Abstract
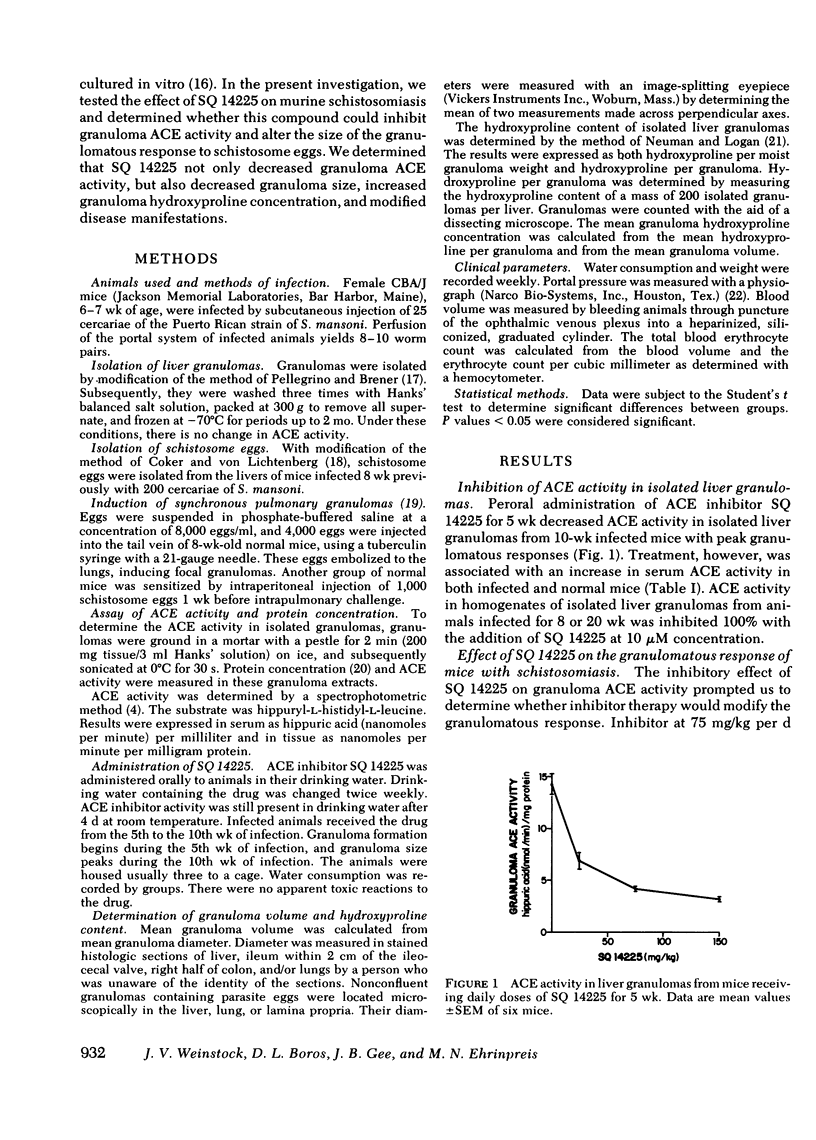
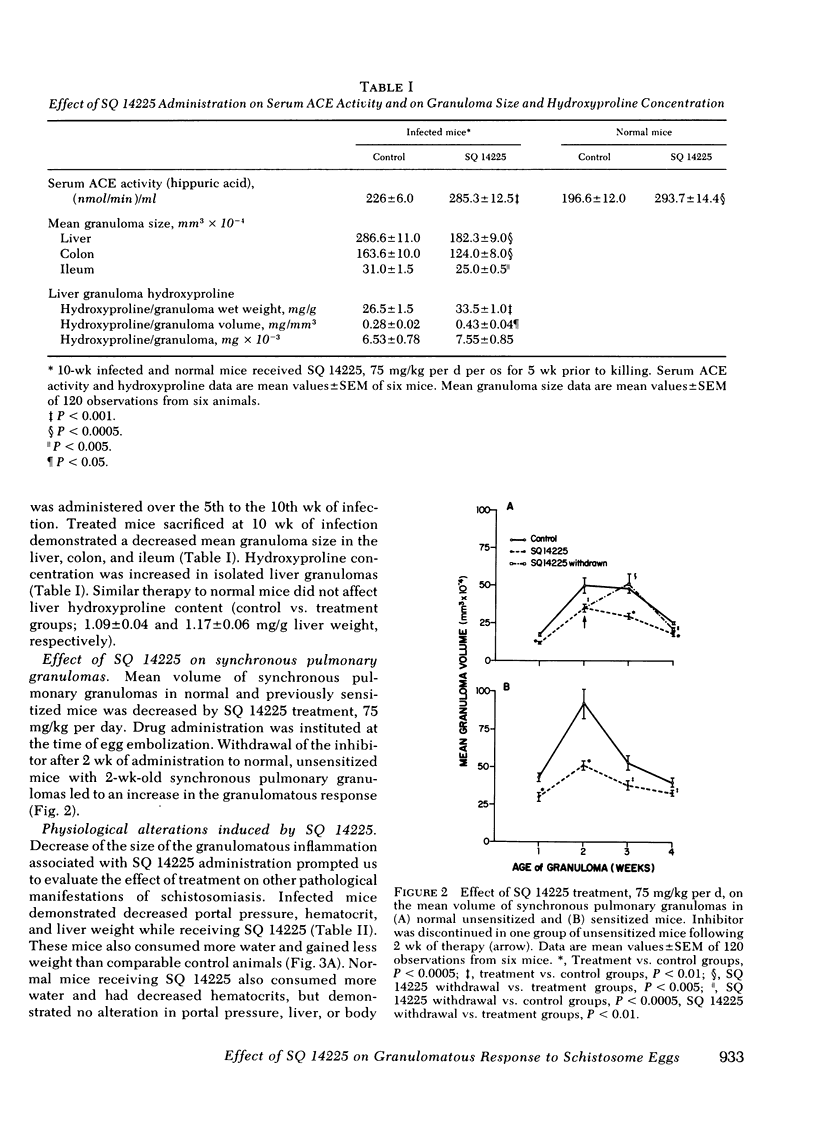
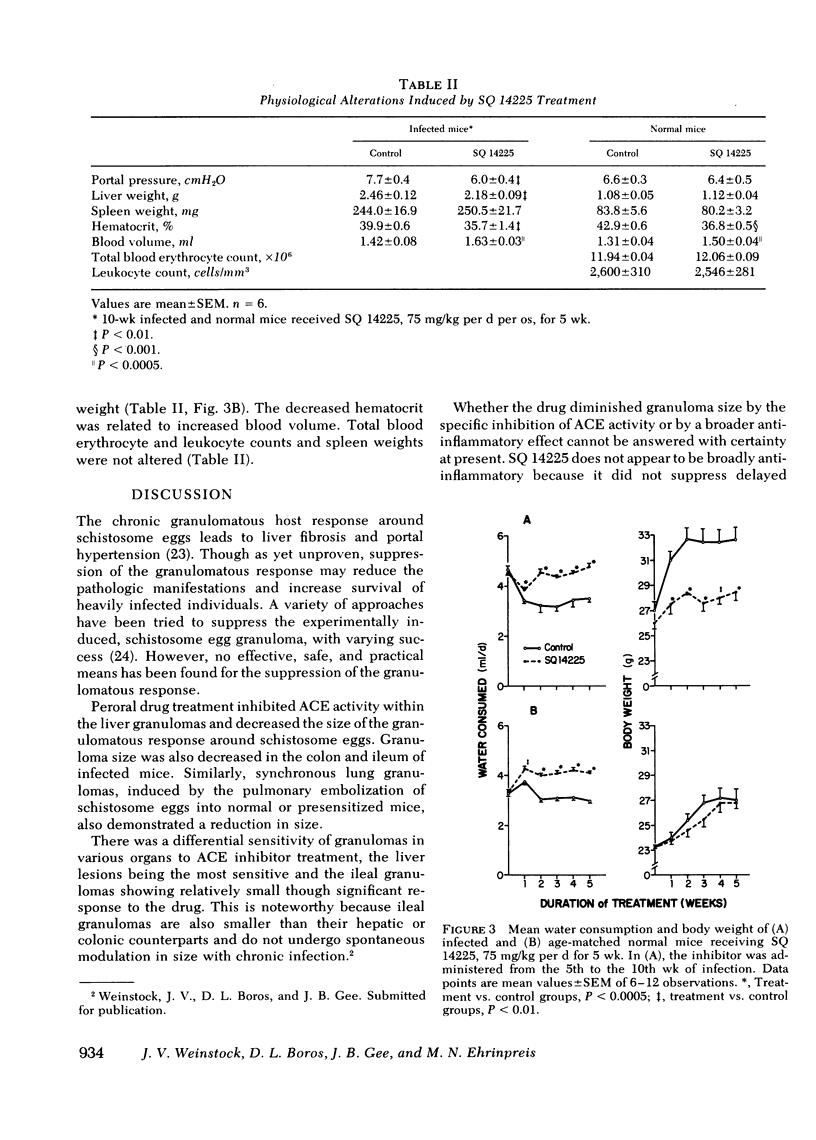
Murine schistosomiasis is a granulomatous disease associated with high serum and granuloma angiotensin I-converting enzyme (ACE) activity. SQ 14225, a specific competitive inhibitor of ACE, was administered to normal mice and mice infected with Schistosoma mansoni to determine whether this compound could inhibit granuloma ACE activity and modify the size of the granulomatous response to schistosome eggs. Peroral administration of SQ 14225 for 5 wk to infected mice with peak granulomatous responses decreased ACE activity in isolated liver granulomas. Treated mice demonstrated a decrease in granuloma size in the liver, colon, and ileum, and hydroxyproline concentration of isolated liver granulomas was increased. Mean diameters of synchronous pulmonary granulomas, induced by the pulmonary embolization of schistosome eggs into normal and sensitized mice, were decreased by a similar dose of SQ 14225. Withdrawal of SQ 14225 from unsensitized mice with 2-wk-old synchronous pulmonary granulomas induced an increase in inflammation. Infected, but not normal mice receiving SQ 14225 demonstrated reduced portal pressure, liver weight, and body weight. Both normal and infected mice experienced dipsogenesis, expanded intravascular volume, and increased serum ACE. These observations suggest that SQ 14225 can partially inhibit the granulomatous response to schistosome eggs and the pathological manifestations of schistosomiasis. It is possible that ACE has an inflammatory role in granulomatous inflammation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENER Z., PELLEGRINO J. Method for isolating schistosome granulomas from mouse liver. J Parasitol. 1956 Dec;42(6):564–564. [PubMed] [Google Scholar]
- Brooks V. L., Malvin R. L. An intracerebral, physiological role for angiotensin: effects of central blockade. Fed Proc. 1979 Aug;38(9):2272–2275. [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- DeRemee R. A., Rohrbach M. S. Serum angiotensin-converting enzyme activity in evaluating the clinical course of sarcoidosis. Ann Intern Med. 1980 Mar;92(3):361–365. doi: 10.7326/0003-4819-92-3-361. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. Endogenous desensitization: changing host granulomatou response to schistosome eggs at different stages of infection with schistosoma mansoni. Am J Pathol. 1968 Feb;52(2):369–379. [PMC free article] [PubMed] [Google Scholar]
- Erdos E. G., Yang H. Y. An enzyme in microsomal fraction of kidney that inactivates bradykinin. Life Sci. 1967 Mar 15;6(6):569–574. doi: 10.1016/0024-3205(67)90090-2. [DOI] [PubMed] [Google Scholar]
- Fregly M. J. Angiotensin-induced thirst: peripheral and central mechanisms. Fed Proc. 1978 Nov;37(13):2667–2668. [PubMed] [Google Scholar]
- Gavras H., Brunner H. R., Turini G. A., Kershaw G. R., Tifft C. P., Cuttelod S., Gavras I., Vukovich R. A., McKinstry D. N. Antihypertensive effect of the oral angiotensin converting-enzyme inhibitor SQ 14225 in man. N Engl J Med. 1978 May 4;298(18):991–995. doi: 10.1056/NEJM197805042981803. [DOI] [PubMed] [Google Scholar]
- Hinman L. M., Stevens C., Matthay R. A., Bernard J., Gee L. Angiotensin convertase activities in human alveolar macrophages: effects of cigarette smoking and sarcoidosis. Science. 1979 Jul 13;205(4402):202–203. doi: 10.1126/science.221980. [DOI] [PubMed] [Google Scholar]
- Igic R., Erdös E. G., Yeh H. S., Sorrells K., Nakajima T. Angiotensin I converting enzyme of the lung. Circ Res. 1972 Sep;31(9 Suppl):51–61. [PubMed] [Google Scholar]
- Jimenez S. A., McArthur W., Rosenbloom J. Inhibition of collagen synthesis by mononuclear cell supernates. J Exp Med. 1979 Dec 1;150(6):1421–1431. doi: 10.1084/jem.150.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. L., Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976 Jul;58(1):240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman J., Beutler E. Elevation of serum angiotensin-converting enzyme in Gaucher's disease. N Engl J Med. 1976 Jun 24;294(26):1442–1444. doi: 10.1056/NEJM197606242942609. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Nosal A., Schlessner A., Sastre-Foken A. Serum angiotensin-converting enzyme for diagnosis and therapeutic evaluation of sarcoidosis. Am Rev Respir Dis. 1979 Aug;120(2):329–335. doi: 10.1164/arrd.1979.120.2.329. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Rea T. H. Serum angiotensin-converting enzyme in leprosy and coccidioidomycosis. Ann Intern Med. 1977 Oct;87(4):423–425. doi: 10.7326/0003-4819-87-4-422. [DOI] [PubMed] [Google Scholar]
- NEUMAN R. E., LOGAN M. A. The determination of hydroxyproline. J Biol Chem. 1950 May;184(1):299–306. [PubMed] [Google Scholar]
- Rea T. H., Lieberman J., Carmel R., Walsh G. P. Serum angiotensin-converting enzyme, transcobalamin and lysozyme in normal and lepromatous armadillos. J Reticuloendothel Soc. 1979 Oct;26(4):367–372. [PubMed] [Google Scholar]
- Silverstein E., Friedland J. Elevated serum and spleen angiotensin converting enzyme and serum lysozyme in Gaucher's disease. Clin Chim Acta. 1977 Jan 3;74(1):21–25. doi: 10.1016/0009-8981(77)90382-5. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Friedland J., Kitt M., Lyons H. A. Increased serum angiotensin converting enzyme activity in sarcoidosis. Isr J Med Sci. 1977 Oct;13(10):995–1000. [PubMed] [Google Scholar]
- Silverstein E., Friedland J., Lyons H. A., Gourin A. Elevation of angiotensin-converting enzyme in granulomatous lymph nodes and serum in sarcoidosis: clinical and possible pathogenic significance. Ann N Y Acad Sci. 1976;278:498–513. doi: 10.1111/j.1749-6632.1976.tb47062.x. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Friedland J., Vuletin J. C. Marked elevation of serum angiotension-converting enzyme and hepatic fibrosis containing long-spacing collagen fibrils in type 2 acute neuronopathic Gaucher's disease. Am J Clin Pathol. 1978 Apr;69(4):467–471. doi: 10.1093/ajcp/69.4.467. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Warren K. S. Hepatosplenic schistosomiasis mansoni: an immunologic disease. Bull N Y Acad Med. 1975 Apr;51(4):545–550. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. The pathogenesis of "clay-pipe stem cirrhosis" in mice with chronic schistosomiasis mansoni, with a note on the longevity of the schistosomes. Am J Pathol. 1966 Sep;49(3):477–489. [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J Pharmacol Exp Ther. 1971 Apr;177(1):291–300. [PubMed] [Google Scholar]


