Abstract
There is extensive evidence that fish from waters with PCB-contaminated sediments accumulate PCBs and related chemicals, and that people who eat fish from contaminated waters have higher body burdens of PCBs and PCB metabolites than those who do not. PCBs and their metabolites are potentially toxic, thus it is important to human health to understand the uptake, biotransformation and elimination of PCBs in fish, since these processes determine the extent of accumulation. The intestinal uptake of PCBs present in the diet of fish into fish tissues is a process that is influenced by the lipid composition of the diet. Biotransformation of PCBs in fish, as in mammals, facilitates elimination, although many PCB congeners are recalcitrant to biotransformation in fish and mammals. Sequential biotransformation of PCBs by cytochrome P450 and conjugation pathways is even less efficient in fish than in mammalian species, thus contributing to the retention of PCBs in fish tissues. A very important factor influencing overall PCB disposition in fish is water temperature. Seasonal changes in water temperature produce adaptive physiological and biochemical changes in fish. While uptake of PCBs from the diet is similar in fish acclimated to winter or summer temperatures, there is evidence that elimination of PCBs occurs much more slowly when the fish is acclimated at low temperatures than at warmer temperatures. Research to date suggests that the processes of elimination of PCBs are modulated by several factors in fish including seasonal changes in water temperature. Thus, the body burden of PCBs in fish from a contaminated location is likely to vary with season.
Keywords: Polychlorinated biphenyls in fish, Seasonal effects on PCB elimination, Temperature effects on PCB disposition in fish, PCB biotransformation, Temperature acclimation
Introduction
The widespread use of polychlorinated biphenyls (PCBs) in the mid-20th century has left a worldwide legacy of pollution. PCBs contaminate the sediments of rivers, lakes, and coastal waterways near production and disposal sites, such as the Great Lakes and the Hudson river in the United States. Although high concentrations of PCBs are found near the sites of production and disposal, PCBs are known to be dispersed throughout the world through volatilization then deposition, a process that accounts for contamination of colder northern latitudes with no nearby sources of production or use of PCBs (Scheringer 2009). In aquatic environments, PCBs are taken up from sediments and suspended particles by phytoplankton and enter the aquatic food web. PCBs are then transferred to fish, shellfish and other wildlife (Bordajandi et al. 2006; Perugini et al. 2006; Rawn et al. 2006; Harvey et al. 2008). Because PCBs and many of their metabolites are more lipid than water-soluble, they are often eliminated very slowly from animals, thus PCBs and some metabolites bioaccumulate and the highest tissue concentrations are reached in top predators (Borga et al. 2001; Kwon et al. 2006; Weijs et al. 2009). Uptake, biotransformation and elimination of PCBs are known to vary between congeners that have differing positions and numbers of chlorine substituents, as well as between animal species (Letcher et al. 2000; James 2001).
Understanding factors that affect the amounts of PCBs and PCB metabolites in fish is important, since ingestion of fish caught from PCB-contaminated habitats is a documented major source of exposure of people to PCBs (Bjermo et al.; Turyk et al. 2006; Weintraub and Birnbaum 2008) and may be a source of exposure of people to PCB metabolites such as PCB-methyl sulfones, hydroxylated PCBs (OH-PCBs) and PCB-quinones, which have favorable properties for absorption (James 2001). Table 1 shows that people who consumed fish caught from the Great Lakes had significantly higher concentrations of PCBs and other persistent organochlorines such as DDE, compared with referents who consumed fish, but not fish from the Great Lakes (Turyk et al. 2006).
Table 1.
Ingestion of sport-caught fish from the Great Lakes results in increased mean serum concentrations, lipid-adjusted, of PCBs (adapted from Turyk et al. 2006)
| Mean value | ||
|---|---|---|
| Referents, n = 92 | Great Lakes sport-caught fish-eaters, n = 95 |
|
| Age (years) | 44.9 | 45.9 |
| BMI, kg/m2 | 26.0 | 27.3 |
| Male gender, percentage | 57.6 | 57.9 |
| Total fish meals in previous year | 36.7 | 51.9a |
| Total Great Lakes sport-caught fish meals in previous year | 0 | 42.8a |
| Years eating Great Lakes sport-caught fish | 0 | 26.2a |
| Lipid-adjusted DDE concentration in serum, ppb | 411 | 673a |
| Lipid-adjusted concentration in serum of non-coplanar PCBs, ppb | 208 | 659a |
| Coplanar PCBs, ppb | 62 | 136a |
Great Lakes fish eaters were significantly different, p<0.05 than referents.
Although many PCB contaminated environments are found in cold latitudes, due to physical processes of dispersal of the PCBs, there has been relatively little consideration of the role of water temperature on the uptake, biotransformation, elimination and residue retention of PCBs and their metabolites from fish. Fish as poikilotherms present some unique responses to temperature that influence xenobiotic presentation to and processing by dispositional systems in fish. This review will examine some of the existing studies of this topic in edible fish and suggest needed further research.
Uptake and tissue distribution of PCBs by fish
PCBs can be taken up from the water, across the gill (McKim and Heath 1983), or from the diet, through the gastrointestinal tract (Carlson and Hites 2005). Diet is an important route of uptake of most PCBs by fish, as is true for other poorly water-soluble and highly lipid soluble xenobiotics, so that the rate and extent of absorption across the intestine largely determines the body burden (James and Kleinow 1994). There is evidence that fish take up PCBs from the diet through diffusion across the upper intestine, as would be expected for lipophilic small molecules (Doi et al. 2000; Nichols et al. 2001). In trout held at 11°C and fed environmentally relevant concentrations of PCBs in the diet, over 90% of the dietary dose was assimilated in 24 hours (Nichols et al. 2001).
Biotransformation of PCBs by fish
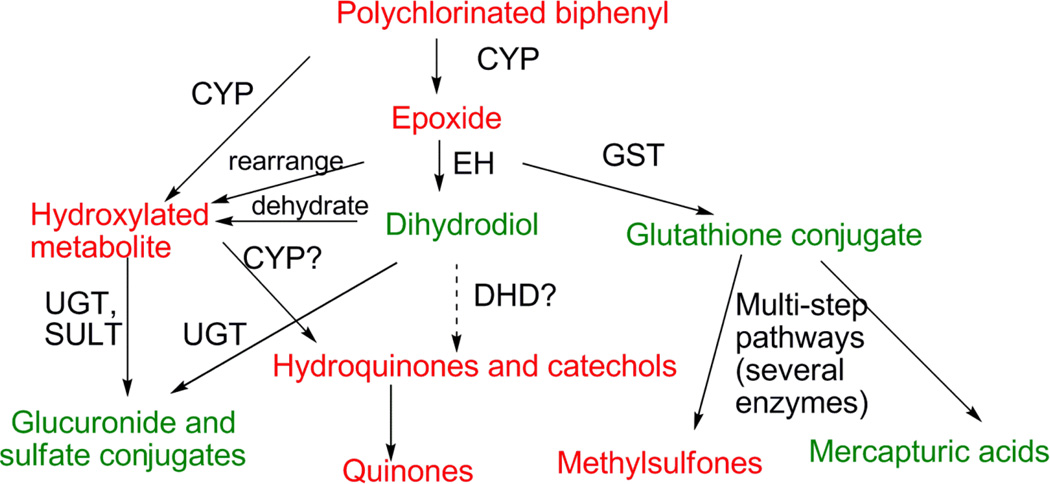
PCBs can be eliminated unchanged from fish, through fecal egestion and diffusion across the gills (Drouillard et al. 2009), however conversion of PCBs to more water-soluble metabolites greatly facilitates elimination. Figure 1 summarizes the biotransformation pathways most likely to be taken by PCBs. The first step in PCB biotransformation is mono-oxygenation, normally catalyzed by one or more members of the cytochrome P450 (P450) superfamily (James 2001). The resulting PCB-epoxide or OH-PCB may then be further metabolized by secondary pathways. PCB-epoxides may be converted to dihydrodiols by epoxide hydrolase, or to glutathione conjugates by glutathione transferase. The glutathione conjugate could be further metabolized to the readily excreted mercapturic acid, but another possibility is conversion in several steps to a methylsulfone metabolite that is more slowly eliminated (Bakke et al. 1982). OH-PCBs can form glucuronide or sulfate conjugates, both of which are expected to be readily excreted.
Figure 1.
Pathways of biotransformation of PCBs. The enzymes catalyzing each reaction are shown: CYP, cytochrome P450; EH, epoxide hydrolase; GST, glutathione transferase; DHD, dihydrodiol dehydrogenase; UGT, UDP-glucuronosyltransferase; SULT, sulfotransferase
P450
Most studies of PCB biotransformation have been conducted in mammals, including rats and several wildlife species. These studies showed that some PCB congeners are very resistant to the first, P450-catalyzed step of biotransformation, while other PCB congeners are better substrates for monooxygenation (Matthews and Dedrick 1984; Birnbaum 1985). PCBs with no vicinal H atoms in the phenyl rings are resistant to P450-catalyzed monooxygenation, whereas PCBs that have vicinal H atoms are more readily biotransformed. The chlorine substitution pattern is another factor in the ease of biotransformation of PCBs. These structural features of the PCB congeners affect the shape of the molecule and the reactivity of the phenyl ring carbons, which in turn determine how readily an individual congener binds to the substrate-binding site of a particular P450 isoform in a suitable orientation for monooxygenation. Although there is some evidence from in vitro studies that co-planar PCBs are metabolized by CYP1 family isoforms (Ishida et al. 1991; Watanabe et al. 2005; Prasad et al. 2007), and non-coplanar PCBs by CYPs in the 2 and 3 families (Ishida et al. 1991; Ariyoshi et al. 1995; Kania-Korwel et al. 2008; Verreault et al. 2009), this has not been extensively studied. The fish species studied to date carry out P450-catalyzed biotransformation of PCBs even more slowly than mammalian species, thus contributing to the retention of PCBs in fish tissues. It has been difficult to study the first step of the reaction, catalyzed by P450, in vitro in fish species, because of generally low activity, even with PCB congeners that are quite readily metabolized in rodent species (James 2001); Prasad et al. 2007). As an example, PCB 77 (3,3’,4,4’-tetrachlorobiphenyl) is comparatively easily metabolized in rats and other mammals, especially after induction of CYP1A1 (McKinley et al. 1993; Chen et al. 2001) but very slowly in fish, even after induction of CYP1A (White et al. 1997; Doi et al. 2000; Schlezinger et al. 2000; Doi et al. 2006). There is evidence that PCB 77 docks to fish CYP1A too far from the active site for efficient catalysis, and this contributes to slow monooxygenation and to uncoupling of the P450 cycle (Schlezinger et al. 2000; Prasad et al. 2007). In vivo studies have demonstrated that fish can convert some PCBs, including PCB 77, to hydroxylated metabolites, through the measurement of OH-PCBs in fish plasma and bile (White et al. 1997; Campbell et al. 2003; Li et al. 2003; Buckman et al. 2006). In situ studies of PCB 77 uptake and metabolism in the isolated perfused channel catfish intestine showed that small amounts of OH-PCBs were present in intestinal mucosa and blood after a one-hour perfusion, indicating slow P450-dependent biotransformation (Doi et al. 2000; Doi et al. 2006). Treatment of trout with a mixture of ortho-substituted PCBs, known to induce CYP3A and CYP2B isoforms in other species (Schuetz et al. 1998; Ngui and Bandiera 1999; Petersen et al. 2007; Yang et al. 2008), resulted in increased biotransformation of several PCBs in trout (Buckman et al. 2007), however the form or forms of P450 responsible are not known.
Glucuronidation and sulfonation of OH-PCBs
In vitro studies showed that several OH-PCBs were glucuronidated in catfish hepatic and intestinal microsomes (James and Rowland-Faux 2003; Sacco et al. 2008). OH-PCBs with two chlorine atoms flanking the OH group were less efficiently glucuronidated than those with just one chlorine adjacent to the OH group (Sacco et al. 2008). The efficiencies of glucuronidation of several OH-PCBs in the catfish liver microsomes were one to two orders of magnitude lower than the same OH-PCBs in rat liver microsomes (Tampal et al. 2002). Sulfonation of two hydroxylated metabolites of PCB 77, namely 2-OH-3,3’,4,4’-tetrachlorobiphenyl and 4-OH-3,3’,4’,5-tetrachlorobiphenyl, was demonstrated in cytosol from channel catfish intestine although the pathway was not studied in depth (James and Rowland-Faux 2003). Although glucuronidation and sulfonation of OH-PCBs occur in catfish, an in situ study of PCB 77 metabolism in catfish intestine showed that unconjugated OH-PCBs were present in blood and tissues (Doi et al. 2000; Doi et al. 2006). Unconjugated OH-PCBs were also found in blood of rainbow trout (Buckman et al. 2006). This suggests that OHPCBs formed in fish are only slowly conjugated by glucuronidation or sulfonation such that part of the OH-PCBs formed in liver or intestine is transferred intact to blood.
Fish exhibit biochemical, structural and functional changes as a function of warm and cold water acclimation
Fish experience change in their thermal environment on a regional and seasonal basis. For strict aquatic poikilotherms, like many fishes, body temperature is determined largely by water temperature (Linthicum and Carey 1972). Under conditions of gradual seasonal temperature changes, cellular structure and function are maintained over a defined range of temperatures through acclimation. As part of this acclimation process, membranes exhibit changes in composition including the quantity and type of unsaturated fatty acids (Hazel 1988), the types and amounts of lipids (Hazel and Zerba 1986; Hazel and Landrey 1988) and the cholesterol/phospholipids ratios (Robertson and Hazel 1995). These processes alter membrane lipid phase behavior through a modification of lipid gel to liquid to gel phase transition temperatures (Cullen et al. 1971; Rilfors et al. 1984; Yeagle 1985; Glaser and Gross 1994; Hazel et al. 1998). At colder temperatures more unsaturated fatty acids are inserted in membranes to lower the lipid melting point, increase membrane fluidity and maintain membrane functionality. Saturated fatty acids tend to solidify at colder temperatures. At warmer temperatures more saturated fatty acids with higher melting points and more cholesterol are present. Cholesterol imparts more rigidity to the membrane. Unsaturated fatty acids at warmer temperatures become too fluid and the membrane ceases to maintain segregation of structure and function between the inside and outside of the cell. Other adaptive changes occur, which maintain functionality at different temperatures, including changes in organelle density (Hazel and Zerba 1986; Egginton and Sidell 1989; Guderley 2004; Tripathi et al. 2005) and organ morphology (Vornanen et al. 2005; Kleinow et al. 2006). As an example, tissue hypertrophy has been demonstrated with cold acclimation in goldfish for the gall bladder (Cremaschi et al. 1973) and liver (Das 1967), and for the intestine of channel catfish (Houpe et al. 1996; Kleinow et al. 2006). In the channel catfish, these hypertrophic changes in the proximal and middle intestines were accompanied by a 43 % increase in intestinal length (Kleinow et al. 2006). Lateral expansion of membranes is known to occur with cold acclimation due to incorporation of less tightly fitting unsaturated fatty acids. For fish muscle, increases in mitochondrial populations (Tyler and Sidell 1984) as well as the volume of the sarcoplasmic reticulum (Penney and Goldspink 1980) have been observed with cold acclimation. These ultrastructural changes may serve to counter the increases in cytosolic viscosity and decreased intracellular diffusion rates by decreasing diffusion distances to organells and increasing the cytosol/organelle membrane surface area interface (Sidell and Hazel 1987). Such hypertrophic effects within cells may also result in an overall increased trans-cellular diffusion distance and associated resistance. These temperature-based changes subsequently influence higher order physiology such as cardiac output (Farrell and Jones 1992), bile flow (Curtis 1983); Curtis et al. 1990) and gastrointestinal clearance rates (Windell et al. 1976), functions that are slowed appreciably at colder temperatures. The net result is that a fish of a given species acclimated at one temperature is not physiologically or biochemically the same animal as fish of the same species acclimated at a divergent temperature.
Membrane bound biotransformation in fish with acclimated temperature change
Temperature induced membrane lipid changes has been shown not only to influence maintenance of membrane permeability, diffusion and water transfer with temperature change (Kleinow et al. 2006), but also membrane bound biotransformation in fish. In vitro P450 biotransformation activities of fish have been shown to exhibit ideal temperature compensation with changes in acclimation temperature congruent with membrane function. Studies of a variety of substrates have shown that fish acclimated to different temperatures have essentially the same in vitro biotransformation activities when assayed at their respective acclimation temperature (Koivusaari et al. 1981; Koivusaari 1983; Koivusaari and Andersson 1984; Ankley et al. 1985; Andersson and Koivusaari 1986; Blanck et al. 1989; Gill and Walsh 1990). Membrane bound biotransformation reactions such as P450 are dependent on lipid character; optimal fluidity and structure for functional considerations including steric, catalytic, binding and transport considerations (Strobel et al. 1970; Nath et al. 2007; Das and Sligar 2009). These biotransformation reactions affect not only xenobiotics such as PCBs, but also endogenous compounds performing essential functions. Teleologically membrane compensation may exist because fish as poikilotherms need to be able to carry out necessary bodily functions over the extent of their thermal range. The range and extent of a fishes’ ability to compensate by altering the degree of fatty acid unsaturation / saturation contributes to the determination of a fishes’ thermal range as well as its optimal environment.
While conservation of biotransformation activity over a range of temperatures has been well documented this only occurs if time is afforded the acclimation process. Where temperature change is acute, membrane-bound biotransformation activities are increased at higher temperatures up to the point where cellular structure is compromised (James et al. 2012). Likewise, activities decrease as membranes solidify at lower temperatures. Furthermore, some biotransformation reactions such as glutathione transferase and sulfotransferase are not membrane-bound. The temperature sensitivity of these pathways has received little attention but they may not be subject to acclimatory compensation.
Temperature alters xenobiotic elimination in fish
Temperature has been shown to have a marked effect on the in vivo disposition of PCBs (Buckman et al. 2004; Buckman et al. 2006; Buckman et al. 2007; Paterson et al. 2007). Fish acclimated to warmer temperatures possess more metabolites and display shorter compound half-lives (t½). Conversely, at colder acclimation temperatures less metabolites are present and compound t½s are longer. This is consistent with numerous temperature studies examining a variety of pharmaceuticals and other xenobiotics (Collier et al. 1978; Varanasi et al. 1981; Salte and Liestol 1983; Kasuga et al. 1984; Jacobsen 1989; van Ginneken et al. 1991; Bjorklund et al. 1992; Kleinow et al. 1994). While these in vivo findings suggest that warmer temperatures result in greater biotransformation and subsequent elimination, in vitro studies with membrane-bound systems suggest activity compensation with temperature change. It is unclear how to reconcile these differences, however, in vitro studies do take the enzymatic process out of context of other mechanisms integrated in vivo with biotransformation. For example, in vivo biotransformation rates depend not only on enzymatic activity, but also on the delivery of substrates and co-factors to the enzymes and removal of products. Substrate, co-factor and product movement are highly dependent on temperature-affected processes such as diffusion and diffusion distance. On the organism level, the apparent xenobiotic parent and metabolite residues in fish rely upon xenobiotic clearance processes known to be temperature dependent (Kleinow et al. 2008). Slower cardiac output, bile flow and other temperature dependent processes are likely to be primary players in the decreased elimination rates observed at colder temperatures. Due to the highly integrative nature of these processes the relative contribution to the in vivo situation has yet to be defined. Multiple cellular or physiological mechanisms may be involved in generating greater PCB residues at colder temperatures.
Lipids, temperature and PCB disposition
Characteristic of PCBs and other non-polar compounds is a close association with lipids in fish (Elskus et al. 2005). Lipid character, content and mobilization are subject to seasonal changes and may influence PCB retention and elimination.
Alterations in the lipid solubility of PCBs have been demonstrated in vitro with changes in chain length and the saturation status of membrane fatty acids (Doi et al. 2000), changes that can occur with acclimation to various water temperatures. PCB 77 was more soluble in micelles prepared from unsaturated (18:2) linoleic acid than in micelles prepared from saturated, shorter chain (12:0) lauric acid (Doi et al. 2000). Similar results were found with Aroclor 1242, which was more soluble in micelles prepared with linoleic acid or oleic acid (18:1) compared with octanoic acid (8:0) (Laher and Barrowman 1983). In vivo PCB retention in fish lipids may be more complex than just solubility concerns. Fatty acid packing densities and fluidity are known to change with temperature in membrane lipids. Saturated fatty acids organize in a tighter membrane packing arrangement than kinked unsaturated fatty acids with double bonds. The partitioning of hydrophobic chemicals has been inversely related to lipid packing densities with unsaturated fatty acids exhibiting greater partitioning (Omann and Lakowicz 1982). In a study of the partitioning of 14 PCBs of varying structures into vesicles of four saturated phosphatidylcholines of varying chain lengths, the fluidity of the membrane bilayer appeared to be more important for the membrane-water partitioning process than the volume of the hydrophobic region, as determined by the membrane lipids alkyl chain length (Dulfer and Govers 1995). Only for PCBs with a large molar volume did steric hindrance appear significant. Perhaps central for times of seasonal temperature variation, significant changes in the partitioning of solutes into membrane lipids have been demonstrated to occur at the gel to liquid phase transition temperatures (Davis et al. 1986). How these features relate to seasonal PCB mobilization and retention, particularly from depot stores is largely unresolved. Several studies have examined the relationship of PCB burdens in fish and their lipids with feeding and energy availability. For Arctic charr dosed with PCBs, the normal winter fast and emaciation typical for this species resulted in a redistribution of the lipophilic PCBs from storage tissues containing high levels of lipid (muscle) to organs such as the liver (Jorgensen et al. 2002; Jorgensen et al. 2006). This redistribution of PCBs was accompanied by induction of hepatic P450 (CYP1A) activities that increased seasonally to maxima in May when lipid mobilization was near completion. Feeding during this same interval prevented lipid mobilization and PCB release from lipid stores, which was otherwise released during fasting. These studies suggest that not only can lipid dynamics mobilize PCBs, but also their mobilization can influence the biotransformation status of the animal and may result in greater metabolism, elimination or both.
Temperature effects on in vivo PCB biotransformation
Very few published studies have examined in vivo quantitative and qualitative changes in PCB biotransformation with temperature in fish. In a study of rainbow trout, it was shown that fish exposed in the diet to environmental mixtures of PCBs at 8°, 12° or 16°C accumulated similar amounts in tissues after 30 days on the contaminated diet (Tables 2 and 3), suggesting no effect of temperature on the uptake and tissue distribution of PCBs from food (Buckman et al. 2007). Table 3 shows the plasma concentrations of PCBs and OH-PCBs in trout held at the three temperatures immediately after 30 days of feeding control or PCB-spiked food. In trout fed the PCB-spiked food, the concentration of OH-PCBs in plasma was significantly higher in fish held at 12°C and 16°C than at 8°C. Sample collection soon after dosing as performed here is likely to influence profiles in the plasma as compared to later time points or samples from other tissues that reflect overall exposure, such as bile. Another consideration relates to conjugation of the OH-PCBs. The method used to quantitate the OH-PCBs did not specifically include a step to hydrolyze sulfate or glucuronide conjugates. Thus, the values shown in Table 3 are likely to represent only unconjugated OH-PCBs. The OH-PCBs measured in plasma are almost certainly formed in the intestine and liver from the dietary PCBs, in P450-dependent reactions. Once formed, the OH-PCBs are subject to glucuronidation and sulfonation in the intestine or liver. Several factors could contribute to the observed temperature-dependent increase in plasma OH-PCB concentration. It is possible that transfer of OH-PCBs from the site of formation into the blood is more rapid at the higher temperatures. Another possibility is that P450-dependent PCB biotransformation at the higher temperatures is not matched by glucuronidation or sulfonation of the bioformed OH-PCB. UDP-glucuronic acid (UDPGA) or PAP-sulfate (PAPS) co-substrates may be reduced in liver at the higher temperatures, thereby reducing glucuronidation or sulfonation efficiency (James et al. 2008; Sacco et al. 2008). Preliminary studies in the channel catfish showed that hepatic UDPGA concentrations were 287 ± 70 nmol/g liver wet weight, mean ± S.D. (n=11 individual fish) in November and December and 132 ± 34 (n=8) in May and June (James et al. 2008; Sacco et al. 2008). Although it is not known what caused the seasonal change in UDPGA concentration, water temperatures were lower in November and December (18–19°C) than in May and June (27–28°C), suggesting the possibility of an influence of water temperature. If trout show similar changes in UDPGA with water temperature or season, this could cause changes in the efficiency of glucuronidation of OH-PCBs formed in the liver.
Table 2.
Uptake of PCBs from the diet by rainbow trout. Values shown are mean concentrations, ng/g, in the food supplied and in the trout carcass after 30 days feeding at the indicated temperatures. The amounts in carcass do not show significant temperature-related changes. Adapted from Buckman et al. 2007
| Number of Cl substituents |
Food | Control Diet, Trout Carcass | PCB-containing Diet, Trout carcass | |||||
|---|---|---|---|---|---|---|---|---|
| Control | PCB | 8° C | 12° C | 16° C | 8° C | 12° C | 16° C | |
| Two | 8 | 180 | 54 | 44 | 41 | 1,200 | 970 | 910 |
| Three | 20 | 780 | 100 | 76 | 74 | 3,700 | 2,900 | 2,800 |
| Four | 64 | 1,300 | 250 | 220 | 200 | 5,200 | 4,000 | 4,000 |
| Five | 27 | 900 | 190 | 160 | 140 | 6,200 | 4,700 | 4,800 |
| Six | 12 | 1,000 | 86 | 64 | 67 | 6,900 | 5,200 | 5,500 |
| Seven | 2.9 | 580 | 22 | 16 | 17 | 4,400 | 3,200 | 3,400 |
| Eight | 2.2 | 180 | 23 | 16 | 18 | 1,900 | 1,300 | 1,500 |
| Nine | 0.31 | 24 | 5.4 | 2.2 | 2.8 | 280 | 180 | 230 |
| Ten | 0.23 | 42 | 6.2 | 2.4 | 3.5 | 890 | 500 | 740 |
Table 3.
Carcass and plasma concentrations, ng/g, of PCBs and OH-PCBs after 30 days of control or PCB-spiked food. Values are mean ± S.E., n = 6. Adapted from Buckman et al., 2007.
| Control diet | PCB-containing diet | |||||
|---|---|---|---|---|---|---|
| 8° C | 12° C | 16° C | 8° C | 12° C | 16° C | |
| Carcass | 1,000 ±330 | 930 ± 210 | 902 ± 132 | 38,447± 10,579 | 28,736± 3,780 | 30,000± 5,100 |
| PCBs | ||||||
| Plasma | 400 ± 100 | 300 ± 50 | 280 ± 32 | 1,700 ± 48 | 1,800 ± 180 | 2,200 ± 120 |
| PCBs | ||||||
| Plasma | 0.53 ± 0.09 | 0.50 ± 0.01 | 0.59 ± 0.04 | 0.77 ± 0.03 | 2.60 ± 0.33* | 5.50 ± 0.52* |
| OH-PCBs | ||||||
Different from fish held at 8°C, p<0.05
PCB elimination and temperature effects in fish
The two recent toxicokinetic studies with PCBs, one with rainbow trout (Buckman et al. 2007), the other with yellow perch (Paterson et al. 2007), have both demonstrated lengthened in vivo PCB mixture t½s at colder temperatures (Tables 4 and 5). While similar in general outcome, the magnitude of response and the type of response differed significantly. The trout study (Buckman et al. 2007) exhibited a smaller change in PCB component t½s with the temperature change used (Table 4) than the perch study (Table 5) (Paterson et al. 2007). Perch eliminated PCBs slowly in fall and spring, not at all in winter, and more rapidly in the summer. In addition, the Paterson study demonstrated a relationship of t½s to temperature as a function of the log Kow of the PCBs examined, whereas the Buckman study did not. While basic differences in study design make rigorous comparisons between studies impossible and ill-advised, differences in design and results may be insightful in regards to the future examination of the processing events involved, the factors determining effect and the magnitude of response expected. Selection of the nature of the thermal regime and magnitude of thermal change relative to the species may have influenced the thermal effects seen. Buckman et al. used separate groups of orally dosed trout held at different, but constant temperatures: 8, 12 or 16°C. Paterson et al. thermally transitioned yellow perch through natural seasonal changes for one year after intraperitoneal dosing of a PCB mixture, allowing phase changes with acclimatory processing after dosing. Mean water temperatures over the year of the Paterson study were 23°C in summer, 10°C in fall, 5°C in winter and 19°C in spring. During this year groups of perch were periodically sampled for analysis. Buckman et al. used a cold-water fatty species, trout, over a colder and a tighter thermal range (8°C) while Paterson et al. used a warmer leaner water species, yellow perch, over a wider temperature range (18°C). Lipid content, energetics and PCB partitioning and mobilization are likely to be different for the two species under the two differing thermal regimes possibly generating the observed differences between these two studies. The static nature of the trout study would appear to relate best to the effects of temperature directly on biotransformation and PCB solubility in lipids of different saturation states, while the dynamic nature of the perch study would also include seasonal lipid alterations and lipid phase transitions.
Table 4.
Reported elimination half-lives of PCB congeners from trout carcass at three different acclimation temperatures. Adapted from Buckman et al. 2007
| Acclimation Temperature |
8° C | 12° C | 16° C |
|---|---|---|---|
| Elimination t½ (days) | 130–263 | 102–210 | 94–190 |
The PCBs were congeners 18, 28, 44, 52, 66, 101, 105, 118, 128, 138, 153, 187, 189, 195, 206, 209.
Table 5.
Effect of season and water temperature on the elimination of PCBs from juvenile yellow perch over an annual cycle. Adapted from Paterson et al. 2007
| Season | Mean Water Temperature °C |
Half-life, days | ||
|---|---|---|---|---|
| Non-metabolized | Metabolized | Range | ||
| Summer | 23.2 | 67 | 49 | 10 to 180 |
| Fall * | 9.5 | 456 ± 125 | 72 to >1,000 | |
| Winter | 5.2 | No elimination | ||
| Spring** | 19.1 | 200 | 150 | |
Water temperature dropped from 24 to 2.7°C.
Water temperature increased from 13 to 21°C
Another possibly informative observation provided by these studies is the relationship of PCB t½s with temperature and compound Kow. Paterson et al. indicated that for metabolized PCB congeners t½s during spring were within a factor of 3 longer than of those calculated for summer regardless of PCB Kow. For persistent PCBs a varying relationship with Kow was observed. Persistent PCBs with Kows less or equal to 6.5 (relatively more water soluble) grouped more with metabolized PCBs as the warming trend with summer resulted in average t½s 3.2 times less than colder temperatures in the spring. In contrast, persistent PCBs with Kows greater than or equal to 6.5 on average exhibited a 9-fold greater elimination rate for this same thermal transition. These data would suggest that dependent on compound character or susceptibility to metabolism, relative water solubility does influence temperature-based changes in PCB retention. However, due to the refractory nature of persistent high Kow PCBs to metabolism and overall slow elimination the many fold response of elimination rates to higher temperatures lends support to the concept that some feature(s) in addition to metabolism is (are) contributing to changes in elimination rates. The trout study did not show an unequivocal relationship of Kow with PCB t½s throughout a wider range of Kows (Buckman et al. 2007). For individual temperatures, PCBs with mid range Kow appeared to exhibit longer t½s as compared with low Kow compounds; however, at higher Kows this was not observed.
Conclusions and Implications for Human Health
The mechanisms underlying these dramatic differences in PCB elimination by fish at different water temperatures and seasons remain to be elucidated in detail. As has been shown for other xenobiotics in fish, the uptake of PCBs from food does not appear to be markedly affected by water temperature, however PCB elimination is altered by changes in acclimation temperature. This suggests that all events important to the dispositional process are not compensated with acclimatory temperature change and that tissue residues of PCBs and metabolites can vary with fish holding temperature. To our knowledge, little attention has been paid to the influence of seasonal variations in assessing the risk of eating fish from contaminated waters. It is known that fish in polar regions, especially those at the top of the food chain, carry higher body burdens of PCBs and other persistent pollutants than fish from warmer regions, but this has been attributed to the presence of airborne pollutants deposited from the atmosphere under cold conditions. While there is no doubt that volatilization of pollutants in warm regions and deposition in cold regions contributes to environmental pollution in cold regions, the persistence of pollutants taken up by coldwater fish is likely to be greater than in warmer regions, leading to the potential for greater bioaccumulation. Thus, consumption of fish from polluted cold environments may result in higher exposure of people to pollutants than consumption of fish from warm-water environments. This is especially true if fish move from a polluted to a non-polluted region. Fish that are transiently exposed to polluted environments in cold-water regions will be likely to carry higher body burdens of pollutants than fish transiently exposed to pollutants in warm-water regions, due to the faster elimination in the warm water conditions. To better predict those conditions and species where temperature change results in significant PCB retention, additional studies integrating fish thermal physiology, lipid processing, energetics and xenobiotic disposition are needed.
Contributor Information
Margaret O. James, Department of Medicinal Chemistry, University of Florida, Gainesville, FL 32605.
Kevin M. Kleinow, Department of Comparative Biomedical Sciences, Louisiana State University, Baton Rouge, LA 70803
References
- Andersson T, Koivusaari U. Oxidative and conjugative metabolism of xenobiotics in isolated liver cells from thermally acclimated rainbow trout. Aquat Toxicol. 1986;8:85–92. [Google Scholar]
- Ankley GT, Reinert RE, Wade AE, White RA. Temperature compensation in the hepatic mixed-function oxidase system of bluegill. Comp Biochem Physiol C. 1985;81:125–129. doi: 10.1016/0742-8413(85)90102-1. [DOI] [PubMed] [Google Scholar]
- Ariyoshi N, Oguri K, Koga N, Yoshimura H, Funae Y. Metabolism of highly persistent PCB congener-2,4,5,2',4',5'-hexachlorobiphenyl, by human CYP2B6. Biochem Biophys Res Commun. 1995;212:455–460. doi: 10.1006/bbrc.1995.1991. [DOI] [PubMed] [Google Scholar]
- Bakke JE, Bergman AL, Larsen GL. Metabolism of 2,4',5-Trichlorobiphenyl by the Mercapturic Acid Pathway. Science. 1982;217:645–647. doi: 10.1126/science.6806905. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. The role of structure in the disposition of halogenated aromatic xenobiotics. Environ Health Perspect. 1985;61:11–20. doi: 10.1289/ehp.856111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermo H, Darnerud PO, Lignell S, Pearson M, Rantakokko P, Nalsen C, Enghardt Barbieri H, Kiviranta H, Lindroos AK, Glynn AK. A Fish intake and breastfeeding time are associated with serum concentrations of organochlorines in a Swedish population. Environ Int. 51C:88–96. doi: 10.1016/j.envint.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Bjorklund HV, Eriksson A, Bylund G. Temperature-related absorption and excretion of oxoloinic acid in rainbow trout (Oncorhynchus mykiss) Aquaculture. 1992;102:17–27. [Google Scholar]
- Blanck J, Lindstrom-Seppa P, Agren JJ, Hanninen O, Rein H, Ruckpaul K. Temperature compensation of hepatic microsomes cytochrome P450 activity in rainbow trout. I. Thermodynamic regulation during water cooling in autumn. Comp Biochem Physiol. 1989;93:55–60. [Google Scholar]
- Bordajandi LR, Martin I, Abad E, Rivera J, Gonzalez MJ. Organochlorine compounds (PCBs, PCDDs and PCDFs) in seafish and seafood from the Spanish Atlantic Southwest Coast. Chemosphere. 2006;64:1450–1457. doi: 10.1016/j.chemosphere.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Borga K, Gabrielsen GW, Skaare JU. Biomagnification of organochlorines along a Barents Sea food chain. Environ Pollut. 2001;113:187–198. doi: 10.1016/s0269-7491(00)00171-8. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Brown SB, Hoekstra PF, Solomon KR, Fisk AT. Toxicokinetics of three polychlorinated biphenyl technical mixtures in rainbow trout (Oncorhynchus mykiss) Environ Toxicol Chem. 2004;23:1725–1736. doi: 10.1897/03-336. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Brown SB, Small J, Muir DC, Parrott J, Solomon KR, Fisk AT. Role of temperature and enzyme induction in the biotransformation of polychlorinated biphenyls and bioformation of hydroxylated polychlorinated biphenyls by rainbow trout (Oncorhynchus mykiss) Environ Sci Technol. 2007;41:3856–3863. doi: 10.1021/es062437y. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Fisk AT, Parrott JL, Solomon KR, Brown SB. PCBs can diminish the influence of temperature on thyroid indices in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2007;84:366–378. doi: 10.1016/j.aquatox.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat Toxicol. 2006;78:176–185. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Campbell LM, Muir DC, Whittle DM, Backus S, Norstrom RJ, Fisk AT. Hydroxylated PCBs and other chlorinated phenolic compounds in lake trout (Salvelinus namaycush) blood plasma from the Great Lakes region. Environ Sci Technol. 2003;37:1720–1725. doi: 10.1021/es026225m. [DOI] [PubMed] [Google Scholar]
- Carlson DL, Hites RA. Polychlorinated biphenyls in salmon and salmon feed: global differences and bioaccumulation. Environ Sci Technol. 2005;39:7389–7395. doi: 10.1021/es048023r. [DOI] [PubMed] [Google Scholar]
- Chen CY, Hamm JT, Hass JR, Birnbaum LS. Disposition of polychlorinated dibenzo-p-dioxins, dibenzofurans, and non-ortho polychlorinated biphenyls in pregnant long evans rats and the transfer to offspring. Toxicol Appl Pharmacol. 2001;173:65–88. doi: 10.1006/taap.2001.9143. [DOI] [PubMed] [Google Scholar]
- Collier TK, Thomas LC, Malins DC. Influence of environmental temperature on deposition of dietary naphthalene in coho salmon (Oncorhynchus kisutch): Isolation and identification of individual metabolites. Comp Biochem Physiol C. 1978;C61:23–28. [Google Scholar]
- Cremaschi D, Smith MW, Wooding FB. Temperature-dependent changes in fluid transport across goldfish gallbladder. J Membr Biol. 1973;13:143–164. doi: 10.1007/BF01868225. [DOI] [PubMed] [Google Scholar]
- Cullen J, Phillips MC, Shipley GG. The effects of temperature on the composition and physical properties of the lipids of Pseudomonas fluorescens. Biochem J. 1971;125:733–742. doi: 10.1042/bj1250733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LR. Glucuronidation and biliary excretion of phenolphthalein in temperature-acclimated steelhead trout (Salmo gairdneri) Comp Biochem Physiol. 1983;C 76:107–111. doi: 10.1016/0742-8413(83)90051-8. [DOI] [PubMed] [Google Scholar]
- Curtis LR, Fredrickson LK, Carpenter HM. Biliary excretion appears rate limiting for hepatic elimination of benzo[a]pyrene by temperature-acclimated rainbow trout. Fundam Appl Toxicol. 1990;15:420–428. doi: 10.1016/0272-0590(90)90028-i. [DOI] [PubMed] [Google Scholar]
- Das A, Sligar SG. Modulation of the cytochrome P450 reductase redox potential by the phospholipid bilayer. Biochemistry. 2009;48:12104–12112. doi: 10.1021/bi9011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AB. Biochemical changes in tissues of goldfish acclimated to high and low temperatures, II. Synthesis of protein and RNA of subcellular fractions and tissue composition. Comp Biochem Physiol. 1967;21:469–485. doi: 10.1016/0010-406x(67)90446-x. [DOI] [PubMed] [Google Scholar]
- Davis SS, James MJ, Anderson NH. The Distribution of Substituted Phenols into Lipid Vesicles. Faraday Discussions. 1986;81:313–327. [Google Scholar]
- Doi AM, Lou Z, Holmes E, Li C, Venugopal CS, James MO, Kleinow KM. Effect of micelle fatty acid composition and 3,4,3', 4'-tetrachlorobiphenyl (TCB) exposure on intestinal [(14)C]-TCB bioavailability and biotransformation in channel catfish in situ preparations. Toxicol Sci. 2000;55:85–96. doi: 10.1093/toxsci/55.1.85. [DOI] [PubMed] [Google Scholar]
- Doi AM, Lou Z, Holmes E, Venugopal CS, Nyagode B, James MO, Kleinow KM. Intestinal bioavailability and biotransformation of 3,3',4,4'-tetrachlorobiphenyl (CB 77) in in situ preparations of channel catfish following dietary induction of CYP1A. Aquat Toxicol. 2006;77:33–42. doi: 10.1016/j.aquatox.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Drouillard KG, Paterson G, Haffner GD. A combined food web toxicokinetic and species bioenergetic model for predicting seasonal PCB elimination by yellow perch (Perca flavescens) Environ Sci Technol. 2009;43:2858–2864. doi: 10.1021/es802567p. [DOI] [PubMed] [Google Scholar]
- Dulfer WJ, Govers HAJ. Membrane Water Partitioning of Polychlorinated-Biphenyls in Small Unilamellar Vesicles of 4 Saturated Phosphatidylcholines. Environmental Science & Technology. 1995;29:2548–2554. doi: 10.1021/es00010a014. [DOI] [PubMed] [Google Scholar]
- Egginton S, Sidell BD. Thermal acclimation induces adaptive changes in subcellular structure of fish skeletal muscle. Am J Physiol. 1989;256:R1–R9. doi: 10.1152/ajpregu.1989.256.1.R1. [DOI] [PubMed] [Google Scholar]
- Elskus AA, Collier TK, Monosson E. Interactions between lipids and persistent organic pollutants in fish. Biochemistry and Molecular Biology of Fishes. TP Mommsen and Moon TW. St. Louis. Elsevier Science. 2005;6:119–152. [Google Scholar]
- Farrell AP, Jones DR. In: The heart Fish Physiology: Cardiovascular Systems. Hoar WS, Farrell AP, editors. XIIA. San Diego: Academic Press; 1992. pp. 1–46. [Google Scholar]
- Gill KA, Walsh PJ. Effects of temperature on metabolism of benzo(a)pyrene by toadfish (Opsanus beta) hepatocytes. Can J. Fish Aquat Sci. 1990;47:831–837. [Google Scholar]
- Glaser PE, Gross RW. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- Guderley H. Metabolic responses to low temperature in fish muscle. Biol Rev Camb Philos Soc. 2004;79:409–427. doi: 10.1017/s1464793103006328. [DOI] [PubMed] [Google Scholar]
- Harvey J, Harwell L, Summers JK. Contaminant concentrations in whole-body fish and shellfish from US estuaries. Environ Monit Assess. 2008;137:403–412. doi: 10.1007/s10661-007-9776-1. [DOI] [PubMed] [Google Scholar]
- Hazel JR. In: Homeoviscous adaptation in animal cell membranes. Advances in Membrane Fluidity - Physiological Regulation of Membrane Fluidity. Aloia RC, Curtain CC, Gordon LM, editors. New York: Liss; 1988. pp. 149–188. [Google Scholar]
- Hazel JR, Landrey SR. Time course of thermal adaptation in plasma membranes of trout kidney I. Headgroup composition. Am J Physiol. 1988;255:R622–R627. doi: 10.1152/ajpregu.1988.255.4.R622. [DOI] [PubMed] [Google Scholar]
- Hazel JR, McKinley SJ, Gerrits MF. Thermal acclimation of phase behavior in plasma membrane lipids of rainbow trout hepatocytes. Am J Physiol. 1998;275:R861–869. doi: 10.1152/ajpregu.1998.275.3.R861. [DOI] [PubMed] [Google Scholar]
- Hazel JR, Zerba E. Adaptation of biological membranes to temperature: molecular species composition of phosphatidylcholine and phosphatidylethanolamine in mitochondrial and microsomal membranes of liver from thermally-acclimated rainbow trout. J Comparative Physiology B. 1986;156:665–674. [Google Scholar]
- Houpe KL, Malo C, Oldham PB, Buddington RK. Thermal modulation of channel catfish intestinal dimensions, BBM fluidity, and glucose transport. Am J Physiol. 1996;270:R1037–R1043. doi: 10.1152/ajpregu.1996.270.5.R1037. [DOI] [PubMed] [Google Scholar]
- Ishida C, Koga N, Hanioka N, Saeki HK, Yoshimura H. Metabolism in vitro of 3,4,3',4'- and 2,5,2',5'-tetrachlorobiphenyl by rat liver microsomes and highly purified cytochrome P-450. J Pharmacobiodyn. 1991;14:276–284. doi: 10.1248/bpb1978.14.276. [DOI] [PubMed] [Google Scholar]
- Jacobsen MD. Withdrawal times of freshwater rainbow trout, Salmo gairdneri Richardson, after treatment with oxolinic acid, oxytetracycline and trimetoprim. J Fish Diseases. 1989;12:29–36. [Google Scholar]
- James MO. PCB: metabolism and metabolites. In: Robertson LW, Hansen LG, editors. Recent advances in the Environmental Toxicology and Health Effects of PCB. Lexington, KY: University of Kentucky Press; 2001. pp. 35–46. [Google Scholar]
- James MO, Kleinow KM. Trophic transfer of chemicals in the aquatic environment. Aquatic Toxicology: Molecular, Biochemical and Cellular Perspectives. DCaO Malins. Boca Raton: GK Lewis publishers; 1994. pp. 1–35. [Google Scholar]
- James MO, Marth CJ, Rowland-Faux L. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquatic Toxicology. 2012;124:72–82. doi: 10.1016/j.aquatox.2012.07.009. [DOI] [PubMed] [Google Scholar]
- James MO, Rowland-Faux L. Hydroxylated PCBs as poor substrates for glucuronidation and sulfonation but good inhibitors of the conjugation of hydroxylated metabolites of benzo(a)pyrene. Fresenius Environmental Bulletin. 2003;12:227–231. [Google Scholar]
- James MO, Sacco JC, Faux LR. Effects of Food Natural Products on the Biotransformation of PCBs. Environ Toxicol Pharmacol. 2008;25:211–217. doi: 10.1016/j.etap.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen EH, Foshaug H, Andersson P, Burkow IC, Jobling M. Polychlorinated biphenyl toxicokinetics and P4501A responses in anadromous Arctic charr during winter emaciation. Environ Toxicol Chem. 2002;21:1745–1752. [PubMed] [Google Scholar]
- Jorgensen EH, Vijayan MM, Killie JE, Aluru N, Aas-Hansen O, Maule A. Toxicokinetics and effects of PCBs in Arctic fish: a review of studies on Arctic charr. J Toxicol Environ Health A. 2006;69:37–52. doi: 10.1080/15287390500259053. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hrycay EG, Bandiera SM, Lehmler HJ. 2,2',3,3',6,6'-Hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem Res Toxicol. 2008;21:1295–1303. doi: 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga Y, Sugitani A, Yamada F, Arai M, Morikawa S. Oxolinic acid residues in tissues of cultured rainbow trout and ayu fish. J Food HygSoc Jpn. 1984;25:512–516. [Google Scholar]
- Kleinow KM, Jarboe HH, Shoemaker KE. Comparative pharmacokinetics and bioavailability of oxolinic acid in channel catfish (Ictalurus punctatus) and rainbow trout (Oncorhynchus mykiss) Can J Fish Aquat Sci. 1994;51:1205–1211. [Google Scholar]
- Kleinow KM, Johnston BD, Holmes EP, McCarrol ME. Rhodamine 123 permeability through the catfish intestinal wall-Relationship to thermal acclimation and acute temperature change. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:205–215. doi: 10.1016/j.cbpc.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Kleinow KM, Nichols JW, Hayton WL, McKim JM, Barron MG. In: Toxicokinetics in Fishes. The Toxicology of Fishes. DiGiulio RT, Hinton DE, editors. Boca Raton: CRC Press; 2008. pp. 55–152. [Google Scholar]
- Koivusaari U. Thermal acclimatization of hepatic polysubstrate monooxygenase and UDP-glucuronosyltransferase of mature rainbow trout (Salmo gairdneri) J Exp Zool. 1983;227:35–42. doi: 10.1002/jez.1402270106. [DOI] [PubMed] [Google Scholar]
- Koivusaari U, Andersson T. Partial temperature compensation of hepatic biotransformation enzymes in juvenile rainbow trout (Slamo gairdneri) during the warming of water in spring. Comp Biochem Physiol B. 1984;78:223–226. [Google Scholar]
- Koivusaari U, Harri M, Hanninen O. Seasonal variation of hepatic biotransformation in female and male rainbow trout (Salmo gairdneri) Comp Biochem Physiol C. 1981;70:149–157. doi: 10.1016/0306-4492(81)90046-0. [DOI] [PubMed] [Google Scholar]
- Kwon TD, Fisher SW, Kim GW, Hwang H, Kim JE. Trophic transfer and biotransformation of polychlorinated biphenyls in zebra mussel, round goby, and smallmouth bass in Lake Erie, USA. Environ Toxicol Chem. 2006;25:1068–1078. doi: 10.1897/05-180r.1. [DOI] [PubMed] [Google Scholar]
- Laher JM, Barrowman JA. Polycyclic hydrocarbon and polychlorinated biphenyl solubilization in aqueous solutions of mixed micelles. Lipids. 1983;18:216–222. doi: 10.1007/BF02534551. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Klasson-Wehler E, Bergman A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. New Types of Persistent Halogenated Compounds. J Paasivirta. Berlin, Springer-Verlag. 2000;3, Part K.:317–357. [Google Scholar]
- Li H, Drouillard KG, Bennett E, Haffner GD, Letcher RJ. Plasma-associated halogenated phenolic contaminants in benthic and pelagic fish species from the Detroit River. Environ Sci Technol. 2003;37:832–839. doi: 10.1021/es026215l. [DOI] [PubMed] [Google Scholar]
- Linthicum DS, Carey FG. Regulation of brain and eye temperatures by the bluefin tuna. Comp Biochem Physiol A Comp Physiol. 1972;43:425–433. doi: 10.1016/0300-9629(72)90201-0. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Dedrick RL. Pharmacokinetics of PCBs. Annu Rev Pharmacol Toxicol. 1984;24:85–103. doi: 10.1146/annurev.pa.24.040184.000505. [DOI] [PubMed] [Google Scholar]
- McKim JM, Heath EM. Dose determinations for waterborne 2,5,2',5'-[14C]tetrachlorobiphenyl and related pharmacokinetics in two species of trout (Salmo gairdneri and Salvelinus fontinalis): a massbalance approach. Toxicol Appl Pharmacol. 1983;68:177–187. doi: 10.1016/0041-008x(83)90002-9. [DOI] [PubMed] [Google Scholar]
- McKinley MK, Kedderis LB, Birnbaum LS. The effect of pretreatment on the biliary excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin-2,3,7,8-tetrachlorodibenzofuran, and 3,3',4,4'-tetrachlorobiphenyl in the rat. Fundam Appl Toxicol. 1993;21:425–432. doi: 10.1006/faat.1993.1118. [DOI] [PubMed] [Google Scholar]
- Nath A, Grinkova YV, Sligar SG, Atkins WM. Ligand binding to cytochrome P450 3A4 in phospholipid bilayer nanodiscs: the effect of model membranes. J Biol Chem. 2007;282:28309–28320. doi: 10.1074/jbc.M703568200. [DOI] [PubMed] [Google Scholar]
- Ngui JS, Bandiera SM. Induction of hepatic CYP2B is a more sensitive indicator of exposure to aroclor 1260 than CYP1A in male rats. Toxicol Appl Pharmacol. 1999;161:160–170. doi: 10.1006/taap.1999.8787. [DOI] [PubMed] [Google Scholar]
- Nichols JW, Fitzsimmons PN, Whiteman FW, Kuehl DW, Butterworth BC, Jenson CT. Dietary uptake kinetics of 2,2',5,5'-tetrachlorobiphenyl in rainbow trout. Drug Metab Dispos. 2001;29:1013–1022. [PubMed] [Google Scholar]
- Omann GM, Lakowicz JR. Interactions of Chlorinated-Hydrocarbon Insecticides with Membranes. Biochimica Et Biophysica Acta. 1982;684:83–95. doi: 10.1016/0005-2736(82)90052-9. [DOI] [PubMed] [Google Scholar]
- Paterson G, Drouillard KG, Haffner GD. PCB elimination by yellow perch (Perca flavescens) during an annual temperature cycle. Environ Sci Technol. 2007;41:824–829. doi: 10.1021/es060266r. [DOI] [PubMed] [Google Scholar]
- Penney RK, Goldspink G. Temperature adaptation of sarcoplasmic reticulum of goldfish. J Thermal Biol. 1980;5:63–68. [Google Scholar]
- Perugini M, Giammarino A, Olivieri V, Di Nardo W, Amorena M. Assessment of edible marine species in the Adriatic Sea for contamination from polychlorinated biphenyls and organochlorine insecticides. J Food Prot. 2006;69:1144–1149. doi: 10.4315/0362-028x-69.5.1144. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Damkier P, Nielsen F, Grandjean P, Weihe P, Brosen K. Polychlorinated biphenyl (PCB) induction of CYP3A4 enzyme activity in healthy Faroese adults. Toxicol Appl Pharmacol. 2007;224:202–206. doi: 10.1016/j.taap.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Prasad JC, Goldstone JV, Camacho CJ, Vajda S, Stegeman JJ. Ensemble modeling of substrate binding to cytochromes P450: analysis of catalytic differences between CYP1A orthologs. Biochemistry. 2007;46:2640–2654. doi: 10.1021/bi062320m. [DOI] [PubMed] [Google Scholar]
- Rawn DF, Forsyth DS, Ryan JJ, Breakell K, Verigin V, Nicolidakis H, Hayward S, Laffey P, Conacher HB. PCB, PCDD and PCDF residues in fin and non-fin fish products from the Canadian retail market 2002. Sci Total Environ. 2006;359:101–110. doi: 10.1016/j.scitotenv.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Rilfors L, Lindblom G, Wieslander A, Christiansson A. Lipid bilayer stability in biological membranes. Biomembranes. 1984;12:205–245. [Google Scholar]
- Robertson JC, Hazel JR. Cholesterol content of trout plasma membranes varies with acclimation temperature. Am J Physiol. 1995;269:R1113–R1119. doi: 10.1152/ajpregu.1995.269.5.R1113. [DOI] [PubMed] [Google Scholar]
- Sacco JC, Lehmler HJ, Robertson LW, Li W, James MO. Glucuronidation of polychlorinated biphenylols and UDP-glucuronic acid concentrations in channel catfish liver and intestine. Drug Metab Dispos. 2008;36:623–630. doi: 10.1124/dmd.107.019596. [DOI] [PubMed] [Google Scholar]
- Salte R, Liestol K. Drug withdrawal from farmed fish. Depletion of oxytetracycyline, sulfadiazine, and trimethophrim from muscular tissue of rainbow trout (Salmo gairdneri) Acta Vet Scand. 1983;24:418–430. doi: 10.1186/BF03546715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheringer M. Long-range transport of organic chemicals in the environment. Environ Toxicol Chem. 2009;28:677–690. doi: 10.1897/08-324R.1. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Keller J, Verbrugge LA, Stegeman JJ. 3,3',4,4'-Tetrachlorobiphenyl oxidation in fish, bird and reptile species-relationship to cytochrome P450 1A inactivation and reactive oxygen production. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125:273–286. doi: 10.1016/s0742-8413(99)00112-7. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidell BD, Hazel JR. Temperature affects the diffusion of small molecules through cytosol of fish muscle. J Exp Biol. 1987;129:191–203. doi: 10.1242/jeb.129.1.191. [DOI] [PubMed] [Google Scholar]
- Strobel HW, Lu AY, Heidema J, Coon MJ. Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem. 1970;245:4851–4854. [PubMed] [Google Scholar]
- Tampal N, Lehmler HJ, Espandiari P, Malmberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem Res Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- Tripathi G, Gaur A, Sharma BM. Temperature related seasonal changes in Golgi complex of brain, heart and intestine of a teleost. J Environ Biol. 2005;26:265–268. [PubMed] [Google Scholar]
- Turyk M, Anderson HA, Hanrahan LP, Falk C, Steenport DN, Needham LL, Patterson DG, Jr, Freels S, Persky V. Relationship of serum levels of individual. PCB dioxin, and furan congeners and DDE with Great Lakes sport-caught fish consumption. Environ Res. 2006;100:173–183. doi: 10.1016/j.envres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Tyler S, Sidell BD. Changes in mitochondrial distribution and diffusion distances in muscle of goldfish upon acclimation to warma nd cold temperatures. J Exp Zool. 1984;232:1–9. [Google Scholar]
- van Ginneken VJ, Nouws JF, Grondel JL, Driessens F, Degen M. Pharmacokinetics of sulphadimidine in carp (Cyprinus carpio L.) and rainbow trout (Salmo gairdneri Richardson) acclimated at two different temperature levels. Vet Q. 1991;13:88–96. doi: 10.1080/01652176.1991.9694290. [DOI] [PubMed] [Google Scholar]
- Varanasi U, Gmur DJ, Reicher WL. Effect of environmental temperature on naphthalene metabolism in juvenile starry flounder (Platichtys stellatus) Arch Environ Contam Toxicol. 1981;10:203–214. doi: 10.1007/BF01055622. [DOI] [PubMed] [Google Scholar]
- Verreault J, Letcher RJ, Sonne C, Dietz R. In vitro metabolism of polychlorinated biphenyls and cytochrome P450 monooxygenase activities in dietary-exposed Greenland sledge dogs. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:91–100. doi: 10.1016/j.cbpc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Vornanen M, Hassinen M, Koskinen H, Krasnov A. Steady-state effects of temperature acclimation on the transcriptome of the rainbow trout heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1177–R1184. doi: 10.1152/ajpregu.00157.2005. [DOI] [PubMed] [Google Scholar]
- Watanabe MX, Iwata H, Okamoto M, Kim EY, Yoneda K, Hashimoto T, Tanabe S. Induction of cytochrome P450 1A5 mRNA, protein and enzymatic activities by dioxin-like compounds, and congener-specific metabolism and sequestration in the liver of wild jungle crow (Corvus macrorhynchos) from Tokyo, Japan. Toxicol Sci. 2005;88:384–399. doi: 10.1093/toxsci/kfi326. [DOI] [PubMed] [Google Scholar]
- Weijs L, Das K, Siebert U, van Elk N, Jauniaux T, Neels H, Blust R, Covaci A. Concentrations of chlorinated and brominated contaminants and their metabolites in serum of harbour seals and harbour porpoises. Environ Int. 2009;35:842–850. doi: 10.1016/j.envint.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Weintraub M, Birnbaum LS. Catfish consumption as a contributor to elevated PCB levels in a non- Hispanic black subpopulation. Environ Res. 2008;107:412–417. doi: 10.1016/j.envres.2008.03.001. [DOI] [PubMed] [Google Scholar]
- White RD, Shea D, Stegeman JJ. Metabolism of the aryl hydrocarbon receptor agonist 3,3',4,4'- tetrachlorobiphenyl by the marine fish scup (Stenotomus chrysops) in vivo and in vitro. Drug Metab Dispos. 1997;25:564–572. [PubMed] [Google Scholar]
- Windell JT, Kitchell JF, Norris DO, Norris JS, Foltz JW. Temperature and rate of gastric evacuation by rainbow trout (Salmo gairdneri) Transact Am Fish Soc. 1976;6:712–717. [Google Scholar]
- Yang F, Xu Y, Pan H, Wu D. Induction of hepatic cytochrome P4501A1/2B activity and disruption of thyroglobulin synthesis/secretion by mono-ortho polychlorinated biphenyl and its hydroxylated metabolites in rat cell lines. Environ Toxicol Chem. 2008;27:220–225. doi: 10.1897/07-108.1. [DOI] [PubMed] [Google Scholar]
- Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]