Abstract
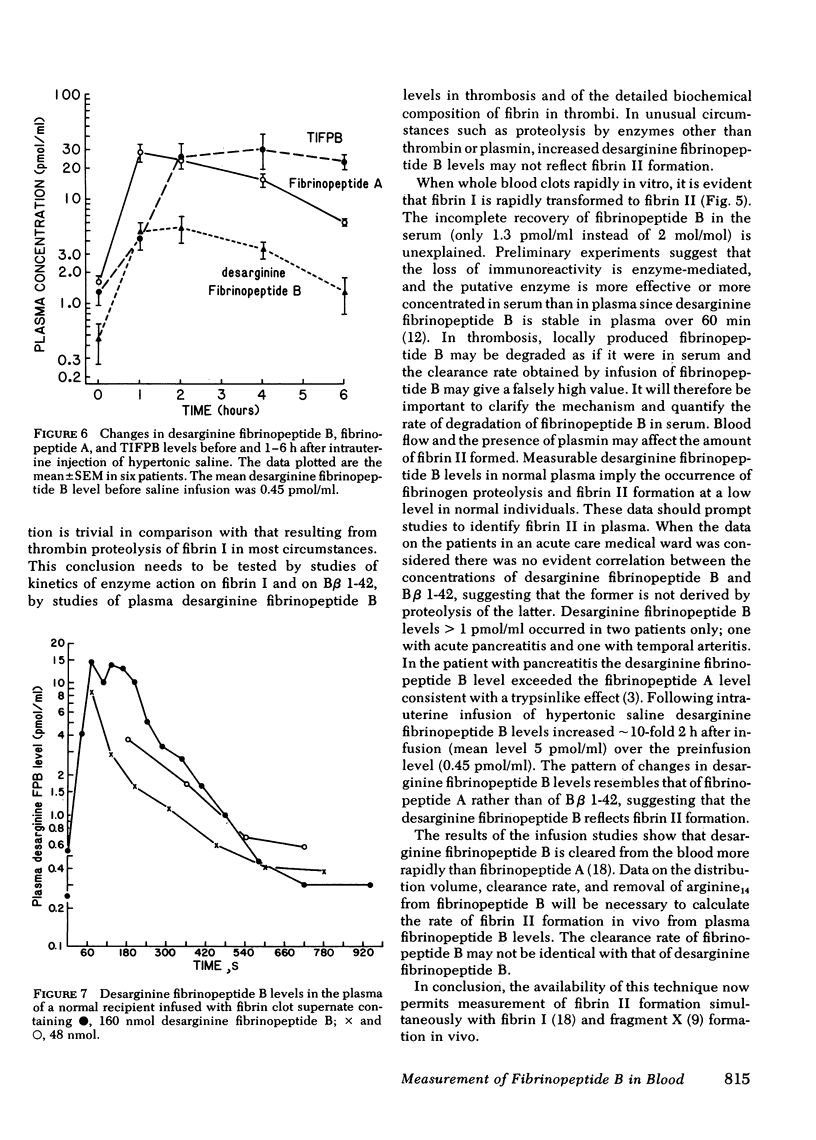
Thrombin converts fibrinogen to fibrin in two steps. First fibrinopeptide A and fibrin I are formed and then fibrinopeptide B (B beta 1-14) and fibrin II. Since it is postulated that fibrin II is important in the genesis of thrombosis, it is of interest to measure fibrinopeptide B in peripheral blood samples. Previous difficulties in interpreting fibrinopeptide B immunoreactivity in plasma resulted from crossreaction of fibrinogen and of plasmin digest peptides B beta 1-42 and B beta 1-21 and from rapid loss of fibrinopeptide B immunoreactivity resulting from cleavage of arginine 14 by blood carboxypeptidase B. We have obviated these difficulties by removing fibrinogen from plasma by precipitation with ethanol and peptides B beta 1-21 and B beta 1-42 by adsorption on bentonite. Fibrinopeptide B is then converted to a desarginine fibrinopeptide B, which is measured in a new specific assay. Studies of the kinetics of fibrinopeptide cleavage showed that when whole blood was allowed to clot in vitro, fibrinopeptide A was cleaved more rapidly than fibrinopeptide B. In 18 patients on an acute care medical ward, desarginine fibrinopeptide B levels were lower than fibrinopeptide A levels and did not correlate with the levels of fibrinopeptide A or B beta 1-42. Desarginine fibrinopeptide B levels were less than 1 pmol/ml in all but two patients. In six patients receiving intraamniotic infusions of hypertonic saline to induce abortion, desarginine fibrinopeptide B levels increased 10-fold from the preinfusion mean level of 0.4 pmol/ml and then decreased. The pattern of changes resembled that of the fibrinopeptide A levels rather than of the B beta 1-42 levels. On the basis of these data it is suggested that plasma desarginine fibrinopeptide B levels reflect fibrin II formation in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilezikian S. B., Nossel H. L., Butler V. P., Jr, Canfield R. E. Radioimmunoassay of human fibrinopeptide B and kinetics of fibrinopeptide cleavage by different enzymes. J Clin Invest. 1975 Aug;56(2):438–445. doi: 10.1172/JCI108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Edman P., Hessel B. Human fibrinopeptides. Isolation, characterization and structure. Biochim Biophys Acta. 1966 Feb 28;115(2):371–396. doi: 10.1016/0304-4165(66)90437-5. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z., Marder V. J., Shainoff J. R. Structure of plasmic degradation products of human fibrinogen. Fibrinopeptide and polypeptide chain analysis. J Biol Chem. 1974 Apr 10;249(7):2294–2302. [PubMed] [Google Scholar]
- La Gamma K. S., Nossel H. L. The stability of fibrinopeptide B immunoreactivity in blood. Thromb Res. 1978 Mar;12(3):447–454. doi: 10.1016/0049-3848(78)90315-8. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Umfleet R. A., Galanakis D. Human fibrinogen heterogeneities. I. Structural and related studies of plasma fibrinogens which are high solubility catabolic intermediates. J Biol Chem. 1972 Aug 25;247(16):5210–5219. [PubMed] [Google Scholar]
- Nossel H. L., Wasser J., Kaplan K. L., LaGamma K. S., Yudelman I., Canfield R. E. Sequence of fibrinogen proteolysis and platelet release after intrauterine infusion of hypertonic saline. J Clin Invest. 1979 Nov;64(5):1371–1378. doi: 10.1172/JCI109594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossel H. L., Yudelman I., Canfield R. E., Butler V. P., Jr, Spanondis K., Wilner G. D., Qureshi G. D. Measurement of fibrinopeptide A in human blood. J Clin Invest. 1974 Jul;54(1):43–53. doi: 10.1172/JCI107749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972 Feb 10;247(3):636–645. [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence studies on plasmin-derived fragments of human fibrinogen: amino-terminal sequences of intermediate and terminal fragments. Biochemistry. 1975 Mar 11;14(5):940–946. doi: 10.1021/bi00676a010. [DOI] [PubMed] [Google Scholar]
- Teger-Nilsson A. C. Degradation of human fibrinopeptides A and B in blood serum in vitro. Acta Chem Scand. 1968;22(10):3171–3182. doi: 10.3891/acta.chem.scand.22-3171. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Thomas D. W., Nossel H. L., Robbins P. F., Mudd M. S. Immunochemical analysis of rabbit antihuman fibrinopeptide B antibodies. Biochemistry. 1979 Nov 13;18(23):5078–5082. doi: 10.1021/bi00590a009. [DOI] [PubMed] [Google Scholar]


