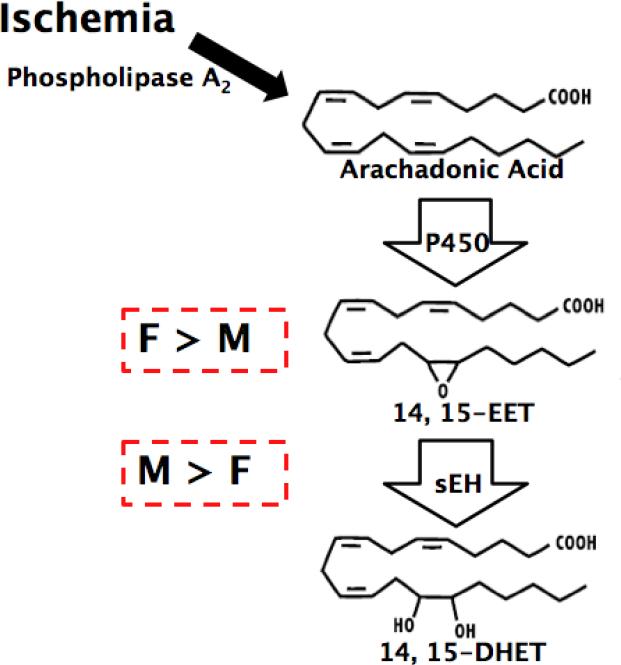
In the rodent brain, global sEH levels have also been shown to be sexually dimorphic [52], leading to differing responses to ischemic conditions, both in vivo and in vitro, between the two sexes. Female brains have a lower expression of sEH and thus decreased DHET but increased circulating EETs levels compared to males [52], as depicted in Figure 1. The protection afforded to females following ischemic insult discussed earlier has been attributed to these differences in sEH expression between the sexes. We have previously shown that cerebral blood flow during occlusion is higher in females than males and that females sustain smaller infarct volumes following MCAO [8, 52]. This sex difference is abolished in mice with targeted deletion of sEH, in which male infarct volume is reduced to female levels [52]. No protection is evident in sEH- null female mice compared to wild- type, presumably because sEH levels are already low in females. Similarly, laser-Doppler perfusion of sEH- null males increases to female levels, with no observable effect of the lack of sEH on female perfusion levels. While the differences in blood flow may account for the vascular effect of sEH on ischemic outcome, we have also observed that cultured male neurons, which have increased sEH expression than females, display increased cell death in response to ischemia indicating that non-vascular mechanisms may also contribute to the sex differences in infarct volume in vivo [53]. These studies therefore suggest that the difference in sEH expression between males and females, is a mechanism involved in the sex- linked differences in outcome following cerebral ischemia.
Fig. 1.
Schematic of sexual dimorphism of sEH and its effect on EETs levels in males and females.
Estrogen has been shown to regulate the expression of sEH, therefore contributing to its role in the sexual dimorphism observed following cerebral ischemia between males and females. Brain tissue from ovariectomized (OVX) rats has increased sEH protein levels compared to tissue taken from OVX rats with estrogen replacement, indicating that estrogen suppresses the expression of sEH [54]. In vivo studies show that following OVX, infarct volume after MCAO is increased to levels similar to those observed in males; OVX therefore effectively abolishes the protection enjoyed by females following cerebral ischemia presumably by increasing sEH levels [52]. Furthermore, the reverse phenomenon is observed in sEH- null mice in which infarct volume is comparable between males, intact females and OVX females; the lack of sEH not only reduces male infarct volume to female levels, but also reduces infarct in females following OVX to levels observed in intact females. Also, OVX decreases laser-Doppler flow levels to those observed in males; deletion of sEH reverses this effect to levels comparable to those in intact females. Estrogen therefore regulates sEH expression and is involved in the sEH-mediated sex differences following cerebral ischemic injury.
sEH expression, as well as being sexually dimorphic in whole brain extracts and cultured neurons, has been shown to be more robustly expressed in male than female cerebral vessels, also EPHX2 mRNA expression is higher in male than female cerebrovascular endothelial cells [43, 55]. Together with the effects of sEH and gender on cerebral blood flow during MCAO, these observations have sparked research into the role of sEH in vascular endothelial cells, both in vitro and in vivo, with the development of a transgenic mouse model with forced human sEH expression exclusively in endothelial cells, driven by the Tie2 promoter (Tie2-sEH) [56].
Surprisingly, in the heart, Edin and colleagues found that mice carrying the Tie2-sEH transgene sustained similar infarct volumes and left ventricular developed pressure (LVDP) following ischemia than wild-type controls in an isolated heart model [56]. However, these studies were carried out only in male mice and, as mentioned, male endothelial cells already express high levels of sEH. It would be interesting to ascertain whether the Tie2-sEH transgene would result in increased cardiac infarct size in female mice following ischemia- reperfusion injury. Using these same mice we have observed increased DHET plasma levels compared to wild-type (unpublished observations). In males, the Tie2-sEH mice also exhibit impaired endothelium- dependent increases in cerebral blood flow, but no observable difference was seen in infarct size following MCAO. Interestingly however, the presence of the Tie2-sEH transgene was detrimental to female mice following MCAO compared to wild-type, who exhibited significant increases in infarct volume in the caudate putamen (unpublished observations). Over-expression of sEH is therefore detrimental to females but not males following ischemic insult, this is the reverse of what is observed in the global sEH-null mice. A possible explanation for this is that the high levels of sEH in male endothelial cells already reach a threshold for the harmful effects on infarct following ischemia, whereas the lower sEH expression in females falls below this threshold, and so females are susceptible to increased damage with the increased sEH expression of the Tie2-sEH mice. These studies suggest that endothelial sEH, and thus EETs, are involved in the gender- specific response to ischemia.
Evidence generated in vitro using endothelial cultures supports this notion. Aortic- derived endothelial cells over-expressing the cytochrome P450 enzyme CYP2J2, which is involved in the generation of EETs from arachidonic acid, protects against hypoxic injury in vitro [57], demonstrating a cytoprotective effect of EETs on endothelial cell survival following ischemic insult. Brain endothelial cells also exhibit the sex difference in cell survival in response to ischemia. Female brain endothelial cells sustain a milder injury following anoxia and reperfusion than male cells in vitro, as measured by propidium iodide and cleaved caspase-3 labeling [55]. This has been attributed to the increased expression of EPHX2 mRNA, which encodes sEH, in males compared to females, corresponding to decreased EETs levels in males than females. To confirm the involvement of sEH, its inhibition in cultures using trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB) abolished the observed sex difference in endothelial cell death [55]. The function of EETs is therefore two-fold in the endothelium; they are involved in endothelium-dependent functions such as vasodilation, as well as being implicated in endothelial survival via cytoprotective mechanisms.
Summary
Premenopausal women are at lower risk of stroke than men of the same age. Rodent models demonstrate that sex differences are also present in outcome to experimentally-induced ischemia, with female gender being protective in terms of both infarct volume and preservation of blood flow. The sexually dimorphic nature of the enzyme sEH, and its influence on EETs levels, contributes to this sex difference. Experiments on ovariectomized and intact female rodents show that estrogen suppresses sEH expression, thereby increasing EETs levels. In vivo and in vitro studies indicate that increased sEH is detrimental to males via both vascular and non-vascular mechanisms. Regarding vascular mechanisms, inhibition of sEH alleviates endothelial dysfunction, as measured by improvement in endothelium- dependent vasodilation. sEH in the endothelial lining of cerebral vessels appears to be involved in endothelial- dependent vasodilation. Endothelial specific over-expression of sEH results in increased infarct volume in females but not in males in vivo, while inhibition of sEH in vitro abolishes the sex difference observed in endothelial cell death following ischemic insult. Endothelial sEH, and its effect on vessel integrity and function, therefore appears to be an important factor in the observed sex difference in outcome following cerebral ischemia, offering an attractive avenue for clinical intervention of stroke.
Acknowledgements
This work was supported by American Heart Association grant 10POST3630019 to CMD and by the Foundation for Anesthesia Education and Research (Rochester, MN, USA) FAER Research Fellowship Grant to SLF. The authors declare that they have no conflict of interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Appelros P, Stegmayr B, Terent A. Sex Differences in Stroke Epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 3.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–26. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2010;35(2):127–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–41. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry K, Wiśniewski HM, Svarzbein L, Baez S. On the relationship of brain vasculature to production of neurological deficit and morphological changes following acute unilateral common carotid artery ligation in gerbils. J Neurol Sci. 1975;25(1):75–92. doi: 10.1016/0022-510x(75)90188-4. [DOI] [PubMed] [Google Scholar]
- 7.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11(2):292–8. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 8.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-Linked Brain Injury in Experimental Stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 9.Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol. 1980;29(3):389–95. doi: 10.1016/0006-2952(80)90518-3. [DOI] [PubMed] [Google Scholar]
- 10.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat. 2007;82(1-4):42–9. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–62. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 12.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276(18):14867–74. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 13.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–95. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007;82(1-4):60–7. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci. 2007;28(9):448–52. doi: 10.1016/j.tips.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD, Simpkins AN, Renic M, Harder DR. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med. 2011;13:e7. doi: 10.1017/S1462399411001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem. 1992;58(2):503–10. doi: 10.1111/j.1471-4159.1992.tb09749.x. [DOI] [PubMed] [Google Scholar]
- 18.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259(4 Pt 2):H1171–7. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- 19.Davis CM, Siler DA, Alkayed NJ. Endothelium-derived hyperpolarizing factor in the brain: influence of sex, vessel size and disease state. Womens Health (Lond Engl) 2011;7(3):293–303. doi: 10.2217/whe.11.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, Hurn PD. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33(6):1677–84. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 21.Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291(2):H537–42. doi: 10.1152/ajpheart.00071.2006. [DOI] [PubMed] [Google Scholar]
- 22.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291(2):H517–31. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 23.Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68(1):18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Medhora M, Dhanasekaran A, Gruenloh SK, Dunn LK, Gabrilovich M, Falck JR, Harder DR, Jacobs ER, Pratt PF. Emerging mechanisms for growth and protection of the vasculature by cytochrome P450-derived products of arachidonic acid and other eicosanoids. Prostaglandins Other Lipid Mediat. 2007;82(1-4):19–29. doi: 10.1016/j.prostaglandins.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25(8):939–48. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- 26.Sura P, Sura R, Enayetallah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. J Histochem Cytochem. 2008;56(6):551–9. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem. 2004;52(4):447–54. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- 28.Iliff JJ, Close LN, Selden NR, Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol. 2007;92(4):653–8. doi: 10.1113/expphysiol.2006/036889. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27(12):1931–40. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27(17):4642–9. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163(2):646–61. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ Res. 1997;81(2):258–67. doi: 10.1161/01.res.81.2.258. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am J Physiol. 1999;277(5 Pt 2):H2098–108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- 34.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87(11):992–8. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 35.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99(4):442–50. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295(5):H2128–34. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39(7):2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282(5):2891–8. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174(6):2086–95. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, Boerwinkle E. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum Mol Genet. 2005;14(19):2829–37. doi: 10.1093/hmg/ddi315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Müller-Myhsok B, Wichmann HE, Meitinger T, Dichgans M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke. 2008;39(5):1593–6. doi: 10.1161/STROKEAHA.107.502179. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Ding H, Yan J, Hui R, Wang W, Kissling GE, Zeldin DC, Wang DW. Genetic variation in cytochrome P450 2J2 and soluble epoxide hydrolase and risk of ischemic stroke in a Chinese population. Pharmacogenet Genomics. 2008;18(1):45–51. doi: 10.1097/FPC.0b013e3282f313e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iliff JJ, Alkayed NJ. Soluble Epoxide Hydrolase Inhibition: Targeting Multiple Mechanisms of Ischemic Brain Injury with a Single Agent. Future Neurol. 2009;4(2):179–199. doi: 10.2217/14796708.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni GH, Chen JF, Chen XP, Yang TL. Soluble epoxide hydrolase: a promising therapeutic target for cardiovascular diseases. Pharmazie. 2011;66(3):153–7. [PubMed] [Google Scholar]
- 45.VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenases are incorporated into phospholipids of vascular endothelial cells. J Biol Chem. 1996;271(24):14001–9. doi: 10.1074/jbc.271.24.14001. [DOI] [PubMed] [Google Scholar]
- 46.Zhang LN, Vincelette J, Chen D, Gless RD, Anandan SK, Rubanyi GM, Webb HK, MacIntyre DE, Wang YX. Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension. Eur J Pharmacol. 2011;654(1):68–74. doi: 10.1016/j.ejphar.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Watkins JB, 3rd, Mangels LA. Hepatic biotransformation in lean and obese Wistar Kyoto rats: comparison to that in streptozotocin-pretreated Sprague-Dawley rats. Comp Biochem Physiol C. 1987;88(1):159–64. doi: 10.1016/0742-8413(87)90061-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, Jin L, Pollock JS, Webb RC, Imig JD. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R188–96. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Quigley JE, Yuan J, Wang MH, Zhou Y, Imig JD. PPAR-alpha activator fenofibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2006;290(6):H2187–95. doi: 10.1152/ajpheart.00937.2005. [DOI] [PubMed] [Google Scholar]
- 50.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275(51):40504–10. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 51.Chanas B, Wang H, Ghanayem BI. Differential metabolism of acrylonitrile to cyanide is responsible for the greater sensitivity of male vs female mice: role of CYP2E1 and epoxide hydrolases. Toxicol Appl Pharmacol. 2003;193(2):293–302. doi: 10.1016/j.taap.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J Cereb Blood Flow Metab. 2009;29(8):1475–81. doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fairbanks SL, Young JM, Nelson JW, Davis CM, Koerner IP, Alkayed NJ. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience. 2012;219:183–91. doi: 10.1016/j.neuroscience.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front Biosci. 2008;13:2833–41. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. ATVB. 2012;32(8):1936–42. doi: 10.1161/ATVBAHA.112.251520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, Foley JF, Torphy R, Ronnekleiv OK, Tomer KB, Lee CR, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25(10):3436–47. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60(2):310–20. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]