Abstract
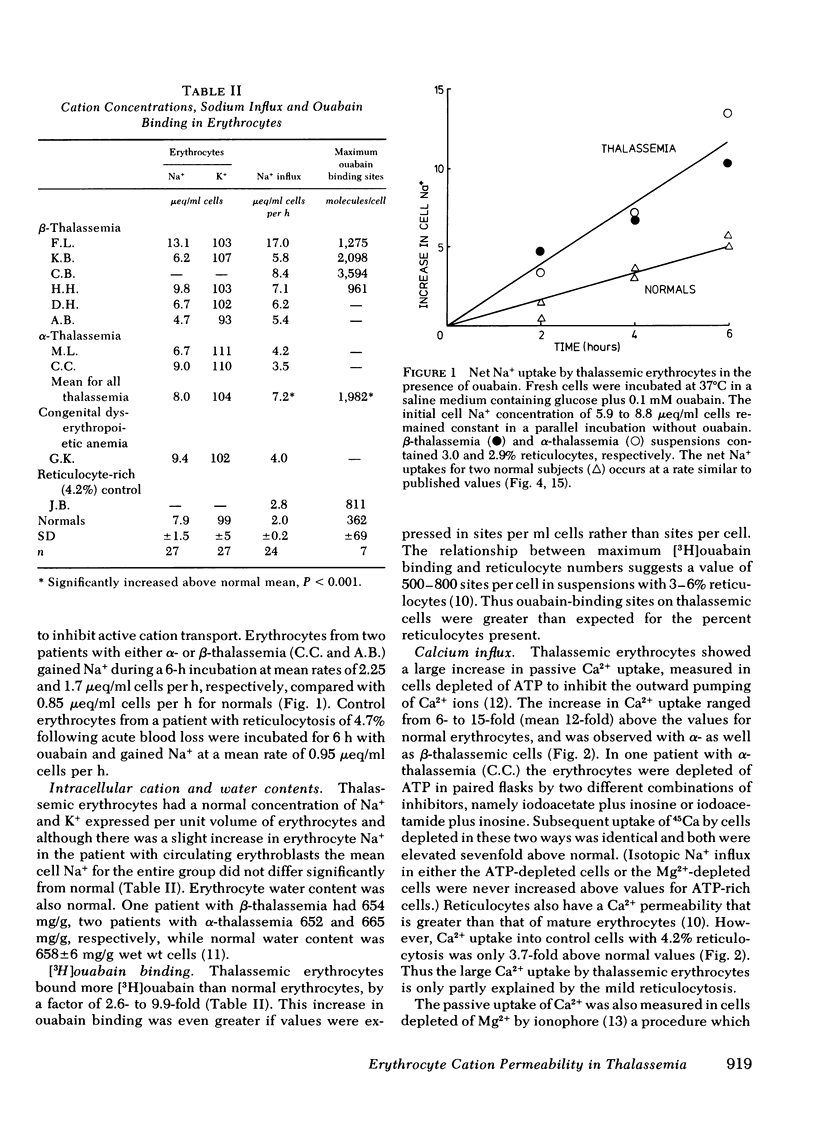
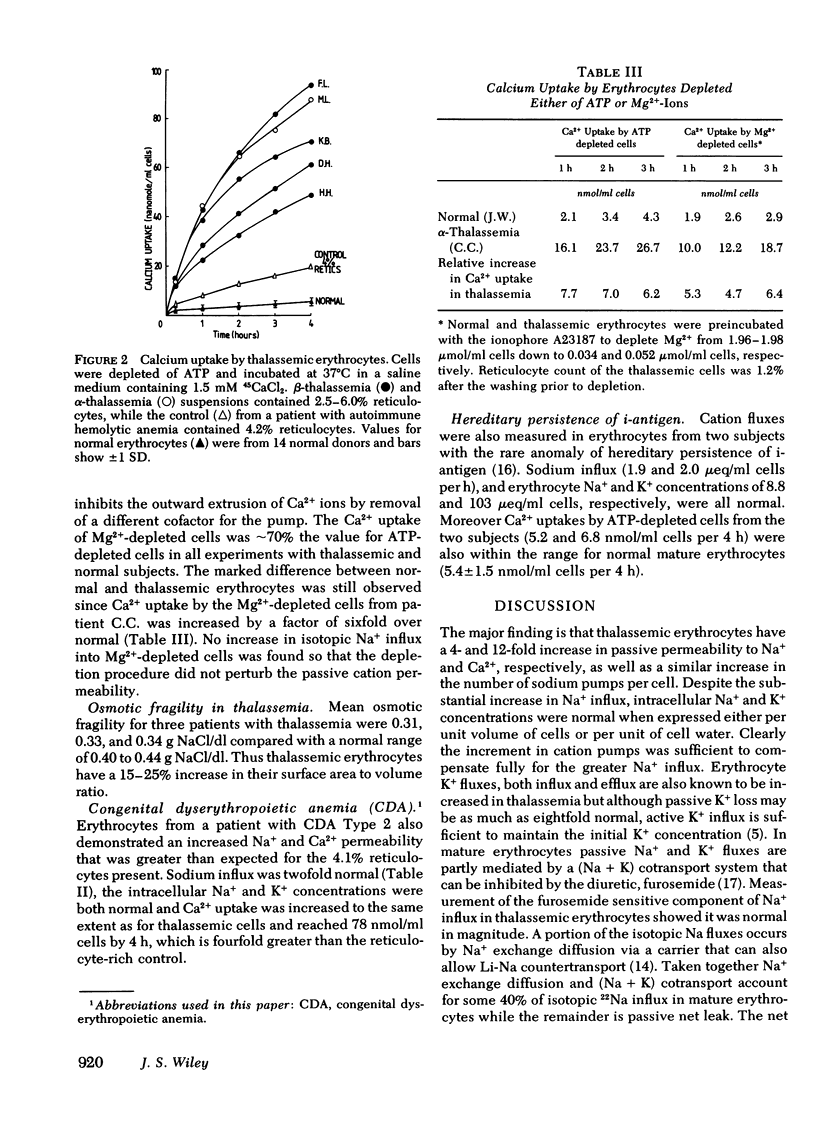
Calcium and sodium permeability of erythrocytes from patients with untransfused α- or β- thalassemia major has been studied and compared to mature erythrocytes or control cells with comparable reticulocytosis. Isotopic Na+ influx was increased a mean fourfold greater than normals and threefold greater than reticulocyte rich control. Passive net leak of Na+ into thalassemic cells incubated with ouabain was also increased corresponding to their greater 22Na+ influx. Erythrocyte Na+ and K+ concentrations and cell water content per unit volume of cells were normal. Quantitation of active cation pumps in the cell membrane by the technique of [3H]ouabain binding showed a 2.6- to 9.9-fold increase above normal. Inward Ca2+ movement was studied in cells with absent Ca2+ pumping produced by depletion of either ATP or Mg2+-ions. Calcium uptake by ATP depleted thalassemic cells was increased 12-fold above normals and 3.6-fold above reticulocyte-rich controls. The Ca2+ uptake by Mg2+-depleted thalassemic cells was also increased above normal confirming that erythrocyte Ca2+ permeability is increased in this disease. Osmotic fragility measurements show that the surface area to volume ratio of thalassemic erythrocytes was increased by 15 to 25% above mature erythrocytes. The increased passive cation permeability of thalassemic erythrocytes cannot be explained by either reticulocytosis or an increased surface area to volume ratio of these cells. Moreover, erythrocyte Na+ and Ca2+ influxes in congenital dyserythropoietic anemia (CDA type 2) were increased 2- and 14-fold, respectively, above normal. The increased cation fluxes and cation pump numbers in thalassemic and congenital dyserythropoietic anemia erythrocytes are consistent with the hypothesis of membrane immaturity arising from rapid marrow transit times, a concept previously advanced to explain the persistence of i-antigen on these cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EYLAR E. H., MATIOLI G. T. GLYCOPROTEIN BIOSYNTHESIS IN HUMAN RETICULOCYTES: A LESION IN THALASSEMIA. Science. 1965 Feb 19;147(3660):869–870. doi: 10.1126/science.147.3660.869. [DOI] [PubMed] [Google Scholar]
- FINCH C. A., STURGEON P. Erythrokinetics in Cooley's anemia. Blood. 1957 Jan;12(1):64–73. [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Flatman P., Lew V. L. Use of ionophore A23187 to measure and to control free and bound cytoplasmic Mg in intact red cells. Nature. 1977 May 26;267(5609):360–362. doi: 10.1038/267360a0. [DOI] [PubMed] [Google Scholar]
- Friedman S., Oski F. A., Schwartz E. Bone marrow and peripheral blood globin synthesis in an American black family with beta thalassemia. Blood. 1972 Jun;39(6):785–793. [PubMed] [Google Scholar]
- GIBLETT E. R., CROOKSTON M. C. AGGLUTINABILITY OF RED CELLS BY ANTI-I IN PATIENTS WITH THALASSEMIA MAJOR AND OTHER HAEMATOLOGICAL DISORDERS. Nature. 1964 Mar 14;201:1138–1139. doi: 10.1038/2011138a0. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Silvers D. N., Rosse W. F. Potassium permeability in -thalassemia minor red blood cells. J Clin Invest. 1972 May;51(5):1043–1050. doi: 10.1172/JCI106895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLCOAT B. L., WATERS A. H. The survival of 51Cr labelled autotransfused red cells in patients with thalassaemia. Australas Ann Med. 1962 Feb;11:55–58. doi: 10.1111/imj.1962.11.1.55. [DOI] [PubMed] [Google Scholar]
- Hillman R. S., Giblett E. R. Red cell membrane alteration associated with "marrow stress". J Clin Invest. 1965 Oct;44(10):1730–1736. doi: 10.1172/JCI105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Joiner C. H. Increased potassium transport and ouabain binding in human Rhnull red blood cells. Blood. 1976 Sep;48(3):457–468. [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Lowenthal R. M., Marsden K. A., Dewar C. L., Thompson G. R. Congenital dyserythropoietic anaemia (CDA) with severe gout, rare Kell phenotype and erythrocyte, granulocyte and platelet membrane reduplication: a new variant of CDA type II. Br J Haematol. 1980 Feb;44(2):211–220. doi: 10.1111/j.1365-2141.1980.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Marsh W. L. Scoring of hemagglutination reactions. Transfusion. 1972 Sep-Oct;12(5):352–353. doi: 10.1111/j.1537-2995.1972.tb04459.x. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Stossel T. B., Gunn R. B., Zarkowsky H. S., Laforet M. T. Influence of hemoglobin precipitation on erythrocyte metabolism in alpha and beta thalassemia. J Clin Invest. 1969 Jan;48(1):33–41. doi: 10.1172/JCI105972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer E. P., Parker J. C. Selective increase of potassium permeability in red blood cells exposed to acetylphenylhydrazine. Blood. 1977 Dec;50(6):1013–1021. [PubMed] [Google Scholar]
- Sarkadi B., Alifimoff J. K., Gunn R. B., Tosteson D. C. Kinetics and stoichiometry of Na-dependent Li transport in human red blood cells. J Gen Physiol. 1978 Aug;72(2):249–265. doi: 10.1085/jgp.72.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gerlóczy A., Gárdos G. Transport parameters and stoichiometry of active calcium ion extrusion in intact human red cells. Biochim Biophys Acta. 1977 Jan 4;464(1):93–107. doi: 10.1016/0005-2736(77)90373-x. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. The silent carrier of beta thalassemia. N Engl J Med. 1969 Dec 11;281(24):1327–1333. doi: 10.1056/NEJM196912112812403. [DOI] [PubMed] [Google Scholar]
- VULLO C., TUNIOLI A. M. Survival studies of thalassemic erythrocytes transfused into donors, into subjects with thalassemia minor and into normal and splenectomized subjects. Blood. 1958 Aug;13(8):803–810. [PubMed] [Google Scholar]
- WIENER A. S., UNGER L. J., COHEN L., FELDMAN J. Type-specific cold auto-antibodies as a cause of acquired hemolytic anemia and hemolytic transfusion reactions: biologic test with bovine red cells. Ann Intern Med. 1956 Feb;44(2):221–240. doi: 10.7326/0003-4819-44-2-221. [DOI] [PubMed] [Google Scholar]
- Wiley J. S. Cation fluxes in Rhnull red cells. Blood. 1978 Mar;51(3):555–557. [PubMed] [Google Scholar]
- Wiley J. S. Co-ordinated increase of sodium leak and sodium pump in hereditary spherocytosis. Br J Haematol. 1972 May;22(5):529–542. doi: 10.1111/j.1365-2141.1972.tb05700.x. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Cooper R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest. 1974 Mar;53(3):745–755. doi: 10.1172/JCI107613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Ellory J. C., Shuman M. A., Shaller C. C., Cooper R. A. Characteristics of the membrane defect in the hereditary stomatocytosis syndrome. Blood. 1975 Sep;46(3):337–356. [PubMed] [Google Scholar]
- Wiley J. S., Shaller C. C. Selective loss of calcium permeability on maturation of reticulocytes. J Clin Invest. 1977 Jun;59(6):1113–1119. doi: 10.1172/JCI108735. [DOI] [PMC free article] [PubMed] [Google Scholar]



