Abstract
The effect of spatial interference on place learning was examined in young and old rats. Rats were trained on a radial eight-arm maze to discriminate between a reward arm and a non-reward arm that either were adjacent to one another (high spatial interference) or separated by a distance of two arm positions (low spatial interference). Each rat was tested until reaching a criterion of nine correct choices out of 10 trials across two consecutive days. The data revealed that old rats committed significantly more errors than young rats when the arms were adjacent and spatial interference was high. However, no group differences were detected when the arms were separated and spatial interference was low. Group differences also were not detected in the number of trials required to reach the learning criterion in either condition. The results indicate that age-related brain changes result in increased errors during place learning, particularly when spatial interference is high, suggesting that spatial pattern separation may be less efficient in aged animals.
Keywords: Aging, Interference, Pattern Separation, Spatial Memory, Hippocampus
1. Introduction
The hippocampus supports several mnemonic processes critical for memory encoding and retrieval. Among these processes is spatial pattern separation, a mechanism suggested to reduce interference among similar memory representations and increase the probability that one place is encoded as separate from another similar place (Gilbert and Brushfield, 2009). The dentate gyrus (DG) and CA3 subregions of the hippocampus have been reported to support pattern separation in studies involving: a) computational models (Kesner, 2007a; Marr, 1971; Mcnaughton and Nadel, 1989; Myers and Scharfman, 2011; O’Reilly and McClelland, 1994; Rolls, 2010; Rolls and Kesner, 2006; Shapiro and Olton, 1994), b) electrophysiological methods in animals (Leutgeb et al., 2007; Leutgeb et al., 2005; Leutgeb et al., 2004; McNaughton et al., 1989; Tanila, 1989), c) neurotoxin induced and temporary lesions in animals (Butterly et al., 2012; Gilbert et al., 2001; Gilbert and Kesner, 2006; Goodrich-Hunsaker et al., 2008; Hunsaker and Kesner, 2008; Lee et al., 2005; McTighe et al., 2009; Morris et al., 2012), d) genetic manipulations in animals (Cravens et al., 2006; Kubik et al., 2007; McHugh et al., 2007), e) neuroimaging methods in humans (Bakker et al., 2008; Carr et al., 2010; Kirwan and Stark, 2007; Lacy et al., 2011; Yassa and Stark, 2011), and f) neuropsychological studies with hippocampal damaged humans (Duff et al., 2012; Kirwan et al., 2012).
Age-related gray and white matter changes have been documented in many regions of the brain (Allen et al., 2005; Driscoll et al., 2009; Kennedy and Raz, 2009; Peters and Rosene, 2003; Ziegler et al., 2008), including the hippocampus (Allen et al., 2005; Good et al., 2001; Driscoll and Sutherland, 2005; Raz et al., 2005; Walhovd et al., 2010). Additionally, fMRI signal intensity has been shown to decline in all subregions (DG, CA3, CA1) of the human hippocampus (Small et al., 2002). Activity in the perforant path input to DG also has been shown to decrease in aged rats (Geinisman et al., 1992) and older humans (Yassa et al., 2011). Similarly, perforant path connections to the DG in old rats are reported to be less excitable and require greater stimulation to achieve long-term potentiation compared to young rats (Burke and Barnes, 2006). In addition, a 40% reduction in DG neurogenesis was reported in middle-aged rats and a reduction of up to 80% in rats at advanced ages (Bizon and Gallagher, 2003). Taken together, the aforementioned evidence suggests that the DG may be especially susceptible to aging effects in humans (Small et al., 2002) and animals (Patrylo and Williamson, 2007; Small et al., 2004). These age-related changes in the hippocampus may affect mnemonic processes such as pattern separation.
Wilson and colleagues (Wilson et al., 2006) proposed that age-related changes in the hippocampus impair the ability to reduce similarity among new input patterns leading to decreased pattern separation efficiency. Pattern separation is hypothesized to become less efficient due to a strengthened processing of stored information at the expense of processing new information. Furthermore, age-related changes in the hippocampus strengthen the autoassociative network of the CA3 subregion that supports pattern completion, a mechanism that allows a complete representation of stored information to be retrieved using partial cues. These age-related changes may cause the CA3 subregion of the hippocampus to become entrenched in pattern completion at the expense of processing new information and pattern separation (Wilson et al., 2006). Yassa et al. (2011) reported similar findings in a study examining circuit-specific disruptions in the hippocampus in young and older adults using high-resolution fMRI and ultrahigh-resolution diffusion imaging. The results revealed that reduced pattern separation activity in the DG/CA3 regions of aged humans was linked to structural changes in the perforant pathway. These changes were suggested to strengthen the processing of stored information while weakening the processing of novel information (Yassa et al., 2011). Therefore, decreased efficiency in pattern separation may be a critical age-related processing deficit.
Given the well-documented role of the DG in the reduction of interference and its susceptibility to age-related changes, recent human studies have begun to investigate the effect of aging on pattern separation. Pattern separation has been shown to be less efficient in older adults on tasks involving spatial locations (Holden et al., 2012; Stark et al., 2010) visual objects (Toner et al., 2009; Yassa et al., 2011), and temporal order of items in a sequence (Tolentino et al., 2012). However, few studies have examined age-related changes in pattern separation in animal models of aging. A recently published study by Marrone et al. (2011) found that older animals recruited distinct populations of granule cells during exploration of the same environment on two different occasions, which the authors interpreted to be an indication of greater pattern separation. However, when the aged animals visited different environments, the age-related increase in pattern separation was no longer apparent. The study also found that increased pattern separation in the similar contexts was correlated with a reduced ability of older animals to disambiguate similar contexts during a sequential spatial recognition task. The authors conclude that spatial memory performance in aged animals is most impaired in situations where interference is increased, presumably due to decreased pattern separation. Kubo-Kawai and Kawai (2007) tested young and older monkeys on a delayed nonmatch-to-position task involving manipulations of the spatial distance between two locations. The data revealed that older monkeys were impaired relative to young monkeys across all spatial separations, with the largest group differences found on trials involving the closest separation. One could speculate that smaller separations were likely to create increased overlap among memory representations, which may result in heightened interference and a greater need for pattern separation. Although this study (Kubo-Kawai and Kawai, 2007) was not designed or interpreted by the authors to assess pattern separation, these findings in aged monkeys are in accordance with the findings from the studies involving older humans (Holden et al., 2012; Stark et al., 2010).
A recently published article by Holden and Gilbert (2012) suggests that less efficient pattern separation may contribute to age-related spatial memory decline. Given that few studies have been conducted to examine spatial pattern separation deficits, particularly in older animals, the present study used a place-learning task dependent on the DG subregion of the hippocampus (Morris et al., 2012) to assess age-related changes in pattern separation in a rodent model of aging.
2. Method
2.1 Subjects
Forty Fischer/Brown Norway (F344/BN) male rats (Harlan Laboratories) approximately 6 months (n = 20) and 24 months (n = 20) of age were used as subjects. Two 24-month-old rats died during testing (separate condition n=1; adjacent condition n=1) and three 6-month-old rats were unable to complete 10 daily trials (separate condition n=1; adjacent condition n=2). These animals completed less than 5 trials during the first two days of testing and were excluded from the statistical analyses. The F344/BN is a hybrid between a female Fisher 344 rat and a male Brown Norway rat. Subjects were housed individually in standard plastic containers located in a temperature-controlled room. Animals were food deprived and maintained at 80–85% of their free-feeding weight. The mean weight was 356.78 g (SE= 15.19, range = 328 – 377 g) for the 6-month-old rats and 560.95 g (SE= 8.67, range = 481 – 620 g) for the 24-month-old rats at the beginning of testing. As described below, each age group was evenly divided and randomly assigned to either an adjacent or separate condition. The colony room was kept on a 12hr:12hr light:dark cycle and all testing was conducted during the light phase. All experimental procedures were reviewed and approved by the Institutional Animal Use and Care Committee at San Diego State University.
2.2 Apparatus
A commercially produced radial eight-arm maze made of aluminum and clear Plexiglas was used as the test apparatus. The maze had a center arena that measured 34.9 cm in diameter surrounded by 34.9 cm high clear Plexiglas mechanical guillotine doors and openings to the arms. Each arm was 69.8 cm in length, 10.5 cm wide, and was bordered by clear Plexiglas walls that were 20.3 cm high. Food wells were located at the end of each arm. The maze was located in the center of a windowless, rectangular shaped room that contained a variety of visual/distal cues. The maze was positioned approximately 40 cm from three of the walls and a white curtain used so that the experimenter was not visible to the animal during testing. Two visual cues approximately 40–60 cm in height were position on each wall approximately 50 cm above the arms. The cues were positioned between two of the arms so that one cue could not be used to locate a particular arm.
2.3 Shaping
To minimize neophobia and maximize exploratory behavior, the experimentally naïve subjects were handled for 10 minutes every day for 14 days prior to testing. Following handling, each rat was allowed to individually explore the test apparatus. During the exploration period, each rat was placed in the center arena with all guillotine doors open and allowed to explore for 5 minutes. For the next two days, Froot Loop cereal (Kellogg’s) was distributed across the surface of the apparatus, including each individual food well, and the animal was allowed to explore each arm of the apparatus to retrieve the food rewards. The food rewards were placed on the maze prior to each exploration period and were not replaced once consumed.
Once each rat was habituated to the apparatus, the rats were randomly assigned to the adjacent condition or separate condition and tested on a paradigm developed by McDonald and White (1995) and subsequently used by Morris et al. (2012).
2.4 Place learning task
For the adjacent condition, one of the eight arms of the radial maze was randomly assigned as the reward arm. The arms positioned immediately to the left and right of the reward arms were the non-reward arms (Figure 1). The separate condition was conducted using an identical procedure and criterion as described below for the adjacent condition except that the reward arm was separated from the two possible non-reward arms by a distance of two arm positions (Figure 1). Prior to the beginning of each testing session, the experimenter raised the door for the reward arm and one of the two non-reward arms. The rat was placed on the center arena facing a randomly chosen closed guillotine door. On each trial, the rat was allowed to choose between the reward arm and one non-reward arm. If the rat entered the reward arm, it was allowed to consume the Froot Loop food reward located in the food well at the end of the arm. However, if the rat entered the non-reward arm (defined as the forelimbs crossing the threshold of the door), then the rat would not receive a food reward and would not be allowed to enter the reward arm. Each of the two non-reward arms was utilized on five of the 10 daily trials in a pseudo-randomly determined order. The same rewarded arm and one of the same two non-reward arms were used on each trial for a particular rat; however, the rewarded arm varied across animals. Each rat received 10 trials per day with a 60 second inter-trial interval used to clean and re-bait the apparatus, during which time the animal was removed and placed in the home cage. Testing was conducted daily and each animal was tested until the animal reached a criterion of nine correct choices out of 10 consecutive trials spread across two consecutive days of testing, or until the animal was tested for 20 consecutive days without reaching the learning criterion.
Figure 1.
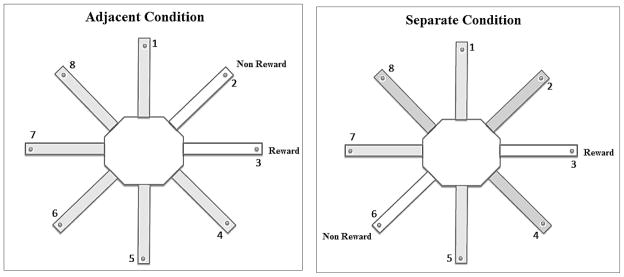
Schematic of a radial eight-arm maze illustrating an example of a reward arm location and one of the two respective non-reward arms in the adjacent condition (left) and the separate condition (right).
A three-location configuration consisting of one reward arm and two different non-reward arms was utilized to ensure that the rats do not adopt an egocentric response strategy that could provide an accurate solution to the task. If a two-location configuration was used, one arm would be designated as the reward arm and another arm always would be designated as the non-reward arm. For example, the reward arm always would be to the right of the non-reward arm. Therefore, regardless of where the rat was placed on the center arena, the animal could reach the reward location by consistently choosing the arm on the right and would not need to attend to extramaze cues to locate the reward arm. This solution was not possible with the current three-location configuration because the non-reward arm was on the left of the reward arm on half of the trials and on the right for the remaining half of the trials. Thus, the rats were unable to adopt an egocentric response strategy and instead were required to distinguish the reward arm from the non-reward arm only on the basis of their locations with respect to the distal cues. On every trial, the maze was rotated randomly to prevent the use of intramaze cues such as odorants, but the location of the external cues remained consistent respective to the rewarded arm. Therefore, the arm that was used as the rewarded arm on Trial 1, would be rotated into a different position for Trial 2 and so forth for all 10 daily trials. As a result, no one arm would smell any more or less like Froot Loops than any other arm across trials, thus minimizing odor cues from the reward as a useful intramaze cue to solve the task.
3. Results
3.1 Errors committed prior to reaching criterion
One-way analysis of variance (ANOVA) tests were used to analyze group differences (6-month-old, 24-month-old) in the number of errors committed prior to reaching the criterion in the adjacent condition and the separate condition. A Bonferroni correction was used to control for multiple comparisons (p-value set at p < .025) and the effect size for significant group differences was calculated using Cohen’s d. As shown in Figure 2, the analysis revealed that 24-month-old rats committed more errors than 6-month-old rats on the adjacent condition F(1, 15) = 10.25, p = .006, d = 1.53. However, no significant group differences were detected on the separate condition F(1, 16) = .98, p = .34.
Figure 2.
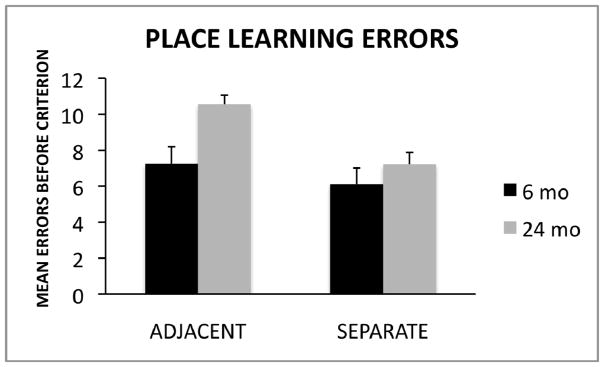
Mean errors committed (± SE) before reaching the learning criterion of nine correct choices out of 10 consecutive trials spread across two consecutive days of testing, for the adjacent and separate conditions for young and aged rats.
To assess whether the adjacent and separate conditions differed in task difficulty, a one-way ANOVA was used to compare the number of errors committed by young rats on the two conditions. The analysis revealed no significant difference between young rats tested on the adjacent (Mn=7.25, SE=.94) and separate (Mn=6.11, SE=.90) conditions F(1,16) = .76, p = .40. The finding that young rats did not differ significantly between the two conditions provides evidence that age-related deficits on the adjacent condition were due to a greater demand for pattern separation rather than a difference in task difficulty compared to the separate condition.
3.2 Trials to criterion
One-way ANOVA tests were used to analyze group differences (6-month-old, 24-month-old) in number of trials required to reach the learning criterion in the adjacent condition and the separate condition. As shown in Figure 3, the analyses revealed no statistically significant differences between 6- and 24-month-old rats in the adjacent condition F(1, 15) = 2.36, p = .14 or the separate condition F(1, 16) = .00, p = 1.00.
Figure 3.
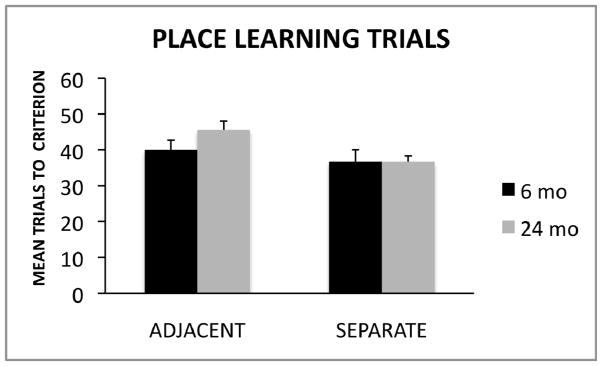
Mean trials (± SE) to reach the learning criterion of nine correct choices out of 10 consecutive trials spread across two consecutive days of testing, for the adjacent and separate conditions for young and aged rats.
3. Discussion
The goal of our study was to examine the effect of spatial interference on place learning for adjacent and separated locations in young and old rats. It was hypothesized that discriminations between adjacent locations involved more spatial interference among distal cues and a greater need for spatial pattern separation than separated locations (also see Morris et al. 2012). As shown in Figure 2, an examination of the total errors committed before reaching the learning criterion revealed that older rats made significantly more errors than young rats in the adjacent condition where spatial interference was high. The effect size for group differences on the adjacent condition was high (d = 1.53). However, no group differences were detected in the separate condition where spatial interference was low. These findings support the hypothesis that age-related differences in performance were larger on the adjacent condition (presumably due to increased spatial interference and increased demand for pattern separation) compared to the separate condition (involving decreased spatial interference and less pattern separation demand). However, as shown in Figure 3, the number of trials required to reach the learning criteria was comparable between young and old rats in both conditions. Taken together, the data suggest that although aged rats may be able to acquire spatial discriminations, age-related changes in the brain may result in less efficient pattern separation resulting in increased likelihood of spatial memory errors, particularly in situations where spatial interference is high.
As reviewed previously, age-related changes have been documented in various regions of the brain including the hippocampus. Studies have shown that aging is likely to result in functional changes within the DG (Small et al., 2002; Patrylo and Williamson, 2007; Small et al., 2004) and alterations in perforant path input to the region (Geinisman et al., 1992; Yassa et al., 2001; Burke and Barnes, 2006). Using the place-learning task employed in the present study, Morris et al. (2012) concluded that the DG is critical for place learning in the adjacent condition when spatial interference is high, presumably due to critical role the DG plays in supporting spatial pattern separation. Given that the present task has been shown to be dependent on the DG subregion and the DG may be particularly susceptible to the effects of aging, the increased errors committed by old rats in the present study may be due to age-related changes in the DG. However, age-related changes in other brain regions also may have been involved. For example, McDonald and White (1995) found that rats with fornix-fimbria lesions required significantly more trials to reach the learning criterion in the adjacent condition but not the separate condition compared to controls. Therefore, future studies are needed to further examine the neural changes associated with aging that may underlie deficits on the present task.
Although only a few behavioral studies have examined the effects of aging on spatial pattern separation, the results point to an age-related decrease in pattern separation efficiency in both older humans (Holden et al., 2012; Stark et al., 2010) and animals (Marrone et al., 2011). Taken together with the present findings, the aforementioned studies suggest that less efficient spatial pattern separation may be critical processing deficit in older humans and animals that may contribute to age-related spatial memory decline (Holden and Gilbert, 2012). These data also provide support for the hypothesis that age-related changes adversely affect pattern separation by weaken the processing of novel information while strengthening the processing of stored information (Wilson et al., 2006; Yassa et al., 2011).
Age-related decline in pattern separation may be due in part to changes in hippocampal neurogenesis. Penner et al. (2011) suggested that age-related memory impairment may be due to subregion-specific epigenetic and transcriptional changes in the hippocampus. Past studies also have reported reduced neurogenesis in the aged hippocampus (Kuhn et al., 1996) that is directly related to decrease hippocampal volume and impaired performance on hippocampal dependent tasks (Driscoll et al., 2006) and may contribute to impaired pattern separation (Aimone et al., 2011; Creer et al., 2010). In particular, Clelland and colleagues (2009) tested mice with ablated hippocampal neurogenesis on a task similar to the one used in the present study. The authors reported that mice with ablated neurogenesis were impaired when the spatial separation between arms was small but not when the separation between arms was increased (Clelland et al., 2009). New evidence suggests that older DG cells may contribute to pattern completion, while newborn neurons may be involved in pattern separation (Nakashiba et al., 2012), as they are particularly involved in mnemonic processes dependent on the DG (Sahay et al., 2011). Thus, interventions that increase neurogenesis during adulthood may have clinical implications for reversing age-related impairments in pattern separation and associated DG dysfunction (Sahay et al., 2011). The development of such interventions may be particularly important given recent evidence in animals suggesting that pattern separation deficits may begin in middle age (Huxter et al., 2012). Creer et al. (2010) reported that voluntary running improved the ability of adult mice to discriminate between two spatially adjacent locations, suggesting an improvement in spatial pattern separation. In addition, the improved spatial pattern separation in animals engaging in voluntary running was correlated with increase neurogenesis. Therefore, exercise may be a potential intervention to combat pattern separation deficits and decreased neurogenesis in adulthood. Unfortunately, voluntary running did not have similar effects on pattern separation or neurogenesis in very old mice (Creer et al., 2010). Given the aforementioned studies, the development of behavioral tasks sensitive to age-related changes in spatial pattern separation may have implications for future studies of neurogenesis.
A number of previous behavioral studies have used 24-month-old Fisher 344 rats (F344) to examine age-related changes in cognition. The present study was conducted using the F344/BN hybrid strain. This strain has been shown to live longer than F344 rats and is recommended by the National Institute on Aging for use in aging research. The 50% survival age for F344/BN male rats is 34 months; however, the 50% survival age for F344 male rats is 24 months (National Institute on Aging). Therefore, the rats in the present study showed significant memory impairments at 24 months of age despite having longer average longevity than other strains of rats used in prior behavioral experiments. These findings are consistent with prior studies reporting significant age-related deficits in F344/BN rats 24–26 months of age on a variety of cognitive tasks, as briefly reviewed by Maasberg et al. (2011). Given the increased longevity in this strain, it could be debatable whether 24-month-old rats are considered “middle-aged” or “old”. As reviewed previously, pattern separation has been suggested to become less efficient in old age. However, recent evidence suggests that pattern separation deficits may begin in middle age (Huxter et al., 2012). Therefore, future longitudinal studies are needed to examine how pattern separation efficiency changes between middle-age and old-age.
In conclusion, our findings add to a relatively small but growing literature reporting decreased efficiency in pattern separation in older humans and animals (Yassa and Stark, 2011; Yassa et al., 2011; Yassa et al., 2011; Holden et al., 2012; Stark et al., 2010; Toner et al., 2009; Marrone and Adams, 2011; Yassa et al., 2010). The present findings also provide further evidence for behavioral age-related learning and memory impairments in the F344/BN hybrid strain. A potential strength of the present study lies in the finding that old rats are impaired on a well-characterized spatial pattern separation task shown to be dependent on the DG subregion of the hippocampus (Morris et al., 2012). Given the wealth of evidence indicating that the DG may play a critical role in pattern separation [for reviews see (Gilbert and Brushfield, 2009; Yassa and Stark, 2011; Schmidt et al., 2012; Kesner, 2007b)], age-related changes in the DG may adversely affect pattern separation. This decreased efficiency in spatial pattern separation has been hypothesized to contribute to spatial memory decline and episodic memory impairment associated with aging (Holden and Gilbert, 2012). Behavioral tasks that measure specific mnemonic processes, such as pattern separation, may be highly sensitive to subtle age-related changes. The observed pattern of age-related change in aged F344/BN rats is reported to closely resemble the changes found in nondemented older humans (Driscoll et al., 2006). Therefore, the present study provides further evidence that this strain of rats, may serve as a useful animal model to study the effects of aging on cognitive function. The present findings may have important implications for designing and testing behavioral interventions to improve pattern separation in older animals to reduce interference, thus improving memory function.
Acknowledgments
The research was supported by NIH grant #AG026505 from NIA to Paul E. Gilbert. The authors thank Savanna Tierney for her assistance with formatting the manuscript.
Footnotes
Disclosure Statement: Other than the NIH grant support to Paul E. Gilbert listed above, there are no conflicts of interest to disclose. All experimental procedures were reviewed and approved by the Institutional Animal Use and Care Committee at San Diego State University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: Relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Butterly DA, Petroccione MA, Smith DM. Hippocampal context processing is critical for interference free recall of odor memories in rats. Hippocampus. 2012;22:906–13. doi: 10.1002/hipo.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65:298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K. CA3 NMCA receptors are crucial for rapid and automatic representation of context memory. Euro J Neurosci. 2006;24:1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–72. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: A multi-level analysis in the rat. Neurosci. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Sutherland RJ. The aging hippocampus: Navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- Duff MC, Warren DE, Gupta R, Vidal JP, Tranel D, Cohen NJ. Teasing apart tangrams: testing hippocampal pattern separation with a collaborative referencing paradigm. Hippocampus. 2012;22:1087–91. doi: 10.1002/hipo.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Prog Neuropsychopharmacol Biol. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Kesner R. The role of the dorsal ca3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker M, Kesner R. The interactions and dissociations of the dorsal hippocampus subregions: How the dentate gyrus, ca3, and ca1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Front Aging Neurosci. 2012;4:9. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012 doi: 10.1002/hipo.22017. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18:955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxter JR, Miranda JA, Dias R. The hippocampal physiology of approaching middle-age: Early indicators of change. Hippocampus. 2012 doi: 10.1002/hipo.22027. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007a;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007b;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Hartshorn A, Stark SM, Goodrich-Hunsaker NJ, Hopkins RO, Stark CE. Pattern separation deficits following damage to the hippocampus. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.06.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- Kubo-Kawai N, Kawai N. Interference effects by spatial proximity and age-related declines in spatial memory by Japanese monkeys (Macaca fuscata): Deficits in the combined use of multiple spatial cues. J Comp Psychol. 2007;121:189–197. doi: 10.1037/0735-7036.121.2.189. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Jerman TS, Kesner RP. 2005 Disruption of delayed memory for a sequence of spatial locations following CA1- or CA3-lesions of the dorsal hippocampus. Neurobiol Learn Mem. 2005;84:138–147. doi: 10.1016/j.nlm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Maasberg DW, Shelley LE, Gracian EI, Gilbert PE. Age-related differences in the anticipation of future rewards. Beh Brain Res. 2011;223:371–375. doi: 10.1016/j.bbr.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Proc R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Adams AA, Satvat E. Increased pattern separation in the aged fascia dentata. Neurobiol Aging. 2011;32:2317.e23–32. doi: 10.1016/j.neurobiolaging.2010.03.021. [DOI] [PubMed] [Google Scholar]
- McDonald R, White N. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Nadel L. Hebb-Marr networks and the neurobiological representation of action in space. In: Gluck MA, Rumelhart DE, editors. Neuroscience and Connectionist Theory. New Jersey: 1989. pp. 1–63. [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. 2009 A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Morris A, Churchwell J, Kesner R, Gilbert P. Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations. Neurobiol Learn Mem. 2012;97:326–331. doi: 10.1016/j.nlm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 back projection. Hippocampus. 2011;21:1190–215. doi: 10.1002/hipo.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Patrylo P, Williamson A. The effects of aging on dentate circuitry and function. Prog Brain Res. 2007;163:679–696. doi: 10.1016/S0079-6123(07)63037-4. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF, Barnes CA. Age related changes in arc transcription and DNA methylation within the hippocampus. Neurobiol Aging. 2011;32:2198–2210. doi: 10.1016/j.neurobiolaging.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Rosene DL. In aging, is it gray or white? J Comp Neurol. 2003;462:139–143. doi: 10.1002/cne.10715. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rolls E. A computational theory of episodic memory formation in the hippocampus. Behav Brain Res. 2010;215:180–196. doi: 10.1016/j.bbr.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Rolls E, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson D, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res. 2012;226:56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Olton DS. Hippocampal function and interference. In: Schacter DL, Tulving E, editors. Memory systems. Massachusetts: MIT Press; 1994. pp. 141–146. [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Stark S, Yassa M, Stark C. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H. Hippocampal place cells can develop distinct representations of two visually identical environments. Hippocampus. 1999;9:235–246. doi: 10.1002/(SICI)1098-1063(1999)9:3<235::AID-HIPO4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tolentino JC, Pirogovsky E, Luu T, Toner CK, Gilbert PE. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn Mem. 2012;19:251–5. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–34. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS, Salmon DP, Fennema-Notestine C Alzheimer’s Disease Neuorimaging Initiative. Multi-modal imaging predicts memory performances in normal aging and cognitive decline. Neurobiol Aging. 2010;31:1107–1112. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: Prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M, Lacy J, Stark S, Albert M, Gallagher M, Stark C. Pattern separation deficits associated with increased hippocampal ca3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M, Mattfeld AT, Stark SM, Stark C. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M, Stark C. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2008;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


