Abstract
The technique of 32P-postlabeling, which was introduced in 1982 for the analysis of DNA adducts, has long been the method of choice for in vivo studies because of its high sensitivity as it requires only <10 μg DNA to achieve the detection of 1 adduct in 1010 normal bases. 32P-postlabeling has therefore been utilized in numerous human and animal studies of DNA adduct formation. Like all techniques 32P-postlabeling does have several disadvantages including the use of radioactive phosphorus, lack of internal standards, and perhaps most significantly does not provide any structural information for positive identification of unknown adducts, a shortcoming that could significantly hamper progress in the field. Structural methods have since been developed to allow for positive identification of DNA adducts, but to this day, the same level of sensitivity and low sample requirements provided by 32P-postlabeling have not been matched. In this mini review we will discuss the 32P-postlabeling method and chronicle the transition to mass spectrometry via the hyphenation of gas chromatography, capillary electrophoresis, and ultimately liquid chromatography which, some 30 years later, is only just starting to approach the sensitivity and low sample requirements of 32P-postlabeling.
This paper focuses on the detection of bulky carcinogen-DNA adducts, with no mention of oxidative damage or small alkylating agents. This is because the 32P-postlabeling assay is most compatible with bulky DNA adducts. This will also allow a more comprehensive focus on a subject that has been our particular interest since 1990.
Keywords: DNA adduct, 32P-postlabeling, Gas chromatography, Capillary electrophoresis, Liquid chromatography, Mass spectrometry
1. Introduction
Exposure to carcinogens can lead to the formation of DNA adducts, a key step towards the onset of disease such as cancer. This is a well-established mechanism and therefore detection and monitoring DNA adducts can serve as an indicator of exposure and disease. While there is great debate as to whether the presence of DNA adducts will lead to disease, it is imperative that early detection methods be developed to improve prognosis and early treatment. DNA adducts are an ideal target for human screening, biomarker discovery, and measurement of exposure, as adducts are often present well before the onset of disease.
1.1. Bulky aromatic DNA adducts
The term “bulky” DNA adducts was derived from the early experiments using 32P-postlabeling that were aimed at the detection of adducts of polycyclic aromatic hydrocarbons (PAHs) whose identity could not be verified with this technique [1]. The term has been extended to include all relatively large aromatic and nonpolar carcinogen adducts. PAHs are a class of compounds produced from the incomplete combustion of organic material. They are found in a wide range of media ranging from cigarette smoke, environmental aerosols, and places of work [2–6]. The most studied PAH is benzo[ a]pyrene (BaP), a well-known human and animal carcinogen (see structure, Fig. 1) [7,8]. BaP requires metabolic activation by CYP450s to its diol epoxide derivative before reacting with DNA [5,9].
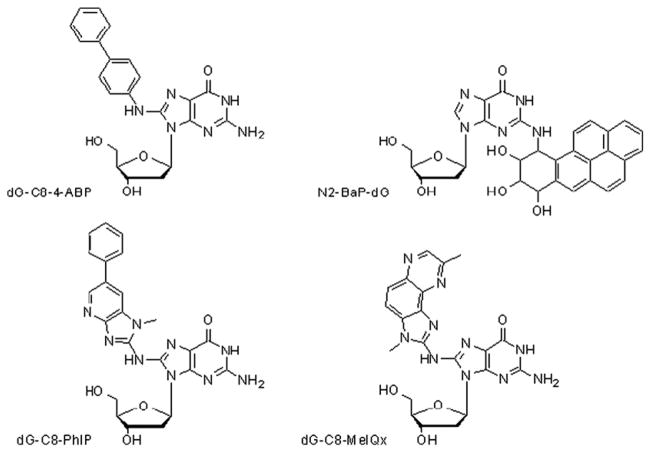
Fig. 1.
Structures of several bulky DNA adducts discussed in this review. From top left to bottom right, the aromatic amine dG-C8-4-ABP, the PAH N2-BaP-dG and two heterocyclic aromatic amines, dG-C8-PhIP and dG-C8-MeIQx.
Heterocyclic aromatic amines (HAAs) are formed by cooking protein-containing foods such as meat [10]. In 1986, Felton et al. [11] determined 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) to be the major HAA in fried beef, at a level of 15 mg/kg (see structure, Fig. 1). PhIP is carcinogenic in mammalian models and requires metabolic activation before it can react with DNA [12,13]. 2-amino-3,8-dimethylimidazo [4,5-f]quinoxaline (MeIQx) is another well-studied carcinogen in this class and when fed to mice was shown to induce liver tumors (see structure, Fig. 1) [14]. The aromatic amine 4-aminobiphenyl (4-ABP) is a known human bladder carcinogen found in nanogram quantities in cigarettes (see structure, Fig. 1) [15,16]. 4-ABP reacts primarily with the C-8 position of guanine residues in DNA to form dG-C8-4-ABP.
DNA adducts are most often detected at the nucleoside or nucleotide (monomer) level. This provides optimal sensitivity and straightforward quantitation. However, DNA adduction is not random, and is highly sequence dependent. Sequence information is lost upon digestion to nucleosides or nucleotides and therefore analysis of oligonucleotides long enough for gene identification is also an important goal. While the bulk of this review is focused on the analysis of nucleotides and nucleosides, a discussion on the current progress toward the analysis of oligonucleotide adducts has been included.
2. Methods
The review by Apruzzese and Vouros [17] includes a very clear description of the need for a sensitive analytical method for the detection and quantitation of DNA adducts. As stated in that review, if an analytical method has a mass detection limit of 10 pg for a particular adduct, and a detection limit of 1 adduct per 108 nucleotides is required, this may require 10 g of tissue (1 g of tissue contains roughly 1 mg of DNA) [17]. For in vivo studies, and particularly human studies in which tissue samples are limited, the analytical method of choice must be superbly sensitive.
DNA adducts are formed at very low levels in living systems. Additionally, the DNA adducts are located amongst unadducted nucleotides generally at a minimal level of 106 times greater abundance. Therefore, an analytical method aimed at detecting DNA adducts in living systems must be very sensitive, highly specific, and require only small amounts of sample. This poses a daunting analytical challenge, and over the years several successful platforms have been developed. These include 32P-postlabeling, gas chromatography mass spectrometry (GC–MS), capillary electrophoresis mass spectrometry (CE–MS), and liquid chromatography mass spectrometry (LC–MS). Each method has its own inherent strengths and weaknesses as we summarize here with a focus on sensitivity and sample requirements, two parameters important for application of the method to human studies, in which adducts are present at low quantities, and sample sizes are often limited. Table 1 summarizes the methods described in this review highlighting sample requirements and sensitivities of each method for different DNA adducts. Fig. 2 graphically represents these qualities.
Table 1.
The DNA adduct detection methods discussed in this review highlighting the targeted adduct, the amount of DNA required, and the sensitivity of the method.
| Platform | DNA Adduct | Sample req. (μg DNA) | Sensitivity (adducts/nuc.) | Ref. |
|---|---|---|---|---|
| 32P | AAP-dG | 1 | 1/1e10 | [19] |
| dG-4-ABP | 3 | 1/1e8 | [38] | |
| IQ-dG | 30 | 1/1e9 | [64] | |
| GC–MS | ||||
| dG-4-ABP | 100 | 0.32/1e8 | [37] | |
| PhIP-dG | 100 | 1/1e8 | [38] | |
| LC–MS | ||||
| BaP-dG | 100 | 0.3/1e8 | [57] | |
| PhIP-dG | 100 | 1/1e7 | [55] | |
| BaP-dG | 100 | 3/1e8 | [30] | |
| MeIQx-dG | 100 | 1/1e8 | [56] | |
| microLC–MS | ||||
| IQ-dG | 500 | 1/1e10 | [54] | |
| AAF-dG | 1000 | 1/1e6 | [40] | |
| PhIP-dG | 1000 | 3/1e8 | [60] | |
| dG-4-ABP | 300 | 5/1e9 | [66] | |
| IQ-dGa | 300 | 2/1e8 | [63] | |
| nanoLC–MS | ||||
| IQ-dG | 300 | 1/1e7 | [61] | |
| IQ-dG | 500 | 5.3/1e9 | [54] | |
| BaP-dG | 1000 | 2/1e8 | [65] | |
| UPLC–MS | ||||
| Methyleugenol-dG | 12.5 | 2/1e8 | [76] | |
| dG-4-ABPb | 10 | 1/1e7 | [73] | |
| LC–MS column switching | ||||
| dG-4-ABP | 100 | 0.7/1e7 | [69] | |
| PhIP-dG | 50 | 1.5/1e8 | [70] | |
| dG-4-ABPc | 5 | 5/1e9 | [71] | |
| AA-I-dGd | 10 | 0.3/1e8 | [74] | |
MicroLC-nanoESI-MS.
MicroUPLC-MS.
Nano-LC-MS Column Switching.
MicroUPLC-MS Column Switching.
Fig. 2.

Plot of the DNA adducts and methods used to detect them. Points closest to the x,y origin represent the most sensitive methods with the smallest sample requirements. The number of DNA adducts per nucleo(side/tide) is plotted (x-axis) vs. the amount of DNA in μg (y-axis). CS stands for column switching.
2.1. The 32P-postlabeling assay
In 1982, Gupta et al. [18] introduced the 32P-postlabeling assay for the detection and quantitation of aromatic DNA adducts. Briefly, this assay works by digesting isolated DNA into 3′-deoxynucleoside monophosphates, transfer of γ-32P from adenosine [γ-32P]triphosphate to generate 3′,5′-[5′-32P]diphosphates using T4 poly-nucleotide kinase [18]. The labeled adducted nucleotides are then separated by thin layer chromatography (TLC) and quantified by scintillation counting. This platform was capable of detecting one adduct in 107–108 nucleotides using only 1 μg of DNA [18].
A key limitation to the 32P-postlabeling assay is the fact that all 3′-nucleoside monophosphates, both DNA and RNA, are substrates for T4 polynucleotide kinase and therefore the assay is dependent upon the ability to remove unadducted nucleotides from the reaction mixture. Successful improvements to this assay were soon developed by including additional cleanup steps before the labeling reaction, which reduced the presence of unmodified nucleotides in the sample.
In 1985, Gupta [19] improved the sensitivity of the 32P-postlabling assay by extracting the adducted nucleotides with 1-butanol in the presence of tetrabutyl-ammonium chloride before the labeling reaction. This extra step extended the detection limit to a single adduct in 109–1010 nucleotides while maintaining the extremely low sample requirements of only 1–10 μg of DNA [19]. In 1986, Reddy and Randerath [20] used Nuclease P1 enrichment to achieve similar sensitivity and in 1988 [21], reversed-phase high performance liquid chromatography (HPLC) was used for the separation of unadducted from adducted nucleotides, as well as for separation of adducts with different structures into individual fractions which were then visualized on TLC plates, significantly diminishing the detection complexity. Fig. 3 shows data comparing butanol and nuclease P1 enrichment in conjuction with 32P-postlabeling in a study of DNA adducts from tobacco chewers [1,21]. In this case, butanol enrichment was proven to be superior to nuclease P1 enrichment in the isolation of adducts from oral mucosa [1,21]. While nuclease P1 treatment works well with PAH adducts, it does not work well with aromatic amines or C8-dG adducts [22–24].
Fig. 3.
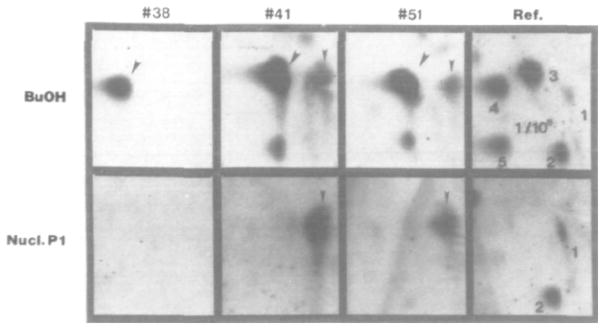
A comparison of bulky DNA adduct recovery utilizing two enrichment procedures, butanol extraction (top) and nuclease P1 treatment (bottom). In this study, oral mucosa DNA from tobacco chewers was investigated. The numbers indicate different subjects and are compared to a positive reference (right panels). Reprinted with permission from [1].
32P-Postlabeling has since been the method of choice, particularly with in vivo studies, due to its high sensitivity (1 adduct in 1010 nucleotides) and low sample requirement (1–10 μg DNA) and has been used in a number of important studies for biomonitoring, measures of exposure, and linking DNA adduction to disease as illustrated in the reviews by Beach and Gupta [1] and Gyorffy et al. [25]. However, 32P-postlabeling, like any analytical method, does have some disadvantages. The assay requires the use of 32P, a radioactive isotope that poses health risks and requires a dedicated, isolated environment [26]. While intrinsically quantitative, internal standards are not available which makes it difficult to account for losses during sample preparation or incomplete labeling, which is likely responsible for the underrepresentation of DNA adducts that have been observed when comparing this assay to other methods [27–29]. Perhaps the most significant disadvantage to this assay is the lack of structural information, which, before structure-based methods were available, often led to ambiguity in the identities of the DNA adducts that were being detected.
2.2. Gas chromatography mass spectrometry
Gas chromatography has been a powerful separation technique since its introduction in the 1950s and its true power was realized when it was hyphenated with electron capture ionization and mass spectrometry, the most common mass analyzers being ion traps or single quadrupoles. GC–MS provides excellent resolution and peak capacity of volatile molecules as well as structural information, screening capabilities, and even exact mass when combined with a high resolution time of flight (TOF) analyzer. In the analysis of DNA adducts, GC–MS suffers from low throughput due to the requirement to derivatize these polar adducts combined with relatively long temperature gradients. In addition, GC is only compatible with volatile molecules, a stipulation that poses problems when trying to analyze polar nucleotides and nucleosides, and indirect strategies must be applied. Despite these limitations, GC–MS has achieved widespread use for the analysis of DNA adducts. In the analysis of PAH adducts, PAHs are liberated from the DNA by acid hydrolysis and then detected as derivatized tetrols. Not long after its introduction, this method was used to confirm the presence of BaP adducts in human placenta [30]. In this study the importance of the structural elucidation capability of GC–MS was clearly illustrated when HPLC–synchronous fluorescence spectroscopy was used to screen for BaP adducts and GC–MS was used to confirm their presence in 10 of 28 samples [30]. This study provided conclusive evidence that maternal exposure to environmental carcinogens can lead to DNA adducts in human placenta [30]. GC–MS was used to show that, after human exposure from tobacco smoke, BaP is transported to the cervix where it is metabolized to BPDE and forms DNA adducts [31]. The authors cite previous studies of DNA adducts in human cervical cells using 32P-post-labeling [31–35]. However, since the analysis was conducted on the released BaP tetrols, the exact structural identity of the DNA adducts remained unknown. Still, GC–MS was able to confirm that adducts derived from BaP were likely at least one of the markers detected by the studies utilizing 32P-postlabeling [31].
GC–MS has proven useful for bulky DNA adducts of different classes. Lin et al. [36] introduced a method for the detection of 4-ABP adducts in human urinary bladder and lung tissues. In this method, 0.05 N NaOH was used to hydrolyze 4-ABP from the DNA molecules [36]. Gas chromatography negative ion chemical ionization mass spectrometry (GC–NICI–MS) was used to detect the liberated carcinogens with a sensitivity as low as 0.32 adducts per 108 nucleotides [36]. This method was validated by comparison with the long established 32P-postlabeling assay and excellent agreement was found between the quantitation results from the two methods (r = 0.999, n = 9) [36].
The alkaline hydrolysis method and GC–NICI–MS was later used by Friesen et al. [37] to detect and quantify the heterocyclic aromatic amine PhIP in various human tissues. Fig. 4 shows the selected ion monitoring (SIM) chromatograms of derivatized PhIP and the internal standard isolated from the DNA of rat lung. Excellent sensitivity was reported for the GC–MS detection of this adduct as well, with an LOD of 1 adduct in 108 nucleotides when using 100 μg of DNA and sensitivities were comparable to that of 32P-Postlabeling (1 adduct in 108 nucleotides) when 3 μg of DNA was used [37]. The same authors also found a good correlation when comparing the GC–MS method with 32P-postlabeling (r2 = 0.83) [37]. As the authors point out, best results were achieved when using at least 100 μg of DNA so this GC–MS method may not be practical for most human studies, in which smaller amounts of DNA can be obtained [37].
Fig. 4.
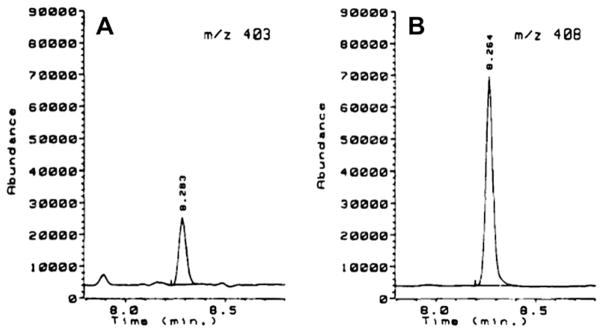
The detection of bis(pentafluorobenzyl) PhIP hydrolyzed from rat lung DNA by GC–MS (A). B shows the deuterated (d5) PhIP internal standard. Reprinted with permission from [38].
The primary limitations preventing GC–MS from becoming the method of choice for the analysis of DNA adducts in biological matrices are the indirect methods by which DNA adducts must be measured (e.g. hydrolysis to tetrols and removal of sugars) and the relatively large amounts of sample needed to reach the sensitivity required to measure adducts at biologically relevant levels [38].
2.3. Capillary electrophoresis mass spectrometry
In 1994, Wolf and Vouros [39] were among the first to use capillary zone electrophoresis (CZE) and continuous flow fast atom bombardment mass spectrometry (FAB-MS) for the detection of low picogram amounts of standard DNA adducts of three different classes (4-ABP, N-acetylaminofluorene (AAF), aminofluorene (AF), anthracene). While low mass sensitivity was achieved, the limitation of CE was realized as the concentration detection limit was on the order of 10−5 M due to the requirement of 5–10 nL injection volumes. The sample stacking technique (concentration of analyte into a plug) proposed by Chien and Burgi [40] was later used to partially overcome this problem and reach concentration detection limits on the order of 10−8 M for adducts of different classes, a nearly 1000× improvement [41,42]. Fig. 5 shows the mass electropherogram of a digested and purified in vitro reaction of BPDE with calf thymus DNA. Multiple reaction products were detected in addition to the expected BaP-dGMP using sample stacking and this demonstrates the powerful resolving capabilities and ability to detect unknown or unexpected DNA adducts, as these results were obtained by scanning a wide mass range from 400 to 1400 m/z [42].
Fig. 5.
The detection of multiple BaP adducts from an in vitro reaction of BPDE with calf thymus DNA using CE–MS following sample stacking. Note the offscale signal for BaP-dGMP, the major adduct in the mixture as a result of the sample enrichment process. Reprinted with permission from [43].
Among the electrophoretic methods, capillary electrochromatography (CEC) is a separation technique that essentially combines traditional HPLC with electrophoretic mobility resulting in less band broadening than that observed with HPLC and, before the advent of ultrahigh pressure liquid chromatography (UHPLC), allowed the use of sub 3 μm particles without the backpressure issues seen with HPLC separation [43]. In 1997, Ding and Vouros [44] explored the fundamentals of CEC for hyphenation with MS in the analysis of model carcinogens, AAF-dG and the PAH benzo[g]chrysene. CEC demonstrated several advantages over HPLC including higher resolution (plate counts of >100,000), faster analysis (seconds vs. minutes) and lower analyte consumption [44]. Utilizing on-column focusing and step gradients the authors achieved concentration detection limits on the order of 10−6 M and shed light on key physical properties for the improvement of sensitivity in CE–MS [44,45].
In a more recent application, Fang et al. [46] used CE–MS to characterize an adduct resulting from the PAH metabolite 9-OH-BaP which had been observed previously in rodent liver and lung tissue by 32P-postlabeling after exposure to BaP [47,48]. This study once again showed the importance of a structure-based method to confirm the identity of unknown adducts.
2.4. Liquid chromatography mass spectrometry
High sensitivity has been achieved in all the methods discussed to this point, but with each method, the researcher must make a sacrifice and either use large amounts of sample or lose the ability for structural identification. Clearly a more effective method would combine all three attributes: high sensitivity, low sample requirements, and structural identification. A number of publications have identified the increasing role that LC–MS would play in the analysis of DNA adducts and the evolution of the technique is summarized next [17,49–53].
2.4.1. Conventional flow liquid chromatography mass spectrometry
HPLC has been used for the analysis of DNA adducts since the early 1990s and hyphenation with MS began to show great promise for unambiguous identification of DNA adducts in complex matrices. The coupling of LC with ESI dramatically increased the utility of LC–MS for the detection and quantitation of DNA adducts. In 2001, Nelson et al. [54] used LC–MS/MS to confirm the bioactivation of N-OH-PhIP and formation of DNA adducts in human prostate and human prostate-derived cells and provide insights into protection against early-stage prostate carcinogenesis. The analytical method provided an LOD of 2 adducts in 107 nucleotides using 100 μg of DNA [54]. Paehler et al. [55] utilized LC–MS/MS using a 1 mm id × 50 mm reversed-phase column and a flow rate of 50 μL/min to identify the major DNA adducts associated with MeIQx exposure, and were the first to provide spectral data on HAA-DNA adducts using 100 μg of DNA to achieve similar sensitivity [26]. In 2005, Beland et al. [56] used HPLC–ESI–MS/MS to detect BaP-dG adducts in lung DNA from mice fed a diet supplemented with coal tar and from human lung tissue samples from both healthy autopsy samples and lung cancer patients. Using 100 μg of DNA, this platform achieved an LOD of 0.3 adducts per 108 nucleotides which enabled the detection of dG-BaP adducts in 1 of the 26 human samples while 32P-post-labeling revealed adducts in 20 of the 26 human samples [56]. These results suggest that BaP-related adducts constituted only a small fraction of the DNA adducts which points to the low specificity of 32P-postlabeling compared to LC–MS/MS [56]. Singh et al. [29] developed a narrow bore LC–ESI–MS/MS method to detect N2-BaP adducts in mouse liver with a detection limit of 3 adducts in 108 nucleotides using 50–100 μg of DNA. A dose-dependent trend was identified and their results correlated well with their analysis of the same sample set using 32P-postlabeling [29]. Singh et al. [57] later undertook a major study of the effects of environmental air pollution on human populations from three cities in Eastern Europe. Interestingly, they found negative correlations between oxidative DNA damage and total PAH levels and BaP adducts [57].
Recent publications show that LC–MS with conventional flow using 1–4.6 mm columns is still a valuable platform for in vivo animal studies. In 2011, Zhang et al. [58] used a stable isotope dilution LC–MS/MS method to detect DNA adducts in oral tissue of mice resulting from exposure to dibenzo[a,l]pyrene (DB[a,l]P). They were able to determine the primary DNA adduct resulting from this exposure as anti-DB[a,l]PDE-N6-dA and quantify its abundance. While the authors do not explicitly state their LOD, the lowest point on their calibration curve was 5 pg in 100 μg DNA [58].
2.4.2. Capillary and nano-flow liquid chromatography mass spectrometry
In the mid 1990s researchers began to capitalize on the advantages offered by low-flow LC and ESI to improve sensitivity of DNA adduct detection, perhaps with inspiration from capillary electroseparation techniques which had already demonstrated the compatibility of MS with these lower flow rates. Probably the earliest use of capillaryLC (capLC) coupled with tandem MS and microESI (μESI) for the analysis of DNA adducts was done by Wolf and Vouros [39]. AAF-DNA adducts were separated by a capillary column with the dimensions of 320 μm i.d. × 15 cm packed with 3 μm C18 beads at a flow rate of 4 μL/min provided by a syringe pump [39]. Continuous flow fast atom bombardment (CFFAB) was used for ionization due to its optimal low flow of ~5 μL/min which was nicely compatible with MS. The authors also investigated the advantages of the different MS scan modes offered by a triple quadrupole mass spectrometer, namely constant neutral loss (CNL) and multiple reaction monitoring (MRM) [39]. Fig. 6 (top) shows the use of CNL for screening a mixture for the presence of AAF adducts while Fig. 6 (bottom) shows the dramatic sensitivity enhancement of MRM for the specific transitions (473 m/z → 357 m/z) and (489 m/z → 373 m/z) [39]. The method suffered from relatively poor sensitivity and large sample requirements, with an LOD in MRM mode of 1 adduct in 106 nucleotides using 1 mg of DNA. However, this study provided an excellent illustration of the applicability of capLC–μESI–MS/MS for the analysis of DNA adducts and the unique features offered by the triple quadrupole analyzer for the analysis of this class of compounds [39]. Rindgen et al. [59] detected dG-PhIP adducts formed in vitro using capillary LC–ESI–MS/MS with an LOD of 80 fmol using 1 mg of DNA (three adducts per 108 nucleosides). 32P-postlabeling was performed in parallel and proved to be much more sensitive using only 3.75 μg of DNA [59]. Still, the LC–MS/MS method proved sufficiently sensitive for in vivo exposure studies in animals and provided important structural information that enabled the detection and quantitation of three distinct PhIP-DNA adducts [59]. Gangl et al. [60] helped to revitalize the interest in capLC by decreasing the flow rate to 0.250 μL/min for nanoESI (referred to in the publication as microelectrospray). The same authors also investigated the sensitivity associated with different tandem MS experiments in the triple quadrupole mass spectrometer. CNL was 1000× less sensitive than single reaction monitoring (SRM) for the analysis of dG-IQ, 1 adduct in 104 nucleotides vs. 1 adduct in 107 nucleotides, respectively, using 300 μg of DNA [60]. This was a very important step because, as also pointed out in other reviews, SRM in a triple quadrupole has become the most accepted MS/MS technique for clinical applications and is regarded as the most sensitive and specific MS method in DNA adduct detection and quantitation [61].
Fig. 6.
One of the earliest examples of the use of capLC coupled with tandem QqQ–MS for the analysis of DNA adducts produced from the reaction of calf thymus DNA with N-acetoxy- AAF. The top panel shows the various AAF adducts detected when screening the digested reaction mixture by CNL. Fig. 7 (bottom) shows the dramatic sensitivity enhancement via the use of MRM for the specific transitions (473 → 357) m/z indicating the presence of AAF-dA adducts and (489 → 373) m/z for the AAF-dG adducts. Peaks denoted A and C are DNA adducts of interest in this study. Modified with permission from [40].
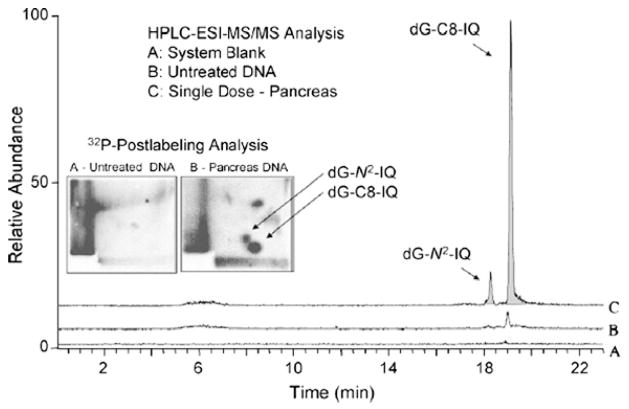
In a further reduction in column diameter, Soglia et al. [62] used capLC–nano-ESI–MS/MS (referred to in the publication as μESI) to achieve an LOQ for dG-C8-IQ (2-amino-3-methylimidazo[4,5-f]quinoline) of 2 adducts in 108 nucleotides. HPLC separation was done on a 75 μm i.d. self-packed reversed-phase column at a flow rate of 200 nL/min for introduction into the mass spectrometer [62]. This method was used to measure adduct formation in rat liver as a function of IQ dose and results were compared with a similar study using 32P-postlabeling [62]. A relatively large quantity (300 μg) of DNA was required to achieve this sensitivity [62]. Fig. 7 shows a comparison between 32P-postlabeling and capLC–nanoESI–MS/MS of dG-IQ adducts from pancreatic tissue of non-human primates [26]. In the study by Gangl et al. [53], the strengths and weaknesses of each method are clear. Both methods had comparable detection limits, roughly 1 adduct in 109 nucleo(sides/tides) which demonstrated the applicability of LC–MS to in vivo human and animal studies just as 32P-postlabeling had been used for decades [53]. However, to achieve this level of sensitivity, the LC–MS platform required 500 μg of DNA compared to the 30 μg used for the 32P-postlabeling assay [53]. The improved TLC separation developed by Turesky et al. [63]was capable of resolving the N2 from C8 isomers, which was readily accomplished with LC [53]. However, the LC–MS assay provided better resolution of the adducts (reversed-phase column compared to TLC plate) as well as molecular weight information allowing for the unambiguous identification of the adducts [53]. Wang et al. [64] used nanoflow LC–ESI with a quadrupole time of flight mass spectrometer (QTOF) for the detection and quantitation of BaP-dN adducts in mouse lung tissue after exposure to asphalt fume. The authors achieved femtomole-level detection limits in part by utilizing a 75 μm id capillary column however a large amount of DNA (1 mg) was still utilized [64]. Perhaps the large sample was required due to the extensive offline cleanup steps aimed at enriching multiple BaP-dN adducts [64]. Ricicki et al. [65] used capLC–μESI–MS/MS to detect and quantify dG-C8-ABP adducts in human pancreatic tissue and achieved a detection limit of 5 fmol dG-C8-4-ABP (5 adducts per 109 nucleosides) with a 320 μm id capillary column and a flow rate of 10 μL/min. This was one of the first studies using LC–MS to study 4-ABP adducts in human samples and demonstrated the viability of this platform for screening human samples. However, in this study sample quantities were not limited and 300 μg of DNA was procured from donated organs for digestion and analysis [65]. For human screening this will rarely be the case as non-invasive techniques are preferred.
Fig. 7.
A comparison of 32P-postlabeling and capillary LC–nanoESI–MS/MS of dG-IQ adducts from pancreatic tissue of non-human primates [27,54]. Reprinted with permission from [27].
2.4.3. Column switching
One of the drawbacks in many of the studies utilizing LC–MS was the fairly large sample requirements, often 100 μg of DNA or more. Sample requirements of this size are simply incompatible with most human studies and so these requirements would have to be significantly decreased to gain acceptance and compete with 32P-postlabeling. One factor dictating the amount of sample that is needed for an analytical method is the cleanup requirement which may involve one or more offline steps that can include solid phase extraction (SPE), liquid–liquid extraction, and protein precipitation [36,39,59,66–68]. Not only does this offline cleanup result in analyte losses, but it also significantly increases analysis time, reducing throughput. In 1997, Vanhoutte et al. [50] showed that a nanoflow ESI–LC–MS system with online column switching could improve the mass detection limit of BPA-dG by 3300×, as compared to conventional ESI (200 nL/min vs. 40 μL/min, respectively). Doerge et al. [69] used narrow bore (2 × 150 mm column) LC–ESI–MS and online column switching to detect 4-ABP adducts in hepatic DNA from mice treated with 4-ABP. A detection limit of 0.7 adducts in 107 nucleotides using 100 μg of DNA and adduction levels ranging from 4.9 and 30 dG-C8-4-ABP adducts in 107 were detected in 0.1 mg 4-ABP/mouse and 1.0 mg 4-ABP/mouse, respectively [69]. The authors demonstrated the exceptional cleanup provided by online column switching and faster overall analysis time by directly injecting the enzymatic DNA hydrolysates without protein precipitation or any offline cleanup [69]. Additionally, as the authors mentioned, improved selectivity in cleanup can be accomplished by using different online cleanup strategies, such as immunoaffinity chromatography [69].
After the initial introduction of online column switching, significant effort was placed into decreasing sample requirements which, in effect, has made LC–MS much more compatible with human studies. In 2010, Singh et al. [70] use narrow bore LC–ESI–MS/MS and online column switching/cleanup for the analysis of dG-C8-PhIP to achieve a detection limit of 1.5 adducts in 108 nucleosides using 50 μg of DNA. This method was used to quantify dG-C8-PhIP in colon tissue of mice treated by oral gavage with PhIP for 5 days [70]. Adduction levels of roughly 15 adducts in 106 were found in these tissues using a very rapid and easily automatable method [70]. Randall et al. [68] utilized an Agilent Technologies microfluidics HPLC chip, which incorporates online SPE, capLC and nanoESI, all with minimal dead volume. With this technology, sample losses were minimized and sensitivity was maximized, along with improving the reproducibility and stability of nanoESI. The true value of this method is the ability to achieve a detection limit for dG-C8-4-ABP of 5 adducts in 109 nucleosides using only 5 μg of DNA (1.25 μg of DNA per injection) [68]. With online sample cleanup and nanoESI, the authors developed an LC–MS platform with sensitivity and low sample requirements competitive with 32P-post-labeling making this applicable to human studies, but with the added benefit of positive adduct identification through structural information (MW, fragmentation). This platform was later used to study the chemopreventative effects of sulforophane, on dG-C8-4-ABP formation in mouse bladder cells, and later to identify the role of Nrf-2 directed cytoprotection against 4-ABP in different mouse tissues [71].
2.4.4. Ultra performance liquid chromatography mass spectrometry
A fledgling technique compared to most of those discussed in this review is UPLC. This technique introduced in 2004 has been quickly gaining popularity due to the fast analysis times (<10 min) and high-resolution separation efficiencies (>100,000 plates) compared to traditional HPLC. This is afforded by using sub 3 μm packing material in the analytical columns and specially designed instrumentation to withstand the high pressure (<15,000 psi). In 2011, Nauwelaers et al. [72] used μUPLC–μESI-LC–MS/MS to study the formation of 4-ABP and several HAA adducts in rat and human hepatocytes. Using only 10 μg of DNA and offline SPE, an LOQ of 1 adduct in 107 nucleosides (assumed from lowest point on calibration curve) the authors compared quantities of DNA adducts in human and rat tissue [72]. They found that rat models may significantly under-represent adduction levels in humans, as HAA adducts were as much as 100-fold higher in human hepatocytes as compared to rat [72]. Yun et al. [73] used μUPLC–MS/MS and online sample enrichment to develop a highly sensitive method with an LOD of 0.3–1 adducts per 108 nucleosides using only 10 μg of DNA. The authors used this platform to quantify aristolactam DNA adducts in the tissues of mice exposed to 8-Methoxy-6-nitrophenanthro-[3,4-d]-1,3-dioxolo-5-carboxylic acid (AA-I), a carcinogen associated with rare cancers of the upper urinary tract [73]. More significantly, they demonstrated the utility of this method for human biomonitoring by analyzing DNA taken from the renal cortex of patients from Taiwan, the country where the largest number of these cancer cases are reported [74]. Levels of dA-AL-I were found ranging from 9 to 338 adducts per 108 nucleosides, while dG adducts were not found [73]. Herrmann et al. [75] used isotope dilution UPLC–MS/MS to investigate the activation of the secondary herbal metabolite methyleugenol by sulfotransferases and subsequent formation of DNA adducts. Using a 2.1 × 100 mm Phenyl column packed with 1.7 μm particles and a flow rate of 300 μL/min, an LOD of 2–6 adducts in 108 nucleosides was achieved starting with only 12.5 μg of DNA [75].
While only a few papers have been published at the time of this review utilizing UPLC–MS for the detection of DNA adducts, this is certain to be a powerful technique which will help bridge the gap in sensitivity, speed of analysis, and low sample requirements between 32P-postlabeling and MS-hyphenated techniques.
3. Mass spectrometry in the analysis of oligonucleotide adducts
The analytical approaches discussed to this point, both 32P-postlabelling and MS methods, although very sensitive and selective, are limited to the determination of DNA damage by the analysis of DNA adducts of monomeric species. In addition to quantitative data, MS-based methods have been successful in providing structural information about the adducts, i.e. linkage to sugar, phosphate or nucleobase. While this information is proving to be of great value in terms of risk assessment, it should not be over-looked that a significant contribution of the mutagenic effect of a carcinogenic agent is also dependent on the sequence selectivity in the adduction process and the location of the carcinogen within a DNA sequence. Thus, a successful technique for predicting the relationship between DNA adduction and cancer is one which would be sensitive enough to quantify the exposure limit, explore whether the covalent interaction is modifying the nucleotide, probe the efficiency of the cellular repair process for removing the DNA adduct and last, but not necessarily least, determine the exact location of adduct attachment on the DNA. It is only by addressing these issues simultaneously that the DNA adduction can be understood and new tools for the early detection of cancer can be found. In this regard the detection and structural characterization of oligonucleotide adducts plays a major role.
Over the last few years there has been rapid progress in the development of sequencing techniques but unfortunately, in these next-generation DNA sequencing or PCR-based techniques, the adduct is lost prior to DNA sequencing [76,77]. It is this inherent failure of these techniques to analyze DNA adducts which has brought MS to the forefront as an efficient technique to sequence DNA adduct fragments. Fragmentation patterns are well defined and nomenclature based on the dissociation of unmodified oligonucleotides introduced by McLuckey can be incorporated for the interpretation of CID spectra of adducted oligonucleotides [78].
For more than a decade now, the Vouros research group has been continuously working to advance the role of mass spectrometry in analyzing oligonucleotide adducts. In 1998, Marzilli et al. [79] analyzed the unmodified oligonucleotide 5′-CCGGAGGCC and the aflatoxin B1 (AFB) modified oligonucleotides using a Finnigan LCQ ion trap mass spectrometer. Later in 2000, Harsch et al. reported the use of LC–MS/MS for the identification of benzo[c]-phenanthrene diol epoxide adducted double-stranded (ds) 12-mer oligonucleotide (TAGTCAAGGGCA, sequence derived from the HPRT gene sequence) using a reversed-phase polystyrene divinylbenzene (PS-DVB) particle column (1.0 × 150 mm, 3 μm) and 20 mMammonium acetate as an ion pairing reagent [80]. Further in 2007, Xiong et al. [81], reported separation and sequencing of (±)-anti-7r,8t-dihydroxy-9t,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene ((±)-anti-BPDE) adducted ds 5′-PO4—ACCCGCGTC157CGCGC-3′ oligonucleotides containing codon 157 of the p53 gene using PS-DVB monolithic columns by ion-pair reversed-phase nanoHPLC coupled to ion trap mass spectrometry. Recently Sharma et al. [82] evaluated a number of ion pairing agents for the separation and sequencing of AAF modified isomeric oligonucleotide adducts. The latter study also discusses optimization of mobile phase conditions for the positional mapping of oligonucleotide adducts individually or in a complex mixture using a PS-DVB monolithic column for reversed-phase ion-pair liquid chromatography electrospray ionization tandem mass spectrometry [82]. In addition, this study also reported using the optimized mobile phase conditions for the positional mapping of oligonucleotide adducts individually or in a complex mixture. Linscheid et al. reported a number of studies starting in 1995 regarding the use of CZE and negative ion ESI–MS for detecting styrene oxide adducted dinucleotides–heptanucleotides [83,84].
In 2007, Chowdhury and Guengerich [85] reported direct detection and mapping of sites of base modification of a (±)-anti-BPDE or 4-ABP adducted double stranded oligonucleotide containing codon 157 of the p53 gene using an Acquity UPLC BEH C18 column (1.7 μm, 1.0 mm × 100 mm) and 10 mM ammonium acetate as the ion pairing reagent. In 2008, Jamin et al. [86] investigated the influence of the local environment and neighboring base effect on the adduction of HAAs, specifically PhIP and IQ on T-rich model single-strand oligonucleotides. Similarly, Chan et al. [87] reported an electrospray ionization tandem mass spectrometry method for the identification and position mapping of unmodified and aristolochic acid modified (5′-TTTATT-3′, 5′-TTTGTT-3′ and 5′-TACATGTGT-3′) oligonucleotides. Anichina et al. [88] used a 4000 QTRAP triple-quadrupole mass spectrometer and a triple TOF 5600 hybrid quadrupole time-of-flight (TOF) mass spectrometer for the analysis of the binding interaction of oligonucleotides with structurally related bromobenzoquinones. Lai et al. [89] reported a similar study on electrospray ionization tandem mass spectrometric characterization of DNA adducts formed by bromobenzoquinones in 2011.
Despite the progress that has been achieved toward LC–ESI–MS/MS analysis of oligonucleotide adduct mixtures manual mass spectral interpretation of the resulting data is a bottleneck limitation. Several groups including the Vouros research group have also been working on the development of data mining software of mass spectrometric data for nucleic acids for the last few years [90–95]. For example GenoMass software, based on the ‘reversed pseudo-combinatorial’ approach developed in the Vouros lab is a fast, automated, efficient, high-throughput data-mining algorithm for mass spectrometric analysis of nucleic acids [94,95]. This peak assignment software can further be utilized for tandem mass spectrometry data computational interpretation of oligonucleotide adducts thus, not only identifying the oligonucleotide but also determining the exact location of the modification [82].
Mass spectrometry coupled to separation techniques like liquid chromatography along with efficient LC–MS/MS data mining software could result in a significant step forward in genomic DNA research. The MS coupling to capLC helps in minimizing ion suppression, which could result from co-eluting similar compounds, or isomeric oligonucleotide adducts. The ability to couple LC–MS/MS with efficient data interpretation software like Geno-Mass is critical to the future of the field because of the huge amount of data needed to be interpreted for the digested genomic DNA. It is only by addressing and solving the challenges associated with building a mass spectrometry-based platform for analyzing digested genomic DNA fragments that the interaction between genes and environment could be understood.
4. Conclusions and future directions
Since its introduction in 1982, 32P-postlabeling has been the preferred method for the analysis of bulky DNA adducts, particularly in human studies due to its low sample requirement of <10 μg and high sensitivity of 1 adduct in 1010 nucleotides. Ambiguity in the identification of DNA adducts has led to the hyphenation of GC, CE and LC with MS to provide molecular weight and structural information for DNA adducts in question. GC–MS is inherently not ideal for the analysis of polar DNA adducts which require derivatization. For adequate sensitivity GC–MS requires relatively large amounts of DNA, as much as 100–300 μg and, in addition, when it comes to bulky adducts, it is effectively an indirect method since the analysis is conducted on the PAH tetrol or other moiety produced by hydrolysis of the adduct. Various CE–MS techniques including CEC and CZE have helped reduce the amount of DNA required for analysis and provided highly efficient separation with plate counts >100,000. CE with its low flow rates is fully compatible with nanoESI–MS and provided increased sensitivity with smaller sample sizes. However, this technique often proved troublesome because of interferences from complex biological matrices. LC–ESI–MS is a highly flexible technique capable of analyzing a wide range of DNA adducts in a variety of different biological matrices. Conventional LC–MS using 1–4.6 mm columns and compatible flow rates still required 100–300 μg of DNA and LODs 100–10,000× higher than 32P-postlabeling. Capillary separation methods combined with micro or nanoESI have produced a significant improvement in sensitivity. UPLC–MS has reduced analysis times and improved separation efficiencies with plate counts of >100,000. Perhaps the most significant development which has helped close the gap between 32P-postlabeling and LC–MS was the introduction of chip-based technologies for online column switching which combine online sample cleanup, capillary separation, and nanoESI. This has enabled detection limits of ~1 adduct in 109 nucleotides using 1–10 μg of DNA.
The continuous developments in mass spectrometers will continue to improve the sensitivity of existing analytical methods. It is clear that technological developments in LC–MS, perhaps combining UPLC with chip-based sample cleanup and capillary chromatography will continue to push the lower limits of detection of DNA adducts while reducing the amount of sample required for analysis making LC–MS the preferred technique for noninvasive human biomonitoring and screening using biological fluids such as urine. Perhaps LC–MS will also provide clinicians with a tool for the early detection of disease and dangerous exposures to environmental carcinogens and the ability to sequence DNA adduction sites may provide a better understanding of the relationship between DNA adduction and disease.
Acknowledgments
This work was supported by NIH RO1 CA693390. This is contribution No. 1023 from the Barnett Institute.
Abbreviations
- PAH
polycyclic aromatic hydrocarbon
- BaP
benzo[a]pyrene
- BPDE
benzo[a]pyrene diol epoxide
- HAA
heterocyclic aromatic amine
- PhiP
2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine
- MeIQx
2-amino-3,8-dimethylimidazo[ 4,5-f]quinoxaline
- 4-ABP
4-aminobiphenyl
- dG
deoxyguanosine
- MS
mass spectrometry
- CE
capillary electrophoresis
- LC
liquid chromatography
- TLC
thin layer chromatography
- HPLC
high performance liquid chromatography
- NICI
negative ion chemical ionization
- TOF
time of flight
- SIM
selected ion monitoring
- CZE
capillary zone electrophoresis
- AAF
N-acetylaminofluorene
- AF
aminofluorene
- dGMP
deoxyguanosine monophosphate
- CEC
capillary electrochromatography
- UHPLC
ultrahigh pressure liquid chromatography
- LOD
limit of detection
- ESI
electrospray ionization
- MS/MS
tandem mass spectrometry
- DB[a,l]P
dibenzo[a,l]-pyrene
- dA
deoxyadenosine
- CNL
constant neutral loss
- MRM
multiple reaction monitoring
- SRM
single reaction monitoring
- IQ
2-amino-3-methylimidazo[4,5-f]quinoline
- QTOF
quadrupole time of flight
- PS-DVB
polystyrene divinylbenzene
- ds
double-stranded
- dN
deoxynucleotide
- SPE
solid phase extraction
- UPLC
ultra performance liquid chromatography
- AA-I
8-methoxy-6-nitrophenanthro-[3,4-d]-1,3-dioxolo-5-carboxylic acid
- AL-I
aristolactam
- QTRAP
quadrupole linear ion trap
References
- 1.Beach AC, Gupta RC. Human biomonitoring and the 32P-postlabeling assay. Carcinogenesis. 1992;13:1053–1074. doi: 10.1093/carcin/13.7.1053. [DOI] [PubMed] [Google Scholar]
- 2.Hemminki K, Grzybowska E, Chorazy M, Twardowska-Saucha K, Sroczynski JW, Putman KL, Randerath K, Phillips DH, Hewer A, Santella RM, et al. DNA adducts in human environmentally exposed to aromatic compounds in an industrial area of Poland. Carcinogenesis. 1990;11:1229–1231. doi: 10.1093/carcin/11.7.1229. [DOI] [PubMed] [Google Scholar]
- 3.Tsapakis M, Stephanou EG. Polycyclic aromatic hydrocarbons in the atmosphere of the eastern Mediterranean. Environ Sci Technol. 2005;39:6584–6590. doi: 10.1021/es050532l. [DOI] [PubMed] [Google Scholar]
- 4.IARC, Monographs programme on the evaluation of the carcinogenic risk of chemicals to humans, Preamble. IARC Monogr Eval Carcinogen Risk Chem Hum. 1986;39:13–32. [PubMed] [Google Scholar]
- 5.Weinstein IB, Jeffrey AM, Jennette KW, Blobstein SH, Harvey RG, Harris C, Autrup H, Kasai H, Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976;193:592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DH, Schoket B, Hewer A, Bailey E, Kostic S, Vincze I. Influence of cigarette smoking on the levels of DNA adducts in human bronchial epithelium and white blood cells. Int J Cancer. 1990;46:569–575. doi: 10.1002/ijc.2910460403. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch-Wenzel RP, Brune H, Grimmer G, Dettbarn G, Misfeld J. Experimental studies in rat lungs on the carcinogenicity and dose-response relationships of eight frequently occurring environmental polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 1983;71:539–544. [PubMed] [Google Scholar]
- 8.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 9.Jennette KW, Jeffrey AM, Blobstein SH, Beland FA, Harvey RG, Weinstein IB. Nucleoside adducts from the in vitro reaction of benzo[a]pyrene- 7,8-dihydrodiol 9,10-oxide or benzo[a]pyrene 4,5-oxide with nucleic acids. Biochemistry. 1977;16:932–938. doi: 10.1021/bi00624a019. [DOI] [PubMed] [Google Scholar]
- 10.Sugimura T, Sato S. Mutagens–carcinogens in foods. Cancer Res. 1983;43:2415s–2421s. [PubMed] [Google Scholar]
- 11.Felton JS, Knize MG, Shen NH, Andresen BD, Bjeldanes LF, Hatch FT. Identification of the mutagens in cooked beef. Environ Health Perspect. 1986;67:17–24. doi: 10.1289/ehp.866717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohgaki H, Takayama S, Sugimura T. Carcinogenicities of heterocyclic amines in cooked food. Mutat Res. 1991;259:399–410. doi: 10.1016/0165-1218(91)90130-e. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi K, Nagao M, Esumi H, Sugimura T. Food-derived mutagens and carcinogens. Cancer Res. 1992;52:2092s–2098s. [PubMed] [Google Scholar]
- 14.Ohgaki H, Hasegawa H, Suenaga M, Sato S, Takayama S, Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) from cooked foods. Carcinogenesis. 1987;8:665–668. doi: 10.1093/carcin/8.5.665. [DOI] [PubMed] [Google Scholar]
- 15.Feng Z, Hu W, Rom WN, Beland FA, Tang MS. 4-aminobiphenyl is a major etiological agent of human bladder cancer: evidence from its DNA binding spectrum in human p53 gene. Carcinogenesis. 2002;23:1721–1727. doi: 10.1093/carcin/23.10.1721. [DOI] [PubMed] [Google Scholar]
- 16.Patrianakos C, Hoffmann D. Chemical studies on tobacco smoke LXIV. On the analysis of aromatic amines in cigarette smoke. J Anal Toxicol. 1979;3:150–159. [Google Scholar]
- 17.Apruzzese WA, Vouros P. Analysis of DNA adducts by capillary methods coupled to mass spectrometry: a perspective. J Chromatogr A. 1998;794:97–108. doi: 10.1016/s0021-9673(97)00820-0. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RC, Reddy MV, Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen–DNA adducts. Carcinogenesis. 1982;3:1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RC. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985;45:5656–5662. [PubMed] [Google Scholar]
- 20.Reddy MV, Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986;7:1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- 21.Chacko M, Gupta RC. Evaluation of DNA damage in the oral mucosa of tobacco users and non-users by 32P-adduct assay. Carcinogenesis. 1988;9:2309–2313. doi: 10.1093/carcin/9.12.2309. [DOI] [PubMed] [Google Scholar]
- 22.Phillips DH, Arlt VM. The 32P-postlabeling assay for DNA adducts. Nat Protoc. 2007;2:2772–2781. doi: 10.1038/nprot.2007.394. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RC, Earley K. 32P-adduct assay: comparative recoveries of structurally diverse DNA adducts in the various enhancement procedures. Carcinogenesis. 1988;9:1687–1693. doi: 10.1093/carcin/9.9.1687. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher JE, Jackson MA, George MH, Lewtas J, Robertson IG. Differences in detection of DNA adducts in the 32P-postlabelling assay after either 1-butanol extraction or nuclease P1 treatment. Cancer Lett. 1989;45:7–12. doi: 10.1016/0304-3835(89)90029-3. [DOI] [PubMed] [Google Scholar]
- 25.Gyorffy E, Anna L, Kovacs K, Rudnai P, Schoket B. Correlation between biomarkers of human exposure to genotoxins with focus on carcinogen–DNA adducts. Mutagenesis. 2008;23:1–18. doi: 10.1093/mutage/gem043. [DOI] [PubMed] [Google Scholar]
- 26.Turesky RJ, Vouros P. Formation and analysis of heterocyclic aromatic amine–DNA adducts in vitro and in vivo. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:155–166. doi: 10.1016/j.jchromb.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 27.Phillips DH. Detection of DNA modifications by the 32P-postlabelling assay. Mutat Res. 1997;378:1–12. doi: 10.1016/s0027-5107(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 28.Beland FA, Doerge DR, Churchwell MI, Poirier MC, Schoket B, Marques MM. Synthesis, characterization, and quantitation of a 4-aminobiphenyl-DNA adduct standard. Chem Res Toxicol. 1999;12:68–77. doi: 10.1021/tx980172y. [DOI] [PubMed] [Google Scholar]
- 29.Singh R, Gaskell M, Le Pla RC, Kaur B, Azim-Araghi A, Roach J, Koukouves G, Souliotis VL, Kyrtopoulos SA, Farmer PB. Detection and quantitation of benzo[a]pyrene-derived DNA adducts in mouse liver by liquid chromatography–tandem mass spectrometry: comparison with 32P-postlabeling. Chem Res Toxicol. 2006;19:868–878. doi: 10.1021/tx060011r. [DOI] [PubMed] [Google Scholar]
- 30.Manchester DK, Weston A, Choi JS, Trivers GE, Fennessey PV, Quintana E, Farmer PB, Mann DL, Harris CC. Detection of benzo[a]pyrene diol epoxide-DNA adducts in human placenta. Proc Natl Acad Sci USA. 1988;85:9243–9247. doi: 10.1073/pnas.85.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melikian AA, Sun P, Prokopczyk B, El-Bayoumy K, Hoffmann D, Wang X, Waggoner S. Identification of benzo[a]pyrene metabolites in cervical mucus and DNA adducts in cervical tissues in humans by gas chromatography–mass spectrometry. Cancer Lett. 1999;146:127–134. doi: 10.1016/s0304-3835(99)00203-7. [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Routledge MN, Jenkins D, Garner RC. DNA adducts in different tissues of smokers and non-smokers. Int J Cancer. 1990;45:673–678. doi: 10.1002/ijc.2910450417. [DOI] [PubMed] [Google Scholar]
- 33.Simons AM, Phillips DH, Coleman DV. Damage to DNA in cervical epithelium related to smoking tobacco. BMJ. 1993;306:1444–1448. doi: 10.1136/bmj.306.6890.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips DH, She MN. DNA adducts in cervical tissue of smokers and non-smokers. Mutat Res. 1994;313:277–284. doi: 10.1016/0165-1161(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 35.Ali S, Astley SB, Sheldon TA, Peel KR, Wells M. Detection and measurement of DNA adducts in the cervix of smokers and non-smokers. Int J Gynecol Cancer. 1994;4:188–193. doi: 10.1046/j.1525-1438.1994.04030188.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin D, Lay JO, Jr, Bryant MS, Malaveille C, Friesen M, Bartsch H, Lang NP, Kadlubar FF. Analysis of 4-aminobiphenyl-DNA adducts in human urinary bladder and lung by alkaline hydrolysis and negative ion gas chromatography–mass spectrometry. Environ Health Perspect. 1994;102(Suppl 6):11–16. doi: 10.1289/ehp.94102s611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friesen MD, Kaderlik K, Lin D, Garren L, Bartsch H, Lang NP, Kadlubar FF. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem Res Toxicol. 1994;7:733–739. doi: 10.1021/tx00042a004. [DOI] [PubMed] [Google Scholar]
- 38.de Kok TM, Moonen HJ, van Delft J, van Schooten FJ. Methodologies for bulky DNA adduct analysis and biomonitoring of environmental and occupational exposures. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:345–355. doi: 10.1016/s0378-4347(01)00543-6. [DOI] [PubMed] [Google Scholar]
- 39.Wolf SM, Vouros P. Application of capillary liquid chromatography coupled with tandem mass spectrometric methods to the rapid screening of adducts formed by the reaction of N-acetoxy-N-acetyl-2-aminofluorene with calf thymus DNA. Chem Res Toxicol. 1994;7:82–88. doi: 10.1021/tx00037a013. [DOI] [PubMed] [Google Scholar]
- 40.Chien RL, Burgi DS. On-column sample concentration using field amplification in CZE. Anal Chem. 1992;64:1046–1050. [Google Scholar]
- 41.Wolf SM, Vouros P. Incorporation of sample stacking techniques into the capillary electrophoresis CF-FAB mass spectrometric analysis of DNA adducts. Anal Chem. 1995;67:891–900. doi: 10.1021/ac00101a016. [DOI] [PubMed] [Google Scholar]
- 42.Barry JP, Norwood C, Vouros P. Detection and identification of benzo[a]pyrene diol epoxide adducts to DNA utilizing capillary electrophoresis-electrospray mass spectrometry. Anal Chem. 1996;68:1432–1438. doi: 10.1021/ac9510245. [DOI] [PubMed] [Google Scholar]
- 43.Reilly JT, Saeed M. Capillary electrochromatography as an alternative separation technique to high-performance liquid chromatography and capillary zone electrophoresis for the determination of drug related impurities in Lilly compound LY300164. Anal Chem. 1998;829:175–186. [Google Scholar]
- 44.Ding J, Vouros P. Capillary electrochromatography and capillary electrochromatography–mass spectrometry for the analysis of DNA adduct mixtures. Anal Chem. 1997;69:379–384. doi: 10.1021/ac9606968. [DOI] [PubMed] [Google Scholar]
- 45.Ding J, Szeliga J, Dipple A, Vouros P. Application of mixed mobile phases and a step gradient method in capillary electrochromatography for the separation of isomeric polycyclic aromatic hydrocarbon–deoxyribonucleoside adduct mixtures prepared in vitro. J Chromatogr A. 1997;781:327–334. doi: 10.1016/s0021-9673(97)00529-3. [DOI] [PubMed] [Google Scholar]
- 46.Fang AH, Smith WA, Vouros P, Gupta RC. Identification and characterization of a novel benzo[a]pyrene-derived DNA adduct. Biochem Biophys Res Commun. 2001;281:383–389. doi: 10.1006/bbrc.2000.4161. [DOI] [PubMed] [Google Scholar]
- 47.Garg A, Beach AC, Gupta RC. Interception of reactive, DNA adduct-forming metabolites present in rodent serum following carcinogen exposure: implications for use of body fluids in biomonitoring. Teratog Carcinog Mutagen. 1993;13:151–166. doi: 10.1002/tcm.1770130402. [DOI] [PubMed] [Google Scholar]
- 48.Ross J, Nelson G, Kligerman A, Erexson G, Bryant M, Earley K, Gupta R, Nesnow S. Formation and persistence of novel benzo(a)pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res. 1990;50:5088–5094. [PubMed] [Google Scholar]
- 49.Farmer PB, Singh R. Use of DNA adducts to identify human health risk from exposure to hazardous environmental pollutants: the increasing role of mass spectrometry in assessing biologically effective doses of genotoxic carcinogens. Mutat Res. 2008;659:68–76. doi: 10.1016/j.mrrev.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Vanhoutte K, Van Dongen W, Hoes I, Lemiere F, Esmans EL, Van Onckelen H, Van den Eeckhout E, van Soest RE, Hudson AJ. Development of a nanoscale liquid chromatography/electrospray mass spectrometry methodology for the detection and identification of DNA adducts. Anal Chem. 1997;69:3161–3168. doi: 10.1021/ac970121q. [DOI] [PubMed] [Google Scholar]
- 51.Giese RW, Vouros P. Methods development toward the measurement of polyaromatic hydrocarbon–DNA adducts by mass spectrometry. Res Rep Health Eff Inst. 1993:1–25. discussion 27–36. [PubMed] [Google Scholar]
- 52.Phillips DH. DNA adducts in human tissues: biomarkers of exposure to carcinogens in tobacco smoke. Environ Health Perspect. 1996;104(Suppl 3):453–458. doi: 10.1289/ehp.96104s3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gangl ET, Turesky RJ, Vouros P. Detection of in vivo formed DNA adducts at the part-per-billion level by capillary liquid chromatography/microelectrospray mass spectrometry. Anal Chem. 2001;73:2397–2404. doi: 10.1021/ac0100401. [DOI] [PubMed] [Google Scholar]
- 54.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 55.Paehler A, Richoz J, Soglia J, Vouros P, Turesky RJ. Analysis and quantification of DNA adducts of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in liver of rats by liquid chromatography/electrospray tandem mass spectrometry. Chem Res Toxicol. 2002;15:551–561. doi: 10.1021/tx010178e. [DOI] [PubMed] [Google Scholar]
- 56.Beland FA, Churchwell MI, Von Tungeln LS, Chen S, Fu PP, Culp SJ, Schoket B, Gyorffy E, Minarovits J, Poirier MC, Bowman ED, Weston A, Doerge DR. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo[a]pyrene-DNA adducts. Chem Res Toxicol. 2005;18:1306–1315. doi: 10.1021/tx050068y. [DOI] [PubMed] [Google Scholar]
- 57.Singh R, Sram RJ, Binkova B, Kalina I, Popov TA, Georgieva T, Garte S, Taioli E, Farmer PB. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat Res. 2007;620:83–92. doi: 10.1016/j.mrfmmm.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Zhang SM, Chen KM, Aliaga C, Sun YW, Lin JM, Sharma AK, Amin S, El-Bayoumy K. Identification and quantification of DNA adducts in the oral tissues of mice treated with the environmental carcinogen dibenzo[a, l]pyrene by HPLC–MS/MS. Chem Res Toxicol. 2011;24:1297–1303. doi: 10.1021/tx200188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rindgen D, Turesky RJ, Vouros P. Determination of in vitro formed DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine using capillary liquid chromatography/electrospray ionization/tandem mass spectrometry. Chem Res Toxicol. 1995;8:1005–1013. doi: 10.1021/tx00050a003. [DOI] [PubMed] [Google Scholar]
- 60.Gangl ET, Turesky RJ, Vouros P. Determination of in vitro- and in vivo-formed DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline by capillary liquid chromatography/microelectrospray mass spectrometry. Chem Res Toxicol. 1999;12:1019–1027. doi: 10.1021/tx990060m. [DOI] [PubMed] [Google Scholar]
- 61.Singh R, Farmer PB. Liquid chromatography–electrospray ionization–mass spectrometry: the future of DNA adduct detection. Carcinogenesis. 2006;27:178–196. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]
- 62.Soglia JR, Turesky RJ, Paehler A, Vouros P. Quantification of the heterocyclic aromatic amine DNA adduct N-(deoxyguanosin-8-yl)-2-amino-3-methylimidazo[4,5-f]quinoline in livers of rats using capillary liquid chromatography/microelectrospray mass spectrometry: a dose-response study. Anal Chem. 2001;73:2819–2827. doi: 10.1021/ac010218j. [DOI] [PubMed] [Google Scholar]
- 63.Turesky RJ, Gremaud E, Markovic J, Snyderwine EG. DNA adduct formation of the food-derived mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in nonhuman primates undergoing carcinogen bioassay. Chem Res Toxicol. 1996;9:403–408. doi: 10.1021/tx950132j. [DOI] [PubMed] [Google Scholar]
- 64.Wang JJ, Marshall WD, Frazer DG, Law B, Lewis DM. Characterization of DNA adducts from lung tissue of asphalt fume-exposed mice by nanoflow liquid chromatography quadrupole time-of-flight mass spectrometry. Anal Biochem. 2003;322:79–88. doi: 10.1016/j.ab.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Ricicki EM, Soglia JR, Teitel C, Kane R, Kadlubar F, Vouros P. Detection and quantification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl adducts in human pancreas tissue using capillary liquid chromatography–microelectrospray mass spectrometry. Chem Res Toxicol. 2005;18:692–699. doi: 10.1021/tx049692l. [DOI] [PubMed] [Google Scholar]
- 66.Gennaro LA, Vadhanam M, Gupta RC, Vouros P. Selective digestion and novel cleanup techniques for detection of benzo[a]pyrene diol epoxide–DNA adducts by capillary electrophoresis/mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:1541–1547. doi: 10.1002/rcm.1516. [DOI] [PubMed] [Google Scholar]
- 67.Saha M, Giese RW. High-performance liquid chromatography versus solid-phase extraction for post-derivatization cleanup prior to gas chromatography-electron-capture negative-ion mass spectrometry of N1, N3-bis-(pentafluorobenzyl)-N7-(2-[pentafluorobenzyloxy]ethyl)xanthine, a product derived from an ethylene oxide DNA adduct. J Chromatogr. 1993;629:35–40. doi: 10.1016/0021-9673(93)80351-8. [DOI] [PubMed] [Google Scholar]
- 68.Randall KL, Argoti D, Paonessa JD, Ding Y, Oaks Z, Zhang Y, Vouros P. An improved liquid chromatography–tandem mass spectrometry method for the quantification of 4-aminobiphenyl DNA adducts in urinary bladder cells and tissues. J Chromatogr A. 2010;1217:4135–4143. doi: 10.1016/j.chroma.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doerge DR, Churchwell MI, Marques MM, Beland FA. Quantitative analysis of 4-aminobiphenyl-C8-deoxyguanosyl DNA adducts produced in vitro and in vivo using HPLC–ES–MS. Carcinogenesis. 1999;20:1055–1061. doi: 10.1093/carcin/20.6.1055. [DOI] [PubMed] [Google Scholar]
- 70.Singh R, Arlt VM, Henderson CJ, Phillips DH, Farmer PB, Gamboa da Costa G. Detection and quantitation of N-(deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine adducts in DNA using online column-switching liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2155–2162. doi: 10.1016/j.jchromb.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding Y, Paonessa JD, Randall KL, Argoti D, Chen L, Vouros P, Zhang Y. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis. 2010;31:1999–2003. doi: 10.1093/carcin/bgq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nauwelaers G, Bessette EE, Gu D, Tang Y, Rageul J, Fessard V, Yuan JM, Yu MC, Langouet S, Turesky RJ. DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem Res Toxicol. 2011;24:913–925. doi: 10.1021/tx200091y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yun BH, Rosenquist T, Sidorenko V, Iden CR, Chen CH, Pu YS, Bonala RR, Johnson F, Dickman KG, Grollman AP, Turesky RJ. Biomonitoring of Aristolactam–DNA adducts in human tissues using ultra-performance liquid chromatography/ion-trap mass spectrometry. Chem Res Toxicol. 2012 doi: 10.1021/tx3000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102:179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrmann K, Engst W, Appel KE, Monien BH. Mutagenesis, Glatt. 2012. H, Identification of human and murine sulfotransferases able to activate hydroxylated metabolites of methyleugenol to mutagens in Salmonella typhimurium and detection of associated DNA adducts using UPLC–MS/MS methods. [DOI] [PubMed] [Google Scholar]
- 76.Sharma VK, Vouros P, Glick J. Mass spectrometric based analysis, characterization and applications of circulating cell free DNA isolated from human body fluids. Int J Mass Spectrom. 2011;304:172–183. doi: 10.1016/j.ijms.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fabris D. A role for the MS analysis of nucleic acids in the post-genomics age. J Am Soc Mass Spectrom. 2010;21:1–13. doi: 10.1016/j.jasms.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 78.McLuckey SA, Habibi-Goudarzi S. Decompositions of multiply charged oligonucleotide anions. J Am Chem Soc. 1993;115:12085–12095. [Google Scholar]
- 79.Marzilli LA, Wang D, Kobertz WR, Essigmann JM, Vouros P. Mass spectral identification and positional mapping of aflatoxin B1-guanine adducts in oligonucleotides. J Am Soc Mass Spectrom. 1998;9:676–682. doi: 10.1016/S1044-0305(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 80.Harsch A, Sayer JM, Jerina DM, Vouros P. HPLC–MS/MS identification of positionally isomeric benzo[c]phenanthrene diol epoxide adducts in duplex DNA. Chem Res Toxicol. 2000;13:1342–1348. doi: 10.1021/tx000140m. [DOI] [PubMed] [Google Scholar]
- 81.Xiong W, Glick J, Lin Y, Vouros P. Separation and sequencing of isomeric oligonucleotide adducts using monolithic columns by ion-pair reversed-phase nano-HPLC coupled to ion trap mass spectrometry. Anal Chem. 2007;79:5312–5321. doi: 10.1021/ac0701435. [DOI] [PubMed] [Google Scholar]
- 82.Sharma VK, Glick J, Vouros P. Reversed-phase ion-pair liquid chromatography electrospray ionization tandem mass spectrometry for separation, sequencing and mapping of sites of base modification of isomeric oligonucleotide adducts using monolithic column. J Chromatogr A. 2012;1245:65–74. doi: 10.1016/j.chroma.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schrader W, Linscheid M. Determination of styrene oxide adducts in DNA and DNA components. J Chromatogr A. 1995;717:117–125. doi: 10.1016/0021-9673(95)00558-9. [DOI] [PubMed] [Google Scholar]
- 84.Schrader W, Linscheid M. Styrene oxide DNA adducts: in vitro reaction and sensitive detection of modified oligonucleotides using capillary zone electrophoresis interfaced to electrospray mass spectrometry. Arch Toxicol. 1997;71:588–595. doi: 10.1007/s002040050431. [DOI] [PubMed] [Google Scholar]
- 85.Chowdhury G, Guengerich FP. Direct detection and mapping of sites of base modification in DNA fragments by tandem mass spectrometry. Angew Chem Int Ed Engl. 2008;47:381–384. doi: 10.1002/anie.200703942. [DOI] [PubMed] [Google Scholar]
- 86.Jamin EL, Arquier D, Tulliez J, Debrauwer L. Mass spectrometric investigation of the sequence selectivity for adduction of heterocyclic aromatic amines on single-strand oligonucleotides. Rapid Commun Mass Spectrom. 2008;22:3100–3110. doi: 10.1002/rcm.3707. [DOI] [PubMed] [Google Scholar]
- 87.Chan W, Yue H, Wong RN, Cai Z. Characterization of the DNA adducts induced by aristolochic acids in oligonucleotides by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3735–3742. doi: 10.1002/rcm.3791. [DOI] [PubMed] [Google Scholar]
- 88.Anichina J, Zhao Y, Hrudey SE, Schreiber A, Li XF. Electrospray ionization tandem mass spectrometry analysis of the reactivity of structurally related bromo-methyl-benzoquinones toward oligonucleotides. Anal Chem. 2011;83:8145–8151. doi: 10.1021/ac201646z. [DOI] [PubMed] [Google Scholar]
- 89.Lai Y, Lu M, Lin S, Wu H, Cai Z. Electrospray ionization tandem mass spectrometric characterization of DNA adducts formed by bromobenzoquinones. Rapid Commun Mass Spectrom. 2011;25:2943–2950. doi: 10.1002/rcm.5191. [DOI] [PubMed] [Google Scholar]
- 90.Oberacher H, Mayr BM, Huber CG. Automated de novo sequencing of nucleic acids by liquid chromatography–tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15:32–42. doi: 10.1016/j.jasms.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 91.Rozenski J, McCloskey JA. SOS: a simple interactive program for ab initio oligonucleotide sequencing by mass spectrometry. J Am Soc Mass Spectrom. 2002;13:200–203. doi: 10.1016/S1044-0305(01)00354-3. [DOI] [PubMed] [Google Scholar]
- 92.Oberacher H, Wellenzohn B, Huber CG. Comparative sequencing of nucleic acids by liquid chromatography–tandem mass spectrometry. Anal Chem. 2002;74:211–218. doi: 10.1021/ac015595a. [DOI] [PubMed] [Google Scholar]
- 93.Oberacher H, Pitterl F. Tandem mass spectrometric de novo sequencing of oligonucleotides using simulated annealing for stochastic optimization. Int J Mass Spectrom. 2011;304:124–129. [Google Scholar]
- 94.Liao Q, Shen C, Vouros P. GenoMass – a computer software for automated identification of oligonucleotide DNA adducts from LC–MS analysis of DNA digests. J Mass Spectrom. 2009;44:549–560. doi: 10.1002/jms.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma VK, Glick J, Liao Q, Shen C, Vouros P. GenoMass software: a tool based on electrospray ionization tandem mass spectrometry for characterization and sequencing of oligonucleotide adducts. J Mass Spectrom. 2012;47:490–501. doi: 10.1002/jms.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]