Abstract
Thrombospondin-1 is a potent suppressor of T cell activation via its receptor CD47. However, the precise mechanism for this inhibition remains unclear. Because H2S is an endogenous potentiator of T cell activation and is necessary for full T cell activation, we hypothesized that thrombospondin-1 signaling through CD47 inhibits T cell activation by antagonizing H2S signaling. Primary T cells from thrombospondin-1 null mice were more sensitive to H2S-dependent activation assessed by proliferation and induction of interleukin-2 and CD69 mRNAs. Exogenous thrombospondin-1 inhibited H2S responses in wild type and thrombospondin-1 null T cells but enhanced the same responses in CD47 null T cells. Fibronectin, which shares integrin and glycosaminoglycan binding properties with thrombospondin-1 but not CD47 binding, did not inhibit H2S signaling. A CD47-binding peptide derived from thrombospondin-1 inhibited H2S-induced activation, whereas two other functional sequences from thrombospondin-1 enhanced H2S signaling. Therefore, engaging CD47 is necessary and sufficient for thrombospondin-1 to inhibit H2S-dependent T cell activation. H2S stimulated T cell activation by potentiating MEK-dependent ERK phosphorylation, and thrombospondin-1 inhibited this signaling in a CD47-dependent manner. Thrombospondin-1 also limited activation-dependent T cell expression of the H2S biosynthetic enzymes cystathionine β-synthase and cystathionine γ-lyase, thereby limiting the autocrine role of H2S in T cell activation. Thus, thrombospondin-1 signaling through CD47 is the first identified endogenous inhibitor of H2S signaling and constitutes a novel mechanism that negatively regulates T cell activation.
Keywords: Thrombospondin-1, CD47, Hydrogen sulfide, T lymphocytes, Extracellular signal-regulated kinase, Redox signaling
1. Introduction
Thrombospondin-1 (TSP1) is a large (450 kDa) matricellular glycoprotein that plays a pivotal role in regulating vascular homeostasis (Bauer et al., 2010; Isenberg et al., 2009), platelet activation (Isenberg et al., 2008), angiogenesis (Carlson et al., 2008; Miller et al., 2009; Roberts et al., 2012), and immunity (Lopez-Dee et al., 2011). TSP1 mediates these activities by binding to other extracellular matrix components and growth factors, mediating activation of latent TGF-β1 (Schultz-Cherry et al., 1993; Sweetwyne et al., 2012), and binding to at least 12 different cell surface receptors(Murphy-Ullrich et al., 2012). These receptors include five integrins (Calzada et al., 2004a; Calzada et al., 2003; Calzada et al., 2004b; Chandrasekaran et al., 2000; Lawler et al., 1988; Staniszewska et al., 2007), CD36 (Dawson et al., 1997), CD47 (Gao et al., 1996), CD148 (Takahashi et al., 2012), calreticulin/low density lipoprotein receptor-related protein-1 (LRP1) (Elzie et al., 2004), proteoglycans (Feitsma et al., 2000), and sulfatides (Guo et al., 1992). Among these, TSP1 has the highest affinity for CD47, and this receptor is both necessary and sufficient for TSP1 to inhibit NO-cGMP signaling (Isenberg et al., 2006).
TSP1 regulates T cell activation and function in a domain specific manner. Although TSP1 enhances some T cell actions via its N-terminal domains, such as α4β1 integrin-dependent adhesion and chemotaxis (Li et al., 2002), the dominant effect of soluble TSP1 is the potent inhibition of TCR-mediated T cell activation (Li et al., 2001). This inhibition requires interaction of the C-terminal domain of TSP1 with a proteoglycan isoform of CD47 on the T cell surface (Kaur et al., 2011; Li et al., 2002). The inhibitory activity of TSP1 does not require β1 integrins (Li et al., 2002) and is independent of TGFβ, based on resistance to TGFβ-function blocking antibodies (Li et al., 2001) and the inhibitory activity of a recombinant signature domain of TSP1 that lacks the TGFβ binding and activation sequences in the type 1 repeats (Ramanathan et al., 2011). Further evidence that CD47 ligation is sufficient to inhibit T cell activation derives from the inhibitory activity of some CD47 antibodies and CD47-binding peptides such as 7N3 (FIRVVMYEGKK), but not the corresponding control peptide FIRGGMYEGKK (Li et al., 2001).
Despite this evidence that CD47 ligation is necessary and sufficient for inhibiting TCR-dependent T cell activation, the lack of a substantial cytoplasmic domain in CD47 for docking of downstream signaling molecules suggests that lateral interactions with other membrane proteins such as growth factor receptors, integrins, PLIC-1, Fas receptor, and SIRPs are generally required for its signaling functions (reviewed in (Soto-Pantoja et al., 2013).
While the proximal intracellular targets of TSP1/CD47–mediated inhibition of T cell activation are not known, this inhibition occurs downstream of the TCR targeting linker for activated T cells (LAT) and Zap70, but upstream of NFAT activation (Li et al., 2001). TSP1 regulates the activation of soluble guanylate cyclase by NO in Jurkat T lymphoma cells in a calcium-dependent manner (Ramanathan et al., 2011), but this pathway cannot account for the broad effects of CD47 signaling on T cell activation as cGMP signaling is not reported to play a major role in T cell activation and is limited to T cell differentiation (Niedbala et al., 2006).
H2S is emerging as an important member of the gasotransmitter family that also includes NO and carbon monoxide (CO). At toxic environmental concentrations (>200 ppm), H2S inhibits mitochondrial cytochrome c oxidase (Reiffenstein et al., 1992). Lower nontoxic concentrations have physiological functions in neuromodulation ((Abe et al., 1996) and reviewed in (Tan et al., 2009)), metabolic hibernation (Blackstone et al., 2005; Blackstone et al., 2007), protection from ischemia/reperfusion injury (Elrod et al., 2007; Fu et al., 2008; Jha et al., 2008; Sivarajah et al., 2006; Tripatara et al., 2008), oxygen sensing (Olson et al., 2009), vasodilatation (Hosoki et al., 1997; Yang et al., 2008), and promotion of angiogenesis (Wang et al., 2010). Like its gasotransmitter cousins NO and CO, H2S has transitioned from being perceived exclusively as toxin to recognition that it is an important endogenous signaling molecule. In common with NO, H2S has been implicated as both a pro- (Bhatia et al., 2005; Collin et al., 2005; Cunha et al., 2008; Zhang et al., 2007) and anti-inflammatory molecule in innate immune cells (Cunha et al., 2008; Li et al., 2007; Sivarajah et al., 2009; Zanardo et al., 2006). Like NO, H2S relies on its distinctive chemistry for signal transduction, which includes modification of specific protein cysteine residues (termed sulfhydration) and ligation of ferric iron, zinc, or copper centers in metalloproteins (Fukuto et al., 2012).
Recently, we reported that H2S is a potentiator of T cell activation in primary murine and human T cells and T cell lines (Miller et al., 2012). Exogenously added H2S, at nanomolar physiological levels (Furne et al., 2008; Shen et al., 2012) enhances both polyclonal and antigen-specific T cell activation. Notably the capacity of T cells to endogenously make H2S via cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) is turned on as a result of T cell activation. Suppression of CBS and CSE expression by siRNA inhibits T cell activation and T cell proliferation, which can be rescued by supplementation with exogenous H2S. Therefore, H2S signaling is a necessary component of T cell activation.
As there are no reported endogenous inhibitors of H2S signaling, we sought to examine the effect of inhibitory TSP1 signaling through CD47 on H2S-mediated T cell activation. TSP1 is a logical candidate for regulation of this pathway given its potent and broad T cell inhibitory effects and its potent regulation of NO gasotransmitter signaling.
2. Results
2.1. TSP1 null T cells are more sensitive to H2S-potentiated activation
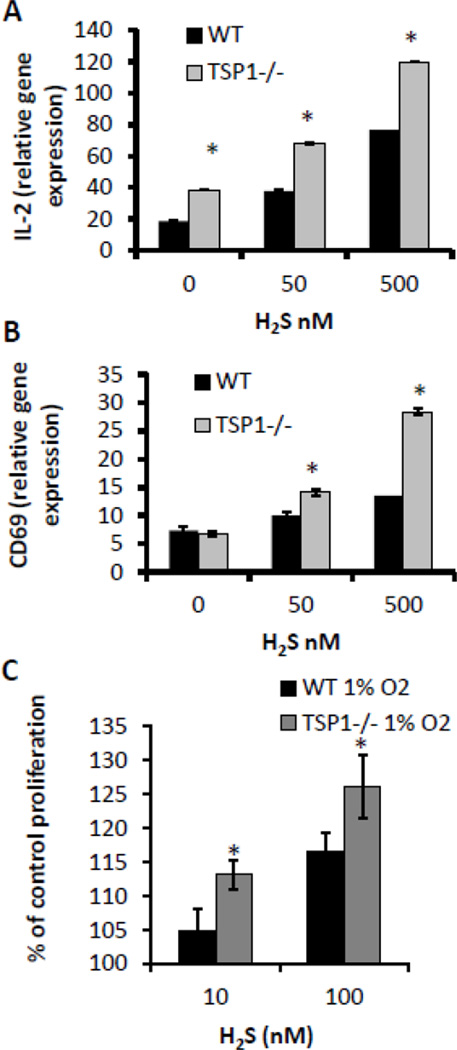
In order to examine the role of TSP1 in H2S-dependent T cell activation, we compared the activation of WT and TSP1 null CD3+ murine T cells via plate-bound anti-CD3 and anti-CD28 antibodies in the presence of H2S. Using IL-2 gene expression as a marker of T cell activation, we observed, as previously, that H2S dose-dependently enhanced IL-2 expression in WT T cells by up to 4-fold over control activated cells not treated with H2S (Fig. 1A). H2S enhancement of IL-2 expression was greater at all H2S concentrations in the activated TSP1 null CD3+ cells (175% at 50 nM, 150% at 500 nM of WT cells) (Fig. 1A). In addition to IL-2, CD69 expression levels were also elevated 4-fold in the presence of 500 nM H2S in TSP1 null activated T cells (Fig. 1B). Likewise we examined the role of endogenous TSP1 on H2S-dependent T cell proliferation (Fig. 1C). TSP1 null murine CD3+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 antibodies in the presence of H2S for 72 hours had significantly greater proliferation at 10 and 100 nM doses of H2S, suggesting that the presence of TSP1 limits H2S signaling in activated T cells.
Figure 1.
H2S-dependent potentiation of T cell activation and proliferation is enhanced in TSP1 null CD3+ cells. (A and B) Murine CD3+ T cells (3×106) were activated with plate-bound anti-CD3/CD28 antibodies in the presence of 50 or 500 nM Na2S or vehicle in 1% O2, and expression of IL-2 and CD69 mRNA was examined by RT-PCR at 4 hours respectively. Data are normalized to a value of 1 for non-activated control for each treatment, n=3, data are shown for a single experiment and are representative of n=2, error bars indicate standard deviation, * denotes p < 0.05. (C) TSP1 null and WT CD3+ cells were activated with plate-bound anti-CD3/CD28 antibodies in the presence of Na2S or vehicle, and proliferation was assessed in a 1% O2 atmosphere via an MTS assay at 72 hours post activation. Data represent net proliferation relative to day 0 and are expressed as a percentage of untreated controls for cell type, n=3, error bars indicate standard deviation, * denotes p < 0.05 compared to vehicle control.
2.2. Exogenous TSP1 limits T cell responses to H2S
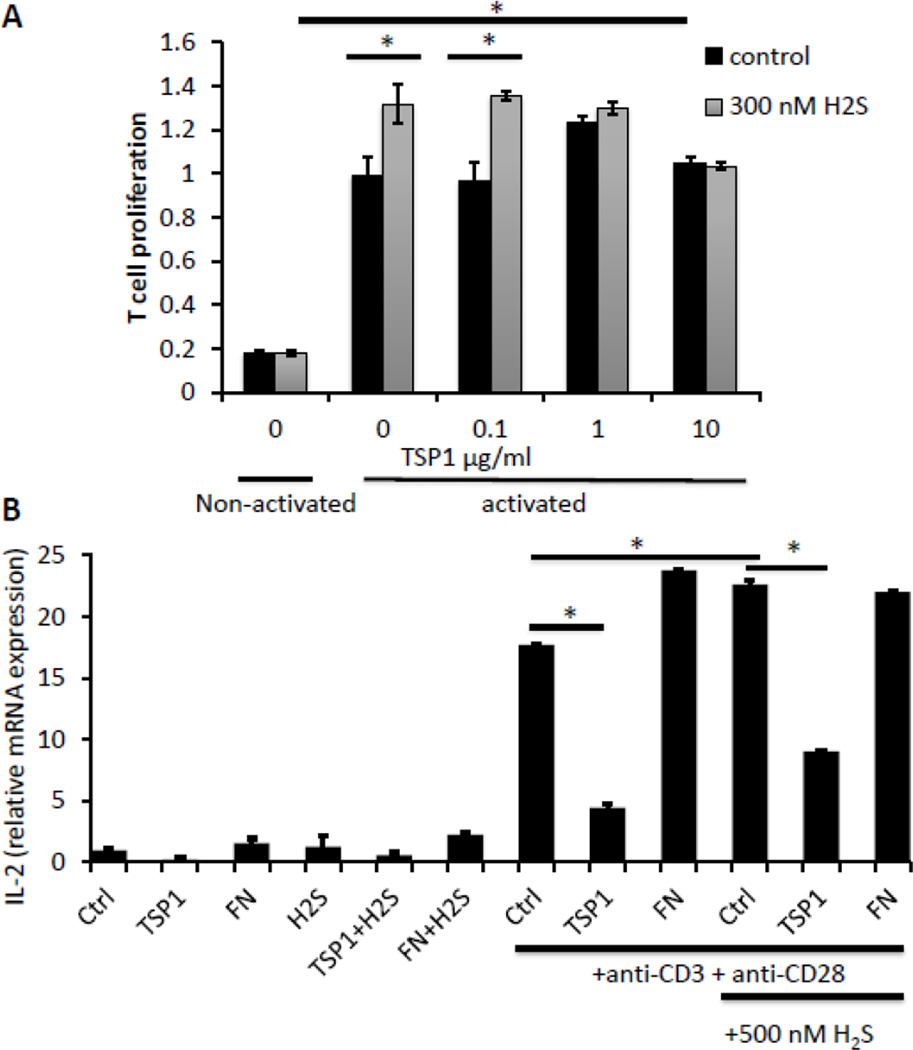
The increased H2S-dependent proliferation of TSP1-null CD3+ T cells was inhibited in a dose-dependent manner by addition of exogenous TSP1 (Fig. 2A). Proliferation of murine TSP1-null CD3+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 antibodies in the presence of H2S for 72 hours at 1% O2 was inhibited back to untreated levels by supplementation of TSP1 at 2.2 (1 μg/ml) and 22 nM. Thus, exogenous TSP1 reverses the enhanced proliferation phenotype of TSP1 null T cells.
Figure 2.
Exogenous TSP1 inhibits H2S-dependent CD3+ T cell proliferation and activation. (A) TSP1 null and CD3+ cells were activated with plate-bound anti-CD3/CD28 antibodies in the presence of 100 nM Na2S and TSP1 (0 to 10 μg/ml), and proliferation was assessed in a 1% O2 atmosphere via an MTS assay at 72 hours post activation. Data represent net proliferation relative to day 0 and are normalized to 100% for untreated anti-CD3/CD28 activated controls for each treatment, n=3, error bars indicate standard deviation, * denotes p < 0.05 compared to vehicle control. (B) Murine CD3+ T cells were incubated without activation or on immobilized anti-CD3 +CD28 in the absence or presence of 500 nM H2S and 1 μg/ml of TSP1 or fibronectin (FN). Cells were incubated for 20 h, and mRNA was isolated for analysis of IL-2 mRNA expression. * denotes p < 0.05 for the indicated comparisons by ANOVA.
To more directly assess the ability of exogenous TSP1 to inhibit T cell activation in the presence of H2S, we assessed the induction of IL-2 mRNA. We previously reported that TSP1 inhibits IL-2 mRNA and protein expression in human T lymphoma cells induced by TCR signaling (Li et al., 2001). Addition of 2.2 nM TSP1 significantly inhibited activation measured by induction of IL-2 mRNA in WT primary CD3+ murine T cells and that stimulated by addition of 500 nM H2S (Fig. 2B). To determine the specificity of this inhibition, we examined fibronectin, a multidomain protein of comparable size that binds similarly to heparin and binds to two β1 integrins on T cells that also recognize TSP1 (Yabkowitz et al., 1993). In contrast to TSP1, fibronectin at the same concentration did not inhibit basal or activation-dependent IL-2 mRNA expression in the absence or presence of 500 nM H2S. Despite sharing several receptor binding specificities with TSP1, fibronectin does not directly interact with two known functional receptors for TSP1 on T cells: CD47 and calreticulin/LRP1 (Li et al., 2005; Li et al., 2006; Li et al., 2002).
2.3. TSP1 limits T cell responses to H2S via CD47
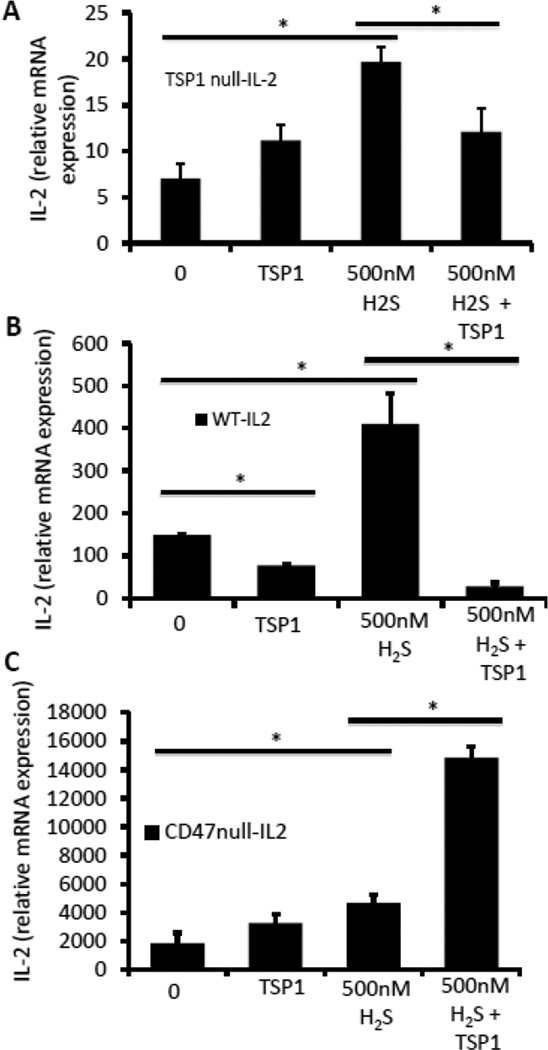
Engaging a proteoglycan isoform of CD47 by TSP1 inhibits T cell activation (Kaur et al., 2011; Li et al., 2002), whereas engaging calreticulin/LRP1 mediates internalization of TSP1 and modulates T cell motility and integrin function (Li et al., 2005; Li et al., 2006a). To determine whether CD47 is necessary for the inhibitory activity of exogenous TSP1, we examined in vitro activated WT, TSP1 null, and CD47 null CD3+ T cells in the presence and absence of 500 nM H2S (Fig. 3). We again measured T cell activation using plate-bound anti-CD3 and anti-CD28 antibodies and observed activation-induced IL-2 mRNA expression in all backgrounds. Baseline activation by anti-CD3 + anti-CD28 was inhibited by addition of 2.2 nM exogenous TSP1 in the WT cells but not significantly altered by the TSP1 in the two null strains. IL-2 mRNA was enhanced more than 2-fold in the presence of 500 nM H2S, and the H2S dependent enhancement of activation was significantly inhibited in the presence of 2.2 nM exogenous TSP1 in the WT and TSP1 null backgrounds but not in CD47 null T cells (Fig 3C). This indicated that CD47 is the necessary receptor for TSP1 to inhibit H2S signaling. Remarkably, the addition of both H2S and TSP1 to activated CD47 null T cells produced a further stimulation of IL-2 expression relative to H2S alone. The positive effect of TSP1 on IL-2 expression in the absence of CD47 is consistent with our previous report that TSP1 similarly increases CD69 expression in CD47-deficient human T cells via an undefined receptor (Kaur et al., 2011) and with the known positive effects of TSP1 receptors such as β1 integrins and calreticulin/LRP1 on T cell functions (Burbach et al., 2007; Li et al., 2005).
Figure 3.
TSP1 Inhibition of H2S signaling is mediated by CD47. (A) Murine TSP1 null, (B) wild-type, or (C) CD47 null CD3+ T cells were activated with plate-bound anti-CD3/CD28 antibodies in the presence of 500 nM Na2S, 2.2 nM of TSP1 or the combination in 1% O2, and gene expression of IL-2 was examined by RT-PCR at 4 hours. Data are normalized to non-activated control for each treatment, and data are shown for a single experiment. Error bars indicate standard deviation, and * denotes p < 0.05 for the indicated comparisons using two way ANOVA or T test.
2.4. CD47 ligation is sufficient for inhibition of T cell activation by H2S
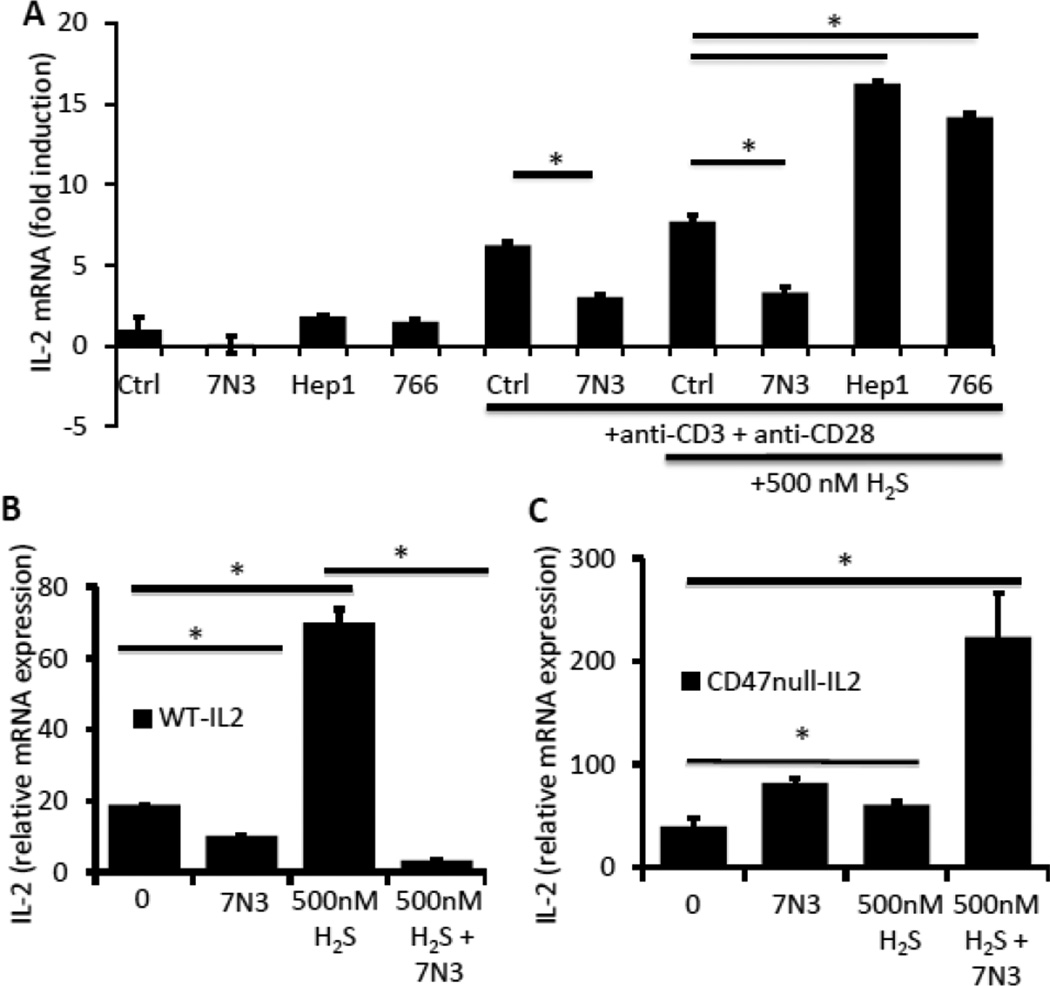
To confirm the role of CD47 and examine possible contributions of other TSP1 receptors such as calreticulin/LRP1, we co-incubated the H2S-exposed CD3+ murine T cells with several functional peptides derived from TSP1. The peptide 7N3 (1102FIRVVMYEGKK1112) is derived from the C-terminal domain of TSP1 and binds to CD47 (Gao et al., 1994; Isenberg et al., 2006). As with full-length TSP1, we observed an inhibition of activation-induced IL-2 mRNA expression and the further stimulation by H2S in WT primary T cell in the presence of 1 µM 7N3 (Fig. 4A). In contrast to 7N3, the calreticulin binding peptide Hep1 (17ELTGAARKGSRRLVKGPD35) from the N-terminal domain of TSP1 (Yan et al., 2011) at 1 μM further enhanced IL-2 expression in cells activated in the presence of 500 nM H2S but did not significantly increase IL-2 expression in nonactivated cells. Similarly, an integrin-binding peptide 766 (87LALERKDHSG96) derived from the N-terminal domain of TSP1 (Calzada et al., 2004a) at 1 μM enhanced IL-2 expression in activated cells treated with H2S.
Figure 4.
Inhibition of H2S signaling is mediated by CD47 binding peptide 7N3. (A) Murine WT CD3+ T cells were incubated in the absence or presence of immobilized anti-CD3 + CD28 and 500 nM H2S in the presence of the indicated TSP1-derived peptides at 1 μM. IL2-mRNA expression was measured after incubating for 20 h in 1% O2. TSP1 peptides: CD47-binding peptide 7N3, calreticulin-binding peptide Hep1 (17ELTGAARKGSRRLVKGPD35), and the integrin-binding peptide 766 (87LALERKDHSG96). (B) Murine wild-type or (C) CD47 null CD3+ T cells were activated with plate-bound anti-CD3/CD28 antibodies in the presence of 500 nM Na2S, 1 μM 7N3 or the combination in 1% O2, and gene expression of IL-2 was examined by RT-PCR at 4 hours. Data are normalized to non-activated control for each treatment and data are shown for a single experiment. Error bars indicate standard deviation, and * denotes p < 0.05 for the indicated comparisons using two way ANOVA.
H2S-dependent induction of IL-2 mRNA in the presence of the CD47 binding peptide 7N3 was significantly inhibited to below control levels in WT T cells, demonstrating that ligation of CD47 is sufficient to inhibit H2S signaling. To confirm that CD47 is the necessary for the inhibitory effect of this CD47 ligand on H2S signaling, we examined its effect on H2S signaling in CD47 null murine CD3+ cells (Fig. 4C). Treatment of CD47 null cells with 7N3 did not inhibit activation due to H2S. Rather, 7N3 potentiated IL-2 mRNA expression induced by CD3+CD28 ligation and that observed by addition of H2S in the CD47 null cells, where we expected 7N3 to have no effect. However, CD47-independent activities of 7N3 and other CD47-binding peptides are well known (Barazi et al., 2002; Tulasne et al., 2001), and the nonspecific positive effect observed in Fig. 4C are consistent with the ability of 7N3 to stimulate integrindependent adhesion of T cells lacking CD47 (Barazi et al., 2002) and the known positive role of integrin activation in T cell activation (Pribila et al., 2004). Despite this off-target activity, these data combined with those in Fig. 3C establish that CD47 is the necessary receptor for TSP1 inhibition of H2S signaling in T cells.
2.5. TSP1 inhibits H2S -induced ERK1/2 phosphorylation in activated T cells
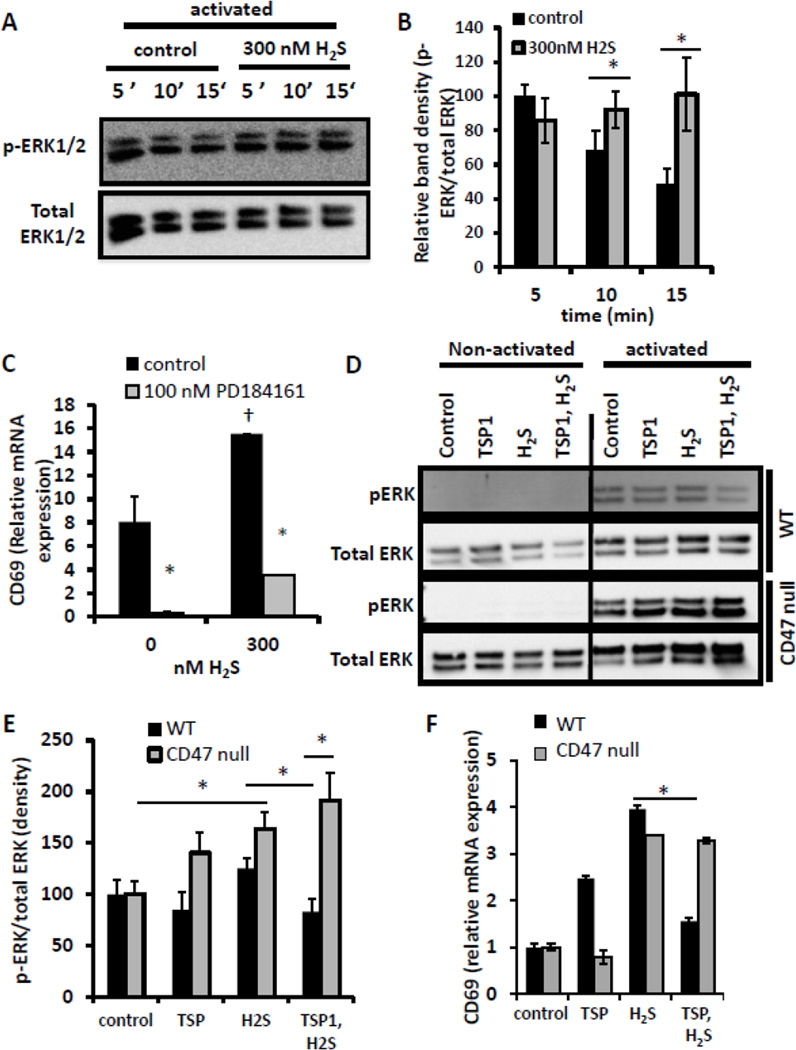
As ERK signal transduction is important in mediating T cell activation and is reported to be a target of H2S signaling in other cell types and tissues (Li et al., 2011), we examined the effect of H2S on T cell activation-induced ERK signaling by monitoring time dependent ERK1/2 phosphorylation in the presence or absence of 300 nM H2S (Fig. 5A,B). Activation of Jurkat T cells induced a robust increase in p-ERK1/2 as early as 5 min following activation. Temporally, this signal peaked at 5 min and declined at 10 and 15 min following activation. H2S co-administration shifted the maximal p-ERK1/2 signal to 10 min. The H2S-induced p-ERK1/2 signal was significantly higher at both 10 and 15 min than the control signal. To our knowledge, this is the first report of H2S modulating ERK signaling at nanomolar levels.
Figure 5.
H2S-mediated ERK phosphorylation is inhibited by TSP1 in a CD47 dependent manner. (A) Jurkat T lymphoma cells (2×106 cells) were activated with plate-bound anti-CD3/CD28 antibodies or control uncoated wells in the presence of 300 nM H2S, and ERK phosphorylation levels were measured by Western blot at the indicated time points and compared to total ERK1/2 staining. (B) Graphical representation of band relative band density in A. (C) Jurkat cells (3×106 cells) were activated with plate-bound anti-CD3/CD28 antibodies in the presence of 300 nM Na2S in 1% O2, and expression of CD69 mRNA was examined by RT-PCR at 4 hours. Data are normalized to non-activated control for each treatment, n=3, error bars indicate standard deviation, * denotes p < 0.05 compared to cells not treated with PD184161. † denotes p < 0.05 compared to cells not treated with H2S. (D) Wild-type and CD47 null Jurkat T cells (2×106 cells) were activated with plate-bound anti-CD3/CD28 antibodies, control uncoated wells in the presence of 300 nM H2S, 2.2 nM TSP1, or the combination, and ERK phosphorylation levels were measured by Western blot at 15 min and compared to total ERK1/2 staining. (E) Graphical representation of band relative band density in A expressed as a percentage of the respective untreated control cells. Western blots are representative of n=2. * denotes p < 0.05. (F) WT and CD47 null CD3+ T cells were activated on immobilized anti-CD3 plus anti-CD28 and treated with 2.2 nM TSP1 and/or 500 nM H2S for 4 h. mRNA was isolated, and expression of CD69 mRNA was analyzed an is presented normalized to cells activated but not treated. * denotes p < 0.05 relative to cells treated with H2S alone.
To test whether increased ERK signaling contributes to H2S-induced potentiation of T cell activation, we examined the activation of Jurkat cells in the presence of an inhibitor of MEK-mediated ERK phosphorylation, PD184161 (Fig. 5C). The TCR-stimulated enhancement of Jurkat cell activation by 300 nM H2S assessed by induction of CD69 mRNA was significantly inhibited in the presence of 100 nM PD184161, suggesting that ERK signaling at least in part mediates the enhancement of T cell activation by H2S.
Previously our lab reported that TSP1 signaling blocked angiogenesis in part by inhibiting NO-induced stimulation of ERK phosphorylation (Ridnour et al., 2005). Here we examined whether TSP1 could also inhibit H2S-dependent increases in T cell activation via suppression of ERK signaling. Due to the significant effect of H2S 15 min following activation in Fig. 5A, we used this time point to examine the effect of TSP1. When human Jurkat T cells were preincubated with 2.2 nM TSP1 15 min prior to addition of H2S, p-ERK1/2 levels were markedly decreased (Fig. 5D, E). Significantly, TSP1 pretreatment inhibited the H2S-mediated enhancement of p-ERK1/2 levels.
We repeated the ERK activation experiments in CD47-deficient Jurkat T cells (clone JinB8 (Reinhold et al., 1999) in order to further assess the role of CD47 in TSP1-mediated inhibition of H2S signaling. H2S-dependent ERK activation is intact in this cell line. However, TSP1 failed to inhibit H2S-stimulated p-ERK1/2 levels (Fig. 5D,E). TSP1 alone elevated p-ERK1/2 in CD47-deficient but not in WT Jurkat cells, which suggests that additional TSP1 receptors mediate positive signaling through ERK and is consistent with our previous report that peptides from the central thrombospondin type 1 repeats activate ERK1/2 phosphorylation in Jurkat cells (Wilson et al., 1999). Changes in CD69 mRNA expression in WT and CD47 null murine T cells paralleled the changes in ERK1/2 phosphorylation induced by H2S and TSP1 (Fig. 5F). H2S increased CD69 expression induced by CD3+CD28 ligation in WT and CD47 null cells, but TSP1 inhibited H2S-induced CD69 expression only in the WT cells. Although CD47-mediated inhibition of H2S signaling probably involves other pathways in addition to ERK1/2, these data further validate the role of CD47 as a mediator of TSP1-dependent inhibition of H2S signaling in T cell activation and identify ERK1/2 as one of its targets.
2.6. TSP1 limits T cell activation-induced up-regulation of H2S production
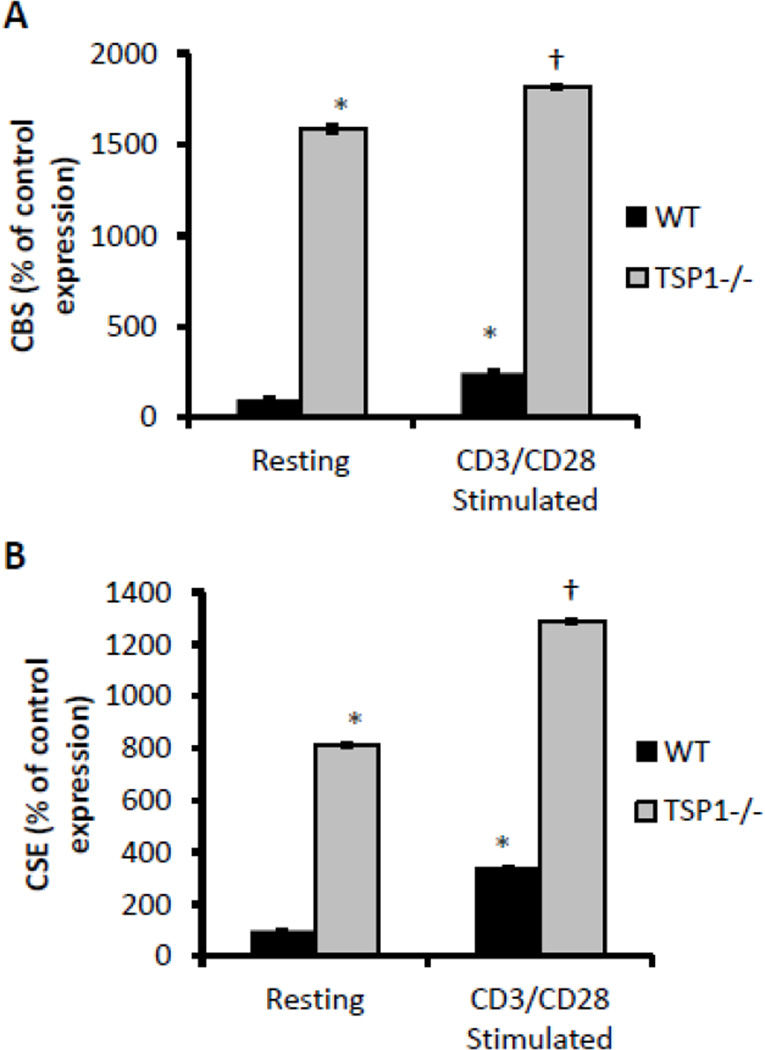
In addition to the role of exogenous H2S, we previously showed that full T cell activation depends on expression of the endogenous H2S-producing enzymes CBS and CSE (Miller et al., 2012). As TSP1 is a potent inhibitor of T cell activation, we examined its role in the expression of endogenous H2S biosynthetic enzymes as a function of T cell activation. Purified CD3+ T cells were activated using platebound anti-CD3 and anti-CD28, and cells were harvested at 24 hours to analyze the expression of CBS and CSE mRNAs. As previously reported, activated WT murine CD3+ T cells show an increase in expression of CBS and CSE mRNA when compared to non-activated cells (Fig. 6A,B). Remarkably, non-activated TSP1-null CD3+ cells showed 15- and 8-fold higher basal CBS and CSE mRNA expression, respectively, relative to WT cells (Fig. 6A,B). As in the wild-type cells, however, the expression level increased in TSP1 null cells with activation to levels significantly higher than in the non-activated control cells. Furthermore, CBS and CSE levels were higher in activated TSP1 null cells than in activated WT cells. This implies that endogenous TSP1 limits the H2S biosynthetic capacity of both resting and activated T cells, possibly contributing to the inhibition of T cell activation by TSP1.
Figure 6.
H2S biosynthetic capacity is unregulated in TSP1 null CD3+ T cells. Murine CD3+ T cells were activated with plate-bound anti-CD3/CD28 antibodies, and expression of CBS (A) and CSE mRNAs (B) were examined in 1% O2 by RT-PCR at 24 h. Data are expressed as a percentage of non-activated control levels for each treatment; n = 3, data are shown for a single experiment and are representative of n=2, error bars indicate S.D. * denotes p < 0.05 compared to resting wild-type control. † denotes p < 0.05 compared to resting TSP1 null control.
3. Discussion
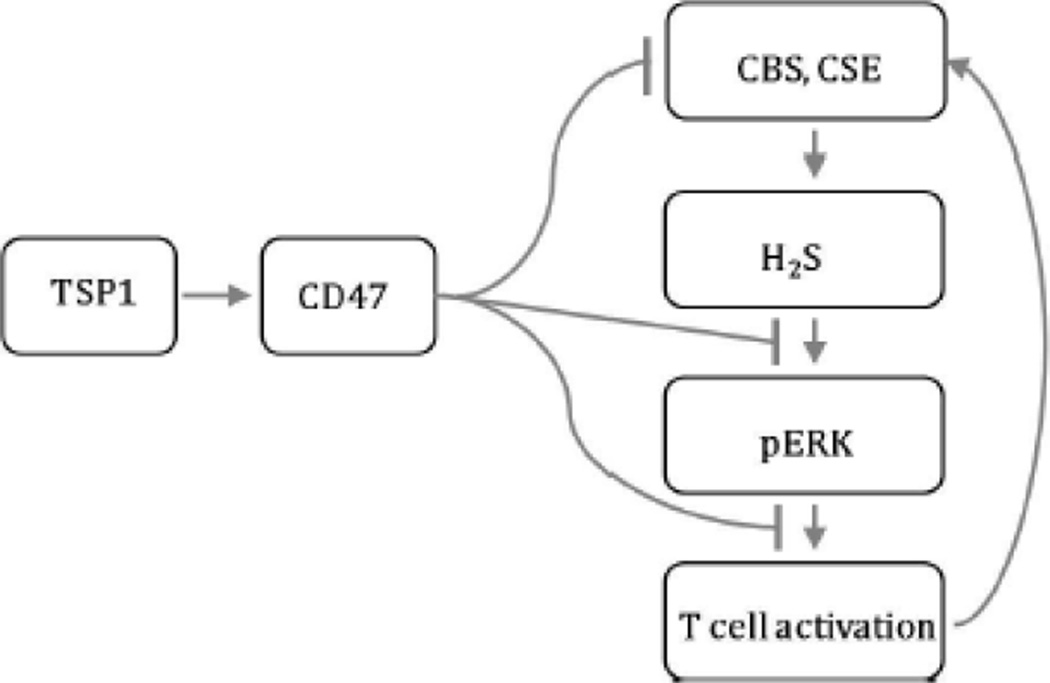
Recently we reported that physiological levels of the gasotransmitter H2S in the nanomolar range function as an endogenous potentiator of T cell activation (Miller et al., 2012). The present work identifies an extracellular matrix signaling pathway that limits this H2S function in T cells (summarized in Fig. 7). We demonstrate that the previously reported potent inhibition of T cell activation by TSP1 (Li et al., 2001) is mediated at least in part through inhibiting T cell responses to H2S and the H2S biosynthetic capacity of T cells. To our knowledge, this is the first report of an endogenous inhibitory signaling pathway that limits H2S signaling and expands the range of signaling functions controlled by the matricellular protein TSP1.
Figure 7.
Schematic of TSP1 inhibition of H2S-dependent T cell signaling through CD47. Exogenously added H2S potentiates TCR-activated ERK phosphorylation to enhance T cell activation. T cell activation in turn stimulates the endogenous production of H2S via transcriptional activation of its biosynthetic enzymes CBS and CSE. The secreted matricellular protein TSP1 engages its high affinity receptor CD47 on the surface of T cells to redundantly inhibit the H2S signaling cascade. TSP1/CD47 signaling potently inhibits T cell activation via inhibition of H2S-mediated ERK phosphorylation and also by limiting the expression of CBS and CSE. TSP1 is the first reported endogenous inhibitor of H2S signaling.
H2S-dependent enhancement of primary murine CD3+ T cell activation and proliferation was increased in TSP1 null cells, but was reversed after the addition of exogenous TSP1, suggesting that endogenously produced TSP1 limits the effect of H2S in the wild-type cells. The IC50 dose of TSP1 based on the data in Figure 2A is somewhere between 0.22 and 2.2 nM. These levels of TSP1 are physiological in plasma and consistent with concentrations needed for the inhibition of NO-cGMP signaling via its high-affinity receptor CD47 (Isenberg et al., 2006). We tested the hypothesis that CD47 is necessary and sufficient for the inhibition of H2S signaling using CD47 null T cells and by replacing TSP1 with a CD47-binding peptide from the C-terminus of TSP1 (7N3), which was sufficient for inhibition of H2S-dependent T cell activation. The specificity of this peptide was validated by using CD47 null CD3+ T cells in place of the wild-type T cells and lack of inhibitory activity for other functional TSP1 peptides that interact with different TSP1 receptors on T cells. The positive effects of peptide 7N3 and TSP1 on H2S-dependent and -independent T cell activation in CD47 null cells are consistent with previous studies and mediated by yet to be identified receptors (Barazi et al., 2002; Kaur et al., 2011; Tulasne et al., 2001).
While H2S likely interacts with multiple signaling pathways to enhance T cell activation (Miller et al., 2012), we examined the specific role of ERK signaling based on several reports of ERK activation by H2S.
We found ERK phosphorylation to be rapid and transient in TCR-activated Jurkat cells, consistent with reports in other cell types. Interestingly, H2S did not simply enhance p-ERK levels, but rather prolonged them. Where maximal phosphorylation occurred at 5 min or earlier in untreated cells, H2S delayed this peak until at least 15 min. This prolongment may indicate that H2S enhances T cell activation by sustaining the ERK signaling pathway. TCR activation initiates the production of H2O2 that limits the activation of the ERK signaling pathway as a negative feedback mechanism (Devadas et al., 2002). The inclusion of the exogenous antioxidants manganese [III] tetrakis (4-benzoic acid) porphyrin (MnTBaP) or N-acetylcysteine or the endogenous overexpression of peroxiredoxin II, all used to reduce O2•− and H2O2 levels, augmented p-ERK levels (Kwon et al., 2003) and produced a qualitatively and temporally similar shift in ERK phosphorylation to that seen in our study, but with concentrations orders of magnitude higher than that of H2S in this study. Perhaps H2S enhances p-ERK levels by reversing or preventing inhibitory thiol oxidation of upstream signaling components responsible for attenuating p-ERK levels.
We previously showed that H2S enhances the reorientation of the microtubule organizing center (MTOC) in T cells and enhances tubulin-dependent cell polarization (Miller 2012). Evidence from several labs indicates a connection between microtubule activity and ERK signaling. MTOC orientation to the virological synapse during the spread of Human T-lymphotropic virus type 1 (HTLV-1) is dependent on ERK signaling (Nejmeddine et al., 2009). Likewise, ERK activity is necessary for NK cell MTOC reorientation and lytic activity (Chen et al., 2007; Li et al., 2008; Nejmeddine et al., 2009). The actions of H2S on the MTOC may be the result of its ability to activate ERK, warranting further investigation into this pathway. In this regard, TSP1 may limit the activity of tumor-infiltrating lymphocytes by down-regulating H2S-dependent ERK signaling.
Interestingly in Fig. 5D,E we observed that TSP1 alone increases p-ERK levels in CD47 null cells. The concentration used (2.2 nM) is below what we should expect to elicit an effect through any known TSP1 receptor other than CD47. These data are consistent with the increased CD69 induction we previously reported when CD47-deficient Jurkat T cells were activated in the presence of TSP1 (Kaur et al., 2011) and indicate that another unrecognized high affinity TSP1 receptor is present on T cells that has an opposing TSP1-dependent signal to CD47. Future studies in our lab will explore this unexpected result.
The increased CBS and CSE expression in TSP1 null CD3+ cells demonstrates that TSP1 is an in situ inhibitor of endogenous H2S production. Interestingly we observed that these enzymes are more highly expressed in the non-activated TSP1 null CD3+ cells at 24 hours, and especially in the case of CBS, a less dramatic increase in expression is observed upon T cell activation. The expression of CBS and CSE is known to be highly dependent on SP1 promoter elements (Ge et al., 2001; Maclean et al., 2004; Yang et al., 2011), and our data may reflect an elevation of SP1 activity in TSP1 null cells. As we also did not sort the CD3+ populations into memory and naïve T cells, the differences in non-activated expression of CBS and CSE in TSP1 and WT cells could reflect differences in T cell population subsets in the spleen and their differences in enzyme expression levels, although no reports of this yet exist. However, we previously found no difference in CD4+ versus CD8+ T cell percentages and no elevation in memory T cells in splenic populations from TSP1-null mice based on CD25 or CD69 expression and the percentage of CD44-high T cells (Kuznetsova et al., 2005).
One pathophysiological condition in which TSP1 may provide a homeostatic role in limiting H2S-dependent T cell signaling is inflammatory bowel disease (IBD). The absence of TSP1 increases the severity of experimental IBD in both acute and chronic models. TSP1 null mice develop colonic focal inflammation after only two days of dextran sodium sulfate exposure in experimental colitis (Punekar et al., 2008). In a mouse model of chronic colitis using multiple cycles of dextran sulfate exposure, the TSP1 null mice exhibit greater disease-associated angiogenesis and tissue inflammation (Zak et al., 2008). Among the possible mechanisms for these findings is decreased immunosuppressive latent TGF-β activation in TSP1 null mice. However, we would also postulate that given the enhanced level of H2S associated with IBD, the lack of TSP1 could result in excessive H2S stimulation of T cell activity.
A specific role of T cell CD47 as a TSP1 receptor in IBD has not been established, but this would be consistent with published evidence that CD47 null mice exhibit exaggerated T cell inflammatory responses (Bouguermouh et al., 2008; Lamy et al., 2007). In contrast, CD47-null mice exhibited a deficit in dendritic cell recruitment in a colitis model that was attributed to the function of CD47 as a counter-receptor for SIRPα (Fortin et al., 2009). The balance between SIRPα-dependent and TSP1-dependent roles of CD47 in IBD will require further study.
4. Experimental Procedures
4.1. Cells and reagents
H2S refers to any of its various protonation states (H2S → HS− + H+ → S2− + H+) with HS− being the predominant form at physiological pH (pKa = 6.8). Na2S and NaHS, the corresponding sodium salts of these anionic forms of H2S, are considered H2S donors at physiological pH and are used as sources of H2S for this study. C57Bl/6 mice were anesthetized and sacrificed by cervical dislocation, and their spleens were harvested for T cell culture. The spleens were gently ruptured in a 40 micron cell strainer (BD Biosciences) and placed over a 50 mL Falcon tube using the back end of a 6 cc syringe plunger. The cells were rinsed through with basal RPMI (0.1% BSA) and centrifuged at 200×g for 5 minutes. CD3+ T cells were purified by using a pan T cell isolation kit II and MACS MS columns (Miltenyi Biotec) without prior red-cell lysis according to the manufacturer’s protocol. The cells were resuspended in 10 ml of RPMI 1640 containing 10% FBS, glutamine and penicillin/streptomycin and plated in a flask for 30 min at 37°C and 5% CO2. Care and handling of animals was in accordance with protocol LP-012 approved by the Animal Care and Use Committee of the National Cancer Institute. TSP1 was purified from human platelets as described here (Miller et al., 2010). Fibronectin was isolated from human plasma by gelatin affinity chromatography as described (Negre et al., 1994). TSP1 peptides 7N3 (1102FIRVVMYEGKK1112), Hep1, and 766 were available from previous studies or purchased from Peptides International (Calzada et al., 2004a). The MEK inhibitor PD184161 was purchased from Cayman Chemical.
Wild-type Jurkat T cells (E6.1, ATCC) were maintained at 2-5x105 cells per ml in RPMI 1640 medium supplemented with glutamine, penicillin/streptomycin, and 10% FBS. Cells were maintained in culture for a maximum of 4 weeks. For cell activation studies, Jurkat cells were resuspended in basal medium (RPMI, glutamine, penicillin, streptomycin, and 0.1% BSA).
Unless specified otherwise, all chemicals were purchased from Sigma (St. Louis, MO). For T cell activation, wells of either 6-well or 96-well plates were coated overnight with a mixture of anti-CD3 and anti-CD28 antibodies (mouse cells: clones 17A2 and 37.51 respectively, BD Biosciences; Jurkat cells: OKT3 and CD28.2, functional grade, eBioscience) at 2 μg/ml and 5 μg/ml respectively for mouse cells and 2 μg/ml and 5 μg/ml in PBS without divalent cations. The following day, the wells were washed wice with PBS to remove unbound antibody, and cells were added in growth medium for stimulation.
The concentrations of H2S used for this study are derived from the EC50 concentrations of H2S (from Na2S) used for stimulation of T cell proliferation derived from our prior work (Miller et al., 2012). Also, the steady state concentration of H2S will depend on how much is added exogenously or made endogenously and the rate of its degradation. The degradation of H2S, both enzymatically and nonenzymatically, depends largely on O2 concentration (Tiranti et al., 2009). Minimizing the O2 levels maximizes available H2S. Due to this, we conducted the experiments at 1% O2, 94% N2 and 5% CO2 unless stated otherwise.
4.2. Gene expression studies
In 6-well plates, 1 – 3×106 cells for each condition were maintained in 1 or 20% O2 for the specified amount of time (4-20 h). The cells were harvested and RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s protocol. cDNA was synthesized from 1 – 5 μg of total RNA using Superscript first strand RT-PCR reagents (Invitrogen) according to the manufacturer’s protocol. qRT-PCR was then performed using the SYBR green kit (Thermo) on the following gene/primer sets: HPRT, IL-2, CD69, CSE, CBS (sequences can be found in (Miller et al., 2012)). HPRT (hypoxanthine phosphoribosyltransferase) was used as the internal control for expression based on previous reports of its superior stability over other commonly used control genes (de Kok et al., 2004). Results were calculated based on the delta-Ct method and normalized to HPRT.
4.3. T cell proliferation
Cells were seeded at 100,000 cells per well in 96 well plates uncoated or pre-coated with anti-CD3/CD28 as specified above and treated as indicated. Proliferation was assessed using cell titer-96 MTS reagent (Promega) after 72 hours of growth according to the manufacturer’s protocol. The formazan signal produced by reduction of [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] inner salt by the cells on day 0 was subtracted from the signal after 72 hours to quantify net proliferation.
4.4. Western blotting
Jurkat cells were resuspended in fresh RPMI-G at 1x106 cells per ml and 2 ml was added to activation plates and incubated at 37°C, 5% CO2 for 5, 10, and 15 min. Cells were removed from the plates into cold 15 ml tubes and were pelleted at 300xg and resuspended in 100 μL of cold modified RIPA lysis buffer (50 mM Tris, pH 7.4 , 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EGTA with 1 mM NaF, 1 mM Na3VO4, and Proteoblock (Fermentas)). The cells then were sonicated for 5 min in a bath sonicator and lysates clarified by centrifugation at 16,000xg for 10 min at 4°C. Lysates then were processed for western blotting. Phospho-ERK1/2 was detected using an antibody from Cell Signaling (#9101) and total ERK with an antibody from Upstate (06-182), both diluted in 5% BSA TBST. Blots were developed with Amersham ECL Plus and imaged with a Kodak digital image station.
Highlights.
Endogenous and exogenous thrombospondin-1 limits the ability of H2S to enhance T cell receptor-mediated T cell activation.
CD47 is necessary for thrombospondin-1 to inhibit activation of T cells by H2S.
Thrombospondin-1 inhibits H2S -induced ERK1/2 phosphorylation in activated T cells.
Thrombospondin-1 limits activation-induced H2S production by inhibiting expression of cystathionine β-synthase and γ-lyase.
Thrombospondin-1 signaling through CD47 is the first identified endogenous inhibitor of H2S signaling.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- ERK
Extracellular signal-regulated kinases
- IL-2
Interleukin-2
- MEK
Mitogen-activated protein kinase kinase
- TCR
T cell antigen receptor
- TSP1
Thrombospondin-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Regulation of integrin function by CD47 ligands. Differential effects on avb3 and a4b1 integrin-mediated adhesion. J Biol Chem. 2002;277:42859–42866. doi: 10.1074/jbc.M206849200. [DOI] [PubMed] [Google Scholar]
- Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. British journal of pharmacology. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- Bouguermouh S, Van VQ, Martel J, Gautier P, Rubio M, Sarfati M. CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol. 2008;180:8073–8082. doi: 10.4049/jimmunol.180.12.8073. [DOI] [PubMed] [Google Scholar]
- Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem. 2004a;279:41734–41743. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, Roberts DD. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by a6b1 integrin. J Biol Chem. 2003;278:40679–40687. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD. α4β1 integrin mediates selective endothelial cell responses to thrombospondins in vitro and modulates angiogenesis in vivo. Circ Res. 2004b;94:462–470. doi: 10.1161/01.RES.0000115555.05668.93. [DOI] [PubMed] [Google Scholar]
- Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran L, He C-Z, Al-Barazi HO, Krutzsch HC, Iruela-Arispe ML, Roberts DD. Cell contact-dependent activation of α3β1 integrin modulates endothelial cell responses to thrombospondin-1. Mol. Biol. Cell. 2000;11:2885–2900. doi: 10.1091/mbc.11.9.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci U S A. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Anuar FBM, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. British journal of pharmacology. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Dal-Secco D, Verri WA, Jr, Guerrero AT, Souza GR, Vieira SM, Lotufo CM, Neto AF, Ferreira SH, Cunha FQ. Dual role of hydrogen sulfide in mechanical inflammatory hypernociception. Eur J Pharmacol. 2008;590:127–135. doi: 10.1016/j.ejphar.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2004;85:154–159. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
- Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Feitsma K, Hausser H, Robenek H, Kresse H, Vischer P. Interaction of thrombospondin-1 and heparan sulfate from endothelial cells. Structural requirements of heparan sulfate. J Biol Chem. 2000;275:9396–9402. doi: 10.1074/jbc.275.13.9396. [DOI] [PubMed] [Google Scholar]
- Fortin G, Raymond M, Van VQ, Rubio M, Gautier P, Sarfati M, Franchimont D. A role for CD47 in the development of experimental colitis mediated by SIRPalpha+CD103- dendritic cells. J Exp Med. 2009;206:1995–2011. doi: 10.1084/jem.20082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem Res Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- Gao A-G, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Gao AG, Frazier WA. Identification of a receptor candidate for the carboxyl-terminal cell binding domain of thrombospondins. J Biol Chem. 1994;269:29650–29657. [PubMed] [Google Scholar]
- Ge Y, Konrad MA, Matherly LH, Taub JW. Transcriptional regulation of the human cystathionine beta-synthase-1b basal promoter: synergistic transactivation by transcription factors NF-Y and Sp1/Sp3. Biochem J. 2001;357:97–105. doi: 10.1042/0264-6021:3570097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo NH, Krutzsch HC, Nègre E, Vogel T, Blake DA, Roberts DD. Heparin- and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion. Proc Natl Acad Sci U S A. 1992;89:3040–3044. doi: 10.1073/pnas.89.7.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD. Identification of CD47 and amyloid precursor-like protein-2 as the major heparan sulfate proteoglycans on T lymphocytes and this isoform of CD47 as the signaling receptor for thrombospondin-1. J Biol Chem. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova SA, Sharrow SO, Lawler J, Roberts DD. CD44 Expression in immune cells is dysregulated in thrombospondin-1 null mice. In: Balazs E, Hascall V, editors. Hyaluronan: Its structure, metabolism, biological activities and therapeutic applications. Edgewater, NJ: Matrix Biology Institute; 2005. pp. 809–812. [Google Scholar]
- Kwon J, Devadas S, Williams MS. T cell receptor-stimulated generation of hydrogen peroxide inhibits MEK-ERK activation and lck serine phosphorylation. Free Radic Biol Med. 2003;35:406–417. doi: 10.1016/s0891-5849(03)00318-6. [DOI] [PubMed] [Google Scholar]
- Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ge B, Nicotra M, Stern JN, Kopcow HD, Chen X, Strominger JL. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:3017–3022. doi: 10.1073/pnas.0712310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Li SS, Forslow A, Sundqvist KG. Autocrine regulation of T cell motility by calreticulin-thrombospondin- 1 interaction. J Immunol. 2005;174:654–661. doi: 10.4049/jimmunol.174.2.654. [DOI] [PubMed] [Google Scholar]
- Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006b;108:3112–3120. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- Li Z, Calzada MJ, Sipes JM, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Interactions of thrombospondins with alpha4beta1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157:509–519. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, He L, Wilson K, Roberts D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol. 2001;166:2427–2436. doi: 10.4049/jimmunol.166.4.2427. [DOI] [PubMed] [Google Scholar]
- Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean KN, Kraus E, Kraus JP. The dominant role of Sp1 in regulating the cystathionine betasynthase-1a and-1b promoters facilitates potential tissue-specific regulation by Kruppel-like factors. J Biol Chem. 2004;279:8558–8566. doi: 10.1074/jbc.M310211200. [DOI] [PubMed] [Google Scholar]
- Miller TW, Isenberg JS, Roberts DD. Molecular regulation of tumor angiogenesis and perfusion via redox signaling. Chem Rev. 2009;109:3099–3124. doi: 10.1021/cr8005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Isenberg JS, Roberts DD. Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol. 2010;159:1542–1547. doi: 10.1111/j.1476-5381.2009.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, Amarnath S, Fowler DH, Roberts DD. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem. 2012;287:4211–4221. doi: 10.1074/jbc.M111.307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Iozzo RV. Thrombospondins in physiology and disease: new tricks for old dogs. Matrix Biol. 2012;31:152–154. doi: 10.1016/j.matbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre E, Vogel T, Levanon A, Guy R, Walsh TJ, Roberts DD. The collagen binding domain of fibronectin contains a high affinity binding site for Candida albicans. J Biol Chem. 1994;269:22039–22045. [PubMed] [Google Scholar]
- Nejmeddine M, Negi VS, Mukherjee S, Tanaka Y, Orth K, Taylor GP, Bangham CR. HTLV-1-Tax and ICAM-1 act on T-cell signal pathways to polarize the microtubule-organizing center at the virological synapse. Blood. 2009;114:1016–1025. doi: 10.1182/blood-2008-03-136770. [DOI] [PubMed] [Google Scholar]
- Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl 3):iii37–iii40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR, Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal. 2009;12:1219–1234. doi: 10.1089/ars.2009.2921. [DOI] [PubMed] [Google Scholar]
- Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- Punekar S, Zak S, Kalter VG, Dobransky L, Punekar I, Lawler JW, Gutierrez LS. Thrombospondin 1 and its mimetic peptide ABT-510 decrease angiogenesis and inflammation in a murine model of inflammatory bowel disease. Pathobiology. 2008;75:9–21. doi: 10.1159/000113790. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Mazzalupo S, Boitano S, Montfort WR. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry. 2011;50:7787–7799. doi: 10.1021/bi201060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Green JM, Lindberg FP, Ticchioni M, Brown EJ. Cell spreading distinguishes the mechanism of augmentation of T cell activation by integrin-associated protein/CD47 and CD28. Int Immunol. 1999;11:707–718. doi: 10.1093/intimm/11.5.707. [DOI] [PubMed] [Google Scholar]
- Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci U S A. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med. 2012;52:2276–2283. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Stein EV, Rogers NM, Sharifi-Sanjani M, Isenberg JS, Roberts DD. Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther Targets. 2013;17:89–103. doi: 10.1517/14728222.2013.733699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, Roberts DD, Mosher DF, Tuszynski GP, Marcinkiewicz C. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Mernaugh RL, Friedman DB, Weller R, Tsuboi N, Yamashita H, Quaranta V, Takahashi T. Thrombospondin-1 acts as a ligand for CD148 tyrosine phosphatase. Proc Natl Acad Sci U S A. 2012;109:1985–1990. doi: 10.1073/pnas.1106171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BH, Wong PT, Bian JS. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochemistry international. 2009;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- Tripatara P, Patel NS, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM, Fantozzi R, Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest. 2008;88:1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- Tulasne D, Judd BA, Johansen M, Asazuma N, Best D, Brown EJ, Kahn M, Koretzky GA, Watson SP. C-terminal peptide of thrombospondin-1 induces platelet aggregation through the Fc receptor gamma-chain-associated signaling pathway and by agglutination. Blood. 2001;98:3346–3352. doi: 10.1182/blood.v98.12.3346. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Cai WJ, Zhu YC. Mechanisms of angiogenesis: role of hydrogen sulphide. Clin Exp Pharmacol Physiol. 2010;37:764–771. doi: 10.1111/j.1440-1681.2010.05371.x. [DOI] [PubMed] [Google Scholar]
- Wilson KE, Li Z, Kara M, Gardner KL, Roberts DD. b1 integrin- and proteoglycan-mediated stimulation of T lymphoma cell adhesion and mitogen-activated protein kinase signaling by thrombospondin-1 and thrombospondin-1 peptides. J Immunol. 1999;163:3621–3628. [PubMed] [Google Scholar]
- Yabkowitz R, Dixit VM, Guo N, Roberts DD, Shimizu Y. Activated T-cell adhesion to thrombospondin is mediated by the α4β1 (VLA-4) and α5β1 (VLA-5) integrins. J Immunol. 1993;151:149–158. [PubMed] [Google Scholar]
- Yan Q, Murphy-Ullrich JE, Song Y. Molecular and structural insight into the role of key residues of thrombospondin-1 and calreticulin in thrombospondin-1-calreticulin binding. Biochemistry. 2011;50:566–573. doi: 10.1021/bi101639y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pei Y, Teng H, Cao Q, Wang R. Specificity protein-1 as a critical regulator of human cystathionine gamma-lyase in smooth muscle cells. J Biol Chem. 2011;286:26450–26460. doi: 10.1074/jbc.M111.266643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak S, Treven J, Nash N, Gutierrez LS. Lack of thrombospondin-1 increases angiogenesis in a model of chronic inflammatory bowel disease. International journal of colorectal disease. 2008;23:297–304. doi: 10.1007/s00384-007-0397-5. [DOI] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2007;292:L960–L971. doi: 10.1152/ajplung.00388.2006. [DOI] [PubMed] [Google Scholar]