Abstract
Ann Kelley was a scientific pioneer in reward neuroscience. Her many notable discoveries included demonstrations of accumbens/striatal circuitry roles in eating behavior and in food reward, explorations of limbic interactions with hypothalamic regulatory circuits, and additional interactions of motivation circuits with learning functions. Ann Kelley's accomplishments inspired other researchers to follow in her footsteps, including our own laboratory group. Here we describe results from several lines of our research that sprang in part from earlier findings by Kelley and colleagues. We describe hedonic hotspots for generating intense pleasure `liking', separate identities of `wanting' versus `liking' systems, a novel role for dorsal neostriatum in generating motivation to eat, a limbic keyboard mechanism in nucleus accumbens for generating intense desire versus intense dread, and dynamic limbic transformations of learned memories into motivation. We describe how origins for each of these themes can be traced to fundamental contributions by Ann Kelley.
Keywords: Eating, Pleasure, Fear, Incentive salience, Nucleus accumbens, Neostriatum, Ventral pallidum, Opioid, Dopamine, Rat
1. Introduction
Our thesis here is that discrete psychological components of motivation and reward affect are to some degree assignable to discrete neurochemical and neuroanatomical mechanisms within brain mesocorticolimbic circuitry. Neural manipulations especially can dissociate and reveal these components, sometimes in surprising ways. For example, some particular psychological components that seem closely interconnected in common experience, such as `wanting' and `liking' for the same reward, may actually be less similar in neural mechanisms than other motivational components that seem psychologically opposite, such as fear and desire. We describe such components here, and highlight how neuroscience studies of motivation and reward can benefit from combining careful behavioral analyses with neural manipulations and mapping of brain mechanisms. Work by the late Ann Kelley and colleagues began many of these efforts, and inspired related studies in our and others' laboratories aimed at identifying the psychological nature of motivation components and the specific neural systems involved.
2. Nucleus accumbens in eating and `liking'
Ann Kelley was a leading pioneer in the neuroscience of reward and motivation. For example, she and her colleagues were among the first to combine research on the anatomy of mesocorticostriatal systems, the role of opioid signals in striatal systems, and their interactions with hypothalamic regulatory circuits in controlling motivated behavior. Those investigations by her laboratory followed her earlier collaborative studies with Iversen and colleagues on classic mesolimbic microinjection effects, and her elegant collaborative neuroanatomical studies with Nauta and colleagues in the late 1970s and early 1980s (Kelley et al., 1980, 1982; Kelley and Iversen, 1978).
One important later theme for the Kelley lab concerned reward circuitry underlying generation of the motivation to eat. By the early 1990s, Bakshi and Kelley (1993b) had shown that microinjections of morphine into either nucleus accumbens (NAc; ventral striatum) or ventromedial regions of neostriatum (dorsal striatum or caudate–putamen) caused robust increases in eating behavior and food intake. Following this discovery, Kelley and colleagues went on to demonstrate that eating induced by mu opioid stimulation of NAc was sensitive to the palatability of the food eaten, preferentially enhancing intake of palatable sweet or high fat foods more than other foods, rather than merely instigating a general drive to ingest or engage in oromotor consummatory acts (Kelley et al., 1996; Zhang et al., 1998; Zhang and Kelley, 1997). Those results from the Kelley lab helped develop the idea that mu opioid signaling in NAc might enhance the hedonic impact of palatable foods to stimulate ingestion (Baldo and Kelley, 2007).
Another important issue for Ann Kelley's work was anatomical heterogeneity and localization of function within subregions of striatal structures. To determine which opioid circuits worked to enhance palatable eating, Zhang and Kelley (2000) conducted an extensive opioid microinjection mapping study of behavioral effects on stimulated eating, comparing regions of NAc and neostriatum. They found that opioid stimulation of eating was supported by the entire NAc shell (both medial shell and lateral shell) and entire NAc core, plus ventrolateral regions of neostriatum. In addition, they showed that mu opioid receptor stimulation in the NAc increased Fos expression in other limbic brain structures, such as lateral hypothalamus and ventral tegmental area, indicating recruitment of distributed brain networks to motivate feeding.
3. Pinpointing opioid hedonic enhancement in NAc: discovery of a `liking' hotspot
Such findings by Ann Kelley and colleagues, together with related work by others (Gosnell and Majchrzak, 1989; Islam and Bodnar, 1990; Simone et al., 1985), inspired many labs to further investigate the role of opioid circuitry in the NAc in palatability. In particular, our lab set out to identify whether and where opioid stimulation would enhance basic positive hedonic reactions of `liking' to palatable tastes, such as sucrose. Initial taste reactivity experiments found that systemic injections of morphine increased hedonic reactions to sucrose solutions (Doyle et al., 1993; Rideout and Parker, 1996) and decreased aversive behaviors to bitter quinine (Doyle et al., 1993; Parker et al., 1992; Rideout and Parker, 1996). The taste reactivity test of orofacial reactions was developed for rodents receiving intra-oral infusions of taste solutions (Grill and Norgren, 1978; Pfaffmann et al., 1977), and was based originally on earlier demonstrations by Steiner (1973) of distinct positive versus negative affective facial expressions in newborn human infants elicited by sweet (e.g., rhythmic lip-licking) versus bitter or sour tastes (e.g., gapes, headshakes). The microstructure of affective orofacial reactions of `liking' versus `disliking' is systematically homologous between rodents, monkeys, apes, and human infants, making taste reactivity a useful tool to empirically study hedonic experiences (Berridge, 2000, 2003; Steiner, 1973; Steiner et al., 2001).
Pecina and Berridge (1995, 2000) approached the localization question for opioid pleasure mechanisms by examining the effects of morphine microinjections on hedonic reactions to sucrose as assessed by the taste reactivity test. First, Pecina and Berridge (1995) found that intracerebroventricular microinjections of morphine into the forebrain lateral ventricles increased hedonic `liking' reactions to a sweet sucrose taste, confirming that opioids promote eating by acting on central brain mechanisms to enhance the sensory pleasure of food. To more directly investigate the localization of substrates for hedonic enhancement, Pecina and Berridge (2000) subsequently made microinjections of morphine directly into brain sites within the medial shell of NAc, one of the areas where Kelley's studies had found mu opioid receptor stimulation to most potently increase eating (Zhang et al., 1998; Zhang and Kelley, 1997, 2000). Peciña and Berridge found that opioid stimulation of the NAc medial Pecina shell was sufficient to enhance hedonic `liking' reactions to sucrose. But not all sites of medial shell were equally effective: a localized hotspot seemed to exist that doubled or tripled `liking' reactions, whereas morphine microinjections at other shell sites did not, even though those sites just as powerfully stimulated eating. This grouping of sites turned out to be clumped in the anterior half of medial shell, as viewed by today's understanding of NAc anatomy.
4. Changing criteria for rostrocaudal boundaries in NAc shell
Peciña and Berridge initially adopted the same stereotaxic coor-Pecina dinates as Kelley and colleagues to target the medial shell (Basso and Kelley, 1999; Kelley and Swanson, 1997; Maldonado-Irizarry et al., 1995; Zhang and Kelley, 2000). Most of their sites were located in what we would now classify as the rostral half of medial shell, even sites intended to be relatively caudal. Indeed, most microin-jection studies from many labs through the 1990s focused primarily on the rostral half of NAc (for example: Burgdorf et al., 2001; Carlezon and Wise, 1996; Duvauchelle et al., 1992; Hyytia and Koob, 1995; Sills and Vaccarino, 1996; Sokolowski and Salamone, 1998). The caudal half was left relatively unexplored until after 2000.
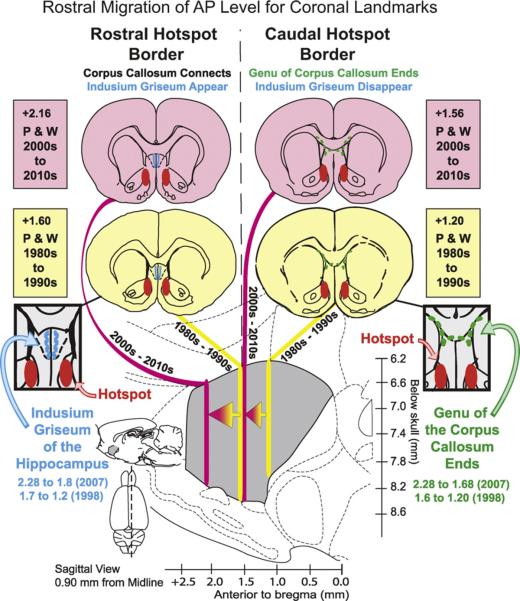
Caudal neglect of NAc until the 21st century may have arisen in part because popular stereotaxic atlas representations of the caudal portion of medial shell were relatively compressed until the year 2000 by comparison to expanded representations in recent years. For example, the entire rostrocaudal extent of NAc shell has grown from under 2 mm in a popular atlas of the 1960s (Pellegrino and Cushman, 1967) to approaching 3 mm in one atlas often used today (Paxinos and Watson, 2007). Even in successive editions of the Paxinos and Watson atlas, the representation NAc shell has grown by nearly half a millimeter from front to back, as represented in serial coronal sections. Most of this apparent growth has been in the caudal half of shell: more sections and a greater anterior–posterior span is now covered by coronal maps for caudal shell, whereas the rostral shell has remained relatively stable. As a result, the represented distance has grown between the anteroposterior (AP) midpoint of medial shell to the caudal edge of medial shell by about 0.5 mm for Paxinos and Watson from 1998 to 2007. The AP midpoint corresponds functionally to the caudal edge of the hedonic hotspot (Fig. 1). An anatomical marker for that AP level for a person inspecting a coronal section is visible in the lateral septum dorsal to the shell: in more rostral sections through the hotspot the septum is penetrated by vertical streaks of the indusium griseum of the hippocampus, but those streaks disappear as one reaches the caudal edge of the hotspot (midpoint of medial shell), and are no longer visible in more posterior sections. An additional midpoint marker is the transition from the genu to the body of the corpus callosum.
Fig. 1.
Rostral shift of anatomical markers for the NAc shell hotspot in the coronal view. Sagittal illustration of how the anteroposterior (AP) value of the rostral and caudal boundaries of the hedonic hotspot (red) have apparently shifted forward, in terms of distance from bregma, based on a comparison of coronal planes in early editions of a popular brain atlas in 1980s and 1990s (yellow; Paxinos and Watson, 1998) to the more recent 2007 edition (pink; Paxinos and Watson, 2007). As one result, anatomical markers for the rostral border of the hotspot, such as the rostral-most extent of the indusium griseum of the hippocampus (blue) where the corpus callosum is first joined, is labeled as 1.6 mm ahead of bregma in 1998 (yellow; Paxinos and Watson, 1998) but as 2.16 mm ahead of bregma in 2007 (pink; Paxinos and Watson, 2007). Concurrently, markers for the caudal border of the hotspot (a point just caudal to the genu of the corpus callosum and the indusium griseum) have changed from 1.2 mm ahead of bregma (yellow; Paxinos and Watson, 1998) to 1.56 mm ahead of bregma (pink; Paxinos and Watson, 2007). The marker referred to as indusium griseum appears as two thin vertical strips, about one mm long, of darker gray matter near the midline located in the medial portion of the dorsal peduncular cortex, extending ventrally from the corpus callosum. The more posterior marker provided by the end of the genu of corpus callosum occurs at the AP point where the rostral genu transitions to the main body of corpus callosum. At that point, the ventral (rostral) tip of indusium griseum is no longer visible in coronal section (though a small dorsal portion of indisium griseum can still be seen just dorsal to the corpus callosum). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Perhaps even more important to early caudal neglect of shell and subsequent correction has been the rostral migration of stereo-taxic coordinates that can be seen in coronal sections of successive Paxinos and Watson editions. For example, an intermediate site in medial shell corresponding to caudal border of hotspot fell in coronal plane that was described as 1.2 mm anterior to bregma in 1998, but is more recently marked as 1.68 mm ahead of bregma in the 2007 edition—a move of nearly half a millimeter. By contrast, sagittal plane maps and coordinates have remained essentially unchanged in Paxinos and Watson since 1998 (e.g., their Figure 165 of Paxinos and Watson, 2007). What this means is that a person looking at a coronal brain slice at the AP midpoint, perhaps marked anatomically by the disappearance of the indusium griseum and the transition from the genu to the body of the corpus callosum (Fig. 1), would conclude that they were 1.2 mm ahead of bregma in 1998, but at 1.68 in 2007. If they plotted the 1.2 mm ahead of bregma position on the sagittal map of Figure 165 in the 1998 edition, their site appears to be in the caudal half of medial shell. But in 2007, plotting the same location as 1.68 mm ahead of bregma on the essentially unchanged corresponding sagittal map moves the site a half-millimeter forward and it is no longer in posterior shell. Thus sites taken as posterior in the 1990s can be recognized now to be only in the center of medial shell. Of course the medial shell has not actually expanded anatomically, nor have these markers moved in the brain, but the change in coronal atlas representations allows a reassessment, and more recent studies have probed caudal zones of shell that once went unmapped in coronal sections.
As a consequence of all this, studies that ostensibly included caudal regions of medial shell (Basso and Kelley, 1999; Kelley and Swanson, 1997) may have actually focused on mid-rostral or central locations (between 1.6 and 2.2 mm rostral to bregma), leaving more caudal regions of medial shell relatively untouched (e.g., 0.45–1.6 mm ahead of bregma).
This rostral NAc tilt applied to Peciña ments in 2000. To map the site of functional hedonic enhancement, Peciña and Berridge used a Fos plume mapping technique, which measures the diameter of drug impact on local neurons surrounding a microinjection. They found that morphine microinjections in a circumscribed area of medial shell generated robust increases in hedonic `liking' responses elicited by sucrose taste, more than doubling control levels, and enhanced chow consumption. Other sites in shell only increased eating but not hedonic responses to taste. The hedonic `liking' enhancements were mapped particularly in what paciña and Berridge then considered to be the caudal region of medial shell (but actually was only the caudal portion of the rostral half of shell). Indeed, in their 2000 paper they called the hedonic site a caudal shell site. At least, it constituted the caudal grouping of their NAc sites (which now would be considered mid-rostral, the other sites being far-rostral).
However, with the emerging realization after 2000 that the NAc medial shell also extended more caudally, it became necessary to remap the hedonic site again including more caudal regions of and Berridge therefore completed a subsequent medial shell. Peciña and Berridge therefore completed a subsequent mapping study using microinjections of the selective mu-opioid agonist, DAMGO, to examine the entire medial shell, including far caudal sites (Pecina and Berridge, 2005). In that second study they found that the hedonic enhancement site (remaining unmoved anatomically) did not extend into the true caudal half of shell after all (though the eating-stimulation zone did), but was rather by our current standards located in the rostral half of medial shell, particularly the midrostral zone (and also positioned in the dorsal half of shell). By measuring the diameters of localized Fos plumes surrounding DAMGO microinjections, and using that as an index of spread of impact for opioid stimulation, Peciña and Berridge calculated the volume of the `liking' enhancement hotspot to be approximately 1 mm3. Therefore the hedonic hotspot was redefined as a cubic millimeter volume in the rostrodorsal quadrant of medial shell, a definition that has persisted to the present (Fig. 2). Beyond locating the hedonic hotspot, another major lesson our lab took from this re-mapping was to always represent localization of function by graphically mapping behavioral effects onto the sites of drug microinjection where they were actually produced. This creates atlas-based pictorial maps of hotspots, gradients or other anatomical distributions of function, rather than rely solely on words to describe neuroanatomical position (because the meaning of words can change regarding anatomical features: such as rostral versus caudal applied to NAc). Showing the mapped position of hotspots graphically, in relation to landmarks and stereotaxic coordinates, may help act as safeguard against having to revise descriptions of a hotspot location in future, even if terms change to designate a particular region.
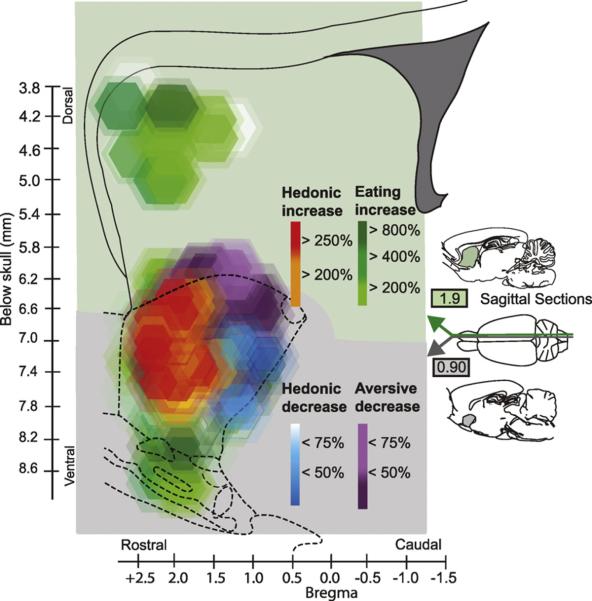
Fig. 2.
Fos plume maps of mu-opioid effects on eating and `liking' in NAc shell. Sagittal illustration of rostral opioid hedonic hotspot in nucleus accumbens (red/orange), as mapped by consequences on hedonic impact and on intake. Symbol sizes show radius of DAMGO microinjection spread based on Fos plumes, and symbol colors indicate where microinjections only generated eating (green), or additionally generated enhanced `liking' (the hedonic hotspot, orange to red), reduced `disliking' or aversion (purple), or reduced `liking' for sucrose task (coldspot, blue). The green in nucleus accumbens extends under all other colors and fills medial shell. The top of map also shows opioid eating generation site in dorsomedial neostriatum generated eating (with no impact on taste `liking' or `disliking'). Only a cubic-millimeter sized hedonic hotspot in NAc shell generates increased `liking', whereas a small blue hedonic “coldspot” in caudal shell oppositely suppresses `liking' reactions to sucrose, and a larger purple zones suppresses `disliking' reactions to quinine. All of these sites still generate eating. Two sagittal planes of different laterality were merged to represent both dorsomedial striatum (1.9 mm from midline) and medial NAc shell (gray, 0.9 mm from midline) in one sagittal map. Based on data from Peciña and Berridge (2006) and DiFeliceantonio et al. (2012). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
5. Anatomical bases of hotspot functional uniqueness
What anatomical basis exists for why the NAc hotspot might be uniquely able to amplify hedonic impact? This issue has recently begun to be explored by neuroanatomists using sensitive tracing techniques. Recent studies by Thompson and Swanson (2010) and by Zahm et al. (2012), have indicated that the NAc hotspot in the rostrodorsal quadrant of medial shell may be distinct anatomically as well as functionally. Both anatomical studies reported the NAc rostrodorsal hotspot to have unique connectivity features that are at least quantitatively and perhaps sometimes qualitatively different from all the rest of NAc (including the rest of medial shell; Fig. 3).
Fig. 3.
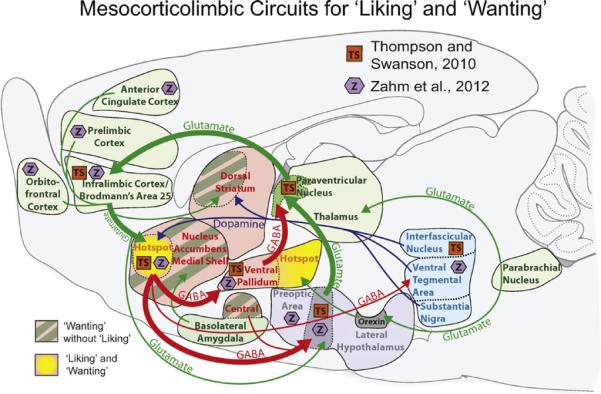
Neural circuits underlying motivated `wanting' and hedonic `liking'. A summary map showing the connections between cortical, limbic, and midbrain nuclei, with a particular focus on the unique connectivity of the NAc hotspot. Thompson and Swanson (TS, orange boxes; 2010) reported that the NAc hotspot is embedded in a closed-circuit loop, receiving corticolimbic inputs from infralimbic cortex, and projecting outputs to restricted subregions of hypothalamus (lateral preoptic area-lateral hypothalamic transition zone) and rostral ventral pallidum. These hypothalamo-pallidal afferents then project to paraventricular nucleus of the thalamus, which then completes the loop by sending efferents to infralimbic cortex. Zahm et al. (Z, purple hexagons; 2012) suggest additional connectivity in a pattern similar to lateral septum. GABAergic projections are indicated in red, hedonic hotspots are marked in yellow, potentiated `wanting' (without `liking') regions are indicated by dark green stripes, glutamatergic projections are bright green, and dopaminergic projections are marked in blue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
For example, Thompson and Swanson reported that the NAc hotspot (rostrodorsal quadrant of medial shell) receives unusually specific inputs from infralimbic cortex (homologous to Brodmann's area 25 or subgenual anterior cingulate cortex in humans) (Barbas et al., 2003; Ongur and Price, 2000; Uylings et al., 2003). Similarly, they reported that the NAc hotspot sends outputs in a unique pattern to restricted subregions of hypothalamus and ventral pallidum, different from the targets of other shell subregions (Thompson and Swanson, 2010). Based on additional mapping of thalamic and prefrontal cortex connections, Thompson and Swanson concluded that the NAc hotspot was embedded in its own special closed-circuit corticolimbic-thalamocortical loop (separate from other segregated loops passing through different NAc shell sub-regions).
Independently, Zahm et al. (2012) recently reported the NAc hotspot of rostral shell to differ from caudal shell, and specifically to share some connection patterns with the lateral septum. Zahm and colleagues suggested the NAc hotspot to be an anatomical transitional zone that merges NAc shell features with features of lateral septum (again, different from the caudal half of medial shell, which they suggest may instead be a transitional zone of NAc with the extended amygdala, including bed nucleus of stria terminalis and central nucleus of amygdala) (Zahm, 2006). While Zahm and colleagues differ from Thompson and Swanson on some points that remain to be resolved, taken as a whole both studies provide initial confirmation that the NAc rostrodorsal quadrant of medial shell is a relatively distinct anatomical entity as well as a functional hotspot.
6. Other hedonic opioid receptors?
Recent work in our lab on the NAc hotspot has further explored the role of the hotspot in determining effects of delta and kappa opioid receptors in the enhancement of hedonic `liking'. This work also follows in the footsteps of Ann Kelley. Bakshi and Kelley (1993a) examined opioid receptor roles in the generation of eating by microinjecting specific agonists for mu, delta or kappa opioid receptors into the NAc and ventral neostriatum. They found that mu agonist microinjections most greatly potentiated feeding, whereas the delta agonist only moderately potentiated feeding, and the kappa agonist failed to stimulate intake. Similar patterns have been replicated (Ragnauth et al., 2000; Zhang and Kelley, 1997). However, recent findings in our lab indicate that the medial shell hotspot may modulate the role of kappa and delta stimulation in `liking' and `wanting', just as it modulates the hedonic role of mu opioid stimulation. Our most recent preliminary results suggest that within the rostrodorsal hotspot kappa and delta receptors also may enhance hedonic impact, though neither do so outside the hotspot (Castro and Berridge, 2012). Further, increased motivation to eat may also be more localized to the hotspot for kappa or delta stimulation than it is for mu opioid stimulation, which can spur intake throughout the entire shell and core. We expect these intriguing possibilities will soon be resolved.
7. Striatopallidal hedonic circuitry: roles of ventral pallidum opioids
The ventral pallidum is the chief anatomical output target of the NAc (Groenewegen et al., 1999), and has emerged recently as an important structure in its own right for the generation of motivation and affect (Inui et al., 2009; Kalivas et al., 1999; Lim et al., 2004; Napier and Mickiewicz, 2010; Smith et al., 2009). Regarding `liking' enhancements in particular, the ventral pallidum has also been shown to contain a hedonic `hotspot', located in its posterior half (Cromwell and Berridge, 1993; Ho and Berridge, 2009; Smith and Berridge, 2005).
Clues that a hedonic hotspot existed in ventral pallidum were first seen in classic 1960s–1970s reports suggesting that lesions to the tissue surrounding and including lateral hypothalamus were sufficient to produce aphagia and disliking behaviors to sucrose (Morgane, 1961; Schallert and Whishaw, 1978; Stellar et al., 1979; Teitelbaum and Epstein, 1962). Those large electrolytic lesions can be seen in retrospect to have damaged ventral pallidum as well as lateral hypothalamus (Berridge, 1996; Smith et al., 2008). In a 1990s excitotoxin lesion study, Cromwell and Berridge (1993) sought to more precisely map the putatively hypothalamic `disliking' region by examining sucrose `disliking' produced by small, discrete cell body lesions in either lateral hypothalamus, globus pallidus, or ventral pallidum. To map the aversion effects, Cromwell measured the diameter of each excitotoxin lesion by quantifying the degree of neuronal death at multiple locations in and near the lesion center. Cromwell and Berridge found that lesions to lateral hypothalamus did indeed produce aphagia, but never `disliking' to sweet solutions, whereas loss of `liking' reactions and replacement with `disliking' gapes only occurred if lesions damaged the ventral pallidum. This ventral pallidum site for damage-induced `disliking' was recently confirmed by Chao-Yi Ho in another excitotoxin study in our lab, and indicated to reside specifically in the posterior half of ventral pallidum (Ho, 2010).
Smith and Berridge (2005) originally identified the hedonic hotspot and mapped its boundaries via opioid enhancements of sensory pleasure, similar to the NAc hotspot. They found that microinjections of DAMGO into the ventral pallidum had bivalent effects on `liking' reactions and eating, depending on the precise site within ventral pallidum. In the roughly cubic-millimeter hotspot within caudal half of ventral pallidum, opioid stimulation doubled hedonic `liking' reactions to sucrose taste as well as increased appetitive eating of chow, again like the NAc hotspot. Conversely, in the rostral half of ventral pallidum mu-opioid stimulation actually decreased `liking' reactions to sucrose and decreased eating and chow intake. Thus, the caudal ventral pallidum contains a distinct opioid hedonic/incentive hotspot that is functionally similar to the NAc hotspot in dorsomedial shell of NAc, plus an oppositely valenced rostral hedonic coldspot for `liking' suppression.
What anatomical basis exists for why the rostral versus caudal subregions of ventral pallidum have opposite opioid effects on hedonic impact and motivation? A potential answer has been suggested by a recent anatomical and electrophysiological study of rostrocaudal differences among neurons within ventral pallidum by Kupchik and Kalivas (2012). Their careful morphological and patch clamp analysis suggested that the caudal ventral pallidum containing the hotspot may comprise mostly neurons that have long aspiny dendrites and are relatively excitable. By comparison, the rostral ventral pallidum (especially rostromedial quadrant) contains neurons with shorter spiny dendrites that are more similar to nucleus accumbens neurons, and are more hyperpolarized and silent (Kupchik and Kalivas, 2012). Such neuronal differences might possibly underlie why opioid hedonic enhancement is functionally localized in ventral pallidum to the caudal hotspot.
The NAc and ventral pallidum are heavily interconnected, with each structure sending GABAergic projections to the other. Although the hotspots may not be directly interconnected anatomically (Thompson and Swanson, 2010), they functionally interact together (Smith and Berridge, 2007; Smith et al., 2011). To explore this interaction, Smith and Berridge (2007) examined whether the two hotspots functionally interacted as a single unit or integrated circuit in enhancing `liking'. They found that recruitment of neurobiological activation occurred after DAMGO microinjection into one hotspot increased local Fos expression, as well as recruiting distant Fos expression in the other structure's hotspot (Smith and Berridge, 2007). Similarly in a later study, NAc hotspot DAMGO microinjection enhanced firing by ventral pallidum hotspot neurons to the taste of sucrose in a sustained pattern that appears to code hedonic impact of the sweet sensation (Smith et al., 2011). Additionally, the hedonic circuit required unanimous opioid participation by the dual hotspots in simultaneous cooperation, further indicating that NAc-VP circuits act together as a single unit to enhance `liking'. When DAMGO was infused into one hotspot, and the opioid antagonist naloxone was simultaneously infused into the other, the endogenous opioid blockade of the second hotspot completely prevented the hedonic enhancement normally produced by opioid stimulation of the first (Smith and Berridge, 2007). Thus blockade in either hotspot vetoes the hedonic enhancement induced by stimulation of the other, presumably by preventing full recruitment of the hedonic circuit as a whole unit. Intriguingly, while `liking' enhancement caused by DAMGO in the NAc hotspot was blocked by ventral pallidum microinjection of naloxone, `wanting' stimulation by the NAc hotspot DAMGO was still preserved: the rats still ate more food. This dissociation indicates that opioid signals that act within the NAc can work to enhance motivational `wanting' for food independently of `liking' under some conditions, even when the source of enhancement begins anatomically in the hotspot, just as opioid stimulation at all NAc sites outside the hotspot selectively stimulates `wanting' without `liking' under all conditions tested so far. The cooperation between NAc-VP may help explain why there are two distinct hedonic hotspots in the brain: the two function together within an integrated circuit that conforms to classic striatopallidal organization. The two hotspots work together in tandem as an integrated functional unit that generates intense hedonic impact for food.
8. Other hedonic mechanisms and circuitry
The magnification of hedonic `liking' for sweet/fatty food can also be enhanced by other neuropeptides and neuromodulators in hotspots, including anandamide, an endocannabinoid (Ho, 2010; Mahler et al., 2007). Endocannabinoid manipulations have been shown to increase eating in a variety of structures, including NAc, in addition to specifically modulating the consumption of palatable food (Cooper, 2004; DiPatrizio and Simansky, 2008a,b; Harrold et al., 2002; Kirkham et al., 2002). Mahler et al. (2007) found that microinjections of anandamide, an endogenous ligand for the CB1 receptor, into the NAc hotspot increase hedonic `liking' reactions to sucrose taste similarly to DAMGO. These results support the idea that endocannabinoids likely modulate appetite and intake in large part by modulating the palatability of food (Kirkham and Williams, 2001).
Recently, our lab has also examined another Ann Kelley theme: interactions of NAc with hypothalamic regulatory circuitry, and particularly the role of hypothalamic orexin/hypocretin in hedonic processing (Ho, 2010; Ho and Berridge, 2009). Orexin/hypocretin-containing neurons are found within a dorsally restricted zone of lateral, perifornical, and dorsomedial hypothalamus, with projections well distributed throughout the brain, including the NAc, ventral pallidum, and parabrachial nucleus (Baldo et al., 2003; Peyron et al., 1998). An anterior subregion of orexin neurons specifically in the lateral hypothalamus has been suggested by Aston-Jones and colleagues to play a special role in food and drug reward (Harris et al., 2005). Orexin neurons receive direct inputs from the orexigenic NPY- and AgRP-containing neurons in the arcuate nucleus, and orexin's role in eating was first observed following demonstrations that intracerebroventricular microinjections of an orexin 1 receptor agonist increased eating (Sakurai et al., 1998). Conversely, administration of an orexin antagonist decreased eating (Haynes et al., 2000; Rodgers et al., 2001).
Kelley and colleagues suggested several years ago a particular circuit route by which lateral hypothalamic orexin neurons might mediate alliesthesia, which is the natural enhancement of food palatability that occurs during hunger states. Kelley et al. (2005a) proposed that orexin neurons act in response to peripheral and central hunger cues via hypothalamic projections to thalamus paraventricular nucleus to activate thalamic glutamate neurons. In turn, they proposed the thalamic paraventricular neurons project to the NAc shell to excite large acetylcholine NAc interneurons. Finally they suggested the acetylcholine interneurons in medial shell then specifically activate nearby intrinsic medium spiny neurons to release enkephalin on neighboring shell neurons. Enkephalin release within the cubic-millimeter hedonic hotspot of rostrodorsal medial shell ought to enhance food `liking', similarly to a DAMGO microinjection. Thus, hunger mediated by orexin activation could amplify `liking' as well as `wanting' for palatable food through the opioid hotspot mechanisms described above.
The circuit described by Kelley and colleagues may well exist, though our lab has additionally found evidence for a more direct circuit in which lateral hypothalamic orexin neuron activation may amplify `liking', via a direct influence of hypothalamic orexin neurons on the ventral pallidum hotspot (Baldo et al., 2003). Ho (2010, 2009) measured `liking' and `disliking' reactions to tastes following microinjection of orexin directly into the ventral pallidum hotspot and found that orexin enhanced `liking' reactions to sucrose similarly to mu opioid stimulation. Her findings indicate that orexin in ventral pallidum may amplify the sensory pleasure of food, which is relevant to orexin roles in motivating behaviors for reward (Cota et al., 2006; Kirkham and Williams, 2001; Lopez et al., 2011).
9. Opioid reward in dorsal neostriatum: above and beyond all ventral striatum
Ann Kelley's mapping of opioid stimulation of intake produced findings that support a role for motivational mechanisms in some regions of neostriatum (dorsal striatum or caudate/putamen). Kelley and colleagues found that microinjections of morphine or DAMGO in ventral regions of neostriatum produced robust eating, similar to NAc (Bakshi and Kelley, 1993a,b; Zhang and Kelley, 2000).
How far dorsal in neostriatum does motivational and eating circuitry extend? The work of Kelley and colleagues recently inspired us to re-examine this question (DiFeliceantonio et al., 2012). Bakshi and Kelley (1993b) first suggested that microinjections of opioid agonists in ventral or middle regions of neostriatum stimulated eating behavior, but that more dorsal regions of neostriatum did not. However, if one looks closely at the histology figure from Zhang and Kelley (2000), one may discern a cluster of sites within the medial dorsal region of neostriatum that appeared to produce increases in eating. We recently probed the medial zone of the most dorsal level of neostriatum with microinjections of DAMGO. We found that DAMGO microinjections at medial sites in dorsal neostriatum can generate robust increases >200% in eating of palatable sweet food (M&M™ chocolate candies), at least in the anterior half of the dorsomedial zone (anteromedial quadrant of dorsal neostriatum) (DiFeliceantonio et al., 2012). This anterior-medial dorsal neostriatum mediation of increased `wanting' to eat was not accompanied by any increases in hedonic `liking' reactions to the tastes of sucrose or of M&M™ candies, as measured by taste reactivity (Fig. 2; DiFeliceantonio et al., 2012). That wanting-without-liking pattern indicates opioid stimulation of this dorsal level of anteromedial neostriatum generates pure motivation without amplifying hedonic impact, similar to other striatal-type areas outside the NAc and ventral pallidum hotspots, including the core and posterior shell of NAc, and even the central nucleus of amygdala (Mahler and Berridge, 2012; Pecina and Berridge, 2005), which has striatal-like anatomical features such as containing predominantly GABAergic neurons (Swanson, 2005).
In addition, using microdialysis techniques conducted in collaboration with Omar Mabrouk and Robert Kennedy of the Chemistry Department at the University of Michigan, we recently found that endogenous enkephalin levels in the same anteromedial quadrant of dorsal neostriatum surged >150% when rats were suddenly allowed to eat palatable chocolate M&M™ candies (DiFeliceantonio et al., 2012). This region of the neostriatum contains “patches” or “striosomes” that are rich in mu opioid receptors, and which receive cortical input projections from limbic regions of prefrontal cortex and may project directly to dopamine neurons in the substantia nigra pars compacta (Eblen and Graybiel, 1995; Fujiyama et al., 2011; Gerfen, 1984; Levesque and Parent, 1998; Ragsdale and Graybiel, 1988, 1990). Enkephalin is likely acting on these mu-opioid receptors in neostriatum patches.
Kelley and colleagues (Kelley et al., 2005a; Will et al., 2007) proposed that enkephalin levels in the NAc and ventral regions of neostriatum controlled short-term food consumption and perhaps short term motivation for food. Our results support their proposal, and suggest this striatal enkephalin role may extend to an anteromedial region even in the most dorsal level of neostriatum.
10. Beyond desire to dread in NAc: rostrocaudal keyboards of appetitive and fearful motivated behaviors in medial shell
Though perhaps somewhat surprising in light of the NAcs well-known association with reward, its medial shell has also emerged as an important neural generator of some negatively valenced motivation states of fear and disgust (Carlezon and Thomas, 2009; Levita et al., 2009, 2012; Reynolds and Berridge, 2001, 2002, 2003; Richard and Berridge, 2011a; Salamone, 1994). In particular, our laboratory has found evidence for a keyboard-like rostrocaudal gradient of motivation generators in medial shell, where localized hyperpolarizations generate intense eating at rostral sites and an equally intense form of active (defensive) fear and even disgust at caudal sites. This organization echoes the rostral hedonic hotspot versus caudal coldspot localization for opioid impact on `liking' reactions, but with even stronger bivalent emotional tone.
Our experiments on the NAc generation of desire and fear stemmed originally as confirmation and hedonic probing of Ann Kelley and colleagues' demonstration of intense eating generated by the rostral-to-middle zones of medial shell after amino acid hyperpolarizations caused by microinjections of an AMPA glutamate antagonist (such as DNQX) or a GABA-A agonist (such as muscimol) (Basso and Kelley, 1999; Kelley et al., 2005b; Kelley and Swanson, 1997; Maldonado-Irizarry et al., 1995; Stratford, 2005; Stratford and Kelley, 1997, 1999; Stratford et al., 1998; Stratford and Wirtshafter, 2011, 2012).
In our lab, Reynolds and Berridge (2001) first confirmed that muscimol microinjection in rostral-to-middle zones of medial shell generated intense eating, and that progressively more caudal microinjections stopped eliciting eating. Yet Reynolds and Berridge also found that caudal microinjections in NAc shell were not behaviorally `silent'. Instead caudal sites elicited intense levels of fearful anti-predator behavior known as defensive treading or defensive burying, in which wild rodents use rapid forepaw movements to throw dirt or debris at a threatening stimulus or predator (i.e. rattlesnake or scorpion) (Coss and Owings, 1978; Londei et al., 1998; Reynolds and Berridge, 2001; Treit et al., 1981), and which lab rats emit to a stationary electrified shock prod or in response to predator odor (De Boer and Koolhaas, 2003; Treit et al., 1981). This anti-predator behavior requires the presence of support stimuli: dirt, sand, wood shavings, cob bedding or some similar loose substrate on the floor of the test chamber for the rat to engage with its forepaws and use in throwing or pushing motions. In a cage empty aside from food, the rat cannot readily display the naturalistic fearful response. That stimulus requirement, plus dependence on caudal sites for generation, is probably why the fearful treading behavior was never reported in NAc studies prior to our lab's findings. Defensive treading generated by caudal shell sites (generally between 0.48 and 1.4 mm ahead of bregma) tends not to be emitted randomly in all directions, but instead is targeted upon a particular location or perceived stimulus that the rat apparently defends itself against: treading and throwing bedding toward the front of the transparent cage (e.g., toward the experimenter, cameras, and lights visible beyond the cage) or toward cage corners where light tends to reflect brightly back into the cage from the curved surface of transparent plastic (Reynolds and Berridge, 2001). Additionally, supporting the interpretation that caudal NAc shell sites release motivated fear, if the experimenter attempts to touch a rat showing defensive treading after a caudal shell microinjection of muscimol or DNQX, the rat is likely to emit other fearful or defensive reactions, such as audible distress vocalizations, frantic leaps and attempts to escape, and even to defensively bite the experimenter's approaching hand, though the same rat at all other times is highly tamed and docile (Reynolds and Berridge, 2001, 2002, 2003).
Microinjections of either AMPA glutamate antagonist DNQX or GABAergic agonist muscimol, both of which likely induce localized inhibitions (disinhibiting downstream targets via release from tonic GABAergic inhibition), generate this same rostrocaudal keyboard pattern of intense eating versus intense fear (Fig. 4) (Faure et al., 2008; Reynolds and Berridge, 2001, 2002, 2003). Importantly, only muscimol's GABAergic inhibition, which likely mimics signaling from intrinsic subcortical GABA-releasing circuitry, generates corresponding changes in pleasure versus disgust along a similar rostrocaudal gradient. Muscimol microinjection in a small vertical strip of rostrodorsal shell enhances “liking” reactions elicited by bittersweet sucrose-quinine taste, whereas more caudal microinjections produce “disliking” or disgust reactions such as gapes (Faure et al., 2010; Reynolds and Berridge, 2002). In contrast, corticolimbic glutamate blockade with DNQX has no effect at any shell site on either “liking” or “disliking” for tastes, despite generating intense motivations related to eating or fear, potentially indicating that top-down cortical glutamate inputs have less access to subcortically generated hedonic processes mediated by intrinsic GABAergic processing within medial NAc shell (Faure et al., 2010).
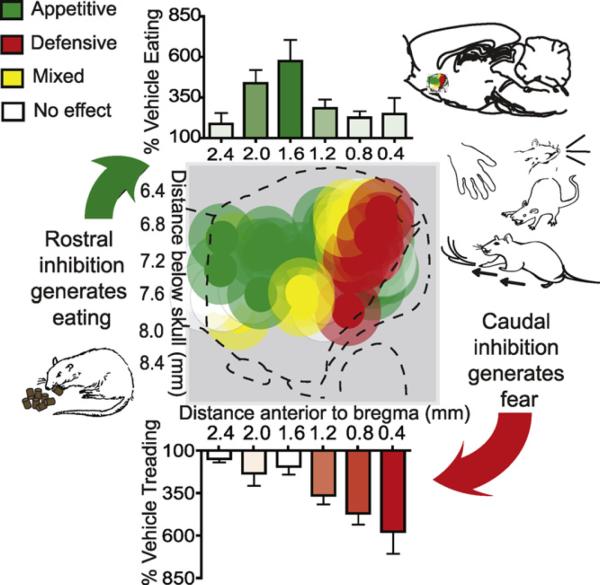
Fig. 4.
Example desire and dread gradient. Sagittal map of intense desire-dread keyboard generated by microinjections that hyperpolarize via glutamate or GABA neurotransmitter signals in NAc medial shell (example from glutamatergic AMPA blockade). DNQX microinjections produced intense appetitive (green), fearful (red) or mixed appetitive and fearful (yellow) motivated behaviors. Rostral DNQX generates purely intense eating, whereas more caudal microinjection generate intense defensive behaviors, including touch-elicited distress vocalizations, escape attempts and bite attempts, and spontaneous defensive treading. Bars above and below the maps show behavioral intensities of eating (above, green) and treading (below, red) as a percent of vehicle level in the same rats (error bars indicate SEM). From Richard and Berridge (2011a,b). Similar patterns of intense behaviors have been found after GABAergic muscimol microinjections and replicated for DNQX in other studies (Reynolds and Berridge, 2001, 2002, 2003, 2008; Faure et al., 2008, 2010). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
The valence of motivated behavior generated by medial shell hyperpolarizations is anatomically biased, such that rostral versus caudal amino acid disruptions typically generate positive versus negative motivational salience, but the behavior produced at a given rostrocaudal site is not absolutely fixed in valence. Environmental and corticolimbic neurobiological factors can retune (or “flip”) the valence of behavior produced from most sites (Reynolds and Berridge, 2008; Richard and Berridge, 2012). Testing in an aversive, stressful environment, with very bright lights and loud rock music (e.g., Iggy Pop sound track; 80–86 decibels) expands the fear-generating zone, such that microinjections at more rostral locations generate fear (Reynolds and Berridge, 2008). Conversely, testing in a familiar and comfortable home-like environment nearly eliminates fear generation from most sites except in the most posterior rim of caudal shell, and expands the appetitive zone, such that more caudal locations generate only eating (Reynolds and Berridge, 2008). However, eating and fear induced by GABAergic muscimol microinjections that mimic intrinsic subcortical NAc circuits are more resistant to environmental retuning than DNQX glutamatergic microinjections: rostral GABA inhibitions always generate intense eating without fear and caudal GABA inhibitions generate fear without eating, regardless of the current emotional environment (Richard et al., 2012). Therefore, in the small rostral NAc shell area where GABA inhibitions increase hedonic `liking' reactions (Faure et al., 2010), GABA inhibitions never generated fear, even in a stressful environment. This perhaps indicates that separate neuronal ensembles or circuits are responsible for positively valenced `liking'/appetitive behaviors versus negatively valenced `disgust'/fear.
Endogenous dopamine also plays a role in the glutamatergic generation of appetitive and fearful motivation by corticolimbic AMPA blockade via DNQX microinjection in NAc shell, as well as in the environment-induced shifts of valence mapping and retuning of particular keyboard sites. Adding a combination of D1 and D2 dopamine antagonists to the DNQX microinjection prevents it from generating either eating or fear (Faure et al., 2008). Importantly, when separately compared, D1 and D2 signals play different roles in enabling motivation generation by NAc glutamate disruption. Eating generated by DNQX in rostral medial shell requires only D1 endogenous dopamine transmission, whereas fearful behavior generated by caudal DNQX requires both D1 and D2 transmission (Richard and Berridge, 2011b). Further, the dopamine receptor requirements for a given site in NAc shell can reverse if a change in environmental ambience shifts the valence of motivation generated by AMPA blockade at the site (Richard and Berridge, 2011b). That is, appetitive eating generated by DNQX never requires D2 dopamine transmission, even when generated by caudal shell in a home-like familiar environment. Conversely, defensive behavior generated by DNQX always requires D2 dopamine transmission, even when generated by rostral shell in a stressful environment (Richard and Berridge, 2011b). This D1 versus D2 dopamine receptor difference may indicate differing recruitment of parallel output pathways from NAc. Dopamine D1 receptors are associated with neurons that give rise to direct pathway projections which target ventral tegmentum, whereas D2 receptors are only associated with `indirect path' projections to ventral pallidum and potentially lateral hypothalamus (Humphries and Prescott, 2010; Lu et al., 1998; Richard and Berridge, 2011b; Zhou et al., 2003). D1 versus D2 dopamine receptors also may differently alter glutamatergic inputs from hippocampus, amygdala, and prefrontal cortex origins (Bamford et al., 2004; Charara and Grace, 2003; Nicola et al., 1996; Pennartz et al., 1992; Richard and Berridge, 2012). These differences in inputs and outputs are consistent with the notion that somewhat separate ensembles of neurons or segregated circuits might be recruited in the NAc generation of desire versus dread.
11. Limbic transformations of memories into motivation
Beyond the generation of motivation by mesocorticolimbic circuits, Ann Kelley and colleagues also explored the interaction between motivation and learning within NAc. She notably explored the role played in appetitive instrumental conditioning by NMDA receptors (Kelley et al., 1997) and protein synthesis (Hernandez et al., 2002), and interactions with dopamine in NAc circuits (Kelley and Delfs, 1991).
On the surface, motivation triggered by Pavlovian cues for reward may often appear to simply be learned (e.g., purely due to conditioned associations or memories triggered by a cue) (Schultz et al., 1997; Wise, 2012). However, learned Pavlovian associations account for only half the input required for the generation of the Pavlovian motivation process of incentive salience (Zhang et al., 2009). The other half comes from the neurobiological state of mesocorticolimbic circuits at the moment of cue re-encounter (Zhang et al., 2009), which is influenced by pharmacological/physiological drug states, appetite/satiety states, stress states, etc. An individual's mesocorticolimbic state combines synergistically with learned CS-UCS associations to generate motivation on the fly, and the result of the combination can raise, lower, or even completely reverse the previously learned motivational value (Zhang et al., 2009).
The strongest proof of principle comes from complete reversal by a shift in neurobiological state of motivation valence for a previously learned cue, from attractive to repulsive or from repulsive to attractive (Fig. 5). For example, we have found that an appropriate physiologically induced state shift within mesocorticolimbic circuitry can dynamically convert Pavlovian cue-triggered motivation from repulsion to incentive salience for a CS associated with disgustingly intense saltiness (Robinson and Berridge, 2010; Tindell et al., 2009). This makes that CS into an instant motivational magnet, even if it was always avoided and learned to be aversive on every previous encounter. Our lab recently demonstrated this psychological transformation in practice by first training a Pavlovian association between a CS (sudden appearance of a noisy metal lever in chamber) with the disgusting UCS experience of tasting a salt solution similar to that of the Dead Sea (9% NaCl; 300% more salty than ordinary sea water) directly into the mouth. Subsequently, a combination of hormone and diuretic drug injections was used to produce a novel physiological state of strong salt appetite. In the new appetite state, we found that the salt CS was suddenly transformed into an attractive motivational magnet, as reflected by the rat's behavior: the earlier-avoided CS now elicited behavioral approach and consummatory nibbles and sniffs upon its very first presentation in extinction, with no re-learning about its salty UCS. The CS transformation occurred to the first re-encounter, without the triple seawater salt solution having ever been experienced as anything other than disgusting. We also found that this dramatic motivation transformation from repulsive to attractive was mediated neurobiologically by intense activation of mesocorticolimbic circuits, as reflected by up to 10-times elevations in expression of Fos protein in neurons belonging to NAc (especially rostral shell), prefrontal cortex, ventral pallidum and ventral tegmentum, triggered by the combination of cue re-encounter with novel physiological appetite state (Robinson and Berridge, 2010).
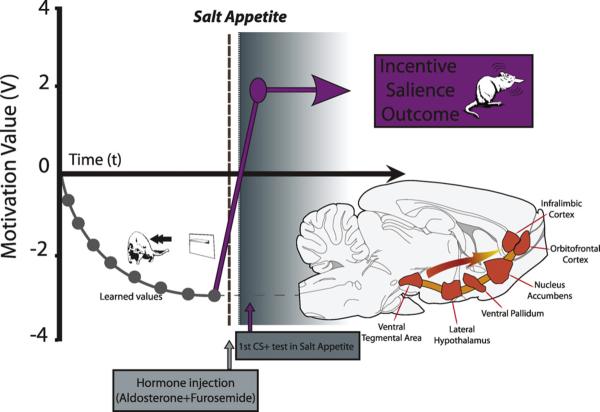
Fig. 5.
Theoretical model of the synergy between learned values and mesocorticolimbic activation. The diagram displays the impact of a sudden change in internal/mesocorticolimbic state (novel salt appetite) on the progressively learned negative value of a Pavlovian CS according to the prediction made by incentive salience theory (Zhang et al., 2009). Incentive salience theory predicts that a change in internal mesocorticolimbic state would be sufficient to drastically change the reward value of a CS from negative to positive without requiring new learning (presentation of the CS alone). Upon the first presentation of the CS for intense saltiness alone (no UCS salt solution), the cue is instantly transformed from previously avoided to jumped upon and avidly nibbled, despite its associated UCS having only ever been experienced as highly disgusting and aversive.
Similar, if not as dramatic, instant re-computations and enhancements of CS motivation value can be accomplished by directly manipulating mesocorticolimbic structures with opioid or dopamine stimulation to alter neurobiological state. Typically, rather than reverse value, this suddenly raises an already moderately high and positive CS motivation value from the level that was previously learned to a new even higher peak that was never learned or experienced prior to the recombination. For example, microinjections of amphetamine or DAMGO into NAc shell or core produces robust increases in cue-triggered `wanting' for sugary rewards as measured in Pavlovian to instrumental transfer (PIT) or in neuronal incentive salience signals (Pecina and Berridge, 2008; Wyvell and Berridge, 2000). Similarly, microinjections of DAMGO or amphetamine raise conditioned reinforcement value or breakpoint values in instrumental tests that might reflect incentive salience (Cunningham and Kelley, 1992; Kelley and Delfs, 1991; Zhang et al., 2003). We have found that DAMGO microinjections in other striatal-type structures such as the central amygdala also increase cue-triggered `wanting' in PIT and likewise increase the motivational magnet strength of an individual's favorite reward cue in a sign-tracking versus goal-tracking test (autoshaping) (DiFeliceantonio and Berridge, 2012; Mahler and Berridge, 2009, 2012). Conversely, microinjections of a dopamine antagonist into NAc core immediately suppress the motivational magnet features of a CS for sugar in sign-tracking rats (Saunders and Robinson, 2012).
The power of a motivational magnet consists of the intensity and frequency of behaviors directed at a cue. Importantly, it is computed on line and is transferable to other reward cues. When in the absence of a goal, for example with intra-oral sucrose delivery, rats that might have ordinarily have been goal-trackers instead engage in sign-tracking behavior (Robinson and Berridge, 2010). Both sign and goal cues can transform into motivational magnets for goal-trackers with certain neurobiological or environmental manipulations (DiFeliceantonio and Berridge, 2012; Mahler and Berridge, 2012; Robinson and Berridge, 2010). In addition, sign-trackers can flip to goal-tracking behavior after some mesolimbic-related pharmacological stimulations or sensitization (Doremus-Fitzwater and Spear, 2011; Holden and Peoples, 2009; Simon et al., 2009), which might boost the incentive salience of UCS-proximal cues such as the goal at the expense of UCS-distal cues such as the Pavlovian CS sign (Smith et al., 2011; Tindell et al., 2006).
Therefore, it seems clear that neurobiological state modulations of mesocorticostriatal circuitry can synergistically combine with previously learned Pavlovian CS-UCS associations to produce sudden changes in psychological incentive salience attributed to the CS at the moment it is re-encountered. These modulations can flexibly change the temptation power of a learned Pavlovian cue for reward either up or down, depending on the neurobiological modulation, and do so even when all learned associations remain unchanged. In other words, there is more to Pavlovian motivation than learning per se.
12. Relation of affective neuroscience functions to brain mechanisms of decisions and learning
Our discussion has focused on the generation of intense levels of motivation (e.g., `wanting' and fear) and hedonic reactions (`liking' and `disgust') by NAc circuitry interacting with related structures such as ventral tegmentum, neostriatum, amygdala and neocortex. How do these topics relate to other psychological functions such as cognitive decision making or to learning of reward predictions or habits? And how do anatomical features such as localized hedonic hotspots or rostrocaudal desire-fear keyboards relate to other features of mesocorticostriatal circuits, such as spiraling connections between midbrain and striatal targets that ascend dorsally from nucleus accumbens to neostriatum (Haber et al., 2000)? We believe that larger perspectives can eventually accommodate all of these functions and features. For example, the generation of `liking' and `wanting' components of reward considered here ordinarily must interact with learning components of reward and related functions (e.g., Pavlovian, instrumental and cognitive mechanisms of learning) to produce real behavior.
Motivational processes such as incentive salience contribute even to cognitive decision making in the sense that subcortically generated `wanting' can bias decisions, including decisions ordinarily controlled by cortex-based circuitry. At intense extremes, motivational processes such as mesolimbic incentive salience or fearful salience can substantially take control of decision-making, producing compulsive forms of addictions or of fearful paranoia. The affective neuroscience functions discussed here co-exist alongside whatever other psychological functions turn out to be mediated by striatal-related circuitry, which by some views would also include prediction error or habit-learning functions (Graybiel, 2008; Schultz et al., 1997).
Neuroanatomically, the distinctive neurobiological substrate features responsible for giving unique hedonic-generating functions to the NAc hotspot or VP hotspot, or responsible for creating rostrocaudal keyboards in NAc shell for generating desire versus fear (Thompson and Swanson, 2010; Zahm et al., 2012), similarly must co-exist with other mesocorticostriatal anatomical wiring features, which include ascending spirals of progressive ventral-to-dorsal information flow (Haber et al., 2000). Although we have focused purely on features underlying the generation of intense motivation and affect, eventually more fully informed perspectives will be able to incorporate all such features and additional functions.
13. Conclusions
`Liking', `wanting' and learning components of reward can be teased apart and to some degree linked to separable brain substrates. Opioid and related hedonic hotspot networks in NAc-pallidal circuits generate and amplify `liking' for sensory pleasures of food. Intense `wanting' to over-consume is generated by larger striatal networks, including even amygdala and the most dorsal levels of neostriatum. `Wanting' is also generated by corticolimbic glutamate interactions with mesolimbic dopamine, but surprisingly overlaps with NAc mechanisms that can equally generate intensely fearful motivations too. Finally, changes in the neuro-biological reactivity of mesocorticolimbic circuitry can completely transform the psychological motivation value of previously learned memories, without any new learning (a transformation potentially relevant to compulsive addictions). Each of these conclusions comes from lines of research in our lab that were originally inspired in large part by earlier ground-breaking findings of Ann Kelley and her laboratory. In short, Ann Kelley was a scientific path finder, whose impressive talent and energy opened up a number of new vistas for research on the brain and motivation. We were fortunate to follow in her scientific path, and like many others in the field we continue to be inspired by her marvelous achievements.
Acknowledgment
Some of the results described here are from work supported by National Institutes of Health grants DA015188 and MH63649 (KCB), F31 MH090602 (JMR), T32 DC00011 (DCC) and T32 DA007267 (AGD).
References
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993a;265:1253–1260. [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology. 1993b;111:207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. Journal of Comparative Neurology. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. Journal of Neuroscience. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behavioral Neuroscience. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience and Biobehavioral Reviews. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain and Cognition. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behavioral Neuroscience. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl. 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. Journal of Neuroscience. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. Comparing mu Delta or kappa Opioid Receptor Activation in the Nucleus Accumbens Hotspot: Enhancement of Food `liking' Versus `Wanting'. Society for Neuroscience; New Orleans, LA: 2012. [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28:1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid palatability-dependent appetite. European Journal of Pharmacology. 2004;500:37–49. doi: 10.1016/j.ejphar.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Coss RG, Owings DH. Snake-directed behavior by snake naive and experienced California ground squirrels in a simulated burrow. Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology. 1978;48:421–435. [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Research Reviews. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Research. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Research. 1992;588:104–114. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JA. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European Journal of Pharmacology. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Berridge KC. Which cue to `want'? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behavioural Brain Research. 2012;230:399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC. Enkephalin surges in dorsal neostriatum as a signal to eat. Current Biology. 2012;22:1918–1924. doi: 10.1016/j.cub.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. Journal of Neuroscience. 2008a;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipatrizio NV, Simansky KJ. Inhibiting parabrachial fatty acid amide hydrolase activity selectively increases the intake of palatable food via cannabinoid CB1 receptors. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008b;295:R1409–R1414. doi: 10.1152/ajpregu.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125:661–667. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacology Biochemistry and Behavior. 1993;46:745–749. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Levitin M, MacConell LA, Lee LK, Ettenberg A. Opposite effects of prefrontal cortex and nucleus accumbens infusions of flupenthixol on stimulant-induced locomotion and brain stimulation reward. Brain Research. 1992;576:104–110. doi: 10.1016/0006-8993(92)90614-f. [DOI] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. Journal of Neuroscience. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. Journal of Neuroscience. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. European Journal of Neuroscience. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Majchrzak MJ. Centrally administered opioid peptides stimulate saccharin intake in nondeprived rats. Pharmacology Biochemistry and Behavior. 1989;33:805–810. doi: 10.1016/0091-3057(89)90474-7. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual Review of Neuroscience. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Annals of the New York Academy of Sciences. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Research. 2002;952:232–238. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regulatory Peptides. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nature Neuroscience. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Ho C-Y. The Ventral Pallidum as a Limbic Pleasure Generator. University of Michigan; Ann Arbor, MI: 2010. [Google Scholar]
- Ho C-Y, Berridge KC. Hotspots for Hedonic `Liking' and Aversive `Disliking' in Ventral Pallidum. 2009 ed. Society for Neuroscience; 2009. 2009 Abstracts. [Google Scholar]
- Holden JM, Peoples LL. Effects of acute amphetamine exposure on two kinds of Pavlovian approach behavior. Behavioural Brain Research. 2009;208:270–273. doi: 10.1016/j.bbr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Progress in Neurobiology. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. European Journal of Pharmacology. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Inui T, Yamamoto T, Shimura T. GABAergic transmission in the rat ventral pallidum mediates a saccharin palatability shift in conditioned taste aversion. European Journal of Neuroscience. 2009;30:110–115. doi: 10.1111/j.1460-9568.2009.06800.x. [DOI] [PubMed] [Google Scholar]
- Islam AK, Bodnar RJ. Selective opioid receptor antagonist effects upon intake of a high-fat diet in rats. Brain Research. 1990;508:293–296. doi: 10.1016/0006-8993(90)90410-d. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Annals of the New York Academy of Sciences. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. Journal of Comparative Neurology. 2005a;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiology and Behavior. 2005b;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. Journal of Pharmacology and Experimental Therapeutics. 1996;278:1499–1507. [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. II. Contrasting effects of amphetamine microinjection into the nucleus accumbens with peptide microinjection into the ventral tegmental area. Psychopharmacology. 1991;103:197–203. doi: 10.1007/BF02244203. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Iversen SD. Behavioural response to bilateral injections of substance P into the substantia nigra of the rat. Brain Research. 1978;158:474–478. doi: 10.1016/0006-8993(78)90693-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-d-aspartate receptor activation in the nucleus accumbens core. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Stinus L, Iversen SD. Interactions between d-ala-metenkephalin, A10 dopaminergic neurones, and spontaneous behaviour in the rat. Behavioural Brain Research. 1980;1:3–24. doi: 10.1016/0166-4328(80)90043-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behavioural Brain Research. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Synergistic effects of opioid and cannabinoid antagonists on food intake. Psychopharmacology. 2001;153:267–270. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. British Journal of Pharmacology. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Kalivas PW. The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: an electrophysiological study. Brain Structure and Function. 2012 doi: 10.1007/s00429-012-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Parent A. Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cerebral Cortex. 1998;8:602–613. doi: 10.1093/cercor/8.7.602. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hoskin R, Champi S. Avoidance of harm and anxiety: a role for the nucleus accumbens. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.04.059. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) Journal of Comparative Neurology. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Londei T, Valentini AM, Leone VG. Investigative burying by laboratory mice may involve non-functional, compulsive, behaviour. Behavioural Brain Research. 1998;94:249–254. doi: 10.1016/s0166-4328(97)00162-9. [DOI] [PubMed] [Google Scholar]
- Lopez CA, Guesdon B, Baraboi ED, Roffarello BM, Hetu M, Richard D. Involvement of the opioid system in the orexigenic and hedonic effects of melanin-concentrating hormone. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;301:R1105–R1111. doi: 10.1152/ajpregu.00076.2011. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. Journal of Neuroscience. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berlin) 2012 doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances `liking' of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. Journal of Neuroscience. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ. Alterations in feeding and drinking behavior of rats with lesions in globi pallidi. American Journal of Physiology. 1961;201:420–428. doi: 10.1152/ajplegacy.1961.201.3.420. [DOI] [PubMed] [Google Scholar]
- Napier TC, Mickiewicz AL. The role of the ventral pallidum in psychiatric disorders. Neuropsychopharmacology. 2010;35:337. doi: 10.1038/npp.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. Journal of Neuroscience. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behavioral Neuroscience. 1992;106:999–1010. doi: 10.1037//0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; New York: 2007. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Central enhancement of taste pleasure by intraventricular morphine. Neurobiology (Bp) 1995;3:269–280. [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic `liking' for food: map based on microinjection Fos plumes. Brain Research. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? Journal of Neuro-science. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Incentive Salience Mediation by Opioid Versus Dopamine in Nucleus Accumbens Shell and Core: Amplified Cue-triggered `Wanting' for Reward. Society for Neuroscience; Washington DC: 2008. [Google Scholar]
- Pellegrino LJ, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. Appleton-Century-Crofts; New York: 1967. [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. Journal of Neurophysiology. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffmann C, Norgren R, Grill HJ. Sensory affect and motivation. Annals of the New York Academy of Sciences. 1977;290:18–34. doi: 10.1111/j.1749-6632.1977.tb39713.x. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Research. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW, Jr., Graybiel AM. Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. Journal of Comparative Neurology. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW, Jr., Graybiel AM. A simple ordering of neocortical areas established by the compartmental organization of their striatal projections. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6196–6199. doi: 10.1073/pnas.87.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. Journal of Neuroscience. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. European Journal of Neuroscience. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. European Journal of Neuroscience. 2011a;33:736–747. doi: 10.1111/j.1460-9568.2010.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. Journal of Neuroscience. 2011b;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Plawecki AM, Berridge KC. Intrinsic GABAergic Hyperpolarization in Nucleus Accumbens Shell Robustly Generates Desire and Dread Despite Environmental Ambience and Without Need of Local Dopamine. Society for Neuroscience; New Orleans, LA: 2012. [Google Scholar]
- Rideout HJ, Parker LA. Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacology Biochemistry and Behavior. 1996;53:731–734. doi: 10.1016/0091-3057(95)02077-2. [DOI] [PubMed] [Google Scholar]
- Robinson MJF, Berridge KC. Instant Incentive Salience: Dynamic Transformation of an Aversive Salt Cue into a `Wanted' Motivational Magnet. Society for Neuroscience; San Diego, USA: 2010. [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. European Journal of Neuroscience. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buck-ingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behavioural Brain Research. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuro-science. 2012 doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Two types of aphagia and two types of sensor-imotor impairment after lateral hypothalamic lesions: observations in normal weight, dieted, and fattened rats. Journal of Comparative and Physiological Psychology. 1978;92:720–741. doi: 10.1037/h0077504. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sills TL, Vaccarino FJ. Individual differences in sugar consumption following systemic or intraaccumbens administration of low doses of amphetamine in nondeprived rats. Pharmacology Biochemistry and Behavior. 1996;54:665–670. doi: 10.1016/0091-3057(96)00024-x. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Bodnar RJ, Goldman EJ, Pasternak GW. Involvement of opioid receptor subtypes in rat feeding behavior. Life Sciences. 1985;36:829–833. doi: 10.1016/0024-3205(85)90206-1. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. Journal of Neuroscience. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal of Neuroscience. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural Brain Research. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacology Biochemistry and Behavior. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symposium on Oral Sensation and Perception. 1973:254–278. [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neuroscience and Biobehavioral Reviews. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Brooks FH, Mills LE. Approach and withdrawal analysis of the effects of hypothalamic stimulation and lesions in rats. Journal of Comparative and Physiological Psychology. 1979;93:446–466. doi: 10.1037/h0077590. [DOI] [PubMed] [Google Scholar]
- Stratford TR. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Research. 2005;1048:241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. Journal of Neuroscience. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. Journal of Neuroscience. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behavioural Brain Research. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behavioural Brain Research. 2011;216:514–518. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behavioural Brain Research. 2012;226:548–554. doi: 10.1016/j.bbr.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. Journal of Comparative Neurology. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychological Review. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. Journal of Neuroscience. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. Journal of Neurophysiology. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacology, Biochemistry & Behavior. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;292:R217–R226. doi: 10.1152/ajpregu.00852.2005. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. Journal of Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional-anatomical `macrosystems'. Neuroscience and Biobehavioral Reviews. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic `hotspot' of Pecina and Berridge with commentary on the transitional nature of basal forebrain `boundaries'. Journal of Comparative Neurology. 2012 doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW. A neural computational model of incentive salience. PLoS Computational Biology. 2009;5:e1000437. doi: 10.1371/journal.pcbi.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behavioral Neuroscience. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology. 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal muopioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120:783–798. doi: 10.1016/s0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]