Abstract
Objective
Stressful life events (SLE) have been associated with increased dementia risk, but their association with cognitive decline has been inconsistent. In a longitudinal population-based study of elderly individuals, we examined the association between SLE and cognitive decline, and the role of potential effect modifiers.
Methods
A total of 2665 non-demented participants of the Cache County Memory Study completed a stress life events questionnaire at Wave 2, and were revisited 4 and 7 years later. The events were represented via several scores: total number, subjective rating (negative, positive, unexpected), and a weighted summary based on their impact. Cognition was assessed at each visit with the modified Mini-Mental State Exam. General linear models were used to examine the association between SLE scores and cognition. Effect modification by age, education, and APOE genotype was tested.
Results
Years of formal education (p = 0.006) modified the effect of number of SLE, and age (p = 0.009) modified the effect of negative SLE on the rate of cognitive decline. Faster decline was observed among those with fewer years of education experiencing more SLE and also among younger participants experiencing more negative SLE. There was no association between other indicators of SLE and cognitive decline. APOE genotype did not modify any of the above associations.
Conclusions
The effects of SLE on cognition in late life are complex and vary by individual factors such as age and education. These results may explain some of the contradictory findings in the literature.
Keywords: cognition, cognitive decline, memory, stress, life events, stressful life events
INTRODUCTION
An individual’s attempt to adapt to the inevitable and varied challenges posed by life necessarily involves some degree of stress. While not all stress is negative, perhaps not surprisingly, numerous studies have implicated greater amounts of stress as a risk factor for poorer physical and mental health (Brosschot et al., 2006; Hammen, 2005; McEwen, 1998; Monroe et al., 2007). Recent work has also suggested that the negative effects of stressful life events (SLE) extend to dementia and cognitive decline. For example, several studies report that the death of a parent in childhood increases the risk of dementia later in life (Norton et al., 2009; Norton et al., 2010 epub; Persson and Skoog, 1996) as do having a greater number of residents in the household (Moceri et al., 2000; Moceri et al., 2001) and having either a spouse or an adult child fall ill (Persson and Skoog, 1996). Moderation by the apolipoprotein E (APOE) gene has been reported for some childhood events such as number of residents in household and other childhood socioeconomic indicators (Borenstein et al., 2005; Moceri et al., 2001). Although the precise mechanisms underlying the association between life events and dementia are unknown, they may reflect the effects of chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis, which has been associated with atrophy in brain structures important in learning and memory (Sapolsky et al., 1986).
The association between SLE and cognitive decline in late life is less clear. In a case-control study of female patients with documented mental deterioration, a greater number and magnitude of SLE had been experienced by cases than controls in the preceding five years (Amster & Krauss, 1974). However, subsequent studies report no effect for number of SLE, but rather, an effect for certain types of events or their impact. A cross-sectional study of older adult females found no association between stress scores derived by expert weightings of events and cognitive decline, but significant associations with subjective ratings of stress. In that study, cognitive decline was inferred from a ratio of intelligence tests assessing fluid versus crystallized abilities. Change in personal health or that of a family member was associated with worse fluid-to-crystallized intelligence ratios (Sands, 1981). A study of elders assessed six years apart found no effect for number of life events, but rather an effect for death of a spouse or child (Grimby and Berg, 1995).
Recent studies suggest that SLE may be associated with better cognitive functioning (Comijs et al. 2011; Rosnick et al., 2007); and certain factors may moderate the association between SLE and cognition. One study found faster cognitive decline with higher stress ratings only among persons with Mild Cognitive Impairment (Peavy et al., 2009). However, in a population-based Dutch study, persons scoring below 25 on the Mini-Mental State Exam at baseline improved on episodic memory tests according to the number of SLE experienced over a three-year interval. In this sample, APOE e4 carriers experiencing negative life events performed worse than non-carriers (Comijs et al. 2011). Another study confirmed the association between stressors and cognitive decline, but also reported greater decline with a decrease in social support (Dickinson et al. 2011).
Many of the above studies have limitations of small samples, limited measures of SLE, cross-sectional designs or limited longitudinal follow-up. Additionally, individual differences in factors known to influence cognition in late life such as age (Christensen, 2001), APOE genotype (Bretsky et al., 2003), and education (Lyketsos et al., 1999) have rarely been studied in conjunction with the effects of SLE and cognition. In this study, we examined whether SLE experienced in late life affect cognitive decline in a population-based sample of non-demented elderly persons. We studied the number of SLE, the degree of stress represented by a weighted summary score, and the subjective ratings of their impact over a follow-up period spanning up to seven years. We further examined whether age, educational attainment, and APOE genotype modified the association between SLE and cognitive decline. We hypothesized that even greater decline would be observed with SLE among older, less educated participants, who were carriers of the APOE e4 allele.
METHODS
Participants and Dementia Screening
The subjects were participants of the Cache County Study on Memory in Aging (CCSMA) who were assessed to be dementia-free and who completed a mail-in life events questionnaire in each of three study waves (Waves 2 through 4) over a period of 7 years. Data from Wave 1 of the CCSMA were excluded since the life events questionnaire was obtained in Waves 2 through 4, thus the “baseline” measures for cognition and stressful life events were collected in Wave 2. To determine dementia status, participants completed a multi-stage protocol described elsewhere (Breitner et al., 1999); (Miech et al., 2002). Briefly, all participants were screened with the Modified Mini-Mental State Exam-Revised [3MS;(Teng and Chui, 1987)], further adapted for this study (Tschanz et al., 2002) or the Informant Questionnaire on Cognitive Decline in the Elderly (Jorm and Jacomb, 1989). Those who screened positive as well as members of a randomly selected, “designated” subsample were asked to complete an informant-based clinical interview about signs and symptoms of dementia. The designated subsample was selected regardless of screening performance and further supplemented with persons above age 89 in Wave 2 and above age 84 in Waves 3 and 4. Those whose interviews were rated with moderate-to-severe cognitive impairment or members of the designated subsample were asked to complete a clinical assessment (CA) consisting of a brief physical and neurological exam, neuropsychological testing, and an informant interview of the subject’s cognitive, functional, and medical history. Neuropsychological tests were interpreted by a study neuropsychologist and together with the clinical data collected at the CA, a geropsychiatrist, neuropsychologist and members of the CA team assigned preliminary diagnoses of dementia for participants who met DSM-IIIR criteria for dementia (American Psychiatric Association, 1987). For those failing to meet DSM-IIIR criteria, the panel assigned diagnoses of non-case for persons without cognitive impairment or noted the presence of a mild cognitive disorder (such as prodromal Alzheimer’s disease) for those with evidence of impairment on neuropsychological testing or activities of daily living. Those with suspected dementia or its prodrome were further assessed with a geropsychiatrist examination, MRI, and standard laboratory tests for dementia. A panel of experts in neurology, geropsychiatry, and neuropsychology reviewed all available clinical data and assigned diagnoses of dementia using DSM-III-R criteria (Association, 1987). All others whose clinical assessment results indicated non-case or mild cognitive disorder and those who screened negative at each stage were considered to be without dementia. Analyses of performance of the screening procedures used in the CCSMA indicate sensitivity for dementia at roughly 85%, indicative of a false negative rate of 15% (Hayden et al., 2003; Khachaturian et al., 2000), however the actual false negative rate is likely lower since screen negative subjects in the designated subsample and those above age 84 were also sent to the CA.
At each subsequent wave, those determined to be without dementia at the preceding wave were recontacted. The procedures were similar to the protocol described above, except for the modification of the 3MS screening cut point (to increase sensitivity for detecting cognitive impairment), and the elimination of the informant interviews in Waves 3 and 4. Study procedures were approved by the Institutional Review Boards of Utah State, Duke, and the Johns Hopkins Universities. For the present analyses, participants had to be non-demented at each wave, have completed the 3MS and the life events questionnaire at Wave 2 (baseline), and also have data on each of the covariates described below.
Cognitive Decline Outcome Measurement
Performance on the Modified Mini-Mental State Examination-revised (Teng & Chui, 1987; Tschanz et al., 2002), served as the primary outcome for cognitive decline. The measure is a 100-point adaptation of the Mini-Mental State Exam (Folstein et al., 1983) and assesses attention, concentration, orientation to time and place, memory, constructional praxis, and expressive and receptive language. A study neuropsychologist trained interviewers in the standardized administration and scoring of the test. Periodic reviews of audio recordings of interviews were conducted to ensure adherence to standardized administration, and scoring was subject to quality assurance review under the supervision of a study neuropsychologist.
Predictors: Assessment of Stressful Life Events
The assessment of SLE was conducted through a mail-in, life events questionnaire left with study participants at the screening visit in Waves 2 and 4, and at the CA visit in Wave 3. The questionnaire consisted of eight items that queried whether the participant had experienced any of the following events in the past three years: death of a spouse, death of a relative or close other, illness of a relative or friend, illness of self, and change in marital, occupational, economic status, or residence. Following an endorsement of an event, participants provided a rating of its valence (positive, negative or neutral), importance (very important, somewhat important or unimportant), and whether the event was anticipated (expected, somewhat expected or unexpected). For analyses, we combined ratings of “important” with “somewhat important” as well as “expected” with “somewhat expected.”
To examine the effects of SLE on cognition, we totaled the number of events across subjective ratings. Because of the significant positive skew of the distribution (64% reported two or fewer SLE at each visit), we summarized participants in categories based on the number as 0 (reference), 1, or 2+.
To examine the differential effects of the severity or impact of stressful events, two approaches were used. In the first approach, we applied weights for events derived from the Geriatric Social Readjustment Rating Scale (Amster & Krauss, 1974). The GSRRS applies different weights to events more likely to occur in late life (Amster & Krauss, 1974). For example, compared to the Holmes-Rahe Social Readjustment Rating Scale (Holmes & Rahe, 1967), the GSRRS applies higher weights for placement in a nursing home vs. moving to a new residence. Fifteen of the 35 events from the GSRRS were listed in our questionnaire, the majority being those with the highest weights on the GSRRS. After applying the specific weight to each event, items were summed to yield a total life event stress score. Multiple events of a given type each contributed to composite scores. For example, if a subject reported two relatives had died, this would contribute 2 points to the total number of SLE, as well as the GSRRS weighting for relative death, multiplied by 2, to the weighted life event stress score. The possible range of scores varied depending on the number of items endorsed, but assuming a single occurrence for each event, the maximum possible would be 559. To examine the effects of significant stressors, we identified persons scoring in the highest 20th percentile (score ≥ 125) and compared change in the 3MS to those with a score of 0.
In a second approach to examine the impact of SLE on cognition, we tallied the number of events described by participants as negative, important, or unexpected. Number of events in each category was summarized as 0 (reference), 1, or 2+. Additionally, to examine the effects of a category tied theoretically to the greatest degree of stress, we categorized participants whose stressors were rated as unexpected, negative, and important. For analysis, we represented the groups as having experienced 0 (reference), 1, or 2+ of these highly stressful events.
Covariates and Possible Effect Modifiers
Demographic covariates and available genotypes tested in statistical models included age, gender, years of formal education (continuous variable representing number of years completed) and APOE genotype (presence or absence of one or more e4 alleles). We tested possible effect modification of the primary predictor variables on cognition and cognitive decline by age, years of education and APOE genotype. Additionally, we controlled for the effects of occupation as another indicator of cognitive reserve. Occupations were grouped into the following categories: never employed (reference), machine trades, agricultural, service, clerical and sales, and professional, technical or managerial occupations.
Statistical Analyses
We provided descriptive statistics and compared those who did vs. did not return a life events questionnaire. For continuous variables, we used analysis of variance and for categorical variables, chi square tests. To examine the effects of stressful events on cognition and rate of cognitive decline, we used general linear models that accommodate repeated measures, incorporating a quadratic (non-linear) term for time. This approach allowed us to model the effects of fixed factors (e.g., number of stressful events) on cognition (3MS score), while accounting for the within-subject correlation across time points. Each SLE variable (number, weighted stress score, and subjective rating of stressors) was treated as a time varying variable. All statistical analyses were conducted using SPSS Version 18 software.
RESULTS
There were 3411 participants who completed Wave 2 screening (baseline for the present analyses), 2324 Wave 3 screening, and 1481 Wave 4 screening. Those who were free of dementia at each wave were 3214 (94%), 2102 (90%) and 1371 (93%), respectively at Waves 2, 3, and 4. Of these, 2665 (83%), 901 (43%) and 1261 (92%) completed and returned the life events questionnaire. The significant reduction in numbers who completed the questionnaire at Wave 3 was due to the smaller subsample approached for a CA, and consisted of 73% of those who completed a CA. Table 1 displays descriptive statistics for those who did and did not return the life events questionnaire according to age, gender, APOE genotype, education, and wave 3MS score. Non-responders were significantly older (with the exception of Wave 3), completed fewer years of education, and scored worse on the 3MS than those who returned the questionnaire (exception, Wave 3; See Table 1). Although we cannot be certain that those missing SLE data at Wave 3 were not biased with respect to number of SLE experienced, a comparison in SLE reports in Waves 2 and 4 by SLE Wave 3 status (missing or non-missing) revealed no significant difference (p > 0.24).
Table 1.
Descriptive statistics of sample
| Wave 2 | Wave 3† | Wave 4 | ||||
|---|---|---|---|---|---|---|
| SLE Q | Missing | SLE Q | Missing | SLE Q | Missing | |
| Males, N (%)†† | 1118 (83%) | 223 (17%) | 418 (49%) | 438 (51%)*** | 535 (94%) | 32 (6%) |
| Females, N (%) | 1545 (83%) | 309 (17%) | 483 (40%) | 714 (60%) | 725 (92%) | 64 (8%) |
| Age, M (SD) | 76.6 (6.0) | 79.1 (7.4)*** | 81.2 (5.2) | 78.8 (5.2)*** | 81.7 (4.5) | 83.8 (5.6)*** |
| Education, M (SD) | 13.5 (2.8) | 12.7 (2.9)*** | 13.8 (3.0) | 13.0 (2.9)*** | 13.9 (2.8) | 12.9 (2.2)*** |
| APOE E4+, N (%)†† | 804 (84%) | 156 (16%) | 294 (47%) | 332 (53%) | 368 (92%) | 30 (8%) |
| E4−, N (%) | 1841 (83%) | 371 (17%) | 604 (43%) | 812 (57%) | 889 (93%) | 66 (7%) |
| 3MS score, M (SD) | 93.0 (6.3) | 88.0 (9.8)*** | 91.0 (6.3) | 91.4 (9.4) | 92.7 (6.3) | 87.6 (8.5)*** |
| # SLE, M (SD) | 1.39 (1.29) | 2.15 (1.48) | 1.9 (1.39) | |||
| # SLE, Mdn | 1.0 | 2.0 | 2.0 | |||
SLE = Stressful life events.
p<0.05;
p<0.01;
p<0.005;
Consists of the subsample of participants who completed a clinical assessment at Wave 3.
Presented are row percentages, so for example, of the males at Visit 1, 83% completed a SLE questionnaire, and 17% did not.
Most participants at each wave reported at least one life event inventoried, with the most common being death of a loved one, followed by illness of a family member or friend. Least common was change in occupational status (See Table 2). Of those experiencing at least one event, the majority of participants rated the events as important (90 – 99%), and unexpected (59 – 68%). The percentage rated negative ranged from 38 to 49% (see Table 3). Only 17–30% reported experiencing events rated as negative, unexpected and important. Due to the limited variability in the ratings of the importance of events, we did not include this variable in modeling change in 3MS. Years of education was not associated with number of reported SLE at each wave (correlations = −0.01 to −0.04, p > 0.12). The mean (SD) of number of stressors reported at for those with < 12 years of education, 12 years of education and > 12 years was 1.47 (1.32), 1.42 (1.35) and 1.39 (1.26) at Wave 2, 2.06 (1.58), 2.12 (1.59) and 2.23 (1.45) at Wave 3, and 1.91 (1.52), 1.88 (1.43) and 1.91 (1.34) at Wave 4.
Table 2.
Stressful life events reported by participants
| Wave 2 | Wave 3† | Wave 4 | ||||
|---|---|---|---|---|---|---|
| Yes, N (%) | No, N (%) | Yes, N (%) | No, N (%) | Yes, N (%) | No, N (%) | |
| Occupational status | 212 (9%) | 2293 (91%) | 88 (10%) | 772 (90%) | 79 (6%) | 1134 (94%) |
| Residence | 107 (4%) | 2478 (96%) | 89 (11%) | 703 (89%) | 124 (10%) | 1080 (90%) |
| Finances | 438 (17%) | 2138 (83%) | 213 (24%) | 661 (76%) | 250 (20%) | 983 (80%) |
| Marital status | 196 (8%) | 2379 (92%) | 95 (11%) | 730 (89%) | 97 (8%) | 1116 (92%) |
| Major illness | 559 (22%) | 1983 (78%) | 310 (34%) | 559 (64%) | 342 (28%) | 863 (72%) |
| Family/friend illness | 836 (38%) | 1369 (62%) | 532 (61%) | 333 (39%) | 687 (58%) | 506 (42%) |
| Death of loved one (not spouse) | 1207 (47%) | 1387 (53%) | 567 (66%) | 299 (34%) | 731 (59%) | 500 (41%) |
| Death of Spouse | 154 (7%) | 2071 (93%) | 78 (9%) | 823 (91%) | 80 (6%) | 1180 (94%) |
Consists of the subsample of participants who completed a clinical assessment at Wave 3.
Table 3.
Summary of stressful life events by Wave
| Total SLE N (%) |
Negative SLE N (%) |
Unexpected SLE N (%) |
Important SLE N (%) |
Important, Negative, Unexpected SLE N (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2+ | 0 | 1 | 2+ | 0 | 1 | 2+ | 0 | 1 | 2+ | 0 | 1+ | 2+ | |
| Wave 1 | 756 (28) |
851 (32) |
1056 (40) |
1258 (63) |
418 (21) |
321 (16) |
883 (44) |
772 (38) |
362 (18) |
195 (10) |
834 (43) |
896 (47) |
2206 (84) |
306 (12) |
122 (5) |
| Wave 2† | 128 (14) |
179 (20) |
591 (66) |
200 (35) |
166 (29) |
207 (36) |
115 (18) |
279 (44) |
245 (38) |
8 (1) |
201 (26) |
551 (73) |
624 (70) |
162 (18) |
108 (12) |
| Wave 3 | 209 (17) |
316 (25) |
735 (58) |
258 (34) |
266 (35) |
228 (30) |
161 (20) |
426 (53) |
214 (27) |
10 (1) |
317 (31) |
707 (68) |
985 (79) |
178 (14) |
90 (7) |
SLE = Stressful life events;
Consists of the subsample of participants who completed a clinical assessment at Wave 3. Percentages that do not sum to 100 are due to rounding.
Applying the weights of the GSRRS to each of the life events questionnaire yielded scores in our sample ranging from 0 to 478. The distribution of scores was positively skewed, with 30% scoring 0 and 90% scoring between 1 and 180.
General Linear Models
Number of SLE
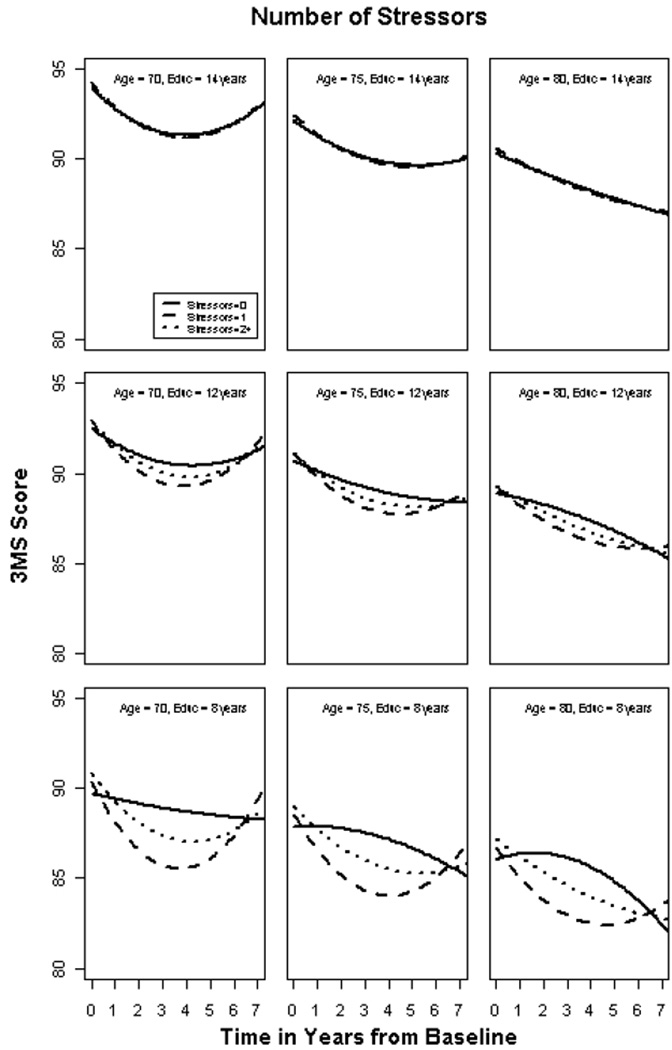
3MS scores declined (p < 0.001) by approximately 3.2 points over the 7-year study interval. There was no overall effect of number of SLE on average 3MS score (p = 0.062). However, there was a significant interaction between number of SLE and rate of 3MS decline that varied by years of education (p = 0.006). SLE predicted more rapid decline among those with fewer years of education, but had little effect among those with more years of education. Figure 1 displays this relationship for three levels of education and age, selected for illustrative purposes. There was no effect modification of number of stressors by APOE genotype (p = 0.364) or age (p = 0.738), although older persons had worse 3MS scores at baseline (see Figure 1). Table 4 displays the results of the multivariate general linear model and significant covariates.
Figure 1 shows model-based relationships between education and stressful life events for a male aged 70, 75 or 80 with 8, 12 or 14 years of education. Experiencing stressful events was associated with worse cognitive outcomes only among those with fewer years of education. The plots also display lower baseline 3MS scores among older individuals. The “improvement” in scores between years 4 and 7 may reflect differential attrition due to mortality, conversion to dementia, or nuances of study design where only a subset of older participants were approached at Year 4 (Wave 3) to complete a life events questionnaire.
Table 4.
Parameter estimates from two general linear models
| Model 1: Total # of Stressful Life Events |
Model 2: Total # of Negative Stressful Life Events |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Stnd Err |
t Statistic |
P | Estimate | Stnd Err |
t Statistic |
P |
| Intercept | 107.46 | 1.80 | 59.74 | <0.001 | 105.78 | 2.14 | 49.44 | <0.001 |
| SLE (1) | 1.20 | 1.15 | 1.04 | 0.297 | 5.16 | 3.46 | 1.49 | 0.136 |
| SLE (2+) | 2.55 | 1.10 | 2.33 | 0.020 | 5.71 | 3.72 | 1.53 | 0.125 |
| Time | −4.22 | 1.80 | −2.34 | 0.020 | 0.24 | 3.15 | 0.08 | 0.94 |
| Time2 | 0.88 | 0.27 | 3.30 | 0.001 | 0.02 | 0.49 | 0.03 | 0.975 |
| Education | 0.71 | 0.07 | 10.26 | <0.001 | 0.65 | 0.05 | 14.02 | <0.001 |
| Female | 2.04 | 0.24 | 8.36 | <0.001 | 2.08 | 0.26 | 8.00 | <0.001 |
| Occupation Professional/technical | 0.65 | 0.42 | 1.53 | 0.126 | 0.66 | 0.45 | 1.48 | 0.14 |
| Clerical | 0.97 | 0.41 | 2.34 | 0.019 | 1.13 | 0.44 | 2.58 | 0.01 |
| Service | −1.17 | 0.49 | −2.37 | 0.018 | −0.97 | 0.53 | −1.84 | 0.066 |
| Agricultural | 0.09 | 0.51 | 0.17 | 0.865 | 0.56 | 0.54 | 1.02 | 0.306 |
| Miscellaneous | 0.23 | 0.48 | 0.49 | 0.623 | 0.36 | 0.51 | 0.71 | 0.48 |
| Age | −0.36 | 0.02 | −20.49 | <0.001 | −0.33 | 0.02 | −13.33 | <0.001 |
| Age*Time | 0.08 | 0.02 | 4.21 | <0.001 | −0.02 | 0.04 | −0.48 | 0.635 |
| Age*Time2 | −0.02 | 0.003 | −5.68 | <0.001 | 0.002 | 0.006 | 0.26 | 0.799 |
| SLE (1)*Time | −5.09 | 1.56 | −3.26 | 0.001 | −8.80 | 4.78 | −1.84 | 0.066 |
| SLE (2+)*Time | −3.30 | 1.28 | −2.58 | 0.010 | −13.54 | 4.58 | −2.96 | 0.003 |
| SLE (1)*Time2 | 0.75 | 0.23 | 3.25 | 0.001 | 1.51 | 0.74 | 2.04 | 0.042 |
| SLE (2+)*Time2 | 0.44 | 0.19 | 2.30 | 0.022 | 2.25 | 0.71 | 3.15 | 0.002 |
| Education*Time | −0.17 | 0.08 | −2.12 | 0.034 | --- | --- | --- | --- |
| Education*Time2 | 0.03 | 0.01 | 2.13 | 0.033 | --- | --- | --- | --- |
| SLE (1)*Education | −0.07 | 0.08 | −0.78 | 0.434 | --- | --- | --- | --- |
| SLE (2+)*Education | −0.18 | 0.08 | −2.24 | 0.025 | --- | --- | --- | --- |
| SLE (1)*Education*Time | 0.35 | 0.11 | 3.12 | 0.002 | --- | --- | --- | --- |
| SLE (2+)*Education*Time | 0.23 | 0.09 | 2.51 | 0.012 | --- | --- | --- | --- |
| SLE (1)*Education*Time2 | −0.05 | 0.02 | −3.12 | 0.002 | --- | --- | --- | --- |
| SLE (2+)*Education*Time2 | −0.03 | 0.01 | −2.24 | 0.025 | --- | --- | --- | --- |
| SLE (1)*Age | --- | --- | --- | --- | −0.07 | 0.05 | −1.74 | 0.102 |
| SLE (2+)*Age | --- | --- | --- | --- | −0.08 | 0.05 | −1.57 | 0.117 |
| SLE (1)*Age*Time | --- | --- | --- | --- | 0.12 | 0.06 | 1.99 | 0.048 |
| SLE (2+)*Age*Time | --- | --- | --- | --- | 0.18 | 0.06 | 3.00 | 0.003 |
| SLE (1)*Age*Time2 | --- | --- | --- | --- | −0.02 | 0.01 | −2.19 | 0.029 |
| SLE (2+)*Age*Time2 | --- | --- | --- | --- | −0.03 | 0.01 | −3.20 | 0.001 |
Note: SLE = Stressful Life Events; Reference category for number of SLE is 0.
Weighted Life Events Stress Score
Compared with those who reported no stressors, there was no effect of the highest weighted life events stress score on average 3MS (p = 0.892) or on the rate of decline (p = 0.160). None of the hypothesized variables [education (p = 0.263), age (p = 0.624) or APOE genotype (p = 0.074)] modified the relationship between weighted stress scores and 3MS decline.
Subjective Rating of SLE
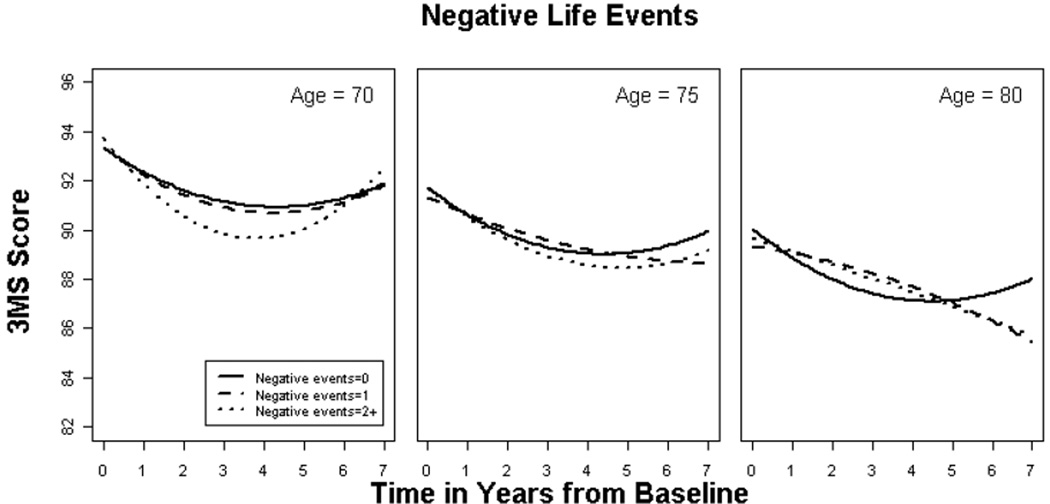
Overall, negatively rated SLE was significantly associated with rate of decline, but this association was modified by age (p = 0.009) such that a greater number of negative SLE predicted faster decline among younger participants (see Figure 2 and Table 4). Neither APOE genotype (p = 0.233) nor education (p = 0.170) modified the association between negative SLE and cognitive decline. There was also no association between number of unexpected events and 3MS score (p = 0.064) or decline (p = 0.521) or effect modification by education (p = 0.922), age (p = 0.533) or APOE genotype (p = 0.699).
Figure 2 shows model-based relationships between negatively rated events and cognitive decline for a male aged 70, 75 or 80 years. Greater number of negative events was associated with worse cognitive outcomes only among younger individuals. Again, the suggested “improvement” in scores between years 4 and 7 may reflect differential attrition or nuances of study design.
The number of events rated as important, negative and unexpected predicted neither overall 3MS (p = 0.112) nor decline (p = 0.427). None of the covariates of age (p = 0.481), APOE genotype (p = 0.732), or education (p = 0.489) modified the relation between SLE and 3MS decline.
DISCUSSION
In this population-based sample of elderly persons without dementia, the effect of stressful life events on cognitive decline was complex and varied by years of formal education and age. Among those with fewer years of education, a greater number of stressful events predicted faster decline, whereas limited effects were observed among those with more education. Consistent with this finding is the notion of resilience to pathological processes for those with greater cognitive or neural reserve, here, reflected by years of education (Stern, 2006). Presumably, those with greater reserve are better able to withstand the degenerative effects of chronic stress on the brain. An alternative explanation is that those with more years of education have access to greater psychosocial, financial or coping resources that may mitigate the effects of stressful events.
Unexpectedly, the number of negative events was associated with faster cognitive decline only among younger persons in this sample. It is unclear what underlies this result, as we expected older persons to be more vulnerable to the effects of stress. However, older participants may have had poorer recall of events or their subjective impact, which would bias our results towards null. Indeed, in our sample, older age was associated with poorer 3MS scores at baseline. Our results may also reflect generational or age differences in how negative experiences are processed. LaBouvie-Vief has found age-related differences in strategies used to regulate emotions, with older persons tending to employ cognitively simpler strategies that are intended to protect the self from unpleasant emotional experiences. Compared to their younger counterparts, elderly persons tend to minimize negative feelings, ignore unpleasant events, and process emotions less deeply (Labouvie-Vief, 2005). Some negative events are also “non-normative” at younger ages, when they are hypothesized to exert even greater stress (Neugarten, 1979; Rook et al., 1989). These and other factors likely contribute to the differential effects stressful events have among older versus younger individuals.
Also unexpected were the results where 3MS scores appeared to “improve” between years 4 and 7 in the analyses (Figures 1 and 2). This phenomenon may reflect differential attrition due to death (Neale et al., 2001) or dementia (the CCSMA follows individuals only to the point of dementia onset). Furthermore, due to nuances of study design, there was greater participation by older participants of the cohort at the first follow-up, whereas the opposite was observed at baseline and the second follow-up. This may have contributed to the apparent “improvement” in scores at the second follow-up, when participants were more broadly representative of the cohort.
Our results are consistent with previous studies showing a deleterious effect of SLE on cognitive decline (Amster & Krauss, 1974; Dickinson et al. 2011; Grimby & Berg, 1995) and may explain some of the conflicting findings in the literature, possibly due to the effect of individual differences that may moderate the effects of SLE. Of interest are the largely negative findings for the subjective appraisal of stressful events, which is inconsistent with theories that suggest the appraisal of events is a critical determinant of their effects (Folkman & Lazarus, 1984). However, it is possible that our measurements were too crude to capture distinctions regarding the severity or degree of impact of events or the outcome, which was limited to a cognitive screening test. We also sampled only 15 of the 35 items reported in the GSSR scale, and used a mail-in questionnaire with highest return rates generally observed among those with higher baseline cognitive scores, younger age, and more years of education. Sample size and high numbers of those missing SLE data, particularly at Wave 3 were limitations which may have contributed to our need to combine ratings of “somewhat” and “very” for unexpected events, precluding an ability to investigate whether higher thresholds on subjective ratings are necessary to trigger adverse cognitive effects. Retrospective report over a three-to-four year interval may also have introduced inaccuracies in the report of the stressors and their impact. Furthermore, we did not collect information on the chronicity and duration of each stressor. This limitation’s significance is underscored by studies implicating the role of chronic stress with neuronal loss in the hippocampus (Sapolsky et al., 1986), whereas acute, low level stress reportedly improves cognitive function, at least over the short term (Dash et al., 2006).
We did not find effect modification of stressful events and APOE genotype in predicting cognitive decline. Some studies report differential risk of AD by APOE genotype for early life conditions such as low socioeconomic occupation of father (Moceri et al., 2001) or number of persons in household (Borenstein et al., 2005), although these are not necessarily “stressful” experiences. In other analyses of our population, we found no effect modification of APOE genotype in predicting dementia with early parental death (Norton et al., in press.). Other studies have also failed to find a modifying role for the APOE gene for life events (Moceri et al., 2000).
The study strengths include its longitudinal design with repeated measurement of SLE, community and population-based sample, the characterization of SLE using three methods, and examination of several variables as potential effect modifiers of the association between stressful events and cognitive decline. Additionally, our sample consisted of relatively highly educated, Caucasian individuals, and therefore study results may not generalize to other ethnic groups.
CONCLUSION
The effects of SLE on cognition in late life are complex and vary according to individual characteristics. Future studies should consider examination of other factors such as coping strategies, personality traits, and social support obtained by alternative methods of assessment such as an interview (e.g., Brown and Harris, 1978) which may yield important dimensions of stressful events that for example, make clearer distinctions between positive and negative effects and allow for the assessment of complex appraisal and coping strategies important in predicting positive and negative emotions (Folkman, 2008). Stress biomarkers (van Eck et al., 1996) may also help identify subgroups with greater vulnerability or resilience to the effects of life stressors.
Key points.
Stressful life events are associated with increased risk for dementia.
There are conflicting results of the effects of stressful life events on cognitive decline in late life.
The effects of stressful life events appear to differ according to individual factors such as age and education.
Acknowledgements
We acknowledge the contributions of the following individuals whose activities have helped to ensure the success of the project: Cara Brewer, B.A., John C.S. Breitner, M.D., MPH, Tony Calvert, B.S., Carol Leslie, M.S., Michelle McCart, Ronald G. Munger, Ph.D., MPH, Georgiann Sanborn, M.S., Nancy Sassano, Ph.D., Sarah Schwartz, M.S., Martin Toohill, Ph.D., Heidi Wengreen, Ph.D., RD, James Wyatt, and Peter P. Zandi, Ph.D., M.P.H.
Supported by NIH grants: R01AG031272, R01AG11380, R01AG18712, R01AG21136
Footnotes
Preliminary results have been presented at the annual meeting of the Gerontological Society of America, November, 2010, New Orleans, LA
Conflict of Interest: The authors have no disclosures representing conflicts of interest.
References
- Amster LE, Krauss HH. The relationship between life crises and mental deterioration in old age. Int J Aging Hum Dev. 1974;5:51–55. doi: 10.2190/JA32-3VFR-29X4-D3Q7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd Ed Revised. Arlington, VA: American Psychiatric Association; 1987. [Google Scholar]
- Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, Mccormick WC, Bowen JD, Mccurry S, Larson EB. Developmental and vascular risk factors for Alzheimer's disease. Neurobiol Aging. 2005;26:325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. New York: Free Press; 1978. [Google Scholar]
- Christensen H. What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry. 2001;35:768–775. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Van Den Kommer TN, Minnaar RW, Penninx BW, Deeg DJ. Accumulated and Differential Effects of Life Events on Cognitive Decline in Older Persons: Depending on Depression, Baseline Cognition, or ApoE {epsilon}4 Status? J Gerontol B Psychol Sci Soc Sci. 2011 doi: 10.1093/geronb/gbr019. [DOI] [PubMed] [Google Scholar]
- Dash PK, Runyan JD, Blum S, Hebert AE, Simos PG, Papanicolaou AC. Putative Brain Mechanisms of the Various Memory Functions. In: Papanicolaou AC, editor. The Amnesias. A clinical textbook of memory disorders. New York: Oxford University Press; 2006. [Google Scholar]
- Dickinson WJ, Potter GG, Hybels CF, Mcquoid DR, Steffens DC. Change in stress and social support as predictors of cognitive decline in older adults with and without depression. Int J Geriatr Psychiatry. 2011 doi: 10.1002/gps.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S. The case for positive emotions in the stress process. Anxiety Stress Coping. 2008;21:3–14. doi: 10.1080/10615800701740457. [DOI] [PubMed] [Google Scholar]
- Folkman S, Lazarus R. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Grimby A, Berg S. Stressful life events and cognitive functioning in late life. Aging (Milano) 1995;7:35–39. doi: 10.1007/BF03324290. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Nortond M, Breitner JC. Characteristics of a two-stage screen for incident dementia. J Clin Epidemiol. 2003;56:1038–1045. doi: 10.1016/s0895-4356(03)00247-6. [DOI] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Gallo JJ, Breitner JC. Performance characteristics of a two-stage dementia screen in a population sample. J Clin Epidemiol. 2000;53:531–540. doi: 10.1016/s0895-4356(99)00196-1. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G. Dynamic integration: Affect, Cogntion, and the Self in Adulthood. In: Morf CCAAO, editor. Current Directions in Personality Psychology. Upper Saddle River, New Jersey: Pearson/Prentice Hall; 2005. [Google Scholar]
- Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. Am J Psychiatry. 1999;156:58–65. doi: 10.1176/ajp.156.1.58. [DOI] [PubMed] [Google Scholar]
- Mcewen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- Moceri VM, Kukull WA, Emanual I, Van Belle G, Starr JR, Schellenberg GD, Mccormick WC, Bowen JD, Teri L, Larson EB. Using census data and birth certificates to reconstruct the early-life socioeconomic environment and the relation to the development of Alzheimer's disease. Epidemiology. 2001;12:383–389. doi: 10.1097/00001648-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Moceri VM, Kukull WA, Emanuel I, Van Belle G, Larson EB. Early-life risk factors and the development of Alzheimer's disease. Neurology. 2000;54:415–420. doi: 10.1212/wnl.54.2.415. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Torres LD, Gotlib IH. Major life events and major chronic difficulties are differentially associated with history of major depressive episodes. J Abnorm Psychol. 2007;116:116–124. doi: 10.1037/0021-843X.116.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale R, Brayne C, Johnson AL. Cognition and survival: an exploration in a large multicentre study of the population aged 65 years and over. Int J Epidemiol. 2001;30:1383–1388. doi: 10.1093/ije/30.6.1383. [DOI] [PubMed] [Google Scholar]
- Neugarten BL. Time, age, and the life cycle. Am J Psychiatry. 1979;136:887–894. doi: 10.1176/ajp.136.7.887. [DOI] [PubMed] [Google Scholar]
- Norton MC, Ostbye T, Smith KR, Munger RG, Tschanz JT. Early parental death and late-life dementia risk: findings from the Cache County Study. Age Ageing. 2009;38:340–343. doi: 10.1093/ageing/afp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MC, Smith KR, Ostbye T, Tschanz JT, Schwartz S, Corcoran C, Breitner JC, Steffens DC, Skoog I, Rabins PV, Welsh-Bohmer KA. Early parental death and remarriage of widowed parents as risk factors for Alzheimer Disease: The Cache County Study. American Journal of Geriatric Psychiatry. 2011;19:814–824. doi: 10.1097/JGP.0b013e3182011b38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MC, Smith KR, Ostbye T, Tschanz JT, Schwartz S, Corcoran C, Breitner JC, Steffens DC, Skoog I, Rabins PV, Welsh-Bohmer KA. Early parental death and remarriage of widowed parents as risk factors for Alzheimer Disease: The Cache County Study. Am J of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e3182011b38. (in press.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy GM, Salmon DP, Jacobson MW, Hervey A, Gamst AC, Wolfson T, Patterson TL, Goldman S, Mills PJ, Khandrika S, Galasko D. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166:1384–1391. doi: 10.1176/appi.ajp.2009.09040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson G, Skoog I. A Prospective population study of psychosocial risk factors for late onset dementia. International journal of Geriatric Psychiatry. 1996;11:15–22. [Google Scholar]
- Rook KS, Catalano R, Dooley D. The timing of major life events: effects of departing from the social clock. Am J Community Psychol. 1989;17:233–258. doi: 10.1007/BF00931009. [DOI] [PubMed] [Google Scholar]
- Rosnick CB, Small BJ, Mcevoy CL, Borenstein AR, Mortimer JA. Negative life events and cognitive performance in a population of older adults. J Aging Health. 2007;19:612–629. doi: 10.1177/0898264307300975. [DOI] [PubMed] [Google Scholar]
- Sands JD. The relationship of stressful life events to intellectual functioning in women over 65. Int J Aging Hum Dev. 1981;14:11–22. doi: 10.2190/7rdc-2ruk-0glr-j2kb. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, Mcewen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- Van ECK M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]