Abstract
Rhodnius prolixus is the vector of Chagas’ disease, by virtue of transmitting the parasite Trypanosoma cruzi. There is no cure for Chagas’ disease and therefore controlling R. prolixus is currently the only method of prevention. Understanding the physiology of the disease vector is an important step in developing control measures. Crustacean cardioactive peptide (CCAP) is an important neuropeptide in insects because it has multiple physiological roles such as controlling heart rate and modulating ecdysis behaviour. In this study, we have cloned the cDNA sequence of the CCAP receptor (RhoprCCAPR) from 5th instar R. prolixus and found it to be a G-protein coupled receptor (GPCR). The spatial expression pattern in 5th instars reveals that the RhoprCCAPR transcript levels are high in the central nervous system, hindgut and female reproductive systems, and lower in the salivary glands, male reproductive tissues and a pool of tissues including the dorsal vessel, trachea, and fat body. Interestingly, the RhoprCCAPR expression is increased prior to ecdysis and decreased post-ecdysis. A functional receptor expression assay confirms that the RhoprCCAPR is activated by CCAP (EC50 = 12 nM) but not by adipokinetic hormone, corazonin or an extended FMRFamide. The involvement of CCAP in controlling heartbeat frequency was studied in vivo and in vitro by utilizing RNA interference. In vivo, the basal heartbeat frequency is decreased by 31% in bugs treated with dsCCAPR. Knocking down the receptor in dsCCAPR-treated bugs also resulted in loss of function of applied CCAP in vitro. This is the first report of a GPCR knock-down in R. prolixus and the first report showing that a reduction in CCAPR transcript levels leads to a reduction in cardiac output in any insect.
Introduction
Neuropeptides and their receptors are vital components in insects since they regulate physiological and behavioral processes associated with development, reproduction and metabolism. Targeting the ligand-receptor interactions is an important strategy for developing therapeutics/pest control agents in the pharmaceutical and agrochemical industries [1]–[6]. These might be used as potential agonist or antagonist of the receptor by interfering with normal functioning within the animal. One such insect for which control is required is the blood-feeding bug, Rhodnius prolixus, which is the vector of Chagas’ disease in Central and South America.
Crustacean cardioactive peptide (CCAP) is a cyclic nonapeptide that is conserved in arthropods. CCAP was first isolated as a cardioaccelerator from the pericardial organs of the shore crab, Carcinus maenas [7], [8]. Later, it was biochemically isolated from the nervous system of insects [9], [10]. The important functions of CCAP in insects have been well documented. For example, CCAP triggers increasing heart rate in Manduca sexta and Drosophila melanogaster [11], [12], controls and regulates ecdysis behavior by modifying central motor programs in M sexta and D. melanogaster [13], modulates oviduct contractions in Locusta migratoria [14], [15], and regulates enzyme secretion in Periplaneta americana [10].
The receptor for CCAP is a G-protein coupled receptor (GPCR). G-protein coupled receptors are important signal transducing receptors since they mediate the effects of many neuropeptides [2], [16]–[18]. GPCRs typically contain 7 alpha-helical transmembrane segments. They can transduce extracellular signals into cellular physiological responses through the activation of a heterotrimeric G protein [2], [16]–[18]. CCAP receptors have been cloned in several insect species [19]–[21]. In D. melanogaster, the CCAP receptor is expressed in all developmental stages, with the highest expression in adult heads [19]. Recently, CCAP receptor expression was observed in all developmental stages of the mosquito with a peak in second instar larvae and pupae [22]. In Tribolium castaneum, two CCAP receptors have been isolated, CCAPR1 and CCAPR2, and only CCAPR2 is essential for eclosion behaviour [21].
In the current study, we have isolated the cDNA sequence of the CCAP receptor in the medically-important blood-gorging bug, Rhodnius prolixus. The R. prolixus CCAP receptor (RhoprCCAPR) was cloned from a CNS cDNA library and in a functional assay shown to be activated by CCAP with an EC50 value of 12.2±1.1 nM. Quantitative real-time PCR (qPCR) analysis revealed spatial expression patterns of RhoprCCAPR in the CNS as well as peripheral tissues of 5th instar R. prolixus. The RhoprCCAPR transcript level increased prior to ecdysis and decreased post-ecdysis. We have also confirmed the cardioacceleratory functions of CCAP in the adult male R. prolixus and demonstrated that its cardioacceleratory effect is abolished when the RhoprCCAPR transcript is knocked down by RNA interference (RNAi).
Materials and Methods
Animals
The R. prolixus was obtained from a colony that is fed once during each instar with defibrinated rabbit blood (Hemostat Laboratories, Dixon, CA, USA; supplied by Cedarlane, Burlington, ON, Canada). Gorging is the stimulus for growth and development to the next instar. The instars used in these experiments were allowed to gorge and then held in an incubator at 30% humidity and 28°C in a 16 h:8 h light/dark cycle.
Isolation of the RhoprCCAPR Transcript from 5th Instar R. prolixus
The sequence of RhoprCCAP receptor was aligned with the corresponding D. melanogaster (AAO66429), Aedes aegypti, (XP_001659389), T. castaneum (ABN79651, ABN79652), and Apis mellifera (XP_001122652) receptors using Clustal W [23]. Conserved regions of amino acid sequences were used in a TBLASTN search against the complete R. prolixus genome database. The contig was constructed and the putative CCAP receptor encoding the nucleotide sequence was obtained. Based on the predicted CCAP receptor sequences, gene specific primers (GSP) were designed (Table 1A). The procedure of cloning the CCAPR cDNA sequence has been performed as previously described [24]. All PCR conditions were identical: 5 min at 95°C, 30 sec at 94°C, 30 sec at 57°C, 60 sec at 72°C and 10 min at 72°C.
Table 1. Gene-specific primers (GSP) for the CCAP receptor in R. prolixus.
| A) Oligo name | Oligo Sequence (5′ to 3′) |
| 5′ RACE primers | |
| CCAPR_FOR1 | AGCACTGGATAATGGACTGG |
| CCAPR_FOR2 | AGCATTTGCAGATTTATCAGTTG |
| CCAPR_FOR3 | ATCGTCTGGATGCAATTACAAG |
| CCAPR_FOR4 | GGTGGAGATAAGGGAGATGAC |
| CCAPR_FOR5 | CCACGTTTATTCAAAGTCTTGC |
| 3′ RACE primers | |
| CCAPR_REV1 | GATTATCTTGGTTACGAATAGTGG |
| CCAPR_REV2 | GGC TAC TGC GAT ATT TGT TTG AG |
| CCAPR_REV3 | TGT CAT CTC CCT TAT CTC CAC C |
| CCAPR_REV4 | TCCATTCAACCGTGATCC |
| CCAPR_REV5 | CCC AAA ACA ATC GCT GC |
| B) Oligo name | Oligo Sequence (5′ to 3′) |
| CCAPR specific primer for qPCR | |
| qPCR _CCAPR_F1 | GCTTAGCACTGGATAATGGACTG |
| qPCR _CCAPR_R1 | TCAATACGCTGATCAGTCCAACT |
| Reference genes for qPCR | |
| qPCR _Actin F1 | AGAGAAAAGATGACGCAGATAATGT |
| qPCR _Actin R1 | CGGCCAAATCCAATCG |
| qPCR _RP48_F1 | GTGAAACTCAGGAGAAATTGGC |
| qPCR _RP48_R1 | GCATCATCAACATCTCTAATTCCTTG |
| C) Oligo name | Oligo Sequence (5′ to 3′) |
| Primers to amplify full ORF | |
| CCAPR_ORF_F1 | TTAGCACTGGATAATGGACTGG |
| CCAPR_ORF_F2 | ATGGACTGGGTTATAAGAGATAATTAC |
| CCAPR_ORF_R1 | CTATTCGTAACCAAGATAATCTCTAAATG |
| CCAPR_ORF_R2 | CACTATTCGTAACCAAGATAATCTCTAA |
| Primers for introduce Kozak sequence | |
| Kozak_CCAPR_ORF_F2 | GCCACC ATGGACTGGGTTATAAGAG |
(A) Primers used for 5′ and 3′ rapid amplification of cDNA ends (RACE). (B) Transcript specific primers for real time PCR for the spatial expression profile. (C) Primers for cloning the complete open reading frame (ORF), and for introducing a Kozak translation initiation sequence at the 5′UTR.
Sequences Analysis
The amino acid sequence of RhoprCCAPR and that of other insect CCAP receptors (Anopheles gambiae AnogaCCAPR1, AGAP001961; AnogaCCAPR2, XP_321100.4; A. aegypti AedaeCCAPR1, XP_001659389.1;AedaeCCAPR2, XP_001659388.1; Culex pipiens CulpiCCAPR1, CPIJ006268; CulpiCCAPR2, XP_001847670.1; Culex quinquefasciatus CulquCCAPR, XP_001847670; D. melanogaster DromeCCAPR, AAO66429.1; D. virilis DroviCCAPR, GJ23325; D. mojavensis DromoCCAPR, GI22912; T. castaneum TricaCCAPR1, ABN79651; TricaCCAPR2, ABN79652; A. florea ApiflCCAPR, Predicted XP_003691184; A. mellifera ApimeCCAPR, XP_001122652.2; Bombyx impatiens CCAPR, Predicted XP_003494126; Megachile rotundata MegroCCAPR, Predicted XP_003700512; Nasonia vitripennis NasviCCAPR, XP_001602277.1; Acyrthosiphon pisum AcypiCCAPR, Predicted XP_003245097, B. mori BommoCCAPR1, NP_001127724.1; BommoCCAPR2, NP_001127746.1) were aligned using Clustal W [23]. Also, the amino acid sequence of RhoprCCAPR was aligned with that of the putative AKH receptor in A. pisum, D. melanogaster and B. mori, as well as the putative corazonin receptor in R. prolixus. The aligned arthropod sequences which were either identical or similar to the consensus sequence, were colored with black or gray, respectively, by using the BOXSHADE 3.21 server (http://www.ch.embnet.org/software/BOX form.html). Phylogenetic analysis of the aligned sequences was produced using Molecular Evolutionary Genetics Analysis (MEGA) (version 4.0.4) [25].
Preparation of the RhoprCCAPR Construct
The coding region of RhoprCCAPR (1,128 bp) (RhoprCCAPR) was amplified from unfed 5th instar CNS cDNA with gene specific primers (Table 1C) using a Q5 High fidelity DNA polymerase (New England Biolabs, Pickering, ON). At the 5′ end before the ATG initiation codon, the Kozak translation initiation sequence (3′ ACCATG-5′) was introduced since this is required for optimal translation by eukaryotic ribosomes (Table 1C) [26]–[28]. The PCR product was run on a 0.8% agarose gel for 40 mins (160V) and the correct size of band was excised and purified (Promega, Madison, WI, USA). This was then subcloned into the pGEM T easy vector (Promega, Madison, WI) to verify its sequence. The insert with the Kozak sequence was excised using restriction enzyme (NotI) and subcloned into the expression vector, pcDNA™3.1(+) (Invitrogen, Carlsbad, CA) to express in Chinese hamster ovary (CHO-K1) WTA11, mammalian cells (Euroscreen S. A., Belgium; provided by Prof. Dr. M. Parmentier and Dr. M. Detheux; Brussels, Belgium).
Functional Analysis of CCAP Receptor
RhoprCCAPR was transiently expressed in CHO-WTA11 cells that stably express promiscuous Gα16 and apoaequorin. This cell line has been used extensively for insect GPCR functional assays [21]. The transfection was performed using X-treme HP DNA transfection reagent (Roche Applied Science, Indianapolis, IN) with the ratio of 3 (transfection reagent) to 1 (RhoprCCAPR/pcDNA™3.1(+) or empty pcDNA™3.1(+) vector) according to the manufacture’s protocol (Roche Applied Science, Indianapolis, IN). Cells were grown in the complete Dulbecco’s Modified Eagle Medium Nutrient Mixture F12-Ham (DMEM/F12, 10% fetal bovine serum, FBA, 100 IU/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA)) in the 5% CO2 37°C incubator. After 48 hours, transfected cells were incubated with 5 µM Coelentarazine for 4 hours (Invitrogen, Carlsbad, CA) at room temperature in the dark. Interaction between ligand and the cloned RhoprCCAPR leads to a bioluminescent response that is mediated by the IP3/Ca2+ signaling pathway. The luminescence assay was performed on opaque 96-well microplates (Corning, Lowell, MA). 50 µl of cells were introduced into each well of the 96-well plate that contained either controls or different concentration of peptides (RhoprCCAP, RhoprCorazonin, RhoprAKH and GNDNFMRFa) (Table 2) and the changes of luminescence were recorded for 20 sec. Wells only containing DMEM/0.1% BSA medium served as a negative control, while wells containing 50nM ATP served as a positive control. All luminescence values were corrected for background values from wells containing only DMEM/BSA medium and all values were calculated as maximum percentage difference. The response for each ligand concentration in replica wells and from at least three replica plates was averaged for analysis. GraphPad prism 5 (version 5.03) was used to analyze and generate the data.
Table 2. Summary of peptides tested on the CCAP functional receptor expression assay.
| Peptide Name | Sequence | EC50 (M) RhoprCCAPR |
| RhoprCCAP | PFCNAFTGC-NH2 | 12.2×10−9 |
| RhoprAKH | pQLTFSTDW-NH2 | Not active |
| Corazonin | pQTFQYSRGWTN-NH2 | Not active |
| ExtendedFMRFa | GNDNFMRF-NH2 | Not active |
cDNA Synthesis from Various Tissues
CNS (brain, sub-oesophageal ganglion, prothoracic ganglion, mesothoracic ganglionic mass and stretches of abdominal nerves) and peripheral tissues (salivary glands, foregut, anterior midgut, posterior midgut, hindgut, Malpighian tubules, pool of tissues including dorsal vessel/trachea/fat body, female reproductive tissue and male reproductive tissue) were extracted from 4th, 5th instar or male adult R. prolixus in physiological saline (NaCl, 150 mmol L−1, KCl, 8.6 mmol L−1, CaCl2, 2 mmol L−1, Glucose, 34 mmol L−1, NaHCO3, 4 mmol L−1, MgCl2, 8.5 mmol L−1, HEPES, 5 mmol L−1, pH 7.0) and stored in RNA later solution (Ambion, Austin, TX). Total RNA was isolated from tissues using the Trizol® reagent (Ambion, Austin, TX) according to the manufacturer’s protocols and quantified using a Nanodrop UV spectrophotometer (Thermo Scientific, Wilmington, Delaware, USA). 200 ng of total RNA from each tissue was used to synthesize cDNA using iScript™ Select cDNA Synthesis Kit (Bio-Rad, Mississauga, ON) according to the manufacturer’s protocols. CNS cDNA was diluted 20-fold using nuclease-free water and subsequently used as template for quantitative PCR (qPCR).
Real Time PCR of RhoprCCAP Receptor
Real time PCR analyses were carried out on a CFX96Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA) using the Ssofast™ EvaGreen supermix (Bio-Rad Laboratories Inc., Hercules, CA, USA). CNS and peripheral tissues from 5th instar R. prolixus were dissected in physiological saline and stored in RNA later solution (Ambion, Austin, TX). Primers for RhoprCCAPR and reference genes (ribosomal protein 49, rp49 and actin 5c) (Table 1B) were designed to amplify target fragments of similar size across all samples [29]. Each primer set was designed with one primer over an exon/exon boundary and the primer efficiency was determined for each target. The amplification conditions were as follows: initial denaturation at 95°C for 30 sec, 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 5 sec, and extension at 72°C for 5 sec. The melting curve analysis was performed and all qPCR products were run on a 1% agarose gel. The relative expression was determined following the ΔΔCt method [30], [31] and fold-differences were normalized to both reference genes, RP49 and actin 5c. qPCRs were repeated for a total of three biological replicates with three technical replicates each that included a no template control and a no reverse-transcriptase control.
Double-stranded RNA Synthesis
RhoprCCAPR transcripts were amplified using PCR from CNS cDNA library. As a control, the ampicillin resistance (ARG) gene was PCR amplified from the pGEM-T Easy Vector system (Promega, Madison, WI, USA). Then, 1 µl of the PCR product was amplified by gene specific PCR primers (Table 3) that were conjugated with 23 bases of the T7 RNA polymerase promoter at the 5′ end (5′-taatacgactcactatagggaga-3′) (Table 3). All PCR amplification conditions were as follows: 5min initial denaturation for 5mins at 94°C, 35 cycles for 30 sec at 94°C, for 30 sec at 58°C, for 60 sec at 72°C, and final extension for 10 min at 72°C. The PCR products were used as a template for double stranded RNA (dsRNA) synthesis using the T7 Ribomax Express RNAi System (Promega, Madison, WI, USA). After synthesis, the dsRNA was precipitated with isopropanol, eluted in DEPC treated water, and then quantified at 260 nm wavelength using nanodrop. The quality of the dsRNA products was verified by 1% agarose gel electrophoresis and kept at −80°C until use. Before injection, the dsRNA was resuspended with DEPC treated water in the 2 µg/µl.
Table 3. Primers used to generate the double strand RNA (dsRNA) of the RhoprCCAPR and the ampicillin resistance gene (ARG).
| RNAi constructs | Oligo Sequence (5′ to 3′) |
| Primers to amplify RhoprCCAPR | |
| dsCCAPR_For1 | CTGGATAATGGACTGGGTTATAAG |
| dsCCAPR_For2 | TATCTGGAGGATCACGGTTG |
| dsCCAPR_Rev1 | GAATAGTGGCTCTGCGTAACG |
| dsCCAPR_Rev2 | TACTGGATTAGCTGCTGAATTGAG |
| Primers to amplify ARG | |
| dsARG_FOR1 | ATGAGTATTCAACATTTCCGTGTC |
| dsARG_FOR2 | CAACAGCGGTAAGATCCTTG |
| dsARG_REV1 | GGCACCTATCTCAGCGATC |
| dsARG_REV2 | AATAGTTTGCGCAACGTTG |
| Primers to generated dsRhoprCCAPR | |
| T7_dsCCAPR_For1 | TAATACGACTCACTATAGGGAGACTGGATAATGGACTGGGTTATAAG |
| T7_dsCCAPR_For2 | TAATACGACTCACTATAGGGAGATATCTGGAGGATCACGGTTG |
| T7_dsCCAPR_Rev1 | TAATACGACTCACTATAGGGAGAGAATAGTGGCTCTGCGTAACG |
| T7_dsCCAPR_Rev2 | TAATACGACTCACTATAGGGAGATACTGGATTAGCTGCTGAATTGAG |
| Primers to generated dsARG | |
| T7_dsARG_FOR1 | TAATACGACTCACTATAGGGAGAATGAGTATTCAACATTTCCGTGTC |
| T7_dsARG_FOR2 | TAATACGACTCACTATAGGGAGACAACAGCGGTAAGATCCTTG |
| T7_dsARG_REV1 | TAATACGACTCACTATAGGGAGAGGCACCTATCTCAGCGATC |
| T7_dsARG_REV2 | TAATACGACTCACTATAGGGAGAAATAGTTTGCGCAACGTTG |
The T7 promoter site is denoted as bold in the sequence of dsCCAPR and dsARG.
dsRNA Delivery
Adult R. prolixus were anesthetized with CO2 for 10 sec and 1 µl of 2 µg of dsCCAPR was injected into the thorax using a 5 µl Hamilton syringe to knock down RhoprCCAPR transcript levels. As a control, groups of R. prolixus were injected with either 1 µl of dsARG or had no injection. R. prolixus were left for 1 hour at room temperature to recover and then placed into an incubator at 28°C on a 16 h:8 h light/dark cycle.
Verification of dsRNA Knockdown Using Real Time PCR
Four CNS or peripheral tissues (pool of tissue containing fat body, trachea and dorsal vessel) were collected from adults that had been injected with dsARG, dsCCAPR as well as no treatment. Total RNA was extracted using the Trizol® reagent (Life Technologies Corporation, Carlsbad, CA, USA) and the cDNA was synthesized using iScript™ Select cDNA Synthesis Kit (Bio-Rad, Mississauga, ON). To verify the efficiency of synthesized dsRNA, qPCR were performed as described above.
Heartbeat Assay
Visual detection of in vivo heartbeat
In this experiment, the heartbeat rate of adult males that had been injected with 2 µg of dsRNA (either dsCCAPR or dsARG) was measured 2 days after injection. Day 2 was chosen since preliminary experiments indicated that greater than 80% knockdown was obtained by this time and this efficiency was similar for days 3 to 5. Animals were immobilized ventral-side down on a Dental wax-coated dissecting dish and their wings were spread gently and held in place by the wax. The heartbeat was observed under a dissecting microscope with a 10× objective focused on the heart through the relatively transparent dorsal cuticle. Each bug was left for 5 minutes under the light of the dissecting microscope and then the heartbeat was counted per minute.
In vitro heartbeat
In vitro heartbeat was monitored as described in Lee and Lange (2011) with minor modifications. The ventral cuticle, digestive and reproductive systems were dissected and removed under RNase free saline. The dorsal vessel and dorsal diaphragm remained attached to the dorsal cuticle. Then, the dorsal cuticle was placed onto a Sylgard-coated dissecting dish and covered with 100 µL of saline. Electrodes attached to an impedance converter (UFI model 2991, Morro Bay, CA, USA) were placed between the fifth and sixth abdominal segments on either side of the dorsal vessel anterior to the alary. The preparation was stabilized in 100 µL of saline for 10 min at room temperature, and then 50 µL of 10−9M CCAP was exchanged for 50 µL of saline. Heartbeat frequency was measured from the traces observed on a Linear Flat-bed single channel chart recorder. The preparation was washed with saline for 5 mins post application of CCAP. Heartbeat frequency was determined for 1 min before and after the application of 10−9M CCAP. The response to 10−9M CCAP was quantified by measuring the increase in frequency compared to saline, which was then expressed as a percentage of the maximum change in frequency for each preparation. Pools of tissues including dorsal vessel/fat body/trachea were collected and the percentage of knockdown was measured by qPCR as indicated above.
3.0. Results
Rhodnius prolixus CCAP receptor
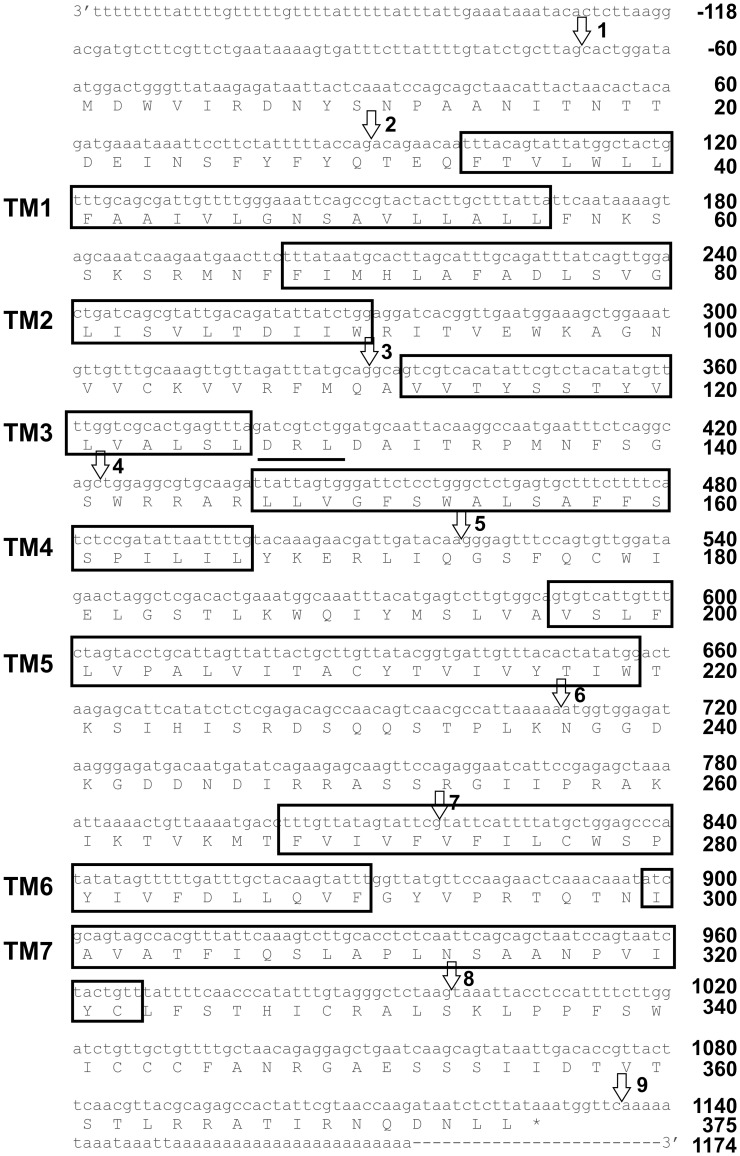
The RhoprCCAPR (Accession number: KC004225) was cloned from a 5th instar cDNA CNS library [see 32] using a modified rapid amplification of cDNA ends (RACE) [32]. The RhoprCCAPR sequence consists of 1279 nucleotides, which code for a polypeptide of 374 amino acid residues (Figure 1). The RhoprCCAPR has 10 exons that are separated by 9 introns and is predicted to have 7 alpha-helical transmembrane segments (TM) in the open reading frame (ORF) with three extra- and three intracellular loops as well as an intracellular C-terminal tail using TMHMM server, v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The sequence analysis of the RhoprCCAPR revealed the characteristics of a rhodopsin-like GPCR [29]. It also showed the presence of a divergent DRL sequence at amino acid residue 127 to 129, instead of the DRY sequence motif that can be found in many GPCRs belonging to the rhodopsin family at the cytoplasmic end of TM3 (Figure 1). Moreover, RhoprCCAPR has a conserved (NSxxNPxxY) motif element in the 7th transmembrane region at amino acid residue 313 to 321 (Figures 1, 2A) [17].
Figure 1. cDNA sequence of the RhoprCCAP receptor and deduced translation in 5th instar R. prolixus.
A) Nucleotide and amino acid sequences of the coding region starts at the nucleotide sequence ATG. Asterisk refers to the stop codon (TGA). The predicted transmembrane domains are box outlined and numbers on the left margin denotes the predicted transmembrane domains (TM1-7). The modified GPCR DRL sequences at amino acid residue 127 to 129 are underlined. The locations of introns (intron 1 to 9) are indicated by arrows.
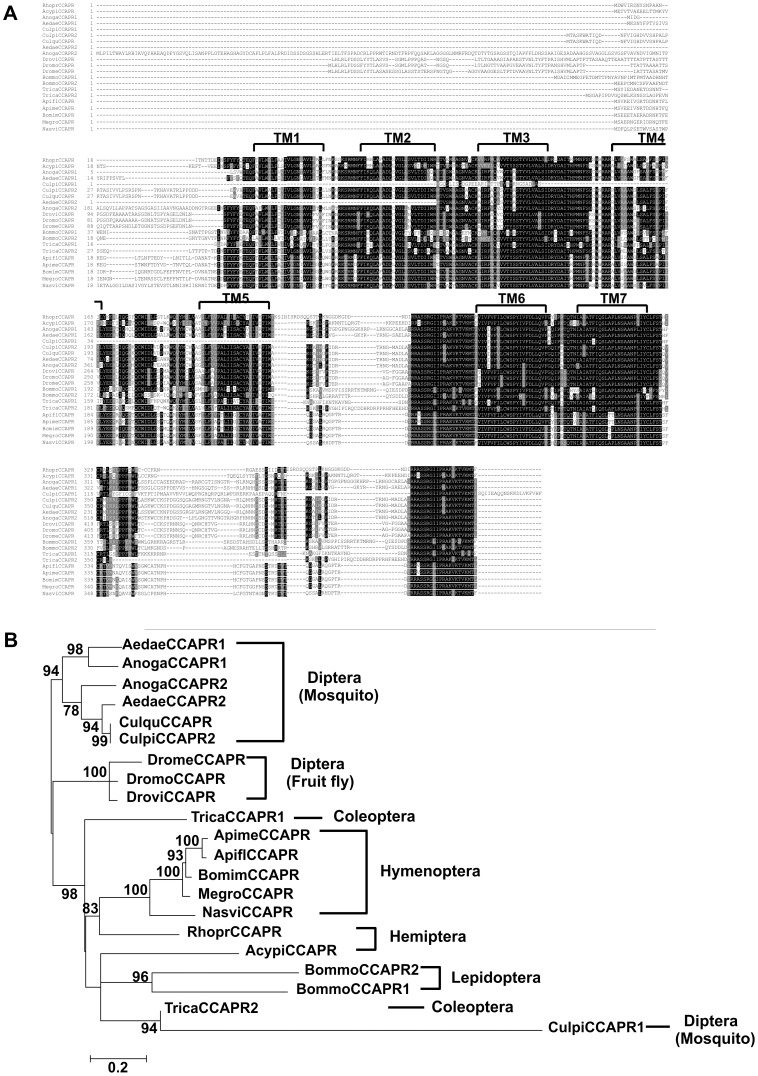
Figure 2. Protein alignment and phylogenetic analysis of CCAP receptors in insects.
A) Amino acid sequence alignment of the CCAP receptors identified or predicted in 21 species in arthropods. The predicted location of the seven transmembrane regions (TM1-TM7) are indicated above each row. Following the 50% majority rule, identical amino acids are shaded in black, and similar amino acids are shaded in gray in column consensus residues. (B) The phylogenetic relationship of the insect CCAP receptors was generated using the Maximum Likelihood method based on the Jones et al. (1992) with frequency model [45]. The numbers at the nodes represent percentage support in 1500 bootstrap replicates. All positions containing gaps and missing data were eliminated. This phylogenetic tree is drawn to scale and the branch lengths are measured in the number of substitutions per site.
Phylogenetic tree
RhoprCCAPR produces only one transcript (Figure 1) as has been observed in several species such as in Diptera (C. quinquefasciatu, D. melanogaster, D. virile, D. mojavensis), Hymenoptera (A. florea, A. mellifera, B. impatiens, M. rotundata, N. vitripennis) and Hemiptera (A. pisum); however, two isoforms of the CCAP receptor have been identified in three species of mosquito (A. gambiae, Aedes aegypti, Culex pipiens) and in T. castaneum and B. mori (Figure 2A, B). Phylogenetic analysis revealed that RhoprCCAPR belongs to the orthologous group of CCAP receptors in Hymenoptera including A. florae, A. mellifera, B. impatiens, M. rotundata and N. vitripennis (Figure 2B). RhoprCCAPR has high amino acid sequence similarity to identified or predicted CCAP receptors in Diptera, Coleoptera, Hymenoptera, Hemiptera, and Lepidoptera, with 55.8% pairwise identity (Figure 2A).
Spatial expression profile of the RhoprCCAPR gene
To identify the potential target sites of RhoprCCAP, the expression patterns of the putative RhoprCCAPR transcript were determined by real-time PCR (qPCR). The RhoprCCAPR gene expression was observed in the CNS, hindgut and female reproductive system (Figure 3). Also, lower transcript levels were observed in the 5th instars salivary glands, the pool of tissues including dorsal vessel/trachea/fat body, and in the male reproductive tissues (Figure 3). On the other hand, very low or nearly undetectable levels of the transcript were observed in foregut, anterior midgut, posterior midgut, and Malphigian tubules (Figure 3).
Figure 3. Expression profile of the RhoprCCAP receptor gene in fifth-instar R. prolixus tissues.
A) RhoprCCAPR transcripts were observed in CNS as well as peripheral tissues. Fold-difference in expression is relative to RhoprCCAPR expression in the salivary glands. Abbreviations: CNS, central nervous system; SG, salivary glands; FG, foregut; AMG, Anterior midgut; PMG, Posterior midgut; HG, hindgut; MTs, Malpighian tubules; DV/TR/FB, dorsal vessel/trachea/fat body; Female RT, female reproductive tissue; Male RT, male reproductive tissue.
Developmental expression profile of the RhoprCCAPR gene
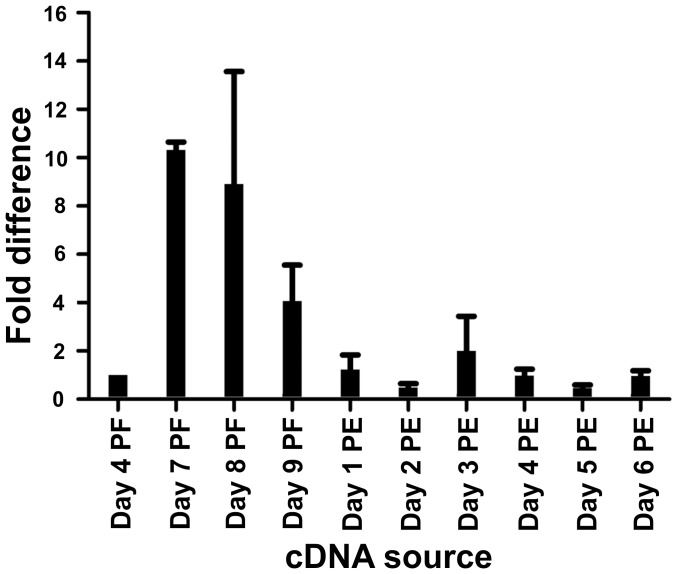
We were specifically interested in the developmental expression patterns of the putative RhoprCCAPR in the pool of tissues including dorsal vessel, trachea and fat body since CCAP is known to be a cardioacceleratory peptide. Our preliminary results showed that RhoprCCAPR transcript levels in day 2, 3 and 4 post-fed were not different. Since increasing heartbeat frequency right before ecdysis is essential in D. melanogaster, we chose day 4 post-fed as the earliest control. The RhoprCCAPR transcript level increased prior to ecdysis in 4th instars, which is at 7 to 9 days post-feeding, and decreased post-ecdysis (Figure 4).
Figure 4. Expression profile of the RhoprCCAP receptor gene during development in a pool of tissues containing dorsal vessel/fat body/trachea of 4th and 5th instars.
RhoprCCAPR expression level was increased prior to ecdysis and decreased post-ecdysis. Specifically, the peak RhoprCCAPR transcript level was observed in Day 7 post-fed (PF). Fold-difference in expression is shown relative to the expression of the RhoprCCAPR transcript in day 4 PF. Abbreviations: PF, post-fed; PE, post-ecdysis.
Functional receptor assays of the RhoprCCAPR
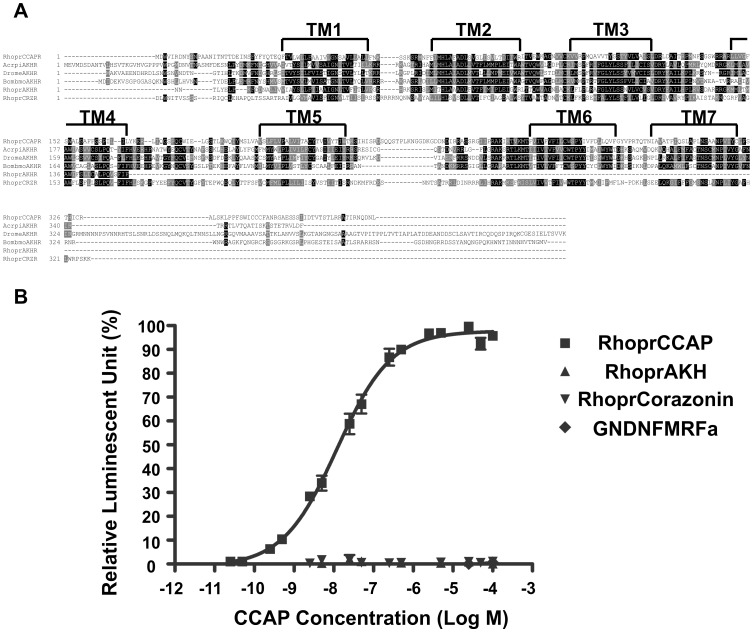
To determine the endogenous ligand for the isolated putative RhoprCCAPR in R. prolixus, we used a calcium mobilization assay which expresses the RhoprCCAPR clone in CHO-K1 cells. Interestingly, the EC50 using CHO-K1 cells was quite high (341±8.8 nM). However, in CHO - WTA11 cells, RhoprCCAPR was dose-dependently activated by CCAP with an EC50 of 12.2±1.1 nM (Figure 5B). The receptor was not activated by other peptides that were tested, including RhoprAKH, RhoprCorazonin and an extended RhoprFMRFa, GNDNFMRFa (Figure 5A, 5B, Table 2). AKH and corazonin were chosen because receptor sequence alignments revealed that the receptors for these peptides may be structurally-related to the CCAP receptor. Control cells that were transfected with an empty pcDNA vector showed no response to the peptides that were used in our assay (data is not shown here). Thus, our control data illustrate that the functional receptor assay system is only activated in cells that are transfected with RhoprCCAPR and not by any endogenous receptors in these CHO cells.
Figure 5. The alignments analysis of putative adipokinetic hormone (AKH) receptors, corazonin receptors and RhoprCCAP receptors as well as the RhoprCCAP receptor expression assay in (CHO-K1) WTA11 cells.
A) Amino acid sequences of receptors for AKH and corazonin were compared to RhoprCCAPR. The RhoprCCAP receptor was aligned with the putative AKH and corazonin receptors in insects. Dark gray shading denotes sequences identical in greater than 50% of that particular column while light gray shading denotes similar residue to column-consensus residue. B) Activity of CCAP, AKH, corazonin and an extended FMRFamide in the RhoprCCAPR functional assay. Dose response curve shows the activity of CCAP on the expressed RhoprCCAPR has an EC50 of 12.25±1.1 nM.
CCAP function in heart rate
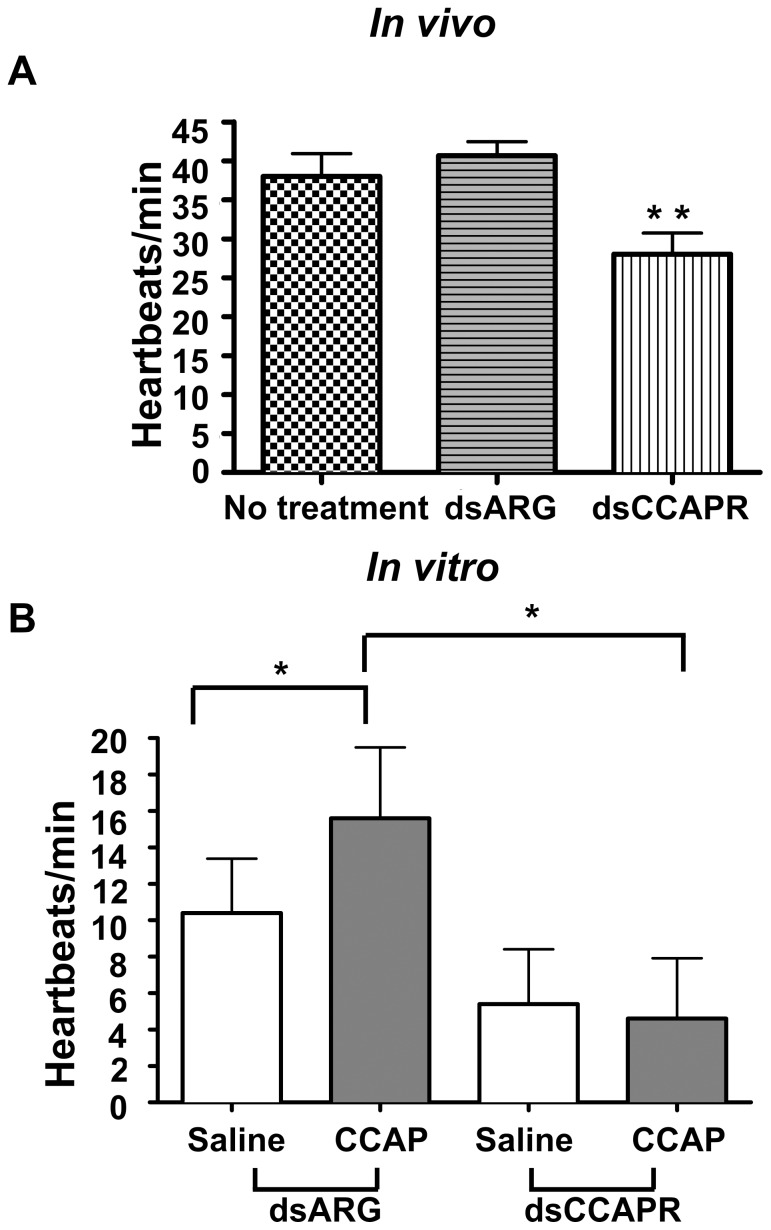
The effect of CCAP on the heartbeat frequency was studied in vivo and in vitro in the adult male R. prolixus treated with control (dsRNA) or dsCCAPR. In vivo heartbeat frequency of dsCCAPR-treated bugs (28.0±2.7 beats/min, n = 10) was significantly decreased by 31.1% compared to the dsARG treated group (40.7±1.8 beats/min, n = 10) (paired t test, p = 0.0005) (Figure 6A). Previously, we have shown that CCAP increases heartbeat frequency in vitro in 5th instar R. prolixus [33]. To verify the results observed in vivo, we investigated whether the reduced heartbeat frequency was due to the absence of the CCAPR. Thus, we again knocked down the RhoprCCAPR mRNA and measured heartbeat frequency in vitro. Our results showed that the heartbeat frequency of the dsARG treated bugs was 10.4±2.9 beats/min in saline and was significantly increased to 15.6±3.9 beats/min in the presence of 10−9 M CCAP (paired t test, p = 0.0376) (Figure 6B). In contrast, the heartbeat frequency of the group that was treated with dsCCAPR was 5.4±3.0 beats/min in saline and was 4.6±3.3 beats/min, in the presence of 10−9M CCAP (paired t test, p = 0.1688) (Figure 6B). When we compared the heartbeat frequency of the two groups in saline that were treated with dsARG or dsCCAPR, the difference was lower, but not statistically significant (unpaired t test, p = 0.1357). However, when the results of after CCAP application was compared between the two groups, the difference was statistically significant (unpaired t test, p = 0.0318) (Figure 6B). The percentage knock-down of the RhoprCCAPR transcription was quantified by qPCR in the pool of tissues (dorsal vessel/trachea/fat body) from these insects and was found to be knocked down by 80.3±1.5% 2 days after injection relative to control dsARG injected bug.
Figure 6. The effects of CCAP on heartbeat in adult male R. prolixus.
2 µg of dsRNA for the ampicillin resistance gene (dsARG) or for the RhoprCCAPR (dsCCAPR) was injected into adult male R. prolixus and 2 days after injection or no treatment heart rate was measured in vivo and in vitro. Values are expressed as the mean ± SEM (n = 5). Asterisk indicates significant differences between dsARG and dsCCAPR-treated groups of animals as determined by t-test (*P<0.05; **P<0.01). A) Heartbeat frequency of adult male R. prolixus was measured in vivo and was significantly lower in bugs in which RhoprCCAPR was knocked down. B) Heartbeat frequency was measured in vitro and found to be lower in dsCCAPR-treated bugs than those treated with dsARF. RhoprCCAPR knocked down bugs were also unresponsive to CCAP. White bars indicate heartbeats in saline and grey bars denote heartbeats in 10 −9 M CCAP.
Discussion
The GPCR superfamily is a critical target for developing pharmacological drug treatments and more than one third of current human drugs act on this family [1], . Identifying agonists or antagonists for this receptor family can lead to treatment for many human diseases, or the development of novel pest-control agents [5], [6], [30].
The CCAP receptor in insects belongs to the GPCR superfamily and it has been characterized in Diptera, Coleoptera, Hymenoptera, Hemiptera and Lepidoptera [19]–[22]. In the present study, we have isolated and characterized the CCAP receptor from R. prolixus. The RhoprCCAPR shows high amino acid sequence similarity to CCAP receptors identified in Hymenoptera, including A. mellifera, A. florea, B. impatiens, M. rotundata and N. vitripennis. Interestingly, two isoforms of the CCAP receptor have been isolated in some Diptera (A. gambiae, A. aegypti, C. pipiens), Coleoptera (T. castaneum) and Lepidoptera (B. mori) but not in other Diptera (Drosophila), Hymenoptera or Hemiptera.
We showed the spatial expression patterns of the CCAPR in R. prolixus and this correlates with our previous immunohistochemical data and physiological roles of CCAP [31]. We have previously shown cells and processes containing CCAP-like immunoreactivity are distributed throughout the CNS and associated with the heart in R. prolixus [31] and CCAP increases heartbeat frequency and contraction in a dose-dependent manner in the heart and hindgut, respectively [31]. Thus the expression of RhoprCCAPR in the CNS and peripheral tissues confirms these findings, suggesting that CCAP controls central and peripheral physiological processes. Expression of CCAPR transcripts has been investigated in D. melanogaster [19], T. castaneum [32], [36] and A. gambiae [22]. Its expression was observed in all developmental stages from embryonic stages to adult in D. melanogaster, A. gambiae and T. castaneum. Specifically, the peak CCAPR expression was observed during late pupal stages in D. melanogaster and A. gambiae while it was observed in the early adult of T. castaneum [19], [21], [22]. The transcript was mainly observed in the head in adult fly and mosquito [19], [22].
Interestingly, we observed additional tissues that expressed the CCAPR, such as the salivary glands, and female and male reproductive systems. This suggests additional targets for the endogenous CCAP. The R. prolixus salivary gland has a double layer of visceral muscle surrounding a large secretory cavity and these muscles are under the control of various neuropeptides and serotonin [32]. Thus, CCAP might also be involved in the control of muscle contraction of the salivary glands or in the process of salivary secretion. Also, the presence of the RhoprCCAPR in the reproductive systems of male and female R. prolixus indicates that CCAP may be involved in reproduction, as it has been shown in L. migratoria [14]. Future studies are required to investigate the other physiological roles of endogenous CCAP at these newly identified target tissues.
To support our phylogenetics and alignment analysis with the expression profile, which suggests that the identified receptor was a CCAP receptor homolog, we expressed this receptor in CHO cells. The expressed CCAP receptor was only activated by low concentrations of RhoprCCAP with EC50 = 12.2±1.1 nM when tested in CHO-WTA11 cells. The improved sensitivity of this cell line over CHO-K cells is due to presence of the promiscuous Gα proteins. Interestingly, although the putative CCAP receptor has been isolated in 20 species of arthropods, the CCAP receptor has only been deorphanized in three species including D. melanogaster, A. gambiae and T. castaneum. In D. melanogaster, the CCAP receptor (CG6111) is activated by low concentrations of CCAP (EC50 of 5.4 x10−10M) [19]. In A. gambiae, the CCAP receptor is activated at an EC50 of 1nM CCAP [20] and in T. castaneum, two CCAP receptors, CCAPR1 and CCAPR2, are activated by CCAP with an EC50 of 624nM and 22nM respectively [21]. The difference in EC50 values may be due to techniques and the expression system that was used, but our EC50 value of 12.2nM is comparable to that found in D. melanogaster and A. gambiae.
We also investigated whether RhoprCCAPR can be activated by other peptides, including corazonin and AKH because the CCAP receptor alignment analysis with corazonin and AKH receptors reveals that they may be structurally-related (31.2% pairwise identity). Also, these peptides are functionally inter-related. For example, CCAP influences AKH release from the corpora cardiaca in Schistocerca gregaria [38] [33]. In M. sexta, AKH mobilize lipids from the fat body during flight or locomotion when heartbeat frequency also increases. Also, CCAP, AKH and corazonin increase heartbeat frequency in some insects [39] [34]. However, this possible functional inter-relationship does not extend to the agonist-binding properties of the corresponding GPCRs, since neither AKH nor corazonin activate the RhoprCCAPR, and nor does an unrelated peptide, an extended FMRFamide.
The crucial roles of CCAP in regulating ecdysis behaviour have been studied in D. melanogaster, Manduca sexta and T. castaneum [36], [40]–[42]. In the moth, assays using the isolated abdominal CNS suggest that CCAP is required for turning off the pre-ecdysis motor program [40] and turning on the ecdysis motor behaviours [40.41]. In Drosophila, lack of CCAP neurons results in the complete failure of pupal ecdysis [42]. Arakane et al (2008) showed that when transcripts levels of CCAP and its CCAP receptor were reduced, ecdysis behaviors were interrupted in T. castaneum [36]. Interestingly, in D. melanogaster, Baker et al. (1999) showed that the Drosophila heartbeat frequency was increased during the last 10 h of adult development and peaked at 1 hour before ecdysis at the white stage [43]. If CCAP has a role in increasing heartbeat frequency prior to ecdysis, then we should expect that the CCAP receptor expression might be up regulated at this time. As anticipated, the CCAP receptor mRNA levels in the pool of tissues (dorsal vessel/trachea/fat body) were increased up to 10 fold prior to ecdysis and decreased post-ecdysis. In addition, CCAP receptor expression was high in the hindgut, a tissue that is regulated at ecdysis for gut emptying and elimination of its cuticular lining. Our results certainly suggest that CCAP might play important roles in R. prolixus ecdysis and future studies will examine this.
The involvement of CCAP in cardiac function has been studied in several insects, including its involvement in adult wing inflation in M. sexta (see 8) In D. melanogaster, although CCAP RNAi injection was not found to have any effect on heartbeat frequency, CCAP cell ablation resulted in a heart rate that was decreased by 37–51%. CCAP cell ablation only affected the anterograde phase of the heartbeat suggesting that CCAP may be involved in regulating the anterograde pacemaker in D. melanogaster. In A. gambiae, silencing the CCAP transcript resulted in a statically significant (6% and 7%) reduction in the total and anterograde heart rate [22]. Previously, we demonstrated that CCAP increases the frequency of the heartbeat in R. prolixus in a reversible, dose-dependent manner [33] and reducing endogenous CCAP receptor levels by RNAi lowers heartbeat in vivo and eliminates the CCAP-induced increase in vitro. The basal heart beat rate in vivo is higher than that observed in vitro, which might imply the absence of endogenous cardioaccelators in the in vitro condition. This is the first report to show that reducing CCAP receptor transcript levels leads to a reduction in the cardiac output in any insect. Interestingly, heartbeat rate is reduced in dsCCAPR-treated bugs indicating that normal heartbeat rate is elevated due to the presence of endogenous CCAP.
The very low or nearly undetectable levels of receptor transcript observed in anterior midgut and posterior midgut in R. prolixus is of some interest, since in P. americana CCAP up-regulates the activity of digestive enzymes in midgut [10], [44]. Thus, exposure of isolated midgut to CCAP increases α-amylase and protease activity. The CCAP may act in a paracrine manner, released from CCAP-containing midgut endocrine cells [10], [44]. Clearly R. prolixus appears to be different, since there does not appear to be CCAP receptors associated with the midgut, and previous studies have failed to find CCAP-like immunoreactive endocrine cells in R. prolixus midgut [33].
R. prolixus is the principal vector of Chagas’ disease, and transmits the parasitic protozoan, Trypanosoma cruzi. Currently, the best solution for disrupting the transmission of this disease is by controlling the vector. Hence, R. prolixus is a useful model organism for studying physiological and neuroendocrine processes but is also medically important. Since GPCRs act as key regulators in the physiology of insects (and other animals) and are critical drug targets in human medicine and in the agricultural industry, understanding and characterizing GPCRs in this insect could lead to control measures against the transmission of Chagas’ disease.
Acknowledgments
We would like to thank Himali Patel for helping with in vitro heart contraction assay and Ian Orchard for advice. The authors gratefully acknowledge M. Parmentier (University of Brussels, Belgium) and M. Detheux (Euroscreen S.A., Belgium) for providing the WTA11 cell line used in this study.
Funding Statement
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Liebmann C (2004) G protein-coupled receptors and their signaling pathways: classical therapeutical targets susceptible to novel therapeutic concepts. Curr Pharm Des 10: 1937–1958. [DOI] [PubMed] [Google Scholar]
- 2.Grimmelikhuijzen CJP, Hauser F (2013) Arthropod Genomics and Pest Management Targeting GPCRs. 165–177.
- 3. Russell JL, Goetsch SC, Aguilar HR, Coe H, Luo X, et al. (2012) Regulated expression of pH sensing G Protein-coupled receptor-68 identified through chemical biology defines a new drug target for ischemic heart disease. ACS Chem Biol 7: 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rozengurt E, Sinnett-Smith J, Kisfalvi K (2010) Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res 16: 2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heilker R, Wolff M, Tautermann CS, Bieler M (2009) G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov Today 14: 231–240. [DOI] [PubMed] [Google Scholar]
- 6. Kawabata A (2001) The G protein-coupled protease receptor PAR (protease-activated receptor) as a novel target for drug development. Yakugaku Zasshi 121: 1–7. [DOI] [PubMed] [Google Scholar]
- 7. Stangier J, Hilbich C, Beyreuther K, Keller R (1987) Unusual cardioactive peptide (CCAP) from pericardial organs of the shore crab Carcinus maenas . Proc Natl Acad Sci U S A 84: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loi PK, Emmal SA, Park Y, Tublitz NJ (2001) Identification, sequence and expression of a crustacean cardioactive peptide (CCAP) gene in the moth Manduca sexta . J Exp Biol 204: 2803–2816. [DOI] [PubMed] [Google Scholar]
- 9. Park Y, Kim YJ, Adams ME (2002) Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci U S A 99: 11423–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakai T, Satake H, Minakata H, Takeda M (2004) Characterization of crustacean cardioactive peptide as a novel insect midgut factor: isolation, localization, and stimulation of alpha-amylase activity and gut contraction. Endocrinology 145: 5671–5678. [DOI] [PubMed] [Google Scholar]
- 11. Tublitz NJ, Evans PD (1986) Insect cardioactive peptides: cardioacceleratory peptide (CAP) activity is blocked in vivo and in vitro with a monoclonal antibody. J Neurosci 6: 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tublitz N (1989) Insect cardioactive peptides: neurohormonal regulation of cardiac activity by two cardioacceleratory peptides during flight in the tobacco hawkmoth, Manduca sexta . J Exp Biol 142: 31–48. [DOI] [PubMed] [Google Scholar]
- 13. Park JH (2003) Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development 130: 2645–2656. [DOI] [PubMed] [Google Scholar]
- 14. Donini A, Agricola H, Lange AB (2001) Crustacean cardioactive peptide is a modulator of oviduct contractions in Locusta migratoria . J Insect Physiol 47: 277–285. [DOI] [PubMed] [Google Scholar]
- 15. Donini A, Ngo C, Lange AB (2002) Evidence for crustacean cardioactive peptide-like innervation of the gut in Locusta migratoria . Peptides 23: 1915–1923. [DOI] [PubMed] [Google Scholar]
- 16. Vaughan M (1998) Signaling by heterotrimeric G proteins minireview series. J Biol Chem 273: 667–668. [DOI] [PubMed] [Google Scholar]
- 17. Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63: 1256–1272. [DOI] [PubMed] [Google Scholar]
- 18. Kobilka BK (2007) G protein coupled receptor structure and activation. Biochim Biophys Acta 1768: 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cazzamali G, Hauser F, Kobberup S, Williamson M, Grimmelikhuijzen CJP (2003) Molecular identification of a Drosophila G protein-coupled receptor specific for crustacean cardioactive peptide. Biochemical and Biophysical Research Communications 303: 146–152. [DOI] [PubMed] [Google Scholar]
- 20. Belmont M, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJ (2006) Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae . Biochem Biophys Res Commun 344: 160–165. [DOI] [PubMed] [Google Scholar]
- 21. Li B, Beeman RW, Park Y (2011) Functions of duplicated genes encoding CCAP receptors in the red flour beetle, Tribolium castaneum . J Insect Physiol 57: 1190–1197. [DOI] [PubMed] [Google Scholar]
- 22. Estevez-Lao TY, Boyce DS, Honegger HW, Hillyer JF (2013) Cardioacceleratory function of the neurohormone CCAP in the mosquito Anopheles gambiae . J Exp Biol 216: 601–613. [DOI] [PubMed] [Google Scholar]
- 23. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 24. Lee DH, Paluzzi JP, Orchard I, Lange AB (2011) Isolation, cloning and expression of the Crustacean Cardioactive Peptide gene in the Chagas' disease vector, Rhodnius prolixus . Peptides 32: 475–482. [DOI] [PubMed] [Google Scholar]
- 25. Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 26. Kozak M (1987) At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 196: 947–950. [DOI] [PubMed] [Google Scholar]
- 27. Kozak M (1990) Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc Natl Acad Sci U S A 87: 8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kozak M (1991) An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol 115: 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paluzzi JP, O'Donnell MJ (2012) Identification, spatial expression analysis and functional characterization of a pyrokinin-1 receptor in the Chagas' disease vector, Rhodnius prolixus . Mol Cell Endocrinol 363: 36–45. [DOI] [PubMed] [Google Scholar]
- 30. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 32. Paluzzi JP, Russell WK, Nachman RJ, Orchard I (2008) Isolation, cloning, and expression mapping of a gene encoding an antidiuretic hormone and other CAPA-related peptides in the disease vector, Rhodnius prolixus . Endocrinology 149: 4638–4646. [DOI] [PubMed] [Google Scholar]
- 33. Lee DH, Lange AB (2011) Crustacean cardioactive peptide in the Chagas' disease vector, Rhodnius prolixus: presence, distribution and physiological effects. Gen Comp Endocrinol 174: 36–43. [DOI] [PubMed] [Google Scholar]
- 34. Schoneberg T, Schultz G, Gudermann T (1999) Structural basis of G protein-coupled receptor function. Mol Cell Endocrinol 151: 181–193. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Q, Nachman RJ, Kaczmarek K, Zabrocki J, Denlinger DL (2011) Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc Natl Acad Sci U S A 108: 16922–16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ, et al. (2008) Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum . Mech Dev 125: 984–995. [DOI] [PubMed] [Google Scholar]
- 37. Orchard I, Brugge VT (2002) Contractions associated with the salivary glands of the blood-feeding bug, Rhodnius prolixus: evidence for both a neural and neurohormonal coordination. Peptides 23: 693–700. [DOI] [PubMed] [Google Scholar]
- 38. Veelaert D, Passier P, Devreese B, Vanden Broeck J, Van Beeumen J, et al. (1997) Isolation and characterization of an adipokinetic hormone release-inducing factor in locusts: the crustacean cardioactive peptide. Endocrinology 138: 138–142. [DOI] [PubMed] [Google Scholar]
- 39. Gade G, Hoffmann KH, Spring JH (1997) Hormonal regulation in insects: facts, gaps, and future directions. Physiol Rev 77: 963–1032. [DOI] [PubMed] [Google Scholar]
- 40. Gammie SC, Truman JW (1997) Neuropeptide hierarchies and the activation of sequential motor behaviors in the hawkmoth, Manduca sexta . J Neurosci 17: 4389–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zitnan D, Adams ME (2000) Excitatory and inhibitory roles of central ganglia in initiation of the insect ecdysis behavioural sequence. J Exp Biol 203: 1329–1340. [DOI] [PubMed] [Google Scholar]
- 42. Park JH, Schroeder AJ, Helfrich-Forster C, Jackson FR, Ewer J (2003) Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development 130: 2645–2656. [DOI] [PubMed] [Google Scholar]
- 43. Baker JD, McNabb SL, Truman JW (1999) The hormonal coordination of behavior and physiology at adult ecdysis in Drosophila melanogaster . J Exp Biol 202: 3037–3048. [DOI] [PubMed] [Google Scholar]
- 44. Sakai T, Satake H, Takeda M (2006) Nutrient-induced α-amylase and protease activity is regulated by crustacean cardioactive peptide (CCAP) in the cockroach midgut. Peptides 27: 2157–2164. [DOI] [PubMed] [Google Scholar]
- 45. Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282. [DOI] [PubMed] [Google Scholar]