Abstract
Numerous inflammatory disorders are associated with elevated levels of xanthine oxidoreductase (XOR) and allied enhancement of reactive species formation contributory to systemic pathology. Despite a long standing association between increased XOR activity and negative clinical outcomes, recent reports describe a paradigm shift where XOR mediates beneficial actions by catalyzing the reduction of NO2− to •NO. While provocative, these observations contradict reports of improved outcomes in similar models upon XOR inhibition as well as reports revealing strict anoxia as a requisite for XOR-mediated •NO formation. To garner a more clear understanding of conditions necessary for in vivo XOR-catalyzed •NO production, this review critically analyzes the impact of O2 tension, pH, substrate concentrations, glycoaminoglycan docking and inhibition strategies on the nitrite reductase activity of XOR and reveals a hypoxic milieu where this process may be operative. As such, information herein serves to link recent reports in which XOR activity has been identified as mediating the beneficial outcomes resulting from nitrite supplementation to a microenviromental setting where XOR can serve as substantial source of •NO.
Keywords: nitrite, xanthine oxidoreductase, nitric oxide, oxygen tension, inflammation, hypoxia
Introduction
Two landmark discoveries placed xanthine oxidoreductase (XOR) at the forefront of free radical biology; 1) XOR was the first demonstration of the biological formation of superoxide (O2•−) and 2) XOR serves as a central source of reactive oxygen species (ROS) in tissue ischemia-reperfusion injury (1, 2). As the redox field progressed, several additional enzymatic and non-enzymatic sources of free radicals and reactive species have been identified yet, to date, XOR remains the most pharmacologically targetable incentivizing extensive exploration of inhibition strategies to address disease processes where oxidative stress is contributory.
Xanthine oxidoreductase is a complex molybdoflavin protein that catalyzes the terminal two reactions in purine degradation (hypoxanthine → xanthine → uric acid) in primates. In humans, XOR is ubiquitously expressed with the splanchnic system displaying the highest specific activity (3). Hypoxia as well as several inflammatory cytokines (TNF-α, IL-1β, IFN-γ), induce XOR expression in tissues and vascular endothelial cells where can be released to the circulation (3, 4). The enzyme is a homodimer of ~300 kD with each subunit consisting of four redox centers: a molybdenum cofactor (Mo-co), one FAD site and two Fe/S clusters. The Mo-co is comprised of a pterin derivative with a cyclized dithiolene side chain and one Mo atom which is pentacoordinated with the dithiolene, two oxygen atoms and a sulfur atom. The Mo-co is the site of purine oxidation while NAD+ and O2 reduction occurs at the FAD. The two Fe/S clusters provide the conduit for electron flux between the Mo-co and the FAD. These Fe/S clusters are both of the ferredoxin type, but are not identical and thus independently distinguishable by their electron paramagnetic resonance spectra (5-7). The enzyme is transcribed as a single gene product in the xanthine dehydrogenase (XDH) form, and as such cellular XOR exists primarily as XDH where substrate-derived electrons reduce NAD+ to NADH, Fig. 1A. However, during inflammatory conditions, reversible oxidation of critical cysteine residues (535 and 992) and/or limited proteolysis converts XDH to xanthine oxidase (XO) (8). In the oxidase form, affinity for NAD+ at the FAD is greatly decreased while affinity for oxygen is enhanced resulting in univalent and divalent electron transfer to O2 generating O2•− and hydrogen peroxide (H2O2), respectively, Fig. 1B (9). However, the relative quantities of O2•− and H2O2 derived from XO are dependent upon O2 tension. For example, at pH 7.4 and 95% O2, XO generates ~68% O2•− and ~32% H2O2 whereas at 1% O2, XO forms ~90% H2O2 and only ~10% O2•− (10). Thus, under conditions such as ischemia and/or hypoxia, where both O2 and pH are lowered, H2O2 formation is favored suggesting that XOR may participate in the numerous signaling cascades where H2O2 has been implicated (11, 12). Therefore, when situated at critical sites in the tissue and vasculature, XO can serve as an abundant source of ROS mediating alterations in vascular function, by reducing •NO bioavailability via direct reaction with O2•−(•NO + O2•− → ONOO−) or indirectly, by mediating redox-dependent cell signaling reactions (13, 14). While the post-translational conversion of XDH to XO has become synonymous with the conversion from a “good” housekeeping enzyme to a “bad” ROS-producing enzyme, it is important to recognize that under certain circumstances XDH can also reduce O2 and generate ROS. Although NAD+ is the preferred electron acceptor for XDH, when levels of this substrate are low, XDH will utilize O2 (15). Such conditions include ischemia or hypoxia either localized, regional or systemic where O2-dependent alterations in cellular respiration lead to decreased mitochondrial NADH oxidation and thus significant decreases in levels of NAD+ (16). Therefore, care should be taken not to exclusively associate XDH as the form of XOR that does not produce ROS, especially under conditions as described above.
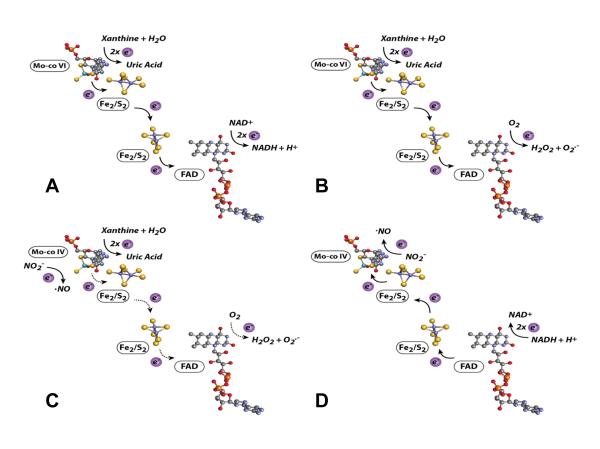
Figure 1. XOR-Catalyzed Reactions.
(A) For XDH, xanthine is oxidized to uric acid and electrons transferred via 2 Fe/S centers to the FAD where NAD+ is reduced to NADH. (B) For XO, xanthine is oxidized to uric acid and electrons are transferred to the FAD where O2 is reduced to O2•− and H2O2. Under normal O2 tension and pH the Mo-co would reside more often in the oxidized +6 (VI) valence as electrons are rapidly transferred to NAD+ (A) or O2 (B) at the FAD. (C) Nitrite (NO2−) undergoes a 1 electron reduction to •NO at the Mo-cofactor of XO (electrons are donated directly to Mo by xanthine). (D) NO2− is reduced to •NO at the Mo-cofactor of XO (electrons are supplied by NADH and transferred retrograde reducing the Mo). Under low O2 tensions and pH the Mo-co would reside more often in the reduced +4 (IV) valence as electrons are more slowly transferred to O2. This decrease in electron flux from the Mo-co to the FAD is depicted in (C) as dashed arrows whereas in (D) NADH-mediated electron donation at the FAD is out-competing O2-mediated electron withdrawal and thus the arrows are solid and indicating flux from the FAD to the Mo.
For decades, the dogma in the redox field has been that inflammation/hypoxia-induced elevation of XO activity equates to elevated XO-derived ROS generation and ultimately to poor clinical outcomes. However, recent reports have forced a reevaluation of this premise by demonstrating a nitrite (NO2−) reductase function for XOR (1 e− reduction of NO2− to •NO) and thus suggesting XOR to be a source of beneficial •NO. For example, under strictly anoxic conditions, using xanthine or NADH as reducing substrates, XO catalyzes the reduction of NO2− to NO• (17-19). The Mo-co has been identified as the site of NO2− reduction where xanthine directly reduces the cofactor (Fig. 1C); alternatively, NADH indirectly provides reducing equivalents via electron donation at the FAD with subsequent retrograde flow to the Mo-co, Fig. 1D (20). In addition to biochemical studies, several reports have demonstrated diminution or ablation of NO2−-mediated beneficial effects upon co-treatment with the pyrazalopyrimidine-based XOR inhibitor allo/oxypurinol, suggesting XOR involvement. For example, inhibition of XOR activity has diminished protective effects of NO2− treatment in animal models of intimal hyperplasia following vessel injury (21), acute lung injury as well as ventilator-induced pulmonary pathology (22, 23), ischemia/reperfusion (I/R)-induced damage in lung transplantation (24), pulmonary hypertension (25), myocardial infarction (26) and renal (27), cardiac (28) and liver (29) I/R injury. It has also been suggested that NO2− concentrations and subsequent •NO levels may be enhanced in an XOR-dependent manner by treatment with nitrate (NO3−) where XOR serves first as a NO3− reductase (NO3− + 1e− → NO2−) and ultimately a NO2− reductase (NO2− + 1e− → •NO) (30). In these experiments treatment of germ-free mice, void of bacterial NO3− reductases, resulted in elevation of plasma NO2− levels that were not observed when mice received co-treatment with allopurinol and thus are consistent with previous biochemical reports demonstrating NO3− reductase activity for XOR (18). In the aggregate, there now exists a new body of evidence suggesting a protective role for XOR under hypoxic and inflammatory conditions in the presence of elevated levels of NO2−.
This concept is provocative and exciting as it serves to shift the paradigm regarding the role of XOR-derived reactive species from deleterious to protective. Yet, the evidence for XOR-mediated •NO formation, especially in vivo, remains significantly underdeveloped for the following reasons: 1) all reports to date have relied upon inhibition of XOR with allo/oxypurinol, an inhibitor known to have off target effects on alternative purine catabolism pathways including those affecting adenosine (31, 32) or 2) by systemic molybdopterin enzyme inactivation upon dietary supplementation with sodium tungstate (NaW), a process that mediates the replacement of the active site Mo with W rendering XOR as well as aldehyde oxidase (AO) and sulfite oxidase (SO) inactive; interestingly, both AO and SO demonstrate NO2− reductase activity (see: Other Molybdopterin-Based Nitrite Reductases), 3) in vitro demonstration of •NO formation from XOR and NO2− has required anoxic conditions and extremely high concentrations of NO2− which are both incompatible with either normal or pathophysiology and 4) the absence of XOR−/− or XOR+/− murine models viable past six weeks of age and/or available tissue-specific XOR conditional knockouts (33, 34). The following sections will critically address these issues and provide new insight to the role of XOR in mediating beneficial actions of NO2−.
Substrates and pH
Under strict anoxic conditions purified XO (potassium phosphate buffer pH 7.4) catalyzes the 1 e− reduction of NO2− to NO• when electrons are provided by xanthine or NADH, Fig. 1C & 1D (17-19). The Mo-co in XO has been identified as the site of NO2− reduction (Km = 2.5 μM) where xanthine (Km = 6.5 μM) directly reduces the cofactor; alternatively, NADH (Km = 121.7 μM) can indirectly provide reducing equivalents via electron donation at the FAD (9, 19). The Km for NO2− at the Mo-co is ~2.5 mM when using xanthine or NADH as the electron donor whereas normal tissue NO2− levels are greater than an order of magnitude lower than this value (~0.3 μM) (35). In animal models of hypoxia NO2− levels in tissue have been reported to decrease (35). Therefore, attainment of NO2− concentrations that would provide ½ maximal rates of NO• production would necessitate: 1) a substantial amount of NO2− be derived from product decomposition of activated NOS, 2) dietary or pharmacological intake and/or 3) possible bacterial contributions (35). In these studies of systemic hypoxia, tissue levels of NO2− decrease yet, nitrate levels increase from a range of 30-40 μM to 120-150 μM depending upon the tissue. This increase in nitrate (NO3−) cannot be overlooked as reports from as early as 1962 have demonstrated XOR-dependent, xanthine and NADH-driven reduction of organic NO3− to NO2− and then to •NO (18, 36-38). In addition, a series of studies have implicated oral bacteria as important bioactivators of NO3− by reducing it to NO2−. For example, when healthy volunteer’s consumed NaNO3− (10mg/kg) in drinking water their plasma and saliva levels of NO2− were significantly elevated whereas those pretreated with chlorhexidine-containing antibacterial mouthwash demonstrated no change in salivary NO3− levels but substantive diminution of detectable NO2− both in the saliva and plasma suggesting a key role for oral bacterial NO3− reductases (39). Similar effects were seen when healthy volunteers were subjected to a mouthwash regimen in the absence of supplemental NO2− and monitored for plasma nitrite levels and blood pressure (40). In this study mouthwash-mediated reduction in oral bacterial NO3− reductase capacity lead to a demonstrable decrease in plasma NO2− as well as blood pressure strongly suggesting that accumulation of NO3− in the salivary gland and its subsequent reduction to NO2− may play a pivotal role in the NO3− → NO2− → •NO pathway.
Kinetic analysis from XOR-driven NO3− reduction produces Km values ranging from 330-500 μM for glycerol trinitrate (GTN) to 32 mM for inorganic nitrate (NaNO3) (18, 36, 37). Again, this raises the question to what biologically significant degree can nitrate mediate •NO production from XOR given the kinetic hurdle that must be overcome? On the other hand, the Km for xanthine at the Mo-co is 6.5 μM and levels of xanthine above 20 μM are reported to induce substrate-dependent inhibition of NO2− reduction (Ki = 55 μM) under anoxia (19). The suggested mechanism for this inhibition is competition where xanthine binding to the oxidized Mo-co blocks access to NO2−. This observation becomes critically important when considering inflammatory/ischemic conditions can result in combined plasma concentrations of hypoxanthine + xanthine ranging from 50-100 μM, levels that would exceed the Ki of 55 μM (41-44). Therefore, one could speculate that a clinical approach based on XOR-mediated •NO generation by enhancement of circulating NO2− and/or NO3− would most likely be buttressed with an adjuvant to reduce ischemia/hypoxia-mediated elevation of hypoxanthine. For example, substantive inhibition of adenosine deaminase (adenosine → inosine) and/or purine nucleoside phosphorylase (inosine → hypoxanthine) may serve to ensure hypoxanthine and xanthine levels are maintained at levels below those that would significantly inhibit the NO2− reductase activity of XOR.
On the other hand, it can be hypothesized that NADH, not hypoxanthine/xanthine, may be the major source of electrons necessary to reduce the Mo-co and drive hypoxic NO2− reduction. Under ischemia or hypoxia, O2-dependent alterations in cellular respiration lead to decreased mitochondrial NADH oxidation and thus significant elevation of NADH levels (16). This enhancement of NADH concentration may serve to efficiently reduce the Mo-co via retrograde electron flow from the FAD in XDH as well as XO; albeit less effectively for the latter. For example, the affinity for NADH at the FAD is substantially greater for XDH (Km = 6.7 μM) than for XO (Km = 121.7 μM) (9, 15, 45). Yet, the overall impact of NADH-dependent retrograde electron loading of XOR may also be reflected in the resultant effect on xanthine. The affinity of xanthine for the Mo-co is dependent on the Mo-co being oxidized. However, conditions where significant contributions to enzyme reduction were being supported by NADH would lead to more time for the Mo-co assuming the reduced state and thus more accessible to NO2−. In fact, when XO-catalyzed NO2− reduction experiments were conducted under 21% O2 conditions, the presence of excessive amounts of NADH (0.5-4 mM) produced •NO formation rates ~70% of those observed under anoxic conditions (46). This scenario of NADH-driven nitrite reduction may therefore serve to counteract the potential substrate-dependent inhibitory effects of ischemia/hypoxia-induced elevation of hypoxanthine/xanthine levels. It would also greatly affect the capacity of O2 to inhibit this process (see: Oxygen Tension).
Another crucial factor that must be considered when examining the mechanics of XOR-dependent NO2− reduction is pH. Hypoxanthine and xanthine oxidation at the Mo-co is a base-catalyzed process with a pH optimum of 8.9 and as such most kinetic studies of XOR have utilized this pH for calculating Km values. The reduction of NO2− at the Mo-co is an acid-catalyzed process with a pH optimum of (6-6.5) (17). Considering in vivo microenvironments encountered during hypoxic inflammatory conditions demonstrate pH’s that fall below 6.9, it is reasonable to anticipate that the capacity of XOR to catalyze •NO formation would be enhanced by slowing the xanthine oxidation process and accelerating NO2− reduction. This was indeed the case as a significant pH-dependent increase in the rate of XO-catalyzed NO2− reduction was observed when pH was lowered (8.0→7.4→6.0→5.0) using either NADH or xanthine as reducing substrates (46). The impact of these observations may be greater than anticipated when considering that acidic pH also leads to decrease in eNOS catalytic activity and resultant diminution of •NO formation; a setting where alternative sources of •NO would be beneficial (47).
Oxygen Tension
Nitrite reductase activity of XO is extremely oxygen sensitive. Several studies have clearly demonstrated XOR-dependent reduction of NO2− to •NO. However, to date and to our knowledge, only two reports indicate operative XO-catalyzed •NO production from NO2− in the presence of O2 from purified XO in buffered solution (17, 46). In one case, experiments revealed measureable •NO formation via ozone-enhanced chemiluminescence detection using NADH as a reducing substrate under conditions of 1.5% O2 and 100 μM NO2− (17). In another report, the effect of various O2 tensions on XO-mediated NO2− reduction were explored by electron paramagnetic resonance (EPR) spin trapping using iron-MGD (N-(Dithiocarbamoyl)-N-methyl-D-glucamine (MGD2-Fe2+)) to measure •NO generation; however, successful •NO detection required the presence of high concentrations of superoxide dismutase (SOD) (400 U/ml) as well as copious amounts of NO2− (2-60 mM) using either xanthine or dihydroxybenzaldehyde (DBA) for reducing substrate (46). It was hypothesized that the presence of SOD prevented diminution of the •NO signal by rapid reaction with O2•−, a process also explored in another report demonstrating O=NOO− formation from reaction of XO with xanthine and NO2− (48). However, amperometric •NO detection studies in our laboratory conducted in a closed system where both O2 and •NO were simultaneously monitored showed no observable •NO production from purified XO until all O2 was consumed when using xanthine as reducing substrate (49). These experiments also revealed that the presence of SOD did not confer the capacity to observe •NO formation under oxygenated conditions (2% O2) suggesting O2•− was not interfering with the ability to detect •NO. However, similar to previous reports, blockade of O2-FAD reaction by treatment with diphenyleneiodium (DPI) did enable detectable •NO formation in the presence of up to 21% O2 (17, 46). Inconsistencies between these studies regarding the extent of O2-mediated effects on XOR-catalyzed •NO formation may be explained, in part, by misinterpretations derived from degradation of the XOR protein during vigorous purging in the flow-through reaction chamber of the chemiluminescent system, the range of NO2− concentrations used (100 μM-20 mM) and/or the variable sensitivity between the different •NO detection methods. For example, enhanced chemiluminescence provides low nM sensitivity whereas EPR spin trapping requires nearly 0.5-1.0 μM for successful assessment. Nonetheless, the various reports do converge at the same conclusion: O2-mediated withdrawal of electrons at the FAD serves to oxidize the Mo-co and inhibit NO2− reduction, Fig. 1C. Furthermore, it can be estimated that rates of production observed under ideal conditions (purified enzyme or tissue homogenate in buffer using xanthine, DBA or NADH as reducing substrates) are in the range of 0.5-2.0 nmoles s−1 mg−1; values similar to those reported for eNOS. This being said, the capacity of XO to produce biologically significant levels of •NO from NO2− in vivo will necessitate shifting preference towards NO2− at the Mo-co from not only acidic pH, elevated NO2− and NADH levels, but substantive hypoxia; most likely O2 concentrations that are lower than the Km for O2 at the FAD cofactor of XO (~27 μM or ~2% O2).
XOR-Vascular Glycosaminoglycan Interactions
While understanding the substrate concentrations, pH and O2 tensions necessary to favor XOR-mediated NO2− reduction over oxidant production is crucial, additional factors may exist that further facilitate this process. For example, binding and immobilization of XO on the luminal surface of vascular endothelial cells induces alterations in XO kinetics that effect substrate affinity as well as the species of oxidant being produced; kinetic alterations that may also impact NO2− reductase activity.
In both animal models and clinical studies of cardiovascular disease, intravenous administration of heparin results in increased plasma XO activity, suggesting XO is bound to the vascular endothelium (50-52). During inflammatory/hypoxic conditions XDH expression is induced resulting elevation of XDH protein abundance and subsequent release into the circulation where it is rapidly (< 1 min) converted to XO and sequestered by negatively charged glycosaminoglycans (GAGs) on the surface of vascular endothelial cells (51, 53). While XO exhibits a net negative charge at physiological pH, cationic amino acid motifs on the surface of the protein result in high affinity for GAGs (Kd = 6 nM) (51, 54, 55). This sequestration of XO by GAGs: 1) substantially amplifies local XO concentration, 2) diminishes rotational and translational mobility and 3) alters XO kinetic properties. For example, due to a decrease in substrate binding affinity, GAG association of XO increases the Km for xanthine 3-fold (6.5 → 21.2 μM) and increases the Ki for allo/oxypurinol by 5-fold (85 → 451 nM) when compared to XO in free in solution (56). In addition, binding of XO to GAGs reduces O2•− production by 34% thus favoring divalent electron transfer to O2 (H2O2 formation) (56). These observations were similar to those obtained when XO was bound to the milk fat globule membrane where immobilization of the enzyme in this setting induced a 2-fold increase in the Km for xanthine while enhancing affinity for NADH by decreasing the Km 3-fold at the FAD (41). Combined, immobilization-induced diminution of affinity for xanthine, enhanced affinity for NADH and substantive reduction in the amount of O2•− being produced would be favorable for NO2− reduction to bioavailable •NO. It is also important to note that a GAG-mediated increase the Ki for allo/oxypurinol has crucial implications for pharmacological intervention studies designed to determine the extent of XO involvement upon treatment with NO2− (see: Inhibitors and XOR Knockouts).
Inhibitors and XOR Knockouts
As noted previously, XOR−/− and XOR+/− mice experience alterations in nutrient absorption and elevated plasma hypoxanthine levels resulting in death from kidney failure before six weeks of age (33, 34). This absence of viable global XOR knockouts as well as the current lack of available tissue-specific conditional knockouts has relegated the use of pharmacologic means to conduct proof of principle experimentation regarding contributory roles of XOR in mediating NO2− reduction. To date, in vitro and in vivo studies have utilized application of allo/oxypurinol or NaW to determine the extent of XOR-mediated contributions to salutary actions resulting from NO2− treatment. This approach has produced results suggestive of an operative NO2− reductase activity for XOR yet, there exist several pitfalls that must be considered. For example, binding of XO to endothelial GAGs confers resistance to inhibition by allo/oxypurinol. A closer look at this issue demonstrates that treatment with allo/oxypurinol at levels (100-400 μM) far above those tolerated clinically (30-90 μM) results in only ~80% inhibition when XO is bound to GAGs, Fig. 2 (56, 57). This level of inhibition, while sufficient to reduce symptoms of gout by lowering plasma uric acid levels, is not optimal for validating roles for XO. In addition, allopurinol is known to mediate off-target effects on alternative purine catabolic pathways that may also contribute to misinterpretation of results (31). Likewise, global XOR knockdown by dietary supplementation with NaW is also wrought with significant off-target implications. Enhancement of circulating W levels results in replacement of the active site Mo in XOR with W rendering it inactive for reactions at the Mo-co (58). However, this is not specific to XOR as W replacement of Mo in other molybdopterin-based enzymes such as aldehyde oxidase (AO) and sulfite oxidase (SO) will also occur. This is important as both of these enzymes have been reported to demonstrate nitrite reductase activity (see: Other Molybdopterin-Based Nitrite Reductases) and thus their inactivation could contribute to observable decreases in NO2− reduction making it difficult to specifically assign contributions to XOR.
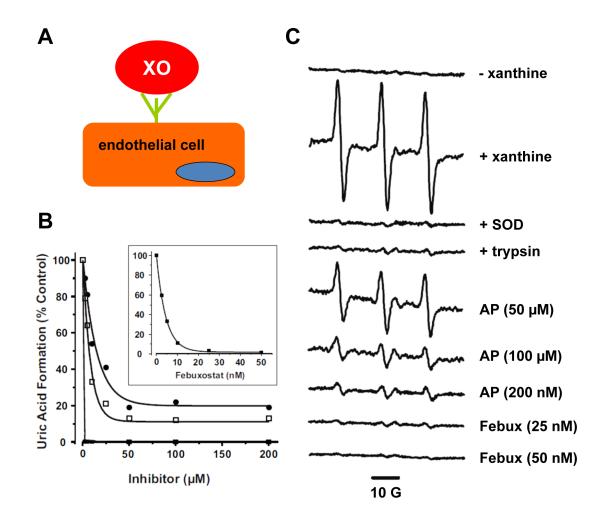
Figure 2. Febuxostat is superior to allo/oxypurinol at inhibiting endothelial cell-bound XO.
(A) Cartoon representation of XO binding to endothelial cell GAGs. (B) BAECs were exposed to purified XO (5 mU/ml) for 20 min at 25 °C and washed extensively to ensure removal of unbound XO. Inhibitors (febuxostat ■, allopurinol □, and oxypurinol •) were added at the indicated concentrations followed by xanthine (100 μM), and uric acid levels assessed after 1 h. Values represent % control (no inhibitor) and equal the mean of at least 3 independent determinations (*p < 0.05). (C) This experiment reveals: 1) the incapacity of allopurinol to inhibit endothelial cell GAG-bound XO at and above clinically-relevant concentrations and 2) the superiority of febuxostat to achieve complete inhibition at concentrations well below those attainable in the clinic. Purified XO was bound to BAEC GAGs (as in A); the cells were then harvested by mechanical dissociation and suspended at 1 × 106 cells/ml in Krebs–HEPES EPR buffer (pH 7.4) treated with Chelex resin and containing 25 μM deferoxamine and 5 μM DTPA to minimize contributions of adventitious metals to the EPR signal. Cell suspensions were exposed to the indicated concentrations of inhibitor followed by xanthine (100 μM) and immediately analyzed by EPR for extracellular O2•− with the cell impermeable spin probe (1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpi-peridine) PPH (50 μM) to ensure the O2•− detected was extracellular and not from intracellular sources. To further confirm that extracellular GAG-bound XO was responsible for the observed PP• signal, XO-loaded BAECs were briefly treated with trypsin to remove the GAG-bound XO, washed and then exposed to xanthine (100 μM) and PPH (50 μM). Validation that the PP• signal was mediated by reaction with O2•− was accomplished by exposure to SOD. Spectra represent XO-loaded cells exposed to PPH and the following agents from top to bottom: (− xanthine); (+ xanthine); (+ xanthine and 100 U/ml SOD); (treated with trypsin to remove extracellular XO and then exposed to xanthine); (+ xanthine and 50, 100, or 200 μM allopurinol (AP)); and (+ xanthine and 25 or 50 nM febuxostat (Febux)). Each spectrum represents five cumulative scans over 1 min from t = 9 to t = 10 min at 37 °C and 21% O2. The spectrometer settings were as follows: field sweep 50 G, microwave frequency 9.78 GHz, microwave power 20 mW, modulation amplitude 2 G, conversion time 327 ms, time constant 655 ms, receiver gain 1 × 105. (54).
These limitations on XO inhibition/inactivation with allo/oxypurinol and NaW affirm the need for a more specific and potent inhibition strategy. To this end, we have identified the XOR-specific inhibitor febuxostat as it demonstrates: 1) over 3 orders of magnitude greater potency than allopurinol (Ki = 0.9 nM vs. 1.6 μM), 2) is not affected by XOR-vascular GAG association and 3) does not alter other purine catabolism pathways (31, 57). Evidence of febuxostat’s superior capacity to inhibit endothelial cell-bound XO is demonstrated in Fig. 2. In this experiment, febuxostat completely inhibits endothelial cell-bound XO-derived O2•− formation at concentrations (25-50 nM) well below the reported plasma Cmax (15 μM) for the clinic whereas levels of allopurinol (200 μM) twice those tolerated clinically do not achieve complete inhibition (31, 32, 59). These findings clearly demonstrate the potential benefit of using febuxostat in experiments designed to examine XOR-dependence of outcomes generated by NO2− treatment.
Taken together, both the use of febuxostat and the development of tissue-specific XOR conditional knockout models will serve to greatly enhance our capacity to elucidate the relative extent of XOR-mediated •NO formation from NO2− and differentiate XOR-mediated effects from those attributable to alternative NO2− reductase processes including other molybdopterin-containing enzymes.
Other Molybdopterin-Based Nitrite Reductases
Humans enzymes containing molybdopterin cofactors include XOR, AO, SO and the newly discovered mitochondrial amidoxime reducing component or mARC (60-62). With the role of XOR’s Mo-co in NO2− reduction during inflammatory/hypoxic conditions gaining relevance, contributions from these other molybdopterin enzymes must not be overlooked. This is exemplified by reports suggesting NO2− reductase activity for both AO and SO and as such necessitating their critical evaluation.
Aldehyde Oxidase
Aldehyde oxidase is a cytosolic enzyme that shares approximately 86% sequence homology with XOR. AO is a homodimer (~300 kD) with each monomer containing a Mo-co, 2 2Fe/2S centers and a flavin cofactor (FAD). Distribution of AO in mammals shows the highest specific activity in liver; however it is also present in lung, spleen, brain, heart, testes and the eye (60, 63). At the Mo-co AO catalyzes the oxidation of variety of aldehydes and aromatic heterocyclic compounds, including several pharmacologic agents, while reducing O2 to O2•− and H2O2 at the FAD (60, 63). Regarding substrates and products, AO differs significantly from XOR by demonstrating promiscuity at the Mo-co and incapacity to function as a dehydrogenase (NAD+ → NADH) at the FAD. However, AO can efficiently catalyze the oxidation of NADH (Km = 11 μM) at the FAD to produce O2•− and H2O2 (63) suggesting that under low O2 tensions NADH may provide electrons for retrograde flow to the Mo-co in a similar manner as seen with XO, Fig. 1D. It is also important to note that, to date, there exist no AO-specific inhibitors and thus in vitro inhibition of AO relies on utilizing compounds with albeit substantive AO inhibitory characteristics (β-estriadol, phenotiazines and raloxifene) but, with significant off-target actions making them less than optimal for in vivo use (64). In addition, the XOR inhibitor allopurinol displays cross-reactivity with AO which further complicates efforts to specifically block AO and/or XOR in model systems (64). Furthermore, there are no antibodies currently available to distinguish between XOR and AO, which again leads to ambiguity regarding immunohistochemical analysis of tissues and forces investigators to rely on activity assays (e.g. uric acid formation from XOR vs. dimethylamino-cinnamaldehyde (DMAC) oxidation for AO) to validate their conclusions. From the information presented above, it is clear that care must be taken to distinguish between actions of XOR and AO as several factors converge to confound interpretation of results. However, unpublished data in our laboratory indicates no reaction between the XOR inhibitor febuxostat and AO suggesting this inhibition approach as a promising new tool for selectively distinguishing catalytic activity of AO from XOR.
With extensive similarity to XOR, it is not surprising that AO has been identified as a NO2− reductase. Both in studies using purified enzyme and in ex vivo tissue analysis, AO demonstrates the capacity to catalyze the 1 e- reduction of NO2− to •NO (65, 66). Studies involving purified AO at physiological pH have shown efficient anaerobic •NO production using either DMAC or NADH as reducing substrate and producing a Km value for NO2− similar to that of XOR (~3 mM) (65). This process was also optimal under slightly acid conditions (between pH 6.0-7.4) using either NADH or DMAC for reducing substrate. Nitrite reduction by AO is significantly oxygen sensitive (Ki = 3.8 μM or <0.5% O2) when providing electrons at the Mo-co with DMAC whereas when the Mo-co is reduced by retrograde flow from reaction of NADH at the FAD, AO-catalyzed •NO formation rates are maintained at nearly 50% of the anoxic conditions at O2 tensions up to 21% and in the presence of 400 U/ml SOD (65). These data are suggestive that conditions in which nitrite reductase activity of AO will be functional are similar to those for XOR including lower pH, hypoxia and elevated levels of NADH.
Sulfite Oxidase
In humans, SO catalyzes the reduction of sulfite to sulfate in the final step of sulfur-containing amino acid catabolism. SO is localized in the intermembrane space of mitochondria with the highest specific activity found in liver, kidney and heart but it is also detectable in spleen, brain, and skeletal muscle (67). Structurally quite different than AO and XOR, SO is a 110 kDa homodimer with each monomer containing an N-terminal b5-type cytochrome heme domain, a central Mo-co domain and a C-terminal dimerization domain (68). In light of these structural differences, SO has recently been identified as a NO2− reductase (62). In these studies the Mo-co of SO catalyzes the reduction of NO2− to •NO using sulfite as a reducing substrate under anoxic conditions and slightly acid pH (6.5). However, more extensive investigation is necessary to determine the physiological significance and the relative impact of SO-derived •NO from NO2− when compared to AO and XOR.
Summary
While it is accepted that tissue production of •NO is primarily dependent on the enzymatic conversion of arginine to citrulline by nitric oxide synthases (NOS), recent studies suggest alternative sources and mechanisms of •NO production may be operative including enzymatic activity of hemoglobin and myoglobin, especially at lower pH’s and O2 tensions typical of ischemic/inflammatory conditions (69-71). However, neither the source of the reducing equivalents used to drive these reactions nor the reductive process(es) have been fully elucidated. Likewise, consideration of XOR as a significant source of •NO during ischemic/hypoxic conditions seems incongruent with numerous studies reporting treatment with allopurinol reducing symptoms and rescuing functional endpoints in similar disease models. If XOR-mediated •NO production is beneficially significant, then it would seem logical that inhibition of this process would produce less than desirable outcomes. However, several recent reports indicate that this is not the case leaving the field again, a bit unclear. Herein, we have discussed several key factors (acidic pH, elevated levels of NADH, hypoxia, GAG immobilization-induced kinetic alterations) that contribute to a microenvironmental setting where XOR-mediated NO2− reduction may be relevant. As tissue O2 concentrations decrease these factors coalesce to create a milieu where the Mo-co assumes a more reduced state Mo(IV) whereas under normal physiologic conditions and O2 tensions, electrons are rapidly removed from the system and thus the Mo-co assumes a more oxidized state Mo(VI), Fig. 3. As O2 levels decrease, approaching and falling below the Km for O2 at the FAD (~27 μM or ~1.5% O2), XOR can shift from a more oxidized to more reduced state Mo(IV). It is also under these same hypoxic conditions (~1.5% O2) that O2 would become limiting as a requisite substrate for nitric oxide synthase necessitating alternative sources become operative. For example, the Km for O2 with eNOS is 23 μM; a value strikingly close to the Km value of 27 μM for O2 and XO (72). It is therefore logical to hypothesize that hypoxia switches XO from oxidant to •NO production. When this does occur, it could also be hypothesized that enhancement of NO2− reaction at the Mo-co would result in further diminution of ROS generation as electrons would be removed from the system and thus unavailable for O2 reduction at the FAD. Hence, this “O2 switch” for XO could result in twice the benefit where deleterious ROS production is critically diminished while beneficial •NO is being generated. The clinical relevance of this concept would, of course, be critically dependent on significant elevation of circulating NO2− via pharmacologic/dietary supplementation combined with concomitant reduction of hypoxanthine levels. However plausible this newly proposed function for XOR may seem, the mechanistic details that underpin key aspects of where, when and how much •NO can be attained via XOR-catalyzed NO2− reduction remain unclear affirming the need for more detailed studies designed to define the biological relevance as well as identify the therapeutic potential. But suffice it to say, if the resultant effects of XOR-mediated reactive species formation were all deleterious, then it would not have survived the evolutionary process.
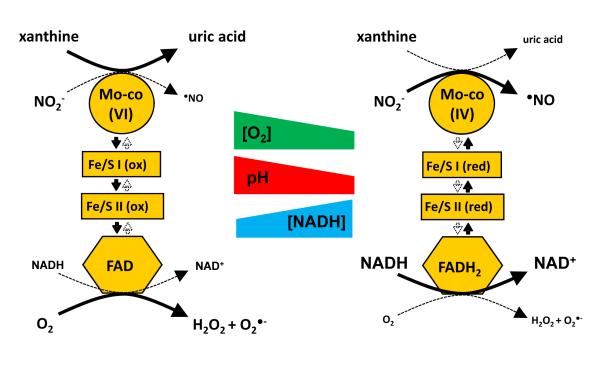
Figure 3. Hypoxic Switch: O2 tensions convert XOR from oxidant to •NO production.
Hypoxia results in the alteration of factors that may facilitate the conversion of XO from oxidant to •NO production including decreasing tissue pH and elevating cellular levels of NADH. Under normal physiologic conditions O2 rapidly removes electrons from XO and thus the Mo-co resulting in a more oxidized Mo-co, Mo(VI) (left). As tissue O2 concentrations decrease additional factors (acidic pH and elevated NADH levels) combine to create a milieu where the Mo-co assumes a more reduced state, Mo(IV) (right). Under more reduced conditions where NADH competes with O2 for reaction at the FAD and where lower pH reduces the rate of xanthine oxidation, NO2− can begin to effectively compete for electrons at the Mo-co (right).
Highlights.
Xanthine oxidoreductase (XOR) catalyzes •NO formation from NO2− under anoxia. Inhibition of XOR diminishes beneficial outcomes mediated by NO2− treatment. However, inhibition of XOR produces salutary outcomes in similar models.
The in vivo significance of XOR-derived •NO formation from NO2− is thus unclear.
We critically review key factors that combine to “switch” XOR from oxidant to •NO production.
Acknowledgements
This work was supported by AHA Scientist Development Grant 10SDG3560005 and University of Pittsburgh, Department of Anesthesiology Development Grant (EEK).
Abbreviations
- BAEC
(bovine aortic endothelial cells)
- DBA
(dihydroxybenzaldehyde)
- DMAC
(dimethylamino-cinnamaldehyde)
- GAGs
(glycosaminoglycans)
- GTN
(glycerol trinitrate)
- H2O2
(hydrogen peroxide)
- •NO
(nitric oxide)
- NOS
(nitric oxide synthase)
- O2•−
(superoxide)
- RNS
(reactive nitrogen species)
- ROS
(reactive oxygen species)
- SOD
(superoxide dismutase)
- XDH
(xanthine dehydrogenase)
- XO
(xanthine oxidase)
- XOR
(xanthine oxidoreductase)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 2.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) Journal of Biological Chemistry. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 3.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 4.Kelley EE, Hock T, Khoo NK, Richardson GR, Johnson KK, Powell PC, Giles GI, Agarwal A, Lancaster JR, Jr., Tarpey MM. Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Radic Biol Med. 2006;40:952–959. doi: 10.1016/j.freeradbiomed.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. Proc Natl Acad Sci U S A. 2000;97:10723–10728. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishino T, Okamoto K. The role of the [2Fe-2s] cluster centers in xanthine oxidoreductase. J Inorg Biochem. 2000;82:43–49. doi: 10.1016/s0162-0134(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki T, Okamoto K, Nishino T, Mizushima J, Hori H. Sequence motif-specific assignment of two [2Fe-2S] clusters in rat xanthine oxidoreductase studied by site-directed mutagenesis. J Biochem. 2000;127:771–778. doi: 10.1093/oxfordjournals.jbchem.a022669. [DOI] [PubMed] [Google Scholar]
- 8.Parks DA, Skinner KA, Tan S, Skinner HB. In: Xanthine oxidase in biology and medicine, In Reactive oxygen species in biological systems. Gilbert DL, Colton CA, editors. Kluwer Academic/Plenum; New York, NY: 1999. pp. 397–420. [Google Scholar]
- 9.Harris CM, Massey V. The Oxidative Half-reaction of Xanthine Dehydrogenase with NAD; Reaction Kinetics and Steady-state Mechanism. Journal of Biological Chemistry. 1997;272:28335–28341. doi: 10.1074/jbc.272.45.28335. [DOI] [PubMed] [Google Scholar]
- 10.Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid.Redox.Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 12.Veal EA, Day AM, Morgan BA. Hydrogen Peroxide Sensing and Signaling. Molecular Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Aslan M, Freeman BA. Oxidant-mediated impairment of nitric oxide signaling in sickle cell disease--mechanisms and consequences. Cell Mol.Biol.(Noisy.-le-grand) 2004;50:95–105. [PubMed] [Google Scholar]
- 14.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris CM, Massey V. The Reaction of Reduced Xanthine Dehydrogenase with Molecular Oxygen. REACTION KINETICS AND MEASUREMENT OF SUPEROXIDE RADICAL. Journal of Biological Chemistry. 1997;272:8370–8379. doi: 10.1074/jbc.272.13.8370. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Stanley WC, Saidel GM, Yu X, Cabrera ME. Regulation of lactate production at the onset of ischaemia is independent of mitochondrial NADH/NAD+: insights from in silico studies. J Physiol. 2005;569:925–937. doi: 10.1113/jphysiol.2005.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of Nitrite to Nitric Oxide Catalyzed by Xanthine Oxidoreductase. Journal of Biological Chemistry. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 18.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the Magnitude and Kinetics of Xanthine Oxidase-catalyzed Nitrite Reduction. EVALUATION OF ITS ROLE IN NITRIC OXIDE GENERATION IN ANOXIC TISSUES. Journal of Biological Chemistry. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 20.Maia LB, Moura JJ. Nitrite reduction by xanthine oxidase family enzymes: a new class of nitrite reductases. J Biol Inorg Chem. 2011;16:443–460. doi: 10.1007/s00775-010-0741-z. [DOI] [PubMed] [Google Scholar]
- 21.Alef MJ, Vallabhaneni R, Carchman E, Morris SM, Jr., Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest. 2011;121:1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: Effect of administration modality. Free Radic Biol Med. 2012;53:1431–1439. doi: 10.1016/j.freeradbiomed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickerodt PA, Emery MJ, Zarndt R, Martin W, Francis RC, Boemke W, Swenson ER. Sodium nitrite mitigates ventilator-induced lung injury in rats. Anesthesiology. 2012;117:592–601. doi: 10.1097/ALN.0b013e3182655f80. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto R, Okamoto T, Nakao A, Zhan J, Wang Y, Kohmoto J, Tokita D, Farver CF, Tarpey MM, Billiar TR, Gladwin MT, McCurry KR. Nitrite Reduces Acute Lung Injury and Improves Survival in a Rat Lung Transplantation Model. Am J Transplant. 2012;12:2938–2948. doi: 10.1111/j.1600-6143.2012.04169.x. [DOI] [PubMed] [Google Scholar]
- 25.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 26.Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J Mol Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 28.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, Zhang JH, Wu YH. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 30.Huang L, Borniquel S, Lundberg JO. Enhanced xanthine oxidoreductase expression and tissue nitrate reduction in germ free mice. Nitric Oxide. 2010;22:191–195. doi: 10.1016/j.niox.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–1847. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Becker MA, Schumacher HR, Jr., Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat Compared with Allopurinol in Patients with Hyperuricemia and Gout. N Engl J Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 33.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine Oxidoreductase Is an Endogenous Regulator of Cyclooxygenase-2. Circ Res. 2004;95:1118–1124. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 34.Vorbach C, Scriven A, Capecchi MR. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes and Development. 2002;16:3223–3235. doi: 10.1101/gad.1032702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 36.Doel JJ, Godber BLJ, Eisenthal R, Harrison R. Reduction of organic nitrates catalysed by xanthine oxidoreductase under anaerobic conditions. Biochim Biophys Acta. 2001;1527:81–87. doi: 10.1016/s0304-4165(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Cui H, Liu X, Zweier JL. Xanthine Oxidase Catalyzes Anaerobic Transformation of Organic Nitrates to Nitric Oxide and Nitrosothiols: Characterization of this mechnism and the link between organic nitrite and guanylyl cyclase activation. Journal of Biological Chemistry. 2005;280:16594–16600. doi: 10.1074/jbc.M411905200. [DOI] [PubMed] [Google Scholar]
- 38.Fridovich I, Handler P. Xanthine oxidase. V. Differential inhibition of the reduction of various electron acceptors. J Biol Chem. 1962;237:916–921. [PubMed] [Google Scholar]
- 39.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med. 2012;55C:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lartigue-Mattei C, Chabard JL, Bargnoux H, Petit J, Berger JA, Ristori JM, Bussiere JL, Catilina P, Catilina MJ. Plasma and blood assay of xanthine and hypoxanthine by gas chromatography-mass spectrometry: physiological variations in humans. J.Chromatogr. 1990;529:93–101. doi: 10.1016/s0378-4347(00)83810-4. [DOI] [PubMed] [Google Scholar]
- 42.Pesonen EJ, Linder N, Raivio KO, Sarnesto A, Lapatto R, Hockerstedt K, Makisalo H, Andersson S. Circulating xanthine oxidase and neutrophil activation during human liver transplantation. Gastroenterology. 1998;114:1009–1015. doi: 10.1016/s0016-5085(98)70321-x. [DOI] [PubMed] [Google Scholar]
- 43.Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am.J.Respir.Crit Care Med. 1997;155:479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- 44.Himmel HM, Sadony V, Ravens U. Quantitation of hypoxanthine in plasma from patients with ischemic heart disease: adaption of a high-performance liquid chromatographic method. J.Chromatogr. 1991;568:105–115. doi: 10.1016/0378-4347(91)80344-c. [DOI] [PubMed] [Google Scholar]
- 45.Briley MS, Eisenthal R. Association of xanthine oxidase with the bovine milk-fat-globule membrane. Biochem J. 1974;143:149–157. doi: 10.1042/bj1430149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the Effects of Oxygen on Xanthine Oxidase-mediated Nitric Oxide Formation. Journal of Biological Chemistry. 2004;279:16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 47.Fleming I, Hecker M, Busse R. Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circ Res. 1994;74:1220–1226. doi: 10.1161/01.res.74.6.1220. [DOI] [PubMed] [Google Scholar]
- 48.Millar TM. Peroxynitrite formation from the simultaneous reduction of nitrite and oxygen by xanthine oxidase. FEBS Lett. 2004;562:129–133. doi: 10.1016/S0014-5793(04)00218-2. [DOI] [PubMed] [Google Scholar]
- 49.Kelley EE, Hundley NJ, Tarpey MM. Oxygen Dependence of XOR Nitrite Reductase Activity. Free Rad. Biol. Med. 2009;47:33. [Google Scholar]
- 50.Granell S, Gironella M, Bulbena O, Panes J, Mauri M, Sabater L, Aparisi L, Gelpi E, Closa D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit Care Med. 2003;31:525–530. doi: 10.1097/01.CCM.0000049948.64660.06. [DOI] [PubMed] [Google Scholar]
- 51.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. Journal of Biological Chemistry. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 52.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 53.Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988;254:G768–774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- 54.Fukushima T, Adachi T, Hirano K. The heparin-binding site of human xanthine oxidase. Biological & Pharmaceutical Bulletin. 1995;18:156–158. doi: 10.1248/bpb.18.156. [DOI] [PubMed] [Google Scholar]
- 55.Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochemical Journal. 1993;289:523–527. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM. Binding of Xanthine Oxidase to Glycosaminoglycans Limits Inhibition by Oxypurinol. Journal of Biological Chemistry. 2004;279:37231–37234. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]
- 57.Malik UZ, Hundley NJ, Romero G, Radi R, Freeman BA, Tarpey MM, Kelley EE. Febuxostat inhibition of endothelial-bound XO: Implications for targeting vascular ROS production. Free Radic Biol Med. 2011;51:179–184. doi: 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson JL, Rajagopalan KV, Cohen HJ. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J Biol Chem. 1974;249:859–866. [PubMed] [Google Scholar]
- 59.Hande K, Reed E, Chabner B. Allopurinol kinetics. Clin Pharmacol Ther. 1978;23:598–605. doi: 10.1002/cpt1978235598. [DOI] [PubMed] [Google Scholar]
- 60.Hille R, Nishino T, Bittner F. Molybdenum enzymes in higher organisms. Coord Chem Rev. 2011;255:1179–1205. doi: 10.1016/j.ccr.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Havemeyer A, Bittner F, Wollers S, Mendel R, Kunze T, Clement B. Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme. J Biol Chem. 2006;281:34796–34802. doi: 10.1074/jbc.M607697200. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Fischer K, Zhao X, Tejero J, Kelley EE, Wang L, Shiva S, Frizzell S, Zhang Y, Basu P, Schwarz G, Gladwin MT. Novel function of sulfite oxidase as a nitrite reductase that generates nitric oxide. Free Rad. Biol. Med. 2010;49:S122. [Google Scholar]
- 63.Kundu TK, Velayutham M, Zweier JL. Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry. 2012;51:2930–2939. doi: 10.1021/bi3000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Obach RS. Potent inhibition of human liver aldehyde oxidase by raloxifene. Drug Metab Dispos. 2004;32:89–97. doi: 10.1124/dmd.32.1.89. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. Journal of Biological Chemistry. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woo WH, Yang H, Wong KP, Halliwell B. Sulphite oxidase gene expression in human brain and in other human and rat tissues. Biochem Biophys Res Commun. 2003;305:619–623. doi: 10.1016/s0006-291x(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 68.Johnson-Winters K, Nordstrom AR, Emesh S, Astashkin AV, Rajapakshe A, Berry RE, Tollin G, Enemark JH. Effects of interdomain tether length and flexibility on the kinetics of intramolecular electron transfer in human sulfite oxidase. Biochemistry. 2010;49:1290–1296. doi: 10.1021/bi9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vitturi DA, Patel RP. Current perspectives and challenges in understanding the role of nitrite as an integral player in nitric oxide biology and therapy. Free Radic Biol Med. 2011;51:805–812. doi: 10.1016/j.freeradbiomed.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bueno M, Wang J, Mora AL, Gladwin MT. Nitrite Signaling in Pulmonary Hypertension: Mechanisms of Bioactivation, Signaling, and Therapeutics. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weitzberg E, Hezel M, Lundberg JO. Nitrate-nitrite-nitric oxide pathway: implications for anesthesiology and intensive care. Anesthesiology. 2010;113:1460–1475. doi: 10.1097/ALN.0b013e3181fcf3cc. [DOI] [PubMed] [Google Scholar]
- 72.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]