Abstract
Background
Chronic alcohol abuse is associated with increased risk for osteoporosis while light to moderate alcohol intake correlates with reduced osteoporosis risk. Addition of alcohol to a liquid diet is often used to model chronic alcohol abuse. Methods to model intermittent drinking (including bindge drinking and light to moderate consumption) include 1) intragastric administration of alcohol by oral gavage or 2) intraperitoneal (ip) administration of alcohol by injection. However, it is unclear whether the latter two methods produce comparable results. The purpose of this investigation was to determine the skeletal response to alcohol delivered daily by oral gavage or ip injection.
Materials and Methods
Ethanol or vehicle was administered to 4-month-old female Sprague Dawley rats once daily at 1.2 g/kg body weight for 7 days. Following necropsy, bone formation and bone architecture were evaluated in tibial diaphysis (cortical bone) and proximal tibial metaphysis (cancellous bone) by histomorphometry. mRNA was measured for bone matrix proteins in distal femur metaphysis.
Results
Administration of alcohol by gavage had no significant effect on body weight gain or bone measurements. In contrast, administration of the same dose of alcohol by ip injection resulted in reduced body weight, total suppression of periosteal bone formation in tibial diaphysis, decreased cancellous bone formation in proximal tibial metaphysis, and decreased mRNA levels for bone matrix proteins in distal femur.
Conclusions
Our findings raise concerns regarding the use of ip injection of ethanol in rodents as a method for modeling the skeletal effects of intermittent exposure to alcohol in humans. This concern is based on a failure of the ip route to replicate the oral route of alcohol administration.
Keywords: rat model, tibia, histomorphometry, gene expression
Introduction
Alcohol has complex, context (age and drinking pattern)-dependent and dose-dependent actions on bone metabolism. Chronic heavy alcohol consumption, either by itself or in combination with co-morbidity factors such as smoking or a poor diet, increases fracture risk (Berg et al., 2008; Chon et al., 1992; Gonzalez-Reimers et al., 2011; Santori et al., 2008; Yuan et al., 2001). In contrast, light to moderate drinking, by attenuating age-related bone loss, may be beneficial for bone health (Cawthon et al., 2006; McLernon et al., 2012; Sommer et al., 2012; Sripanyakorn et al., 2009; Tucker et al., 2009). The mechanisms by which alcohol acts on the skeleton to confer either detrimental or beneficial effects are poorly understood. As a consequence, many uncertainties remain regarding the apparent dose-dependent effects of alcohol on the skeleton. These uncertainties are due, in part, to limitations inherent to observational studies performed in humans. It is especially difficult to seperate the specific contribution of alcohol from other co-morbidity factors known to influence bone health (Kanis et al., 1999). Thus, relevant well validated animal models are critical for characterizing the specific effects and mechanisms mediating the actions of alcohol on bone metabolism.
A complete understanding of the complex effects of alcohol on bone growth and maintenance requires careful evaluation of the skeletal response to a wide range of drinking patterns. Based on a limited number of dose-response and time-course studies, peak blood alcohol concentrations and duration of tissue exposure to alcohol are important variables influencing alcohol-mediated changes in bone metabolism (Turner and Sibonga, 2001; Turner et al., 1998). Additionally, the effects of alcohol on bone are quite rapid and often of short duration (Marrone et al., 2012; Turner et al., 1998). Thus, amount and interval of time between repeat exposures to alcohol may be important variables influencing the precise effects of alcohol on bone.
Lieber and colleagues developed a liquid diet in which alcohol replaces carbohydrates isocalorically (Lieber et al., 1989). We have routinely utilized the Lieber-DeCarli liquid diet to investigate the dose-dependent effects of alcohol on bone metabolism in growing and adult rats. Notably, the changes in bone architecture and turnover observed in skeletally mature male and female rats following long-duration (4 months) delivery of alcohol at 35% caloric intake replicates many of the changes observed in chronic alcohol abusers (Turner, 2000). However, the Lieber-DeCarli diet was designed to model the effects of chronic alcohol abuse and is not ideal for modeling intermittent exposure to alcohol (e.g., binge drinking and light to moderate alcohol intake).
Two methods commonly used to model intermittent alcohol consumption include intraperitoneal (ip) injection and intragastric delivery by oral gavage. Intraperitoneal injection is a convenient way to deliver intermittent alcohol over a wide range of doses. However, discrepancies have been reported between the skeletal effects of oral and ip delivery of alcohol in studies designed to model binge drinking (Lauing et al., 2008; Sampson et al., 1999) and some investigators believe that gavage administration replicates the tissue level effects of drinking with a higher degree of fidelity than ip injection (Luz et al., 1996). Unfortunately, differences in protocols (e.g., dose, age, gender) among studies prevent direct comparison of the two methods. We therefore performed studies to compare the skeletal effects of daily administration of ethanol (1.2 g/kg body weight) for 1 week by oral gavage to ip injection using sexually mature female rats as a model. The results demonstrate that the two methods of alcohol delivery differ dramatically in their effects on bone formation and on mRNA levels for bone matrix proteins.
Methods
Experiment 1; Skeletal response to alcohol administered by gavage
This study was designed to determine the effects of ethanol administered daily by gavage on bone metabolism. Sixteen 4-month-old female Sprague-Dawley rats were obtained from Harlan (Madison, WI) and divided randomly into either a control or an ethanol group (n = 8 animals/group). Ethanol (1.2 g/kg body weight) was administered orallly by gavage diluted with distilled water for a total volume of 1 ml. Control rats received distilled water only. The rats were treated for 7 days. Fluorochromes were administered by tail vein injection to label mineralizing bone at treatment initiation (calcein, 20 mg/kg) and 2 days before sacrifice (tetracycline, 20 mg/kg). The rats were weighed, anesthetized with CO2, bled by cardiac puncture, and sacrificed by decapitation. Tibiae were removed and placed in 70% ethanol for histomorphometric analysis. Femora were frozen in liquid nitrogen and stored at −84°C prior to RNA analysis.
Experiment 2; Skeletal response to alcohol administered by ip injection
The study design of this experiment was identical to that of Experiment 1 with the exception that ethanol (1.2 g/kg body weight) was diluted in saline and administered by ip injection. The controls received saline ip.
Bone Histomorphometry
Cortical bone from the tibial diaphysis was prepared for histomorphometric analysis as described (Iwaniec et al., 2008; Kidder and Turner, 1998). Briefly, 150 μm thick cross-sections were cut just proximal to the tibia-fibula synostosis with a low speed saw (Isomet, Buehler, Lake Bluff, IL) equipped with a diamond wafer blade. The sections were ground to a thickness of 15 – 20 μm on a roughened glass plate and mounted in glycerin for microscopic examination under visible and ultraviolet illumination. The following measurements were performed: cross-sectional area (cortical bone and medullary area, mm2), cortical bone area (mm2), cortical thickness (μm), medullary area (mm2), periosteal double label perimeter (double label perimeter/bone perimeter, %), periosteal mineral apposition rate (calculated as the mean distance between two fluorochrome markers that comprise a double label divided by interlabel time, μm/d), and periosteal bone formation rate (calculated as double label perimeter x mineral apposition rate, μm2/μm/y).
For evaluation of cancellous bone, the proxmal tibia was dehydrated in a graded series of ethanol, embedded without demineralization in a mixture of methylmethacrylate:2-hydroxyethyl-methacrylate (12.5:1) to retain the fluorochrome labels, and sectioned at a thickness of 5 μm (2065 Microtome, Reichert-Jung, Heidelberg Germany). A standard sampling site was established in the secondary spongiosa of the metaphysis 1 mm distal to the calcein label that was deposited at the metaphyseal growth plate, its center perpendicular to the long axis of each bone and extending 2 mm proximal to the starting point. This method adjusts for longitudinal growth, such that only the portion of the secondary spongiosa present throughout the experiment is sampled. The sampling site extends bilaterally but excludes cortical edges. A total metaphyseal area of 2.9 mm2 was sampled for each specimen.
Cancellous bone was measured using unstained sections as described (Iwaniec et al., 2008; Kidder and Turner, 1998). Static cancellous bone endpoints included bone area fraction (bone area/tissue area, %) and the derived architectural indices of trabecular number (mm−1), trabecular thickness (μm), and trabecular separation (μm). Fluorochrome-based indices of bone formation included double label perimeter (double label perimeter/bone perimeter, %) and mineral apposition rate (mean distance between two fluorochrome markers that comprise a double label divided by interlabel time, μm/d). Bone formation rate was calculated using a bone perimeter referent (μm2/μm/y). All data are reported using standard nomenclature (Parfitt et al., 1987).
RNA Analysis
Frozen distal femur metaphyses were individually homogenized in guanidine isothiocyanate using a Spex freezer mill (Edison, NJ). Total cellular RNA was extracted and isolated using a modified organic solvent method and yields determined spectrophotometrically at 260 nM. Northern analysis was performed for prepro-α2 (1)subunit of type 1 collagen (collagen), osteonectin and osteocalcin as described in detail (Turner et al., 1998).
Statistical Analysis
For each experiment, differences between treatment groups were determined using a t-test (SPSS 17.0, SPSS Inc., Chicago, IL). If t-test assumptions of homogeneity of variance were not met, a Mann-Whitney U test was applied. Differences were considered significant at P < 0.05. All data are reported as mean ± SE.
Results
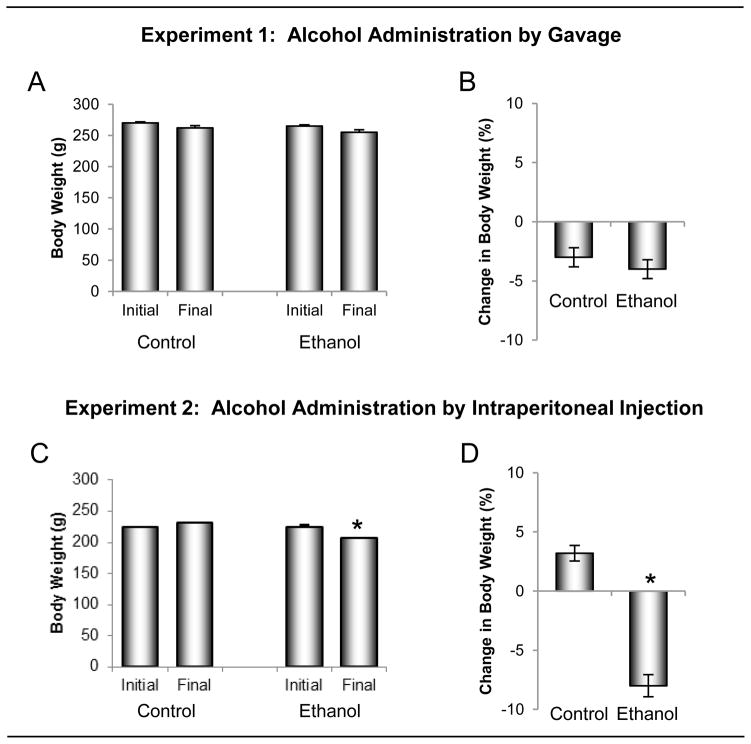
The effects of ethanol administered by oral gavage versus ip injection on body weight change are shown in Figure 1. Body weight did not differ between groups prior to initiation of treatment in either the gavage (Experiment 1) or ip (Experiment 2) studies. Significant changes in body weight were not detected in rats gavaged daily for 1 week with either distilled water (−8 g, −3%) or ethanol solution (−10 g, −4%). Likewise, significant changes in body weight were not detected in rats injected ip with saline (+7 g, +3%). However, body weight decreased (−17 g, −8 %, p<0.05) following ip injection of ethanol solution. Significant differences in uterine weight were not detected between ethanol-treated and control rats following either gavage or ip administration of ethanol (data not shown).
Figure 1.
The effects of ethanol administered by oral gavage (A and B) versus intraperitoneal (ip) injection (C and D) on body weight change during the 1 week treatment interval. Four-month-olds rats (n = 8/group) were administered ethanol (1.2 g/kg body weight) either orally by gavage or ip by injection. Animals were sacrificed after 7 days of treatment. Ethanol administered via gavage had no significant effect on body weight change whereas ip injection of ethanol reduced body weight. Data are mean ± SE. *Different from baseline (initial body weight), p <0.05.
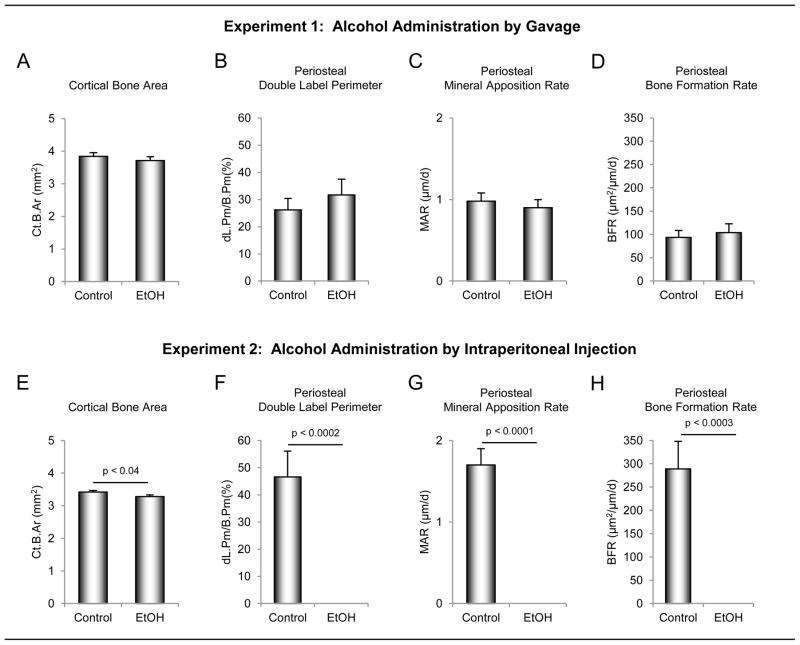
The effects of ethanol administered by oral gavage versus ip injection on static and dynamic cortical bone histopmorphometry in the tibial diaphysis are shown in Figure 2. Whereas differences in cortical bone area were not detected rats administered alcohol orally (Figure 2A), cortical bone area was lower in rats administered alcohol ip (Figure 2E). Neither treatment influenced tibial cross-sectional area, cortical thickness, or medullary area (data not shown). Gavage had no effect on indices of periosteal bone formation (double label perimeter (Figure 2B), mineral apposition rate (Figure 2C) and bone formation rate (Figure 2D)). In contrast, ip administration of ethanol reduced periosteal bone formation to values below detection level (Figure 2F–H).
Figure 2.
The effects of ethanol administered by oral gavage (A – D) versus intraperitoneal (ip) injection (E – H) on static and dynamic cortical bone histomorphometry in the tibial diaphysis. Four-month-olds rats (n = 8/group) were administered ethanol (1.2 g/kg body weight) either orally by gavage or ip by injection. Animals were sacrificed after 7 days of treatment. Cortical bone area was reduced following ip injection (E). Ethanol administered via gavage had no significant effect on indices of periosteal bone formation [double label perimeter (B), mineral apposition rate (C) and bone formation rate (D)] whereas ip injection of ethanol reduced periosteal bone formation to values below detection level (F – H). Data are mean ± SE. Ct.Ar, cortical bone area; dL.Pm/B.Pm, double labeled perimeter/bone perimeter; MAR, mineral apposition rate; BFR/B.Pm, bone formation rate/bone perimeter.
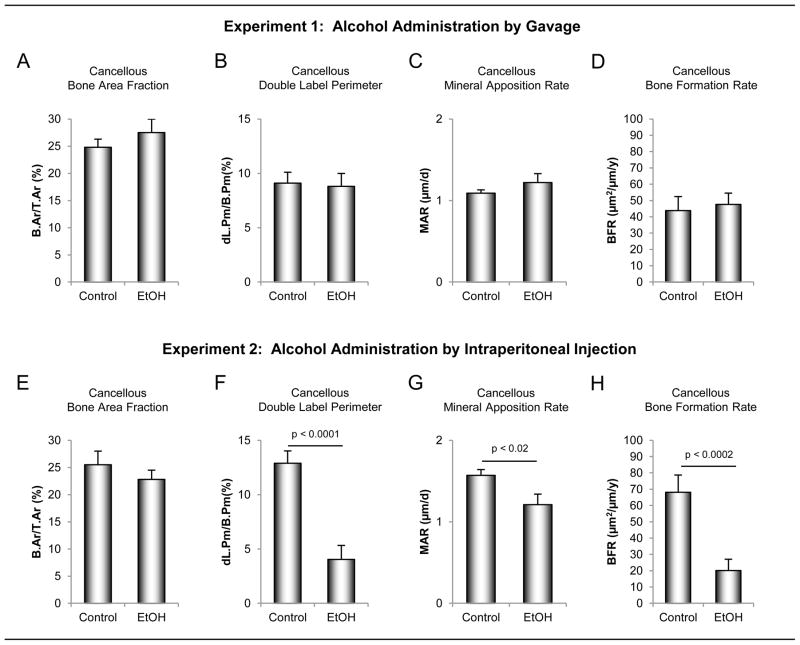
The effects of ethanol administered by oral gavage versus ip injection on static and dynamic cancellous bone histomorphometry in the proximal tibial metaphysis are shown in Figure 3. Neither oral nor ip-administered ethanol had an effect on cancellous bone area fraction (bone area/tissue area) (Figure 3A and E) or indices of bone architecture (trabecular number, thickness and separation; data not shown). Delivery of ethanol by gavage had no effect on dynamic indices of bone formation (double label perimeter (Figure 3B), mineral apposition rate (Figure 3C) and bone formation rate (Figure 3D)). In contrast, double label perimeter (Figure 3F), mineral apposition rate (Figure 3G) and bone formation rate (Figure 3F) were lower in rats administered ethanol ip.
Figure 3.
The effects of ethanol administered by oral gavage (A – D) versus intraperitoneal (ip) injection (E – H) on static and dynamic cancellous bone histomorphometry in the proximal tibial metaphysis. Four-month-olds rats (n = 8/group) were administered ethanol (1.2 g/kg body weight) either orally by gavage or ip by injection. Animals were sacrificed after 7 days of treatment. Neither oral nor ip admininstration of ethanol had a significant effect on cancellous bone area fraction (A and E). Ethanol administered via gavage had no significant effect on dynamic indices of bone formation [double label perimeter (B), mineral apposition rate (C) and bone formation rate (D)] wheras ip administration of ethanol resulted in reduced double label perimeter (F), mineral apposition (G) and bone formation rate (H). Data are mean ± SE. B.Ar/T.Ar, bone area/tissue area; dL.Pm/B.Pm, double labeled perimter/bone perimeter; MAR, mineral apposition rate; BFR/B.Pm, bone formation rate/bone perimeter.
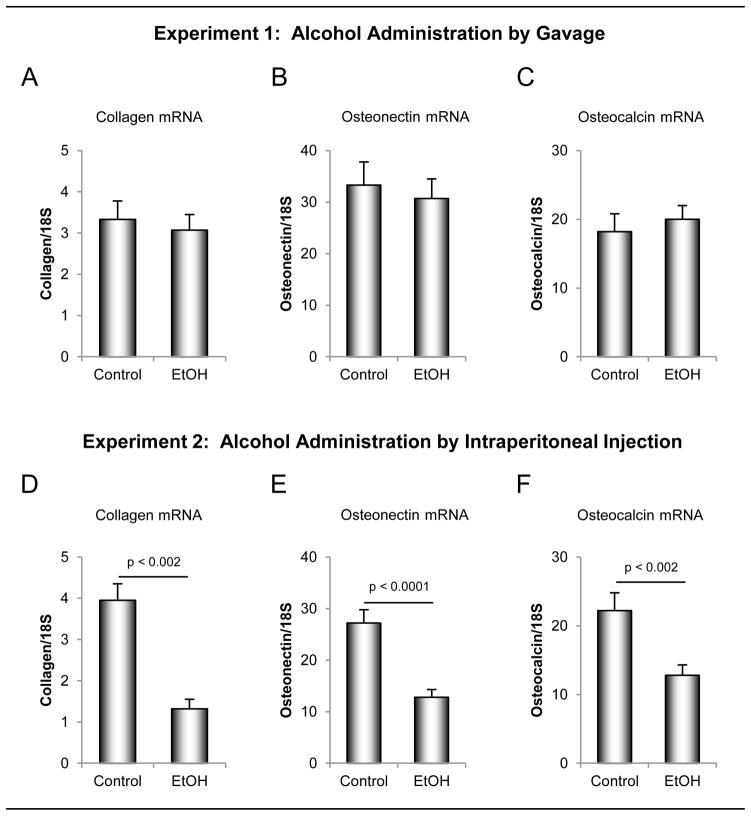
The effects of ethanol administered by oral gavage versus ip injection on mRNA levels for bone matrix proteins in the distal femur metaphysis are shown in Figure 4. Ethanol delivered by gavage had no effect on steady-state mRNA for type 1 collagen (Figure 4A), osteonectin (Figure 4B) or osteocalcin (Figure 4C). In contrast, mRNA levels for all 3 matrix proteins were lower in rats administered ethanol ip (Figure 4D – F).
Figure 4.
The effects of ethanol administered by oral gavage (A –C) versus intraperitoneal (ip) injection (D – F) on mRNA levels for bone matrix proteins in the distal femur metaphysis. Four-month-olds rats (n = 8/group) were administered ethanol (1.2 g/kg body weight) either orally by gavage or ip by injection. Animals were sacrificed after 7 days of treatment. Ethanol administered via gavage had no significant effect on mRNA for prepro-α2 (1)subunit of type 1 collagen (A), osteocalcin (B) or osteonectin (C) wheras ip administration of ethanol resulted in reduced mRNA levels for all 3 matrix proteins (D – F). Data are mean ± SE.
Discussion
Administration of alcohol (1.2 g/kg body weight) for 1 week by once daily oral gavage had no significant effect on body weight, dynamic indices of bone formation at representative cortical and cancellous sites in tibia, or on mRNA levels for bone matrix proteins in distal femur. In contrast, daily ip injection of an identical dose of alcohol for 1 week decreased body weight, extinguished cortical bone formation, reduced cancellous bone formation, and reduced mRNA levels for bone matrix proteins.
Osteopenia induced by chronic heavy alcohol consumption in rats is due in part to depressed bone accrual (Hogan et al., 1997; Howe et al., 2011; Maddalozzo et al., 2009; Sampson et al., 1997; Turner et al., 1987). In the present study, we compared the effects of intermittent alcohol administration on bone formation in rats using fluorochrome-based dynamic histomorphometry and mRNA levels for bone matrix proteins. The dynamic histomorphometry utilized a standard double label technique in which sequential labels are administered at the start of treatment and 5 days later. Thus, the method evaluated bone formed during the initial 5 days of treatment. The complete suppression of periosteal bone formation and the reduction in cancellous bone formation indicate that the inhibitory effects of alcohol administered ip are rapid. The mRNA levels for bone matrix proteins correlate with bone formation rates measured by dynamic histomorphometry and provide an index of bone formation at necropsy (day 7 of treatment) (Turner et al., 1992; Turner and Spelsberg, 1991). The reduction in mRNA levels for bone matrix proteins provide additional support for a reduction in bone formation following administration of alcohol ip, compared to no change in bone formation when alcohol was delivered by gavage. Consistent with our conclusions, Sampson et al. reported no detrimental effects of a similar amount of alcohol (1.1 g/kg body weight) on bone in growing rats using a model for binge drinking in which alcohol was administered by gavage on either 2 consecutive days/week or 5 consecutive days/week for 6 and 7 weeks, respectively (Sampson et al., 1999).
Dose-response studies have been performed to determine blood alcohol concentration (BAC) in rats following oral or ip administration of alcohol. In our hands, BAC was found to increase linearly with dose following ip administration of ethanol from 0.3 to 1.7 g/kg body weight (Turner et al., 1998). The dose used in the present study (1.2 g/kg) resulted in a BAC of 94 mg/dL at 1 hr following ip administration (Turner et al., 1998). We did not measure BAC following gavage but Walker and Ehlers (Walker and Ehlers, 2009) performed detailed studies evaluating method of delivery, age and alcohol dose on BAC. These authors reported that, compared to gavage, ip administration results in similar peak BAC and alcohol clearance when administered at 0.75 g/kg or 1.5 g/kg but higher peak BAC and slower alcohol clearance when administered at 3 g/kg. Based on these observations, it is unlikely that the differences in skeletal repsonse associated with route of alcohol administration observed in our study were due to differences in BAC.
We administered alcohol at 1.2 g/kg in the present study because the peak BAC achieved following ip or oral delivery at this dose is similar to values observed in rats fed a liquid diet in which alcohol contributed 35% of caloric density (Reed et al., 2002; Sampson et al., 1998). Although a comparable peak BAC was likely achieved in this study by gavage and ip delivery to that achieved using a liquid diet (~100 mg/dL), addition of alcohol to the diet (35% caloric density) results in a 10-fold higher (~12 g/kg) daily exposure to alcohol than once daily administration by gavage or ip injection.
Relatively few studies exist comparing tissue level effects of alcohol delivered ip with oral administration (Knapp et al., 2001; Lee and Rivier, 2003; Morales-Gonzalez et al., 1999; Ogilvie et al., 1997). Maternal alcohol exposure, regardless of delivery route, reduced fetal arterial blood acceleration and velocity time integral from umbilical to cerebral arteries (Bake et al., 2012). In contrast, Ciccocioppo et al. reported that conditioned taste aversion to ethanol in alcohol-preferring rats is influenced by the method of alcohol delivery (Ciccocioppo, 1999), as is hepatic metabolism of ethanol during liver regeneration (Morales-Gonzalez et al., 1999). Luz et al. concluded that ip injection of alcohol alters energy balance by an indirect pathway not observed when alcohol is administered by gavage (Luz et al., 1996). Interestingly, intragastric administration of alcohol (3 g/kg) resulted in greater inhibition of liver regeneration than ip administration. In contrast, ip administration of alcohol resulted in weight loss (Morales-Gonzalez et al., 1999; Luz et al., 1996). These differences may be best explained by BAC-independent, tissue-speciifc, delivery-associated effects of alcohol. To our knowledge, the comparative effects of ip and oral alcohol on bone have not been previously reported.
In the current study, once daily gavage with alcohol had no significant effect on body weight. In contrast, ip administration of alcohol resulted in a significant decrease in body weight. These findings are in agreement with Luz et al. who reported that administration of alcohol by gavage had no effect on energy balance whereas ip alcohol administration resulted in a negative energy balance (Luz et al., 1996). Callaci and colleagues, modeling binge drinking using ip alcohol administration delivered on 2 consecutive days followed by 5 days of abstinence, report decreased weight gain (Lauing et al., 2008). These changes in energy status may be relevant to the ip-specific skeletal changes observed in our study as the growing skeleton is exquisitely sensitive to changes in energy balance (Devlin et al., 2010).
Alternatively, ip administration of alcohol may result in local inflammation (Boe et al., 2010; Emanuele et al., 2005) which in turn could results in systemic bone loss (Desimone et al., 1993). Notably, the concentration of alcohol delivered into the peritoneal cavity following ip administration is typically 100–400 times BAC. In this regard, we have shown that acute ip administration of alcohol (1.2 g/kg) results in a transient increase in the proinflammatory cytokine tumor necrosis factor-α (TNF-α) in femur (Turner et al., 1998). Additionally, ip alcohol transiently reduced mRNA levels for the important immune modulators macrophage migration inhibitory factor (MMIF), interlukin-6 (IL-6) and interferon-γ (INF-γ). In contrast, we did not detect changes in expression levels of these cytokines following short-term (1 week) or long-term (16 weeks) administration of alcohol as part of a liquid diet where alcohol contributed 35% caloric intake (unpublished data).
In summary, the effects of intermittent alcohol on bone formation depend upon the method of alcohol administration. Our findings suggest that the antiosteogenic effects of alcohol administered once daily via ip injection do not reflect the actions of dietary alcohol administered by gavage. Because factors such as amount, concentration and frequency of alcohol delivered ip may influence the specificity and magnitude of the response, our findings may not be extrapolatable to other protocols in which alcohol is administered ip to rodents. However, they clearly demonstrate the necessity for method validation, especially if the goal of the research is to model intermittant drinking in humans.
Acknowledgments
Support: This work was supported by NIH grant AA011140 (to RT Turner)
Literature Cited
- Bake S, Tingling JD, Miranda RC. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res. 2012;36:748–758. doi: 10.1111/j.1530-0277.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Jr, Malik R, Arnsten JH. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe DM, Richens TR, Horstmann SA, Burnham EL, Janssen WJ, Henson PM, Moss M, Vandivier RW. Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol Clin Exp Res. 2010;34:1723–1732. doi: 10.1111/j.1530-0277.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon PM, Harrison SL, Barrett-Connor E, Fink HA, Cauley JA, Lewis CE, Orwoll ES, Cummings SR. Alcohol intake and its relationship with bone mineral density, falls, and fracture risk in older men. J Am Geriatr Soc. 2006;54:1649–1657. doi: 10.1111/j.1532-5415.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- Chon KS, Sartoris DJ, Brown SA, Clopton P. Alcoholism-associated spinal and femoral bone loss in abstinent male alcoholics, as measured by dual X-ray absorptiometry. Skeletal Radiol. 1992;21:431–436. doi: 10.1007/BF00190985. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R. Conditioned taste aversion induced by ethanol in alcohol-preferring rats: influence of the method of ethanol administration. Pharmacol Biochem Behav. 1999;64:563–566. doi: 10.1016/s0091-3057(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Desimone DP, Greene VS, Hannon KS, Turner RT, Bell NH. Prostaglandin E2 administered by subcutaneous pellets causes local inflammation and systemic bone loss: a model for inflammation-induced bone disease. J Bone Miner Res. 1993;8:625–634. doi: 10.1002/jbmr.5650080514. [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Kovacs EJ, Emanuele MA. The impact of burn injury and ethanol on the cytokine network of the mouse hypothalamus: reproductive implications. Cytokine. 2005;30:109–115. doi: 10.1016/j.cyto.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reimers E, Alvisa-Negrin J, Santolaria-Fernandez F, Ros-Vilamajo R, Martin-Gonzalez MC, Hernandez-Betancor I, Garcia-Valdecasas-Campelo E, Gonzalez-Diaz A. Prognosis of osteopenia in chronic alcoholics. Alcohol. 2011;45:227–238. doi: 10.1016/j.alcohol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Hogan HA, Sampson HW, Cashier E, Ledoux N. Alcohol consumption by young actively growing rats: a study of cortical bone histomorphometry and mechanical properties. Alcohol Clin Exp Res. 1997;21:809–816. [PubMed] [Google Scholar]
- Howe KS, Iwaniec UT, Turner RT. The effects of low dose parathyroid hormone on lumbar vertebrae in a rat model for chronic alcohol abuse. Osteoporos Int. 2011;22:1175–1181. doi: 10.1007/s00198-010-1304-4. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Wronski TJ, Turner RT. Histological analysis of bone. Methods Mol Biol. 2008;447:325–341. doi: 10.1007/978-1-59745-242-7_21. [DOI] [PubMed] [Google Scholar]
- Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, Dequeker J, Dilsen G, Gennari C, Vaz AL, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Perez Cano R, Rapado A, Ribot C. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9:45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- Kidder LS, Turner RT. Dietary ethanol does not accelerate bone loss in ovariectomized rats. Alcohol Clin Exp Res. 1998;22:2159–2164. [PubMed] [Google Scholar]
- Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcohol Clin Exp Re. 2001;25:1662–1672. [PubMed] [Google Scholar]
- Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–656. doi: 10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. Long-term influence of an initial exposure to alcohol on the rat hypothalamic-pituitary axis. Alcohol Clin Exp Res. 2003;27:1463–1470. doi: 10.1097/01.ALC.0000086065.06203.DD. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- Luz J, Griggio MA, Plapler H, De-Meo-Bancher M, Carvalho-Kosmiskas JV. Effects of ethanol on energy balance of rats and the inappropriateness of intraperitoneal injection. Alcohol. 1996;13:575–580. doi: 10.1016/s0741-8329(96)00070-5. [DOI] [PubMed] [Google Scholar]
- Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, Iwaniec UT. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int. 2009;20:1529–1538. doi: 10.1007/s00198-009-0836-y. [DOI] [PubMed] [Google Scholar]
- Marrone JA, Maddalozzo GF, Branscum AJ, Hardin K, Cialdella-Kam L, Philbrick KA, Breggia AC, Rosen CJ, Turner RT, Iwaniec UT. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause. 2012;19:974–979. doi: 10.1097/gme.0b013e31824ac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLernon DJ, Powell JJ, Jugdaohsingh R, Macdonald HM. Do lifestyle choices explain the effect of alcohol on bone mineral density in women around menopause? Am J Clin Nutr. 2012;95:1261–1269. doi: 10.3945/ajcn.111.021600. [DOI] [PubMed] [Google Scholar]
- Morales-Gonzalez JA, Gutierrez-Salinas J, Yanez L, Villagomez-Rico C, Badillo-Romero J, Hernandez-Munoz R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: role of route and timing of administration. Dig Dis Sci. 1999;44:1963–1974. doi: 10.1023/a:1026601814082. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Reed AH, McCarty HL, Evans GL, Turner RT, Westerlind KC. The effects of chronic alcohol consumption and exercise on the skeleton of adult male rats. Alcohol Clin Exp Res. 2002;26:1269–1274. doi: 10.1097/01.ALC.0000023984.47311.6E. [DOI] [PubMed] [Google Scholar]
- Sampson HW, Chaffin C, Lange J, DeFee B., 2nd Alcohol consumption by young actively growing rats: a histomorphometric study of cancellous bone. Alcohol Clin Exp Res. 1997;21:352–359. [PubMed] [Google Scholar]
- Sampson HW, Gallager S, Lange J, Chondra W, Hogan HA. Binge drinking and bone metabolism in a young actively growing rat model. Alcohol Clin Exp Res. 1999;23:1228–1231. doi: 10.1111/j.1530-0277.1999.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Sampson HW, Hebert VA, Booe HL, Champney TH. Effect of alcohol consumption on adult and aged bone: composition, morphology, and hormone levels of a rat animal model. Alcohol Clin Exp Res. 1998;22:1746–1753. [PubMed] [Google Scholar]
- Santori C, Ceccanti M, Diacinti D, Attilia ML, Toppo L, D’Erasmo E, Romagnoli E, Mascia ML, Cipriani C, Prastaro A, Carnevale V, Minisola S. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31:321–326. doi: 10.1007/BF03346365. [DOI] [PubMed] [Google Scholar]
- Sommer I, Erkkila AT, Jarvinen R, Mursu J, Sirola J, Jurvelin JS, Kroger H, Tuppurainen M. Alcohol consumption and bone mineral density in elderly women. Public Health Nutr. 2012 Jul 17;:1–9. doi: 10.1017/S136898001200331X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripanyakorn S, Jugdaohsingh R, Mander A, Davidson SL, Thompson RP, Powell JJ. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J Bone Miner Res. 2009;24:1380–1388. doi: 10.1359/JBMR.090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Jugdaohsingh R, Powell JJ, Qiao N, Hannan MT, Sripanyakorn S, Cupples LA, Kiel DP. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clin Nutr. 2009;89:1188–1196. doi: 10.3945/ajcn.2008.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–1701. [PubMed] [Google Scholar]
- Turner RT, Greene VS, Bell NH. Demonstration that ethanol inhibits bone matrix synthesis and mineralization in the rat. J Bone Miner Res. 1987;2:61–66. doi: 10.1002/jbmr.5650020110. [DOI] [PubMed] [Google Scholar]
- Turner RT, Kapelner SN, Spelsberg TC. Tissue-specific expression of bone proteins in femora of growing rats. Am J Physiol. 1992;263:E724–729. doi: 10.1152/ajpendo.1992.263.4.E724. [DOI] [PubMed] [Google Scholar]
- Turner RT, Sibonga JD. Effects of alcohol use and estrogen on bone. Alcohol Res Health. 2001;25:276–281. [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Spelsberg TC. Correlation between mRNA levels for bone cell proteins and bone formation in long bones of maturing rats. Am J Physiol. 1991;261:E348–353. doi: 10.1152/ajpendo.1991.261.3.E348. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wronski TJ, Zhang M, Kidder LS, Bloomfield SA, Sibonga JD. Effects of ethanol on gene expression in rat bone: transient dose-dependent changes in mRNA levels for matrix proteins, skeletal growth factors, and cytokines are followed by reductions in bone formation. Alcohol Clin Exp Res. 1998;22:1591–1599. doi: 10.1111/j.1530-0277.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Dawson N, Cooper GS, Einstadter D, Cebul R, Rimm AA. Effects of alcohol-related disease on hip fracture and mortality: a retrospective cohort study of hospitalized Medicare beneficiaries. Am J Public Health. 2001;91:1089–1093. doi: 10.2105/ajph.91.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]