Abstract
In this review we look into the historical development of open abdomen management. Its indication has spread in 70 years from intra-abdominal sepsis to damage control surgery and abdominal compartment syndrome. Different temporary abdominal closure techniques are essential to benefit the potential advantages of open abdomen management. Here, we discuss the different techniques and provide a new treatment strategy, based on available evidence, to facilitate more consistent decision making and further research on this complicated surgical topic.
Keywords: open abdomen, abdominal trauma, septic abdomen, damage control surgery, temporary abdominal closure
“Study the past if you would define the future” (Confucius, Chinese philosopher, 551–479 BC)
Background
The management of the open abdomen was introduced in the English literature by Ogilvie in 1940 [1]. Since then it has been much debated. Its indication has varied from a last resort option in abdominal catastrophes to a preferred initial treatment strategy in damage control surgery (DCS) for both trauma and non-trauma patients. Mortality of patients with abdominal sepsis has remained as high as 20–60% [2–5]. Open abdomen management (OAM) strategies could possibly play an important role in improving survival in this difficult group of patients. Its management includes dealing with DCS principles, intra-abdominal hypertension/abdominal compartment syndrome, and complications as bowel fistulization. There are many techniques described as temporary abdominal closure (TAC), but none has been proven to be superior. Level one evidence is almost impossible to achieve in this heterogeneous group of patients and (non-sponsored) published treatment strategies are scarce. As a result of this lack of evidence, although the principles of OAM seem to be generally accepted, it has not led to a change of practice. Research in 2006 and 2007 showed that surgeons and ICU staff still did as they had always done without any institutional policies regarding OAM [6,7].
In our own analysis of 154 patients treated within a 10-year period with a septic abdomen, we could not prove any benefit of open abdomen management (unpublished data). However, this was not because of the procedure itself, but because of a lack of a systematic approach in the management of this serious surgical problem. The use of open abdomen (OA) and TAC techniques is still dependent on the individual surgeon’s decision and experience and is not standardized. New treatment protocols are hard to implement and it is difficult to convince surgeons to perform this surgery differently. This experience has led us to look back and study the past to see if we could find more consistent directives for the future.
In this review we present a historical perspective on the evolution of OAM and we propose an OAM algorithm based on the literature and our own experience. With this algorithm we aim to establish a reference technique and strategy to accommodate further development and research.
Search Strategy and Selection Criteria
Data for this review were identified by a Pub Med search from 1940 to March 2011. Search terms included “open abdomen”, “management”, “damage control surgery”, “temporary abdominal closure”, and “septic abdomen”. In addition, references from relevant articles were searched to identify additional relevant studies. Although we limited the main search to publications in English and Dutch, frequently cited articles in other languages were also included. Several review articles were included because they provide comprehensive historical overviews. Articles focused on pediatric patients only were excluded, as were articles discussing abdominal wall reconstruction only or the treatment of entero-atmospheric fistulas in general.
The History of Open Abdomen Management
1940–1990
In one of the first known publications in the English literature on OAM, Ogilvie described the use of a “double sheet of light canvas or stout cotton cut rather smaller than the defect in the muscles, and sutured into place with interrupted catgut sutures” in abdominal war wounds that could not be closed primarily [1]. In other cases, Ogilvie described the use of Vaseline-impregnated gauze swabs over exposed viscera, their edges tucked well under those of the defect, after which the sides of the incision were brought together with strips of Elastoplast® or stitches. In a later publication, Ogilvie advocated the same technique in the staged treatment of infected abdominal wounds, leaving the abdomen open after the initial operation in order to close it only after between 1 to 4 days [8]. In that article he compared the septic abdomen to any other septic wound, leaving it open to drain and saving the abdominal wall by not suturing it in order to be able to close it at a later stage.
It took almost 40 years for further studies on OAM to appear [9]. Steinberg described the management of 14 patients with acute generalized peritonitis. After the initial operation, the abdomen was left open with gauze packs on the viscera. After 48–72 hours the packs were removed and wires previously placed through the abdominal wall were tied. He reported 1 intra-abdominal abscess as a post-operative complication and 1 death (7%) [9]. A second descriptive study by Duff et al. described OAM as a last resort treatment of diffuse intra-abdominal sepsis when all other treatment options had failed and the abdomen could no longer be closed. They observed a mortality rate of 39% and concluded it was a feasible technique [10]. Others supported the concept and OAM gradually became accepted as a technique to achieve adequate drainage of the septic abdominal cavity, thereby decreasing mortality rates from >50% to about 38% [11–13]. Anderson, however, found a very high mortality rate of 60% when treating patients with abdominal sepsis using the same technique as Steinberg [14]. Problems such as evisceration, entero-atmospheric fistulization, fluid loss, and potential contamination, as well as complex wound management, were also recognized [15–17]. Therefore, OAM was mostly considered to be a last resort treatment in selected cases of heavily contaminated peritoneal cavities [12].
In this same time period a new technique in dealing with sever intra-abdominal sepsis was introduced in both the German and English literature – the so called “etappen lavage” or planned relaparotomy [18,19]. One of the first publications on planned relaparotomy versus the more traditional “on-demand” strategy was published by surgeons in Belgium in 1983 [19]. In this retrospective study, 42 patients were treated with a planned relaparotomy every 2–3 days until macroscopical abdominal contamination had cleared. With this new strategy mortality was reduced from 73% to 36%. It was advocated that planned relaparatomies should be performed only when adequate debridement could not be achieved. However, planned relaparotomy became a generally used technique in every case of septic abdomen. The abdomen was closed between planned re-explorations until it could no longer be closed. The open abdomen was then packed with gauze (soaked in saline) or sutured with nonabsorbable mesh to prevent evisceration [11,17,20]. These first techniques of TAC or dressing play an important role in the development of the main complication of OAM – the development of entero-atmospheric fistulas, but this was not yet recognized.
New techniques were introduced to cope with the problems associated with OAM. These techniques included Marlex® zippers [21,22], plastic bags (the Bogota technique) [23], Velcro adhesive sheets [24], absorbable mesh [25], and the “sandwich technique” [16]. This last technique was a polypropylene mesh that was sutured to the fascia wall with 2 suction drains and Op-site® (Smith and Nephew) on top. At the final stage, povidone-iodine gauze dressings were packed on the polypropylene mesh and left to granulate [16]. Hedderich et al. described the use of Marlex® mesh with a zipper, facilitating re-opening of the abdomen and then closing it again without resuturing the abdominal wall [22]. However, these were merely small descriptive studies with heterogeneous groups of patients. The need for stratification and standardization using scoring systems to better compare results was expressed [11].
Garcia Sabrido et al. conducted one of the first attempts to prospectively stratify 64 patients with intra-abdominal sepsis (IAS) and used a standardized zipper technique [21]. They found a decreased mortality rate in OAM compared to an estimated mortality based on APACHE II scores. The next year Ivatury et al. used the same scoring system to add objectivity but used a different standardized strategy [25]. The disadvantages of reported complications such as fistulization and infection of retained nonabsorbable mesh were now recognized [22,26]. Therefore, they used absorbable mesh instead. With this strategy they reported a 74% success rate in eradicating sepsis and again suggested leaving the abdomen open at the first operation when there was gross contamination [25].
1990–2010
Indications for OAM
Intra-abdominal sepsis
The discussion on planned versus on-demand relaparotomy strategies continued and characterized the next timeframe. Whether the abdomen should be left open in between the planned relaparatomies has only been part of the discussion, but could be very crucial. Some kept the abdomen closed in between procedures; others used various TAC techniques such as retention sutures, slide fasteners, zippers, and Velcro adhesive sheets. Some improved outcome [24,27], but others could not achieve these positive results [2] or described the opposite [28]. All studies, however, had major shortcomings in study design, description of techniques, and complications, making comparison impossible. This was also the conclusion of a meta-analysis of the literature in 2002 [29]. Nevertheless, the combination of planned relaparotomy and OAM remained a mainstay of the approach of many surgeons to severe intra-abdominal sepsis (SIAS), with varying results [30–33].
The first randomized trial was published in 2007 and showed no statistical differences in mortality or morbidity between patients treated with a planned relaparotomy strategy keeping the abdomen closed as long as possible, compared with an on-demand strategy [4]. This group did find that the on-demand group had significant shorter median intensive care unit stays, shorter median hospital stays, and a significant reduction in medical costs. Therefore, they concluded that on-demand relaparotomy was the preferred surgical treatment strategy in dealing with SIAS.
Robledo et al. compared open versus closed abdominal management in 40 patients with severe secondary peritonitis [34]. They found no significant difference in mortality rates (55% open versus 30% closed). However, the relative risk and odds ratio for death in the open group (1.83 and 2.85, respectively) led to termination of the study at the first interim analysis. These 2 randomized trials motivated others to abandon primary OA approaches [35,36]. However, the results of the latter study need to be reconsidered when considering the method of TAC. Robledo et al. used the sandwich technique with non-absorbable mesh sutured to the fascia (with or without omentum protecting the bowel), on top of which gauze soaked in iodine-povidone was placed. By this time, use of this technique had already been shown to be an unwise strategy, as described earlier [22,26].
Furthermore, in the on-demand strategy for IAS, the decision to perform a relaparotomy and its timing are essential. Factors indicative of progressive or persistent organ failure during early postoperative follow-up were shown to be the best indicators for ongoing infection and were associated with positive findings at relaparotomy [37]. But clear-cut scoring systems never became available. It therefore remains a difficult decision that requires an around the clock, dedicated multidisciplinary team. Planned relaparotomy has therefore not lost its indication for selected patients. It seems logical that in these indications the abdomen is then best left open to preserve the fascia for later closure.
Abdominal Compartment Syndrome
In the meantime, more interest developed in the importance of intra-abdominal pressure (IAP) and abdominal compartment syndrome (ACS). In the late 1990s, intra-abdominal hypertension (IAH) and ACS were defined as indicators for OAM in both trauma and general patients [38,39]. However, it was clear that not all TAC techniques prevented IAH or the development of ACS [39,40]. Therefore, continued monitoring of IAP and abdominal perfusion pressure (APP) was advised [40,41] and this still applies to current practice. ACS has been extensively researched and defined by the World Society on the Abdominal Compartment Syndrome (WSACS). The relevant definitions have been listed in Table 1. It has become clear that patients with ACS should undergo urgent decompressive laparotomy [42]. According to the guidelines published by the WSACS (www.wsacs.org), Grade 3 and 4 IAH need more urgent and precise treatment. Sepsis, massive fluid resuscitation, and massive transfusion were recognized as risk factors of ACS and predominant causes of increased IAP [43]. Management of IAH and ACS in both trauma and IAS patients was recently found to significantly improve survival in a prospective observational study on 478 patients [44]. In this study they used a treatment algorithm according to the guidelines of the WSACS and the use of this algorithm was identified as an independent predictor of survival.
Table 1.
Definitions.
| Open abdomen | Non-closure of fascia and skin |
| Normal intra abdominal pressure (IAP) | 5–7 mmHg in critically ill adults |
| Intra Abdominal Hypertension (IAH) | Sustained or repeated pathological elevation in IAP ≥12 mmHg |
| IAH grade 1 | 12–15 mmHg |
| IAH grade 2 | 16–20 mmHg |
| IAH grade 3 | 21–25 mmHg |
| IAH grade 4 | >25 mmHg |
| Abdominal Compartment Syndrome | Sustained IAP >20 mmHg (with or without an abdominal perfusion pressure <60 mmHg) that is associated with new organ dysfunction/failure |
| Primary ACS | Associated with injury or disease in the abdomino-pelvic region |
| Secondary ACS | Without the presence of intra-abdominal injury |
| Recurrent ACS | Condition in which ACS develops after previous surgical or medical treatment of primary or secondary ACS |
Although more than one-third of patients undergoing acute abdominal general surgery will develop IAH, and one-third of those will develop ACS [43], a survey in 2009 showed that still only one-third of surgeons routinely measure IAP [43]. This situation needs to improve. It is recommended that all patients in ICU after emergency general surgery or massive fluid resuscitation should have IAP measurement performed every 6 hours.
Damage Control Surgery
OAM had been accepted as a strategy in treating intra-abdominal sepsis, but a major change occurred in the 1990s when the indication spread to a new group of patients. In 1993, the term damage control surgery (DCS) for trauma patients was introduced [45]. It was defined as initial control of hemorrhage and contamination, followed by intra-peritoneal packing and rapid TAC, allowing for resuscitation to normal physiology in the intensive care unit and subsequent definitive re-exploration. Although not yet precisely defined in that article, the ‘lethal triad of death’, consisting of coagulopathy, acidosis, and hypothermia, is suggested as a useful guideline indicating patients who will benefit from this approach. More recently, the following clinical parameters were defined as guidelines to consider DCS and OAM: acidosis (pH ≤7.2), hypothermia (temperature ≤35°C), and clinical coagulopathy or massive transfusion (≥10 units packed RBC) [42,46]. In this first study, the abdomen was temporarily closed between subsequent explorations by means of towel clips or sutures to skin and fascia, or with a prosthetic silo [45]. The rapidly accepted and applied concept lead to an increase in OAM and, hence, to an increase in studies on the best TAC technique.
As the same underlying principles seemed to apply to general surgical patients [47], extending DCS to non-trauma abdominal surgery was also explored. In 2004 Finlay et al. described their study on the use of DCS in the management of critically ill general surgical patients, using early OAM and re-applying TAC not every 48 hours, but once every 3–5 days [48]. In this study they observed a mortality rate of 7.1%, which was significantly lower than the predicted mortality of 64.5% for sepsis and 49.6% for ruptured AAA using POSSUM and P-POSSUM scores. Although others supported this concept [46,49–51], it was acknowledged that patients with IAS are significantly less likely to have fascial closure than trauma patients [49,52]. As failure to achieve primary fascial closure is associated with significantly more morbidity and complications, caution in extending the principle to non-trauma patients was still emphasized [53]. Improvements in TAC techniques, however, led to higher fascial closure rates [54]. Recent studies have indeed shown DCS to be a feasible technique in patients with generalized peritonitis [55,56].
Thus, 70 years after its first introduction, the indications for OAM had evolved from intra-abdominal sepsis to DCS and IAH in trauma as well as in emergency and vascular surgery. A summary of its indications is listed in Table 2.
Table 2.
Indications for open abdomen management.
| Cases where the abdomen cannot be closed |
| Loss of abdominal wall e.g. necrotizing fasciitis |
| Inability to close e.g. because of tertiary peritonitis or bowel edema |
| Cases where the abdomen should not be closed |
| Damage Control Surgery |
| Facilitation of re-exploration in abdominal sepsis, when source control hasn’t been accomplished in the initial operation |
| Bowel ischemia |
| Abdominal Compartment Syndrome |
| Surgeon suspicion for intra abdominal hypertension e.g. anticipated to require large volume fluid resuscitation because of shock |
| Combined group |
Temporary Abdominal Closure (TAC) Techniques
With time it has become clear that the final result of OAM is also associated with the design and materials used for TAC. The ideal features of the perfect TAC device are listed in Table 3. Nursing problems, controlling fluid loss, and preventing injury to the viscera were considered as most important. From the early 2000s onwards, a new problem with OAM was recognized: the resulting large ventral abdominal wall hernias. In the existing treatment strategies, the abdomen needed to be closed within a window of 5–7 days, otherwise it was considered unlikely to close at all. In these cases, a planned ventral hernia was created by placing split skin grafts on the viscera with or without a mesh. Abdominal wall reconstruction was planned several months later. This procedure has obvious disadvantages, with high morbidity and a negative effect on quality of life [57]. Failure to primarily close the abdomen is also associated with a significantly higher risk for entero-atmospheric fistula [58]. The ideal TAC device, therefore, not only needs to prevent loss of abdominal domain but also to preserve the fascia/abdominal wall integrity to achieve better primary fascial closure rates [59]. It should also prevent IAH or the development of ACS [39,40].
Table 3.
Ideal features of the temporary abdominal closure device (TAC).
| Contain abdominal contents |
| Protect from external contamination and injury |
| Preserve the integrity of the abdominal wall and support final closure |
| Prevent adherence of the viscera to the abdominal wall and closure material |
| Prevent intra abdominal hypertension |
| Minimize loss of abdominal domain |
| Be easily and rapidly performed |
| Provide easy re-entry |
| Prevent fluid loss |
| Facilitate nursing care |
| Be inexpensive and cost effective |
| Allow patient transport |
When during OAM it is necessary to place (temporary) stomas, they should be placed as laterally as possible to allow maximal medial mobility of the abdominal wall during closure of the OA [60,61].
Many different techniques have been introduced during the past 10 years. Numerous reports exist on all these different techniques [38,39,62–69], but patient groups remained small, with a high heterogeneity in both patients and diseases, making comparison of techniques and outcome impossible. This problem was also recognized by Boele van Hensbroek et al. in their systematic review including 51 studies through 2008 [52]. They found no randomized controlled trials or other comparative studies, limiting the level of evidence of their results. Therefore, their observation that the highest fascial closure rates were seen in the artificial bur (90%), dynamic retention sutures (DRS) (85%), and V.A.C.® (60%) and the lowest mortality rates were seen in the artificial bur (17%), V.A.C.® (18%), and DRS (23%), must be interpreted with great care. Even so, this study should be regarded as the best available level of evidence.
The most important TAC techniques will be discussed here.
Negative Pressure Therapy Techniques
Vacuum pack technique
Brock introduced the vacuum pack technique in 1995 [62]. It was the first technique that used vacuum and has remained until now one of the preferred techniques, if not the current standard of care [42]. It consists of a 3-layer construction: a fenestrated polyethylene sheet between the abdominal viscera and the anterior parietal peritoneum; a moist, surgical towel over the sheet with 2 suction drains; and an adhesive drape over the entire wound, including a wide margin of surrounding skin. The drains are then connected to wall suction, providing 100–150 mmHg continuous negative pressure. One of the main advantages is that it prevents injury to the abdominal wall by not suturing it, preserving it for later closure [10,16,62]. It also is safe, inexpensive, and controls fluid loss. The use of a sterile surgical gown or gauzes wrapped in adhesive drape instead of the fenestrated polyethylene sheet has also been reported [70]. A disadvantage of the technique is that the prevention of loss of abdominal domain seems limited. In a systematic review, vacuum pack showed a 52% primary fascial closure rate [52].
Vacuum-assisted closure and V.A.C.®
To achieve higher fascial closure rates, a modification of the vacuum pack was described by Garner et al. and Miller et al: the vacuum-assisted fascial closure (VAFC) or vacuum-assisted wound closure (VAWC) [71,72]. This system resembles the later developed commercially available V.A.C.® Abdominal Dressing System (KCI, USA). In these first studies, they used a polyurethane sponge (fabricated by KCI Medical) over a non-adhesive polyethylene sheet and used a special pump as the vacuum source instead of wall suction. They also attempted partial suturing of the abdominal wall, placing sutures at the proximal and distal edges after each procedure, and subsequently used smaller pieces of foam. In 2004, the same group presented the results of a prospective study of 53 trauma patients treated with VAFC in a standardized treatment algorithm. They achieved an 88% closure rate, of which 48% was after ≥9 days (range 3–21 days) [73]. The principles of a protective, non-adherent layer between fascia and bowel and the early initiation of partial sutures to achieve higher fascial closure rates were used by others, achieving high fascial closure rates of 65–100% [59,74,75]. All literature on V.A.C.® has recently been critically reviewed by Stevens [76]. Until February 2009 he found 1 randomized controlled trial [77], 3 prospective studies [67,71,73], and several case studies. He found that V.A.C.® therapy increases successful primary fascial closure rates up to 21 days (level 3 evidence). Fistulae were reported only in the minority of wounds and he found no evidence that these are consequential to, as opposed to coincident with, V.A.C.® use. He did, however, express the need for further studies on cost/benefit evaluations and we would like to support this. Subsequent prospective studies also confirmed the use of V.A.C.® to be safe in sepsis patients [78–80].
Mesh
The sandwich technique and zipper technique
As described earlier, these were some of the first described standardized techniques with good results [16,21,22,81]. However, concerns of high fistula rates associated with the placement of non-absorbable mesh placed on unprotected viscera limited general adoption of these techniques [38,82].
Artificial bur device or Wittmann Patch®
The previously described Velcro adhesive sheet technique was first described in 1990 [24,83] and improved into the commercially available Wittmann-Patch® (NovoMedicus, Germany). It consists of 2 adhering sheets of biocompatible polymeric material with hooks on one side and a meshwork of loops on the other. The sheets are sutured to opposite fascial edges; to close the abdomen, the overlapping sheets are compressed to stick together. The sheets are covered by a surgical towel, a suction tube, and an adherent plastic drape. The suction tube is connected to a suction source to create negative pressure. The sheets can be easily pulled apart to allow for re-exploration and tightened every time to allow for gradual closure of the abdominal wall. In a systematic review, it had the highest fascial closure rate (90%) [52].
The use of other prosthetic mesh such as GORE-TEX® DUALMESH® has also been reported in some studies [84]. However, it is a costly technique that does not preserve the fascia. The use of biological prostheses such as acellular dermal matrix can be used to close remaining small abdominal wall defects after OAM [60,61,85,86].
In general, primary fascial closure within the initial admission is associated with the best outcome [59]. In order to achieve this, a non-adherent layer should be placed between the viscera and the abdominal wall, preventing adhesions and preserving the peritoneal space and abdominal wall [25,86,87]. At every subsequent procedure, partial sutures of the abdominal wall at the proximal and distal edges should be attempted, but not under tension [59,60,73–75]. A source of vacuum should be used to control fluid loss and possibly aid in prevention of abdominal domain [60]. Gauze and non-absorbable mesh placed directly on the bowels are associated with the occurrence of entero-atmospheric fistulas (up to 75%) [42]. Therefore, permanent mesh (e.g., polypropylene) or gauze should not be used in direct contact with the viscera [60,61,88,89].
The Bogota Bag
Suturing a 3-L urologic irrigation bag to the fascia or skin was first used simultaneously in several institutions in Colombia in 1984 [90]. It became known worldwide when published in the English literature after Feliciano [91] and Mattox [90] observed Oswaldo Borraez using the technique in Bogota, Colombia. The technique was named ‘the Bogota bag’ [23,92,93]. This technique, however, does not preserve the fascia and might not prevent IAH [89]. In a systematic review, it showed a weighted mortality rate of 41% [52]. Several modifications of the technique have therefore been reported, including the use of double sheets and suction tubes, with good results [94–97] but continuous IAP measurement is necessary.
Dynamic Retention Sutures (DRS)
To further improve fascial closure rates, combinations of techniques combined with retention sutures as well as specially designed dynamic retention suture systems (e.g., ABRA®) have been studied. An RCT comparing a combination of V.A.C.® and retention sutures with V.A.C.® alone in a total of 30 patients with abdominal sepsis was done by Pliakos et al. [98]. They achieved significantly higher closure rates in patients treated with the combination (93.3%). However, their results are limited by the small study group and lack of long-term follow-up. A prospective analysis of a combination of V.A.C.® and mesh-mediated fascial traction was described in 111 patients (trauma and non-trauma). They achieved fascial closure rates of 76.6% in intention-to-treat analysis and 89% in per-protocol analysis. They had a 7.2% fistula rate and a 29.7% in-hospital mortality rate. Intestinal fistula was an independent factor associated with failure of fascial closure. Age and failure of fascial closure were independently associated with in-hospital mortality [54]. The ABRA® system was described by Reimer et al. [99], who achieved a delayed fascial closure rate of 61% starting an average of no less than 18 days after the initial operation. They did, however, have a 26% hernia rate in follow-up.
In conclusion, a combination of techniques including retention sutures and vacuum seems to increase delayed fascial closure rates. Care must be taken to preserve the abdominal wall and prevent IAH in using these techniques.
Conclusions
In this review we have given a historical perspective on the evolvement of OAM. Over the years it has developed from a last resort treatment strategy in abdominal catastrophes to a preferred treatment strategy in ACS and DCS in trauma and non-trauma patients. It is likely to further decrease mortality in critically ill or injured patients. It also poses great challenges in dealing with morbidity due to entero-atmospheric fistulas and abdominal wall hernias. The treatment of these fistulas and abdominal wall reconstruction are beyond the scope of this review. However, it is clear that the main goal of OAM is to preserve abdominal domain and prevent fistulization to achieve primary fascial closure. The technique of TAC is therefore very important in keeping complications as low as possible. A combination of vacuum techniques and retention sutures may achieve the highest delayed fascial closure rates. But above all, preventing and treating ongoing MOF in these patients necessitates a dedicated multidisciplinary team.
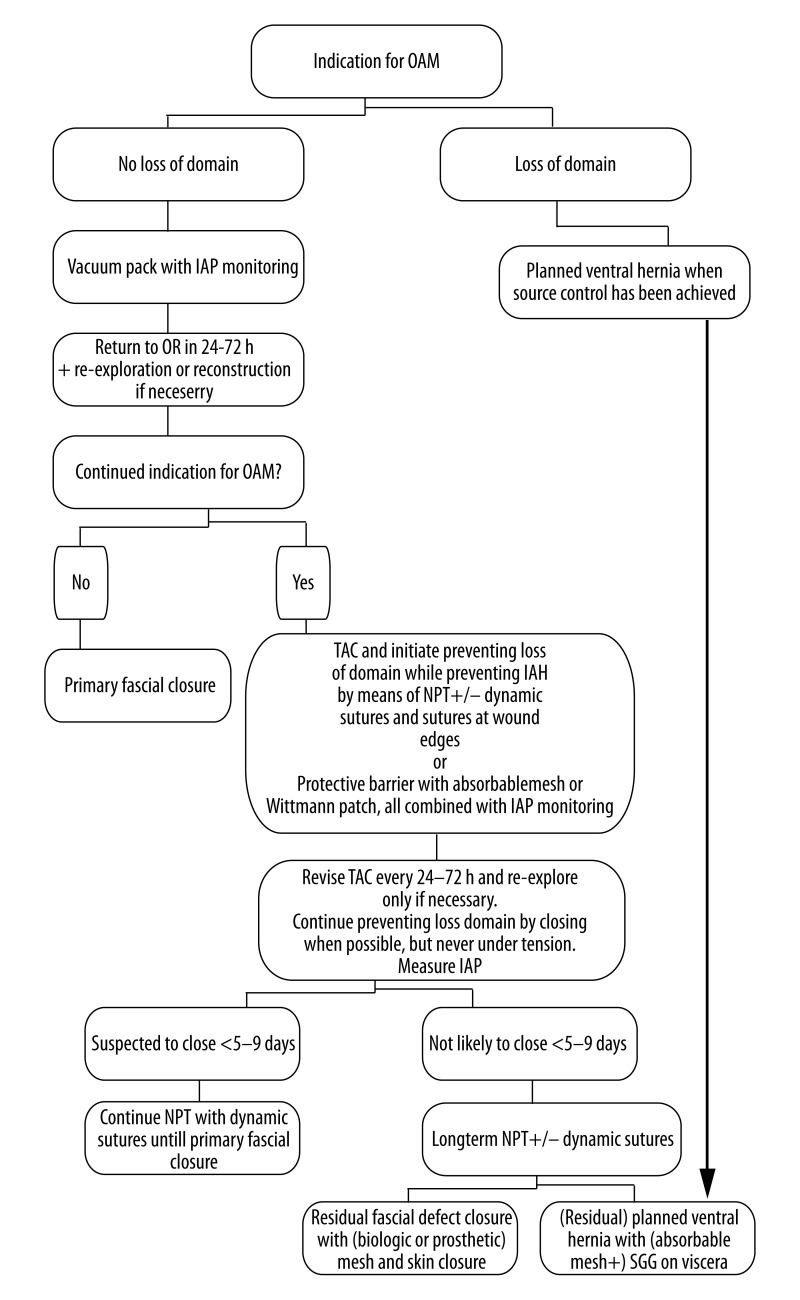
In our own experience OAM is currently still mostly used without sound indications and rational and using a large variety of non-standardized home-made TAC techniques. As a result, improvement in care of the critically ill or injured patients with OAM is hard to prove or implement. Standardization of indications, techniques, and uniform definitions will facilitate and better validate further research. Björck et al. have started with proposing a classification system of OA, which is shown in Table 4[47]. Based on historical experience as presented in the literature, we propose the OAM algorithm shown in Figure 1. We chose the vacuum pack as an initial primary TAC device because it is cheap, safe, and considered as the current standard of care. Intra-abdominal pressures should be measured every 6 hours. When there is an indication for continued OAM after 24–72 hours, the focus should be on maintaining abdominal wall integrity by using one of the described techniques. The mentioned timeframe of 5–9 days is of course arbitrary, but in our experience it usually becomes clear in this time period whether early primary closure will be successful or not. If one is able to prevent loss of domain beyond this time frame with the techniques described (NPT with dynamic sutures, absorbable mesh with a protective barrier or the Wittman patch), in some cases primary fascial closure can still be achieved after 10 days. If not, the residual fascial defect probably has to be closed with mesh (biological or prosthetic), split skin grafts, component parts separation techniques, or a combination of these.
Table 4.
Classifications of open abdomens (OA).
| Grade 1A | Clean OA without adherence between bowel and abdominal wall or fixity (lateralization of the abdominal wall) |
| Grade 1B | Contaminated OA without adherence/ fixity |
| Grade 2A | Clean OA developing adherence/ fixity |
| Grade 2B | Contaminated OA developing adherence/ fixity |
| Grade 3 | OA complicated by fistula formation |
| Grade 4 | Frozen OA with adherent/ fixed bowel, unable to close surgically, with or without fistula |
Figure 1.
Open abdomen management algorithm.
By proposing this algorithm we hope to offer a more standardized management strategy and a tool for use in dealing with this difficult group of patients. Above all, we hope that this algorithm can contribute to putting the potentially worthwhile technique of the open abdomen in a clear perspective to further facilitate research. We are sure that the technique of OAM will then prove its worth.
Footnotes
Source of support: Self financing
Statement
All authors confirm that they have not received any support in the form of grants, etc.
References
- 1.Ogilvie WH. The late complications of abdominal war wounds. Lancet. 1940;2:253–56. [Google Scholar]
- 2.Christou NV, Barie PS, Dellinger EP, et al. Surgical Infection Society intra-abdominal infection study. Prospective evaluation of management techniques and outcome. Arch Surg. 1993;128(2):193–98. doi: 10.1001/archsurg.1993.01420140070011. [DOI] [PubMed] [Google Scholar]
- 3.Lamme B, Boermeester MA, Belt EJT, et al. Mortality and morbidity of planned relaparotomy versus relaparotomy on demand for secondary peritonitis. Br J Surg. 2004;91(8):1046–54. doi: 10.1002/bjs.4517. [DOI] [PubMed] [Google Scholar]
- 4.van Ruler O, Mahler CW, Boer KR, et al. Comparison of on-demand vs planned relaparotomy strategy in patients with severe peritonitis: a randomized trial. JAMA. 2007;298(8):865–72. doi: 10.1001/jama.298.8.865. [DOI] [PubMed] [Google Scholar]
- 5.Bosscha K, Hulstaert PF, Visser MR, et al. Open management of the abdomen and planned reoperations in severe bacterial peritonitis. Eur J Surg. 2000;166(1):44–49. doi: 10.1080/110241500750009690. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick AW, Laupland KB, Karmali S, et al. Spill your guts! Perceptions of Trauma Association of Canada member surgeons regarding the open abdomen and the abdominal compartment syndrome. J Trauma. 2006;60(2):279–86. doi: 10.1097/01.ta.0000205638.26798.dc. [DOI] [PubMed] [Google Scholar]
- 7.Karmali S, Evans D, Laupland KB, et al. To close or not to close, that is one of the questions? Perceptions of Trauma Association of Canada surgical members on the management of the open abdomen. J Trauma. 2006;60(2):287–93. doi: 10.1097/01.ta.0000203579.62446.75. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvie WH. Surgical Lessons of War applied to Civil Practice. Br Med J. 1945;1(4400):619–23. doi: 10.1136/bmj.1.4400.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg D. On leaving the peritoneal cavity open in acute generalized suppurative peritonitis. Am J Surg. 1979;137(2):216–20. doi: 10.1016/0002-9610(79)90148-x. [DOI] [PubMed] [Google Scholar]
- 10.Duff JH, Moffat J. Abdominal sepsis managed by leaving abdomen open. Surgery. 1981;90(4):774–78. [PubMed] [Google Scholar]
- 11.Open Management of the Septic Abdomen [editorial] Lancet. 1986;2:138–39. [PubMed] [Google Scholar]
- 12.Broomé A, Hansson L, Lundgren F, Smedberg S. Open Treatment of Abdominal Septic Catastrophies. World J Surg. 1983;7:792–96. doi: 10.1007/BF01655223. [DOI] [PubMed] [Google Scholar]
- 13.Mughal MM, Bancewicz J, Irving MH. “Laparostomy”: a technique for the management of intractable intra-abdominal sepsis”. Br J Surg. 1986;73(4):253–59. doi: 10.1002/bjs.1800730405. [DOI] [PubMed] [Google Scholar]
- 14.Anderson ED, Mandelbaum DM, Ellison EC, et al. Open Packing of the Peritoneal Cavity in Generalized Bacterial Peritonitis. Am J Surg. 1983;145:131–35. doi: 10.1016/0002-9610(83)90179-4. [DOI] [PubMed] [Google Scholar]
- 15.Schein M, Saadia R, Decker GG. The open management of the septic abdomen. Surg Gynecol Obstet. 1986;163(6):587–92. [PubMed] [Google Scholar]
- 16.Schein M, Saadia R, Jamieson JR, Decker GA. The “sandwich technique” in the management of the open abdomen. Br J Surg. 1986;73(5):369–70. doi: 10.1002/bjs.1800730514. [DOI] [PubMed] [Google Scholar]
- 17.Mastboom WJ, Kuypers HH, Schoots FJ, Wobbes T. Small-bowel perforation complicating the open treatment of generalized peritonitis. Arch Surg. 1989;124(6):689–92. doi: 10.1001/archsurg.1989.01410060055011. [DOI] [PubMed] [Google Scholar]
- 18.Teichmann W, Wittmann D, Andreone P. Scheduled Reoperations (Etappenlavage) for Diffuse Peritonitis. Arch Surg. 1986;121:147–52. doi: 10.1001/archsurg.1986.01400020033002. [DOI] [PubMed] [Google Scholar]
- 19.Penninckx FM, Kerremans RP, Lauwers PM. Planned Relaparotomies in the Surgical Treatment of Severe Generalized Peritonitis from Intestinal Origin. World J Surg. 1983;7:762–66. doi: 10.1007/BF01655218. [DOI] [PubMed] [Google Scholar]
- 20.Schein M, Saadia R, Freinkel Z, Decker G. Aggressive treatment of severe diffuse peritonitis: a prospective study. Br J Surg. 1988;75(2):173–76. doi: 10.1002/bjs.1800750230. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Sabrido JL, Tallado JM, Christou NV, et al. Treatment of severe intra-abdominal sepsis and/or necrotic foci by an “open-abdomen” approach. Zipper and zipper-mesh techniques. Arch Surg. 1988;123(2):152–56. doi: 10.1001/archsurg.1988.01400260032002. [DOI] [PubMed] [Google Scholar]
- 22.Hedderich GS, Wexler MJ, McLean AP, Meakins JL. The septic abdomen: open management with Marlex mesh with a zipper. Surgery. 1986;99(4):399–408. [PubMed] [Google Scholar]
- 23.Howard CA, Turner WW. Successful treatment of early, postoperative, necrotizing infection of the abdominal wall. Crit Care Med. 1989;17(6):586–87. doi: 10.1097/00003246-198906000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990;14(2):218–26. doi: 10.1007/BF01664876. [DOI] [PubMed] [Google Scholar]
- 25.Ivatury RR, Nallathambi M, Rao PM, et al. Open management of the septic abdomen: therapeutic and prognostic considerations based on APACHE II. Crit Care Med. 1989;17(6):511–17. doi: 10.1097/00003246-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Voyles CR, Richardson JD, Bland K, et al. Emergency Abdominal Wall Reconstruction with Polypropylene Mesh Short-term Benefits Versus Long-term Complications. Ann Surg. 1981;194(2):219–23. doi: 10.1097/00000658-198108000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sökmen S, Atila K, Bora S, et al. Evaluation of prosthetic mesh closure in semiopen-abdomen patients. Hernia. 2002;6(3):124–29. doi: 10.1007/s10029-002-0072-2. [DOI] [PubMed] [Google Scholar]
- 28.Adkins AL, Robbins J, Villalba M, et al. Open abdomen management of intra-abdominal sepsis. Am Surg. 2004;70(2):137–40. [PubMed] [Google Scholar]
- 29.Lamme B, Boermeester MA, Reitsma JB, et al. Meta-analysis of relaparotomy for secondary peritonitis. Br J Surg. 2002;89(12):1516–24. doi: 10.1046/j.1365-2168.2002.02293.x. [DOI] [PubMed] [Google Scholar]
- 30.Schein M. Planned Reoperations and Open Management in Critical Intra-abdominal Infections: Prospective Experience in 52 Cases. World J Surg. 1991;15(4):537–45. doi: 10.1007/BF01675658. [DOI] [PubMed] [Google Scholar]
- 31.Hau T, Ohmann C, Wolmershauser A, et al. Planned Relaparotomy vs Relaparotomy on Demand in the Treatment of Intra-abdominal Infections. Arch Surg. 1995;130:1193–97. doi: 10.1001/archsurg.1995.01430110051009. [DOI] [PubMed] [Google Scholar]
- 32.Wittmann D. Operative and nonoperative therapy of intraabdominal infections. Infection. 1998;26(5):335–41. doi: 10.1007/BF02962267. [DOI] [PubMed] [Google Scholar]
- 33.Schein M. Surgical management of intra-abdominal infection: is there any evidence? Langenbecks Arch Surg. 2002;387(1):1–7. doi: 10.1007/s00423-002-0276-z. [DOI] [PubMed] [Google Scholar]
- 34.Robledo FA, Luque-de-león E, Suárez R, et al. Open versus Closed Management of the Abdomen in the Surgical Treatment of Severe Secondary Peritonitis: A Randomized Clinical Trial. Surg Infect. 2007;8(1):63–71. doi: 10.1089/sur.2006.8.016. [DOI] [PubMed] [Google Scholar]
- 35.Boermeester MA. Surgical approaches to peritonitis. Br J Surg. 2007;94(11):1317–18. doi: 10.1002/bjs.6041. [DOI] [PubMed] [Google Scholar]
- 36.Kiewiet JJS, van Ruler O, Reitsma JB, Boermeester MA. Treatment of secondary peritonitis: slow progress. Ned Tijdschr Geneeskd. 2009;153:A386. [PubMed] [Google Scholar]
- 37.van Ruler O, Lamme B, Gouma DJ, et al. Variables associated with positive findings at relaparotomy in patients with secondary peritonitis. Crit Care Med. 2007;35(2):468–76. doi: 10.1097/01.CCM.0000253399.03545.2D. [DOI] [PubMed] [Google Scholar]
- 38.Smith LA, Barker DE, Chase CW, et al. Vacuum pack technique of temporary abdominal closure: a four-year experience. Am Surg. 1997;63(12):1102–7. [PubMed] [Google Scholar]
- 39.Sugrue M, Jones F, Jahangir Jannua K. Temporary abdominal closure: a prospective evaluation of its effects on renal and respiratory physiology. J Trauma. 1998;45(5):914–21. doi: 10.1097/00005373-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Gracias VH, Braslow B, Johnson J, et al. Abdominal compartment syndrome in the open abdomen. Arch Surg. 2002;137(11):1298–300. doi: 10.1001/archsurg.137.11.1298. [DOI] [PubMed] [Google Scholar]
- 41.Duchesne JC, Baucom CC, Rennie KV, et al. Recurrent abdominal compartment syndrome: an inciting factor of the second hit phenomenon. Am Surg. 2009;75(12):1193–98. [PubMed] [Google Scholar]
- 42.Diaz JJ, Cullinane DC, Dutton WD, et al. The management of the open abdomen in trauma and emergency general surgery: part 1-damage control. J Trauma. 2010;68(6):1425–38. doi: 10.1097/TA.0b013e3181da0da5. [DOI] [PubMed] [Google Scholar]
- 43.Sugrue M, Buhkari Y. Intra-abdominal pressure and abdominal compartment syndrome in acute general surgery. World J Surg. 2009;33(6):1123–27. doi: 10.1007/s00268-009-0040-4. [DOI] [PubMed] [Google Scholar]
- 44.Cheatham ML, Safcsak K. Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med. 2010;38(2):402–7. doi: 10.1097/ccm.0b013e3181b9e9b1. [DOI] [PubMed] [Google Scholar]
- 45.Rotondo MF, Schwab CW, McGonigal MD, et al. “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury”. J Trauma. 1993;35(3):375–82. [PubMed] [Google Scholar]
- 46.Waibel BH, Rotondo MF. Damage control in trauma and abdominal sepsis. Crit Care Med. 2010;38(9 Suppl):S421–30. doi: 10.1097/CCM.0b013e3181ec5cbe. [DOI] [PubMed] [Google Scholar]
- 47.Björck M, Bruhin A, Cheatham M, et al. Classification – important step to improve management of patients with an open abdomen. World J Surg. 2009;33(6):1154–57. doi: 10.1007/s00268-009-9996-3. [DOI] [PubMed] [Google Scholar]
- 48.Finlay IG, Edwards TJ, Lambert AW. Damage control laparotomy. Br J Surg. 2004;91(1):83–85. doi: 10.1002/bjs.4434. [DOI] [PubMed] [Google Scholar]
- 49.Tsuei BJ, Skinner JC, Bernard AC, et al. The open peritoneal cavity: etiology correlates with the likelihood of fascial closure. Am Surg. 2004;70(7):652–56. [PubMed] [Google Scholar]
- 50.Cipolla J, Stawicki SP, Hoff WS, et al. A proposed algorithm for managing the open abdomen. Am Surg. 2005;71(3):202–7. doi: 10.1177/000313480507100305. [DOI] [PubMed] [Google Scholar]
- 51.Stawicki SP, Brooks A, Bilski T, et al. The concept of damage control: extending the paradigm to emergency general surgery. Injury. 2008;39(1):93–101. doi: 10.1016/j.injury.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Boele van Hensbroek P, Wind J, Dijkgraaf MGW, et al. Temporary closure of the open abdomen: a systematic review on delayed primary fascial closure in patients with an open abdomen. World J Surg. 2009;33(2):199–207. doi: 10.1007/s00268-008-9867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen JO, Loudon MA. Damage control surgery in a non-trauma setting. Br J Surg. 2007;94(7):789–90. doi: 10.1002/bjs.5922. [DOI] [PubMed] [Google Scholar]
- 54.Acosta S, Bjarnason T, Petersson U, et al. Multicentre prospective study of fascial closure rate after open abdomen with vacuum and mesh-mediated fascial traction. Br J Surg. 2011;98(5):735–43. doi: 10.1002/bjs.7383. [DOI] [PubMed] [Google Scholar]
- 55.Perathoner A, Klaus A, Mühlmann G, et al. Damage control with abdominal vacuum therapy (VAC) to manage perforated diverticulitis with advanced generalized peritonitis – a proof of concept. Int J Colorectal Dis. 2010;25(6):767–74. doi: 10.1007/s00384-010-0887-8. [DOI] [PubMed] [Google Scholar]
- 56.Kritayakirana K, Maggio P, Brundage S, et al. Outcomes and complications of open abdomen technique for managing non-trauma patients. J Emerg Trauma Shock. 2010;3(2):118. doi: 10.4103/0974-2700.62106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheatham ML, Safcsak K, Llerena LE, et al. Long-term physical, mental, and functional consequences of abdominal decompression. J Trauma. 2004;56(2):237–41. doi: 10.1097/01.TA.0000109858.55483.86. [DOI] [PubMed] [Google Scholar]
- 58.Teixeira PGR, Salim A, Inaba K, et al. A prospective look at the current state of open abdomens. Am Surg. 2008;74(10):891–97. doi: 10.1177/000313480807401002. [DOI] [PubMed] [Google Scholar]
- 59.Miller RS, Morris JA, Diaz JJ, et al. Complications after 344 damage-control open celiotomies. J Trauma. 2005;59(6):1365–71. doi: 10.1097/01.ta.0000196004.49422.af. [DOI] [PubMed] [Google Scholar]
- 60.Schecter WP, Ivatury RR, Rotondo MF, Hirshberg A. Open abdomen after trauma and abdominal sepsis: a strategy for management. J Am Coll Surg. 2006;203(3):390–6. doi: 10.1016/j.jamcollsurg.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Campbell A, Chang M, Fabian T, et al. Management of the open abdomen: from initial operation to definitive closure. Am Surg. 2009;75(11 Suppl):S1–22. [PubMed] [Google Scholar]
- 62.Brock WB, Barker DE, Burns RP. Temporary closure of open abdominal wounds: the vacuum pack. Am Surg. 1995;61(1):30–35. [PubMed] [Google Scholar]
- 63.Barker DE, Green JM, Maxwell RA, et al. Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg. 2007;204(5):784–92. doi: 10.1016/j.jamcollsurg.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 64.Hadeed JG, Staman GW, Sariol HS, et al. Delayed primary closure in damage control laparotomy: the value of the Wittmann patch. Am Surg. 2007;73(1):10–12. [PubMed] [Google Scholar]
- 65.Olejnik J, Sedlak I, Brychta I, Tibensky I. Vacuum supported laparostomy – an effective treatment of intraabdominal infection. Bratisl Lek Listy. 2007;108(7):320–23. [PubMed] [Google Scholar]
- 66.Gäddnäs F, Saarnio J, Ala-Kokko T, et al. Continuous retention suture for the management of open abdomen: a high rate of delayed fascial closure. Scand J Surg. 2007;96(4):301–7. doi: 10.1177/145749690709600408. [DOI] [PubMed] [Google Scholar]
- 67.Perez D, Wildi S, Demartines N, et al. Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg. 2007;205(4):586–92. doi: 10.1016/j.jamcollsurg.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Petersson U, Acosta S, Björck M. Vacuum-assisted wound closure and mesh-mediated fascial traction--a novel technique for late closure of the open abdomen. World J Surg. 2007;31(11):2133–37. doi: 10.1007/s00268-007-9222-0. [DOI] [PubMed] [Google Scholar]
- 69.van As AB, Navsaria P, Numanoglu A, McCulloch M. Modified sandwich vacuum pack technique for temporary closure of abdominal wounds: an African perspective. Acta Clin Belg Suppl. 2007;(1):215–19. doi: 10.1179/acb.2007.62.s1.029. [DOI] [PubMed] [Google Scholar]
- 70.van Wessem KJP. Possible devices to temporary cover the open abdomen: pros and cons. Acta Chir Belg. 2010;110(5):499–503. [PubMed] [Google Scholar]
- 71.Miller PR, Thompson JT, Faler BJ, et al. Late fascial closure in lieu of ventral hernia: the next step in open abdomen management. J Trauma. 2002;53(5):843–49. doi: 10.1097/00005373-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Garner GB, Ware DN, Cocanour CS, et al. Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg. 2001;182:630–38. doi: 10.1016/s0002-9610(01)00786-3. [DOI] [PubMed] [Google Scholar]
- 73.Miller PR, Meredith JW, Johnson JC, Chang MC. Prospective Evaluation of Vacuum-Assisted Fascial Closure After Open Abdomen. Ann Surg. 2004;239(5):608–16. doi: 10.1097/01.sla.0000124291.09032.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cothren CC, Moore EE, Johnson JL, et al. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg. 2006;192(2):238–42. doi: 10.1016/j.amjsurg.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Scott BG, Welsh FJ, Pham HQ, et al. Early aggressive closure of the open abdomen. J Trauma. 2006;60(1):17–22. doi: 10.1097/01.ta.0000200861.96568.bb. [DOI] [PubMed] [Google Scholar]
- 76.Stevens P. Vacuum-assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J. 2009;6(4):259–66. doi: 10.1111/j.1742-481X.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bee TK, Croce MA, Magnotti LJ, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma. 2008;65(2):337–42. doi: 10.1097/TA.0b013e31817fa451. [DOI] [PubMed] [Google Scholar]
- 78.Wondberg D, Larusson HJ, Metzger U, et al. Treatment of the open abdomen with the commercially available vacuum-assisted closure system in patients with abdominal sepsis: low primary closure rate. World J Surg. 2008;32(12):2724–29. doi: 10.1007/s00268-008-9762-y. [DOI] [PubMed] [Google Scholar]
- 79.Amin AI. Topical negative pressure in managing severe peritonitis: A positive contribution? World J Gastroenterol. 2009;15(27):3394. doi: 10.3748/wjg.15.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padalino P, Dionigi G, Minoja G, et al. Fascia-to-fascia closure with abdominal topical negative pressure for severe abdominal infections: preliminary results in a department of general surgery and intensive care unit. Surg Infect. 2010;11(6):523–28. doi: 10.1089/sur.2010.042. [DOI] [PubMed] [Google Scholar]
- 81.Cuesta MA, Doblas M, Castafieda L, Bengoechea E. Sequential Abdominal Reexploration with the Zipper Technique. World J Surg. 1991;15(1):74–80. doi: 10.1007/BF01658968. [DOI] [PubMed] [Google Scholar]
- 82.Losanoff JE, Richman BW, Jones JW. Temporary abdominal coverage and reclosure of the open abdomen: frequently asked questions. J Am Coll Surg. 2002;195(1):105–15. doi: 10.1016/s1072-7515(02)01149-3. [DOI] [PubMed] [Google Scholar]
- 83.Aprahamian C, Wittmann DH, Bergstein JM, Quebbeman EJ. Temporary abdominal closure (TAC) for planned relaparotomy (etappenlavage) in trauma. J Trauma. 1990;30(6):719–23. doi: 10.1097/00005373-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Vertrees A, Greer L, Pickett C, et al. Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg. 2008;207(6):801–9. doi: 10.1016/j.jamcollsurg.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Fayman MS, Schein M, Saadia R. Abdominal wall reconstruction after open management of the septic abdomen. S Afr J Surg. 1990;28(2):62–65. [PubMed] [Google Scholar]
- 86.Scott BG, Feanny MA, Hirshberg A. Early definitive closure of the open abdomen: a quiet revolution. Scan J Surg. 2005;94(1):9–14. doi: 10.1177/145749690509400104. [DOI] [PubMed] [Google Scholar]
- 87.Ivatury RR, Kolkman KA, Johansson K. Management of open abdomen. Acta Clin Belg Suppl. 2007;(1):206–9. [PubMed] [Google Scholar]
- 88.Kaplan M, Banwell P, Orgill D, et al. Guidelines for the Management of the Open Abdomen. Wounds Suppl. :2005. [Google Scholar]
- 89.Sugrue M, D’Amours SK, Kolkman KA. Temporary abdominal closure. Acta Clin Belg Suppl. 2007;(1):210–14. doi: 10.1179/acb.2007.62.s1.028. [DOI] [PubMed] [Google Scholar]
- 90.Mattox K. Introduction, background, and future projections of damage control surgery. Surg Clin North Am. 1997;77(4):753–59. doi: 10.1016/s0039-6109(05)70581-8. [DOI] [PubMed] [Google Scholar]
- 91.Feliciano D, Mattox K, Moore EE. Trauma. 6th edition. 2008. p. 860. [Google Scholar]
- 92.Borraez OA. Abdomen abierto: la herida más desafiante. Rev Colomb Cir. 2008;23(4):204–9. [in Spain] [Google Scholar]
- 93.Ghimenton F, Thomson SR, Muckart DJ, Burrows R. Abdominal content containment: practicalities and outcome. Br J Surg. 2000;87(1):106–9. doi: 10.1046/j.1365-2168.2000.01337.x. [DOI] [PubMed] [Google Scholar]
- 94.Navsaria PH, Bunting M, Omoshoro-Jones J, et al. Temporary closure of open abdominal wounds by the modified sandwich-vacuum pack technique. Br J Surg. 2003;90(6):718–22. doi: 10.1002/bjs.4101. [DOI] [PubMed] [Google Scholar]
- 95.Joglar F, Agosto E, Marrero D, et al. Dynamic retention suture closure: modified Bogotá bag approach. J Surg Res. 2010;162(2):274–78. doi: 10.1016/j.jss.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 96.Goodman MD, Pritts TA, Tsuei BJ. Development of a novel method of progressive temporary abdominal closure. Surgery. 2010;148(4):799–805. doi: 10.1016/j.surg.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 97.Kirshtein B, Roy-Shapira A, Lantsberg L, Mizrahi S. Use of the “Bogota bag” for temporary abdominal closure in patients with secondary peritonitis. Am Surg. 2007;73(3):249–52. doi: 10.1177/000313480707300310. [DOI] [PubMed] [Google Scholar]
- 98.Pliakos I, Papavramidis TS, Mihalopoulos N, et al. Vacuum-assisted closure in severe abdominal sepsis with or without retention sutured sequential fascial closure: a clinical trial. Surgery. 2010;148(5):947–53. doi: 10.1016/j.surg.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 99.Reimer MW, Yelle J-D, Reitsma B, et al. Management of open abdominal wounds with a dynamic fascial closure system. Can J Surg. 2008;51(3):209–14. [PMC free article] [PubMed] [Google Scholar]