Abstract
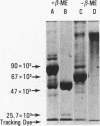
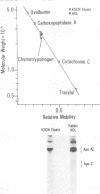
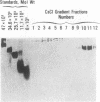
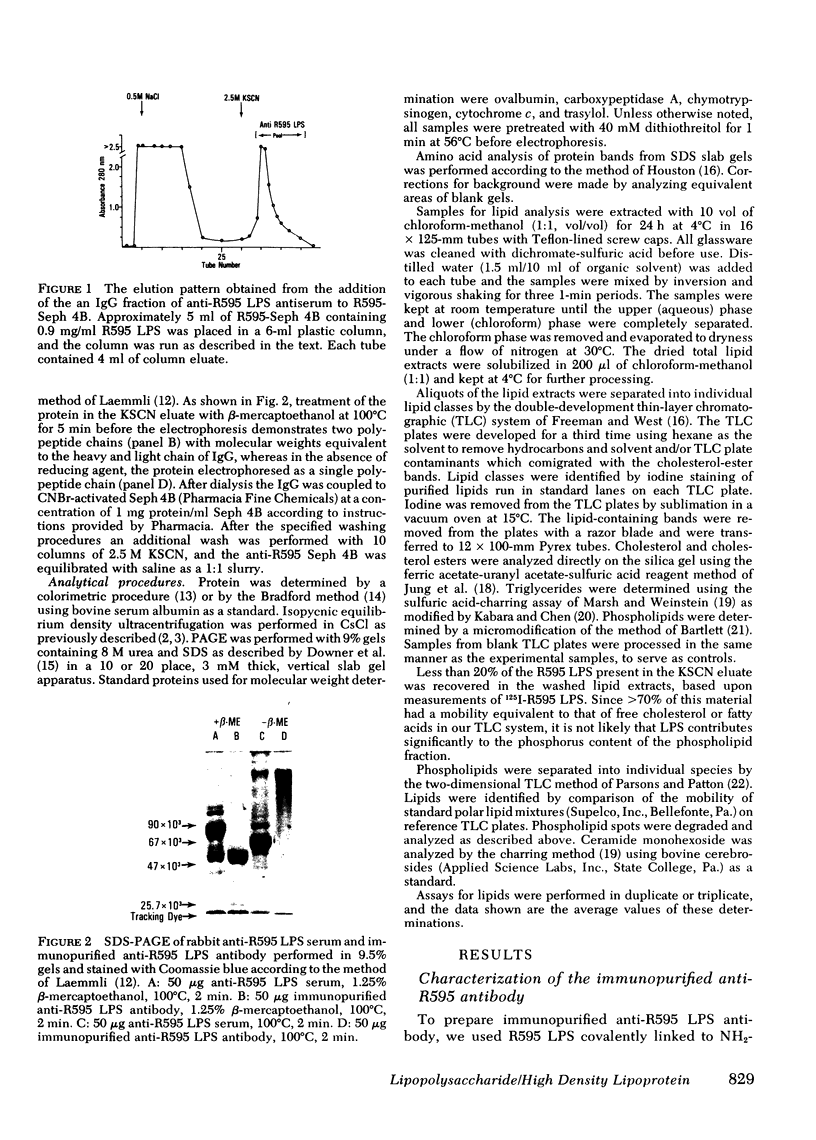
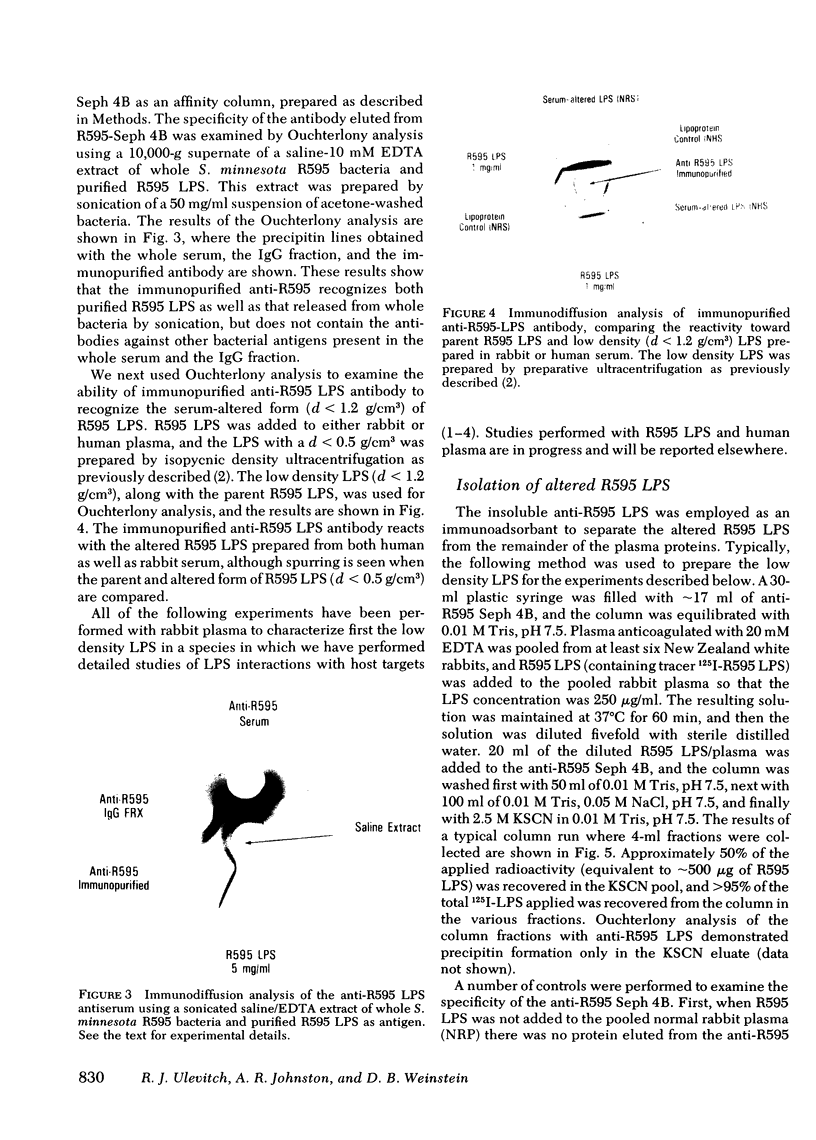
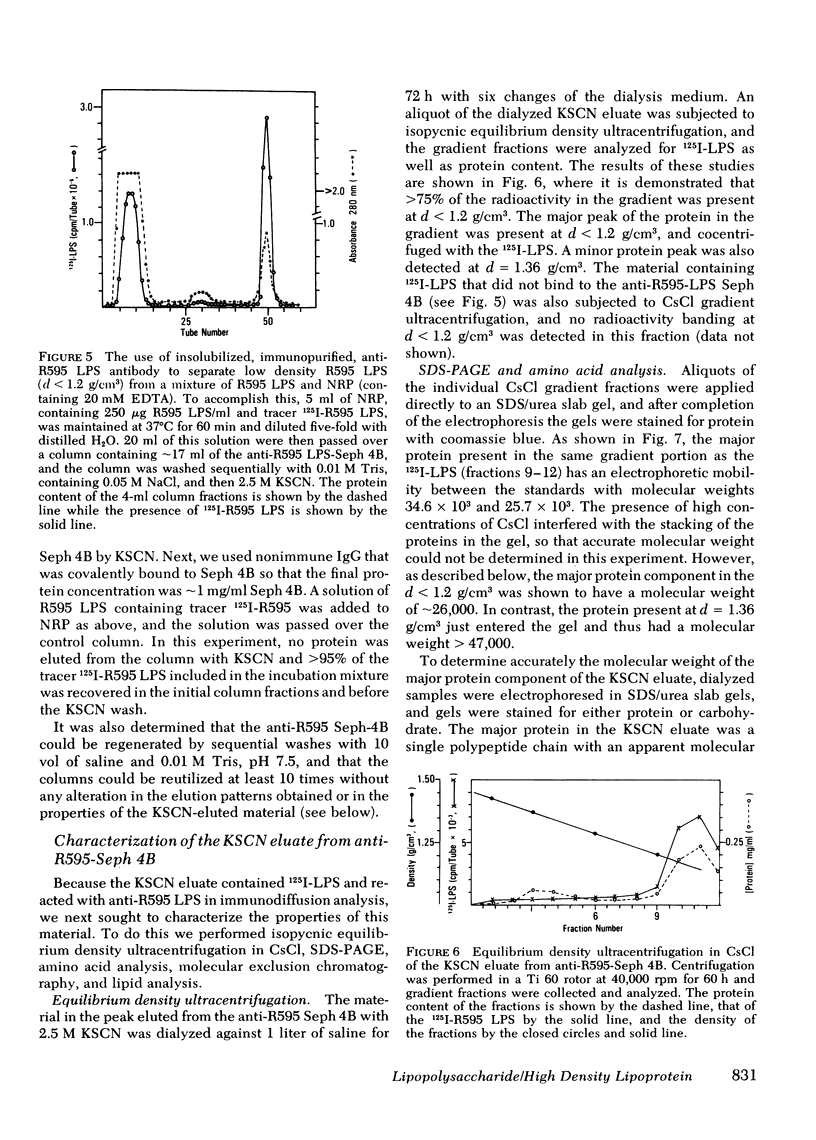
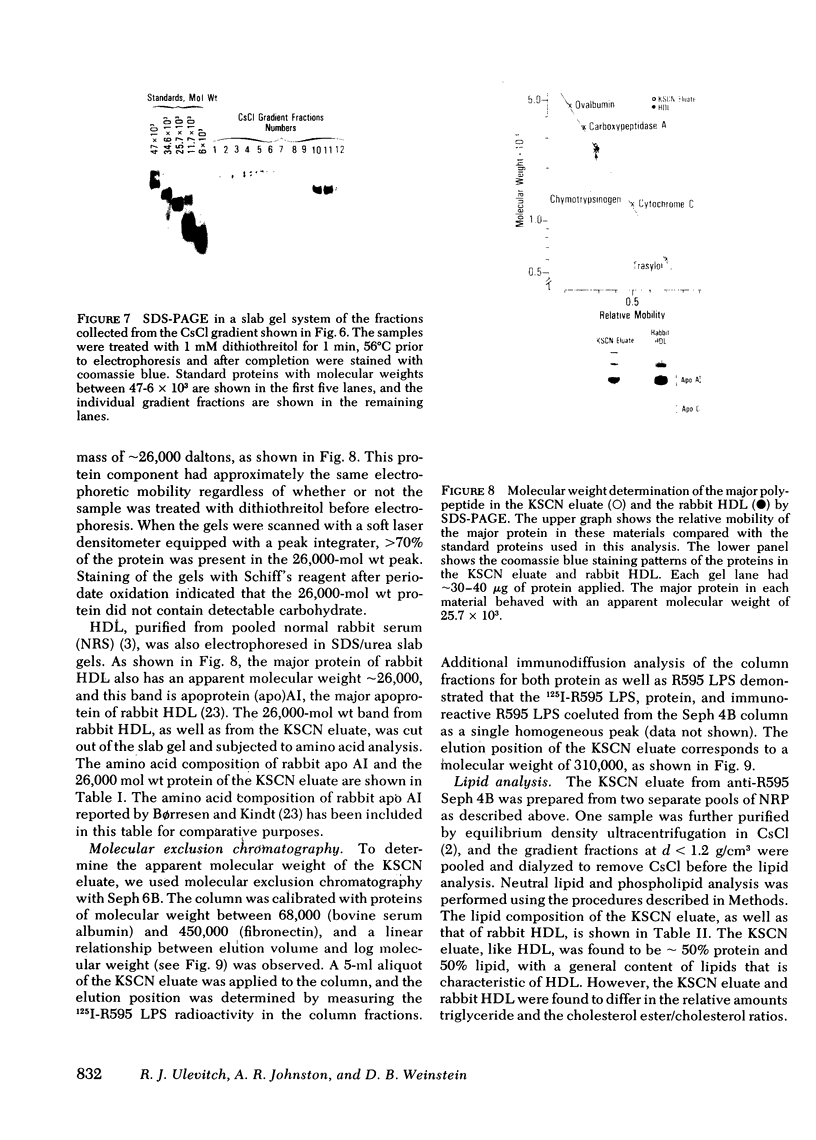
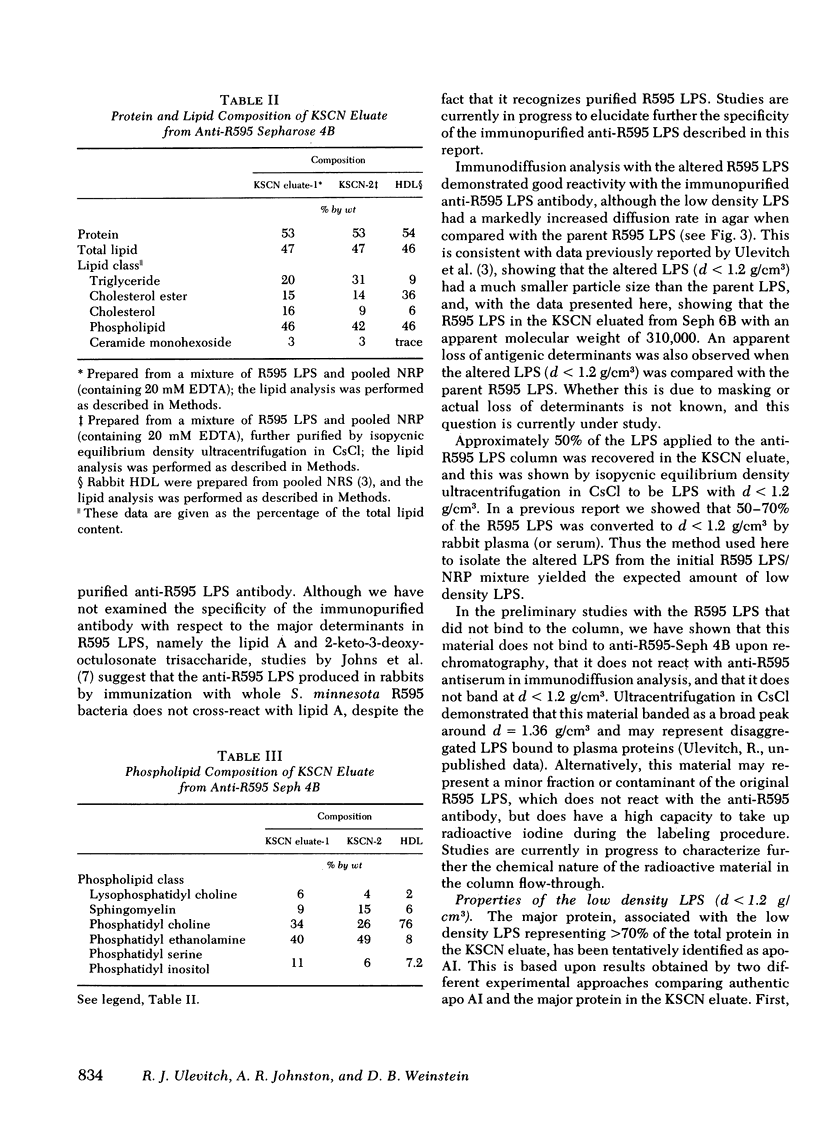
The addition of bacterial lipopolysaccharide (LPS) from Salmonella minnesota R595 to rabbit plasma results in a marked reduction of the hydrated buoyant density of the parent R595 LPS, from 1.38 to less than 1.2 g/cm3. Using immunopurified anti-R595 LPS antibody covalently linked to Sepharose 4B, we were able to separate the altered R595 LPS (d less than 1.2 g/cm3) from the remainder of the plasma proteins by elution of the bound material with 2.5 M KSCN. The KSCN eluate was shown to have a d less than 1.2 g/cm3 and to contain both R595 LPS as well as protein and lipid characteristic of high density lipoprotein (HDL). The major protein in the KSCN eluate is a single polypeptide chain with an apparent molecular weight of 26,000 in sodium dodecyl sulfate and an amino acid composition essentially identical to that of apoprotein AI, the major protein of rabbit HDL. The lipid composition of the KSCN eluate is similar to that of HDL, although marked differences in the cholesterol ester/cholesterol ration and the phosphatidyl choline/phosphatidyl ethanolamine ratio were observed when the KSCN eluate and rabbit HDL were compared. The formation of this R595 LPS-protein-lipid complex in plasma accounts for the marked reduction in buoyant density found when LPS is added to plasma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Børresen A. L., Kindt T. J. Purification and partial characterization of the apoA-I of rabbit high density lipoprotein. J Immunogenet. 1978 Feb;5(1):5–12. doi: 10.1111/j.1744-313x.1978.tb00625.x. [DOI] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- Chobanian J. V., Tall A. R., Brecher P. I. Interaction between unilamellar egg yolk lecithin vesicles and human high density lipoprotein. Biochemistry. 1979 Jan 9;18(1):180–187. doi: 10.1021/bi00568a027. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Houston L. L. Amino acid analysis of stained bands from polyacrylamide gels. Anal Biochem. 1971 Nov;44(1):81–88. doi: 10.1016/0003-2697(71)90348-4. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Bruins S. C., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Serological response to lipid A and the lipopolysaccharide of Re mutants. Infect Immun. 1977 Jul;17(1):9–15. doi: 10.1128/iai.17.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. H., Biggs H. G., Moorehead W. R. Colorimetry of serum cholesterol with use of ferric acetate/uranyl acetate and ferrous sulfate/sulfuric acid reagents. Clin Chem. 1975 Sep;21(10):1526–1530. [PubMed] [Google Scholar]
- Kabara J. J., Chen J. S. Microdetermination of lipid classes after thin-layer chromatography. Anal Chem. 1976 May;48(6):814–817. doi: 10.1021/ac60370a019. [DOI] [PubMed] [Google Scholar]
- Kim Y. C., Nishida T. Nature of the interaction of dextran sulfate with high and low density lipoproteins in the presence of Ca2+. J Biol Chem. 1979 Oct 10;254(19):9621–9626. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh J. B., Weinstein D. B. Simple charring method for determination of lipids. J Lipid Res. 1966 Jul;7(4):574–576. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Nicoll A., Miller N. E., Lewis B. High-density lipoprotein metabolism. Adv Lipid Res. 1980;17:53–106. doi: 10.1016/b978-0-12-024917-6.50008-2. [DOI] [PubMed] [Google Scholar]
- Nishikawa A. H., Bailon P. Affinity purification methods. Improved procedures for cyanogen bromide reaction on agarose. Anal Biochem. 1975 Mar;64(1):268–275. doi: 10.1016/0003-2697(75)90428-5. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Barclay M., Barclay R. K., Fetzer V. A., Good J. J., Archibald F. M. Lipid composition of human serum lipoproteins. Biochem J. 1967 Aug;104(2):340–352. doi: 10.1042/bj1040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney J. B. Characterization of the high-density lipoprotein and its major apoprotein from human, canine, bovine and chicken plasma. Biochim Biophys Acta. 1980 Mar 21;617(3):489–502. doi: 10.1016/0005-2760(80)90015-6. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Hogan V., Askinazi L., Small D. M. Interaction of plasma high density lipoproteins with dimyristoyllecithin multilamellar liposomes. Biochemistry. 1978 Jan 24;17(2):322–326. doi: 10.1021/bi00595a020. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Small D. M. Body cholesterol removal: role of plasma high-density lipoproteins. Adv Lipid Res. 1980;17:1–51. [PubMed] [Google Scholar]
- Tall A. R., Small D. M. Solubilisation of phospholipid membranes by human plasma high density lipoproteins. Nature. 1977 Jan 13;265(5590):163–164. doi: 10.1038/265163a0. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Polyacrylamide-protein immunoadsorbents prepared with glutaraldehyde. FEBS Lett. 1972 Jun 1;23(1):24–28. doi: 10.1016/0014-5793(72)80274-6. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
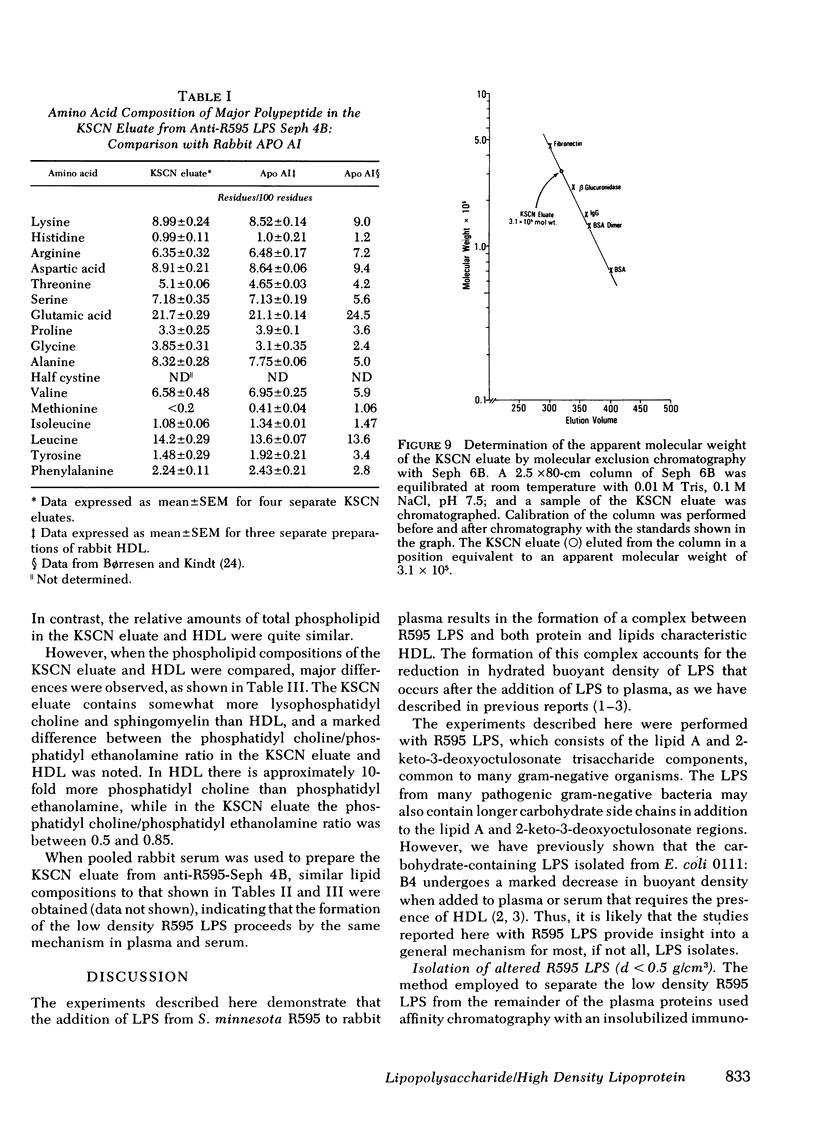
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]