Abstract
The aim of the present study was to evaluate the effects of various physical interventions on the function of epididymal rat spermatozoa and determine whether there are correlations among these functional parameters. Epididymal rat spermatozoa were subjected to various mechanical (pipetting, centrifugation and Percoll gradient separation) and anisotonic conditions, and sperm motility, plasma membrane integrity (PMI), mitochondrial membrane potential (MMP) and intracellular reactive oxygen species (ROS) were evaluated. Repeated pipetting caused a loss in motility, PMI and MMP (P < 0.05). Minimal centrifugation force (200g) had no effect on motility, PMI and MMP, whereas an increase in the centrifugation force to 400g or 600g decreased sperm function (P < 0.005). Percoll gradient separation increased total motility, PMI and MMP (P < 0.05). However, the spermatozoa that were subjected to mechanical interventions showed high susceptibility to a ROS stimulant (P < 0.005). Anisotonic conditions decreased motility, PMI and MMP, and hypotonic conditions in particular increased basal ROS (P < 0.05). In correlation tests, there were strong positive correlations among total motility, PMI and MMP, whereas ROS showed no or negatively weak correlations with the other parameters. In conclusion, the physical interventions may act as important variables, affecting functional parameters of epididymal rat spermatozoa. Therefore, careful consideration and proper protocols for handling of rat spermatozoa and osmotic conditions are required to achieve reliable results and minimise damage.
Additional keywords: mitochondrial membrane potential, osmotic condition, physical interventions
Introduction
The laboratory rat has been one of the most important species in biomedical and genomic research. Rats are widely used as animal models in studies for human biology, toxicology and biomedicine (Lazar et al. 2005; Si et al. 2006). In particular, cryopreservation of rat spermatozoa has great importance for genome banking of transgenic and mutant rat lines, as well as for studies of rat sperm biology, because sperm cryopreservation is a simple and cost-effective method to maintain strains (Nakatsukasa et al. 2003; Varisli et al. 2009b). However, rat spermatozoa are very sensitive to mechanical stresses associated with sperm handling and osmotic stress (Si et al. 2006; Varisli et al. 2009a). Therefore, optimal manipulation conditions for rat spermatozoa are required for successful reproductive, cryobiological, cellular and molecular studies by minimising changes in sperm function caused by external damage (Varisli et al. 2009a). During the course of handling upon recovery, spermatozoa are often placed into various media and undergo multiple pipetting and centrifugation combined with gradient separation to obtain a motile sperm population for further reproductive or cryopreservation studies. However, there is a potential for substantial loss of motility because of mishandling of the sperm samples, which may ultimately cause errors in experimental results.
Several previous studies have found that rodent spermatozoa may be susceptible to mechanical stresses, such as pipetting, mixing and centrifugation (Katkov and Mazur 1998, 1999). Because centrifugation and resuspension of the resulting pellet subject cells to mechanical forces due to close packing, they may adversely affect cell viability (Katkov and Mazur 1998). Centrifugation damages the human sperm acrosome and causes significant loss of sperm motility (Mack and Zaneveld 1987; Alvarez et al. 1993) and enzymatic activity in mouse spermatozoa (Benau and Storey 1987). Moreover, centrifugation can stimulate reactive oxygen species (ROS) production in human spermatozoa and may be detrimental because of the production of a burst of ROS (Aitken and Clarkson 1988).
Percoll has been most extensively used as the density gradient centrifugation medium to remove the non-viable sperm fraction. However, the effect of Percoll on sperm function has been contentious (Henkel and Schill 2003). Percoll adheres to the sperm membranes and may alter them by removing coating envelopes (Tanphaichitr et al. 1988). Therefore, intensive washing of the spermatozoa after sperm separation with Percoll has been recommended (Arcidiacono et al. 1983). This requires additional centrifugation and can be harmful to the spermatozoa because of the action of ROS (Aitken and Clarkson 1988). Conversely, another study in boar spermatozoa reported that Percoll separation could compensate by eliminating dead cells and cells with cytoplasmic droplets and debris that generate a high proportion of ROS (Matás et al. 2011).
It has been reported that osmotically induced changes in volume can cause stress in rodent spermatozoa (Willoughby et al. 1996; Songsasen and Leibo 1997; Koshimoto et al. 2000). Rodent spermatozoa in particular have limited tolerance for changes in cell volume, with increasing volume changes causing loss of cell viability (Agca et al. 2002; Walters et al. 2005; Si et al. 2006). Osmotic stress caused by cryopreservation procedures results in a substantial degree of sublethal and lethal damage (Correa et al. 2007). When cryoprotective agents (CPAs) are added and removed from cells during cryopreservation, the cells undergo transient volume changes as a result of the anisotonic nature of these solutions. However, cells undergo a series of further osmotic volume changes as extracellular ice forms at subzero temperatures, as well as during the thawing and dilution process (Mazur 1984), before they reach osmotic equilibrium. These osmotic changes during the freezing and thawing process can cause irreversible damage to spermatozoa (Agca et al. 2005; Meyers 2005).
It has been known that increased ROS have adverse effects on sperm function (Agarwal et al. 2008; Tremellen 2008). It was recently reported that the osmotic stress induced by cryopreservation also causes oxidative stress to spermatozoa (McCarthy et al. 2010). High levels of ROS increase mitochondrial membrane permeability, interrupting respiratory chain and ATP production and decreasing phosphorylation of axonemal proteins (O’Flaherty et al. 2006), thus affecting sperm quality (Suresh et al. 2010). In addition to ROS evaluation, changes in mitochondrial membrane potential (MMP) could be a good indicator of a functional impairment because the mitochondria of the sperm midpiece generate energy to support motility (Ericsson et al. 1993). However, the effects of various mechanical stresses and anisotonic conditions on MMP and ROS of epididymal rat spermatozoa have not been studied. There is still a lack of information regarding the mechanism by which rat spermatozoa are affected by physical interventions.
The primary aim of the present study was to examine the effects of physical stress factors on MMP and intracellular ROS. First, the effects of pipetting, centrifugation and Percoll gradient separation on sperm function were evaluated. Second, using sucrose as a non-permeating CPA, hypotonic and hypertonic conditions were modelled to experimentally test osmotically induced rat sperm function. In addition, correlations among sperm viability parameters were evaluated to better understand their relationships.
Materials and methods
Animals
Twenty-three sexually mature male rats (12–15 weeks old; outbred Sprague-Dawley strain) were used as sperm donors. Rats were housed in conventional rat cages at 20–25°C in a controlled light environment (10 h dark–14 h light) and provided free access to water and standard rodent chow. Rats were housed in accordance with the policies of the University of Missouri Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals.
Sperm collection
Unless stated otherwise, all chemicals were purchased from Sigma Chemical (St Louis, MO, USA). Male rats were killed by CO2 inhalation. The cauda epididymides were excised and then placed in 35-mm Petri dishes containing HEPES-buffered Tyrode lactate (TL-HEPES) solution (300 mOsmol kg−1, pH 7.4; Bavister et al. 1983) containing 3 mg mL−1 bovine serum albumin (BSA) and 0.11 mg mL−1 pyruvic acid. Each cauda epididymis was then cut at several places using a fine scissors to allow the spermatozoa to swim out for 10 min at 37°C. The sperm suspension was then gently drawn into a plastic Samco transfer pipette (San Fernando, CA, USA) with an inner diameter of 2 mm and placed in 1.5-mL tubes. The initial sperm concentrations were approximately 50 × 106 spermatozoa mL−1 and only semen with ≥70% motility was used for the present study.
Experimental design 1: mechanical stresses in spermatozoa
Spermatozoa (n = 15 rats) were diluted with TL-HEPES at concentrations of 2 × 106 spermatozoa mL−1 for the control, pipetting and centrifugation treatment groups, whereas raw spermatozoa, without dilution, were used for Percoll gradient separation. First, sperm motility, plasma membrane integrity (PMI), MMP, and intracellular ROS were compared between the control group (diluted sperm without treatment) and the group subjected to pipetting. Second, these sperm parameters were compared between the control and centrifugation (including Percoll gradient) groups. All sperm treatments and evaluations were conducted at 37°C.
Pipetting treatment
A 1-mL aliquot of diluted semen was transferred to a 1.5-mL Eppendorf centrifuge tube and subjected to six successive gentle pipettings using pipette tips with a 1 mL capacity (Pipetman P-1000; Gilson, Middleton, WI, USA).
Centrifugation treatment
A 1-mL aliquot of diluted semen was transferred to a 1.5-mL Eppendorf tube and centrifuged at 200g, 400g or 600g average force for 10 min. After the centrifugation procedure, the supernatant was gently removed and 1 mL TL-HEPES solution was slowly added to resuspend the sperm pellet by gentle rotation of the tube.
Percoll gradient separation
The sperm separation procedure via the Percoll gradient was performed as described previously (Parrish et al. 1995) with some modification. Briefly, a concentrated Solution I (31 mM KCl, 800 mM NaCl, 3 mM NaH2PO4 and 100 mM HEPES) was adjusted to pH 7.3 with 1 M NaOH. The concentrated Solution II was then freshly made by adding the following chemicals to the concentrated Solution I (final concentrations in mM): CaCl2 20; MgCl2 4; lactic acid 216; NaHCO3 250. To prepare isotonic Percoll solution (100% Percoll solution), anisotonic Percoll solution was mixed 12:1 with concentrated Solution II (300 mOsmol kg−1). To prepare 90% Percoll solution, the 100% isotonic Percoll solution was mixed 9:1 with TL-HEPES; to prepare the 45% Percoll solution, the 90% Percoll solution was mixed 1:1 with TL-HEPES. The Percoll solutions were equilibrated at 37°C under 5% CO2 in air for 2 h. The raw semen was layered on a discontinuous gradient of 45% and 90% Percoll. The gradient consisted of 150 μL rat spermatozoa layered over 0.5 mL of 45% Percoll and 0.5 mL of 90% Percoll in a 1.5-mL Eppendorf centrifuge tube. The tubes were then centrifuged at 600g average force for 15 min at 30°C. At the end of the centrifugation procedure, the supernatant was gently removed and 1 mL TL-HEPES solution was added to the tube containing the sperm pellet to resuspend it by gentle rotation. The resuspended spermatozoa were then washed by centrifugation at 200g for 5 min and were adjusted to a concentration of 2 × 106 spermatozoa mL−1 with TL-HEPES for further evaluation.
Experimental design 2: osmotic stress in spermatozoa
Hypotonic solution (150 mOsmol kg−1, pH 7.4) was prepared by adding appropriate amounts of sucrose to NaCl-free TL-HEPES solution, whereas the hypertonic solution (450 mOsmol kg−1, pH 7.4) was made by adding sucrose to isotonic TL-HEPES solution (300 mOsmol kg−1, pH 7.4). The osmolalities of the solutions were measured using freezing point depression (VAPRO 5520; Wescor, Logan, UT, USA) with an accuracy of ±5 mOsmol. Prior to use, all solutions were supplemented with 3 mg mL−1 BSA and 0.11 mg mL−1 pyruvic acid. Sperm suspensions (10-μL aliquots; from n = 8 rats) were added to each of three 1.5-mL Eppendorf centrifuge tubes containing isotonic (control), hypotonic and hypertonic solutions. The spermatozoa were equilibrated in these solutions with different osmolalities for 5 min and were then returned to near isotonicity (290–300 mOsmol kg−1) by the addition of an appropriate volume of TL-HEPES solution. Sperm suspensions were equilibrated for 5 min and parameters of sperm were evaluated after each treatment. All experiments were performed at room temperature.
Computer-assisted sperm analysis
Computer-assisted sperm analysis (CASA; M2030; Hamilton Thorne Biosciences, Beverly, MA, USA) was used to determine total motility, progressive motility and average path velocity (VAP) by using fixed-depth (80 μm), dual-sided sperm-counting chambers (2 × CELL; Hamilton Thorne Biosciences) at 37°C. Six fields were counted for each sample. The settings and definition of sperm motion parameters for CASA have been described previously (Varisli et al. 2009a). Briefly, progressive sperm were defined as ratio of motile spermatozoa with VAP >100 μm s−1 and straightness (STR) >50% to total number of spermatozoa.
Evaluation of sperm PMI
A SYTO 10/propidium iodide (PI) stain (L7013 and L7011, respectively; Molecular Probes, Eugene, OR, USA) was used to determine rat sperm PMI according to the manufacturer’s instructions with some modifications. Briefly, 200-μL aliquots of sperm suspension (2 × 106 spermatozoa mL−1) were mixed with A SYTO 10 (final concentration 1:1000 dilution of SYTO stock solution provided by the manufacturer) and PI (final concentration 1 μM). The mixture was incubated at 37°C for 15 min in 5% CO2 and was then analysed by flow cytometry. Spermatozoa that were SYTO+/PI− were considered to have an intact plasma membrane.
Evaluation of sperm MMP
Sperm MMP was evaluated using JC-1 fluorescent dye (M34152; Molecular Probes) using the method described previously (Guthrie and Welch 2006). The JC-1 dye can be used to distinguish spermatozoa with poorly and highly functional mitochondria. In poorly functional mitochondria, JC-1 remains in the monomeric state and fluoresces green (Jmono). However, in highly functional mitochondria, JC-1 forms an aggregate (Jagg+) that fluoresces orange. For evaluation of MMP in viable spermatozoa, 200-μL aliquots of sperm suspension (2 × 106 spermatozoa mL−1) were mixed with JC-1 (final concentration 0.5 μM) and PI (final concentration 2 μM). The mixture was incubated at 37°C for 30 min in 5% CO2 and was then analysed by flow cytometry. The labelled spermatozoa were classified on the basis of the intensity of the orange colour as follows (Fig. 1): spermatozoa that were bright orange (bright Jagg+; high MMP); spermatozoa that were faint orange (faint Jagg+; intermediate MMP); and the sum of the two populations (Jagg+) in the total and viable (PI−) sperm fraction. The percentage of bright Jagg+ spermatozoa and mean Jagg fluorescence intensity (JaggMFI) were evaluated in total and viable sperm populations. In addition, to determine the distribution of spermatozoa with high MMP in the population of viable spermatozoa, the ratio of bright Jagg+ to viable spermatozoa (%) was calculated as follows: (bright Jagg+/PI− spermatozoa [%])/(PI− spermatozoa [%]) × 100.
Fig. 1.
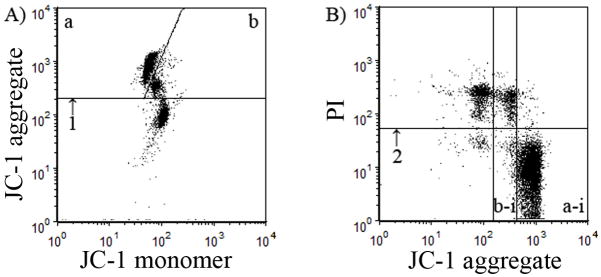
Representative flow cytometric dot plots of epididymal rat spermatozoa stained with JC-1 and propidium iodide (PI). (a) Mitochondrial membrane potential (MMP) was evaluated according to the JC-1 aggregate fluorescence intensity. Cells above the line at (iii) were considered to be Jagg+ spermatozoa (i.e. those spermatozoa in which JC-1 had formed an aggregate that fluoresced orange), whereas below the line were cells considered to be Jagg− (or Jmono) spermatozoa (i.e. those in which JC-1 remained in the monomeric state and fluoresced green). The Jagg+ sperm were then divided into bright Jagg+ (i) and faint Jagg+ (ii) sperm. (b) To evaluate mitochondrial function in the viable sperm population, PI was combined with JC-1 staining. Cells above the line at (iv) were considered viable (PI−) spermatozoa, with viable spermatozoa classified into bright Jagg +/PI− (vi) and faint Jagg +/PI− (v) spermatozoa.
Determination of intracellular ROS before and after induction of ROS
Basal (unstimulated) intracellular ROS levels were measured in the absence of the common inducer of ROS production tert-butyl hydroperoxide (TBHP; I36007; Molecular Probes). Stimulated intracellular ROS was measured by incubating spermatozoa with TBHP (final concentration 100 μM) at 37°C for 30 min in 5% CO2 to induce ROS.
Production of ROS was measured by using an oxidation-sensitive fluorescent probe, namely 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; I36007; Molecular Probes), and methods based on the ROS-dependent oxidation of DCFDA to DCF. Briefly, DCFDA (final concentration 10 μM) and PI (final concentration 1 μM) were added to the sperm suspension (2 × 106 spermatozoa mL−1). The mixture was incubated at 37°C for 40 min in the dark and the labelled spermatozoa were analysed by flow cytometry (Fig. 2). Dead (PI+) sperm were excluded by using a counterstain dye (PI). Intracellular ROS levels were evaluated on the basis of mean DCF fluorescence intensity (DCFMFI), with a higher DCFMFI considered to be an indicator of higher intracellular ROS. The DCFMFI per total (i.e. sum of viable and dead) and viable spermatozoa, and the percentage of viable spermatozoa were analysed before and after TBHP treatment. To evaluate susceptibility of rat spermatozoa to external ROS induction (ROS generating rate), the fold increase in DCFMFI after TBHP treatment was calculated as follows: (stimulated DCFMFI)/(basal DCFMFI). In addition, the decreased rate in viability after TBHP treatment was calculated as: 100 − ([% viability after TBHP treatment]/[% viability before TBHP treatment] × 100).
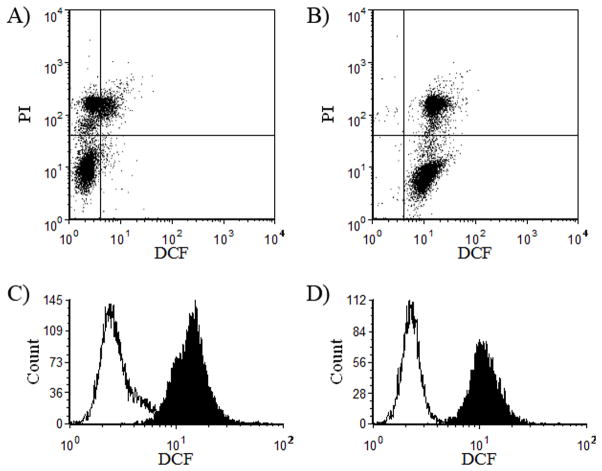
Fig. 2.
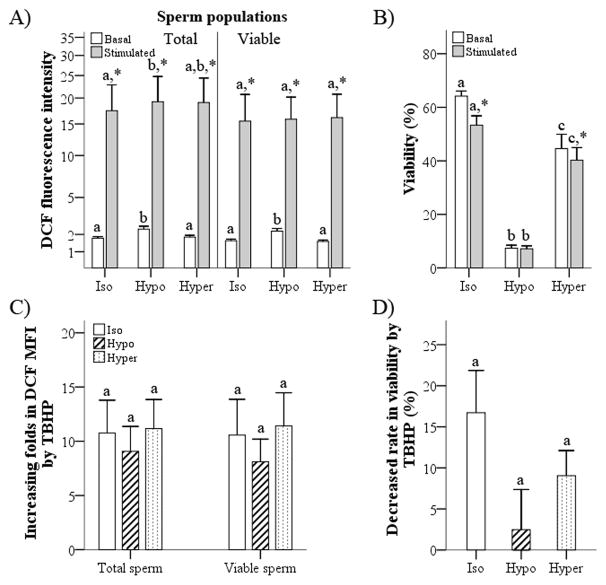
Flow cytometric analysis of epididymal rat spermatozoa stained with 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) and propidium iodide (PI). (a, b) Dot plot cytograms before (a) and after (b) stimulation with tert-butyl hydroperoxide (TBHP). In these cytograms, the lower quadrants represent viable (PI−) spermatozoa and the upper quadrants represent dead (PI+) spermatozoa. (c, d) Intracellular reactive oxygen species levels in spermatozoa were evaluated according to mean DCF fluorescence intensity (DCFMFI) before (open) and after stimulation (shaded) with TBHP in total (c) and viable (d) sperm populations.
Flow cytometric analysis
Samples were acquired using a FACSCalibur flow cytometer (Becton Dickinson, San José, CA, USA) equipped with a 15-mW air-cooled 488-nm argon ion laser. A detailed description of fluorescences, machine set up and data acquisition according to the MIFlowCyt Standards (Lee et al. 2008) can be found in the documentation available as Supplementary Material to this paper. FL1 (SYTO 10, Jmono and DCF) signals were detected through a 530/30-nm band-pass filter, the FL2 (Jagg) signal was detected through a 585/42-nm band-pass filter and the FL3 (PI) signal was detected through a 670-nm long-pass filter in logarithmic scales. A total of 10000 individual sperm-sized events was recorded in list mode at a flow rate of <400 events s−1 and analysed using Cell Quest Pro software (Becton Dickinson). The sperm population was first gated on the basis of the forward and side scatter (FSC/SSC) properties in linear mode. To assess and exclude non-DNA-containing alien particles, such as cytoplasmic droplets, cell debris or diluent components, material unstained by the nuclear probes SYTO 10 and PI was back-gated to a light scatter plot, where it distributes within the sperm light-scatter region. Using the data obtained from the procedure, the percentages of all data were then corrected for non-sperm particles (Petrunkina et al. 2010; Petrunkina and Harrison 2011).
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA) to determine the effects of physical stress factors on motility, PMI, MMP and intracellular ROS. The Shapiro–Wilk test was used to assess the normal distribution of the parameters. For comparisons between pipetting and control groups, paired t-tests were performed for all sperm parameters. For comparisons of different centrifugation factors with the control group, the Mann–Whitney U-test was performed after each value from different centrifugation factors were normalised against control. For comparisons among osmotic conditions, one-way repeated-measures analysis of variance was used for data with a normal distribution and, if there were significant differences, Bonferroni adjustment for multiple comparisons was used for post hoc analysis. Otherwise, the non-parametric Friedman test was used for data that were not normally distributed. When significant differences were found with the Friedman test, post hoc analysis with Wilcoxon’s signed-rank test was conducted with Bonferroni correction. For comparisons before and after treatment with TBHP, paired t-tests or Wilcoxon’s signed-rank test was performed according to the results of normality analysis. Correlations among sperm functional parameters were evaluated using Pearson’s or Spearman’s correlation depending on the normality of the data. Statistical significance was set at P < 0.05 and all tests were two-tailed. Values are presented as the mean ± s.e.m.
Results
Effect of mechanical stresses on rat sperm function
Pipetting resulted in a loss of total and progressive motility and PMI (P < 0.005; Table 1). Pipetting also decreased the mitochondrial function of rat spermatozoa, evidenced as decreases in the percentage of bright Jagg+ and bright Jagg+/PI− spermatozoa (P <0.001), the ratio of bright Jagg+ to viable spermatozoa (P < 0.05) and JaggMFI per total spermatozoa (P < 0.05; Table 1).
Table 1.
Effect of pipetting on motility, plasma membrane integrity and mitochondrial membrane potential in epididymal rat spermatozoa
| Sperm functional parameter | Control | Pipetting |
|---|---|---|
| Motility characteristics | ||
| Total motility (%) | 81.83 ± 2.60 | 63.78 ± 4.41* |
| Progressive motility (%) | 24.19 ± 1.11 | 18.74 ± 1.13* |
| Average path velocity (μm s−1) | 132.21 ± 3.07 | 126.04 ± 6.67 |
| Intact plasma membrane (%) | 65.95 ± 3.56 | 47.39 ± 3.76* |
| High MMP (intensity of orange JC-1 staining) | ||
| Bright Jagg+ (%) | 62.33 ± 5.12 | 39.02 ± 3.81* |
| Bright Jagg+/PI− (%) | 59.45 ± 4.76 | 35.71 ± 3.45* |
| Ratio of bright Jagg+ to viable spermatozoa (%) | 91.74 ± 2.32 | 81.20 ± 4.51* |
| JaggMFI/total spermatozoa | 374.40 ± 52.58 | 264.01 ± 33.98* |
| JaggMFI/viable spermatozoa | 635.67 ± 57.83 | 612.78 ± 83.00 |
Data are the mean ± s.e.m. (n = 9 for motility and plasma membrane integrity; n = 8 for mitochondrial membrane potential [MMP]). Within rows, different superscript letters indicate significant differences (P < 0.05).
P < 0.05 compared with control.
Jagg, formation of an aggregate by JC-1 that fluoresces orange; MFI, mean fluorescence intensity; PI, propidium iodide
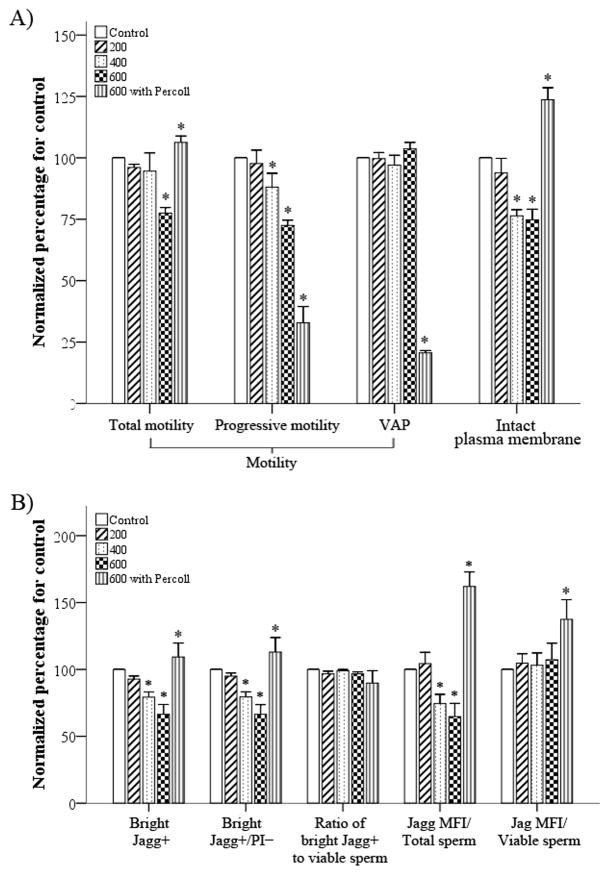
The minimal centrifugation force of 200g did not affect motility, PMI or MMP (P > 0.05), whereas increasing centrifugation forces decreased sperm function, with a loss of total motility after centrifugation at 600g and losses in progressive motility, PMI and MMP after centrifugation at 400g and 600g (P < 0.005; Fig. 3). However, Percoll gradient separation at 600g increased the percentage of total motile spermatozoa and the number of spermatozoa with an intact plasma membrane and high MMP (P < 0.05), but decreased progressive motility and VAP (P < 0.001; Fig. 3).
Fig. 3.
Effects of different centrifugation regimens on the (a) motility, plasma membrane integrity (PMI) and (b) mitochondrial membrane potential (MMP) of epididymal rat spermatozoa. Spermatozoa were subjected to different centrifugation treatments (200g, 400g, 600g or 600g with Percoll gradient medium) for evaluation of motility (a), PMI (a) and MMP (b). T-motility, total motility; P-motility, progressive motility; VAP, average path velocity; Jagg, those spermatozoa in which JC-1 had formed an aggregate that fluoresced orange; MFI, mean fluorescence intensity; PI, propidium iodide. Data are the mean ± s.e.m. (n = 10 for 200g; n = 4 for 400g and 600g; n = 8 for 600g with Percoll gradient medium). *P < 0.05 compared with control.
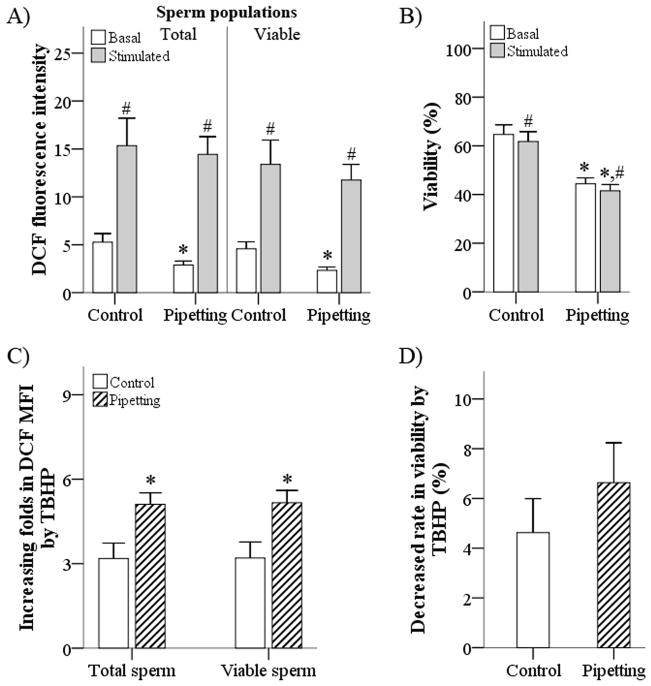
Basal DCFMFI (basal intracellular ROS) was significantly decreased by pipetting (P < 0.005), whereas stimulated intracellular ROS did not differ significantly different between the pipetting and control groups in either the total or viable sperm populations (Fig. 4a). Rat spermatozoa that were subjected to pipetting and those in the control group were susceptible to TBHP treatment, exhibiting an increase in DCFMFI (P < 0.005; Fig. 4a) and a decrease in the percentage of viable spermatozoa (P < 0.05; Fig. 4b) after stimulation with TBHP compared with untreated samples. In particular, spermatozoa that were subjected to pipetting were highly susceptible to the induction of intracellular ROS formation, exhibiting a higher rate of ROS generation in response to TBHP compared with the control group (P < 0.005; Fig. 4c). However, there were no significant differences in the decreases in viability after TBHP treatment between the two groups (P > 0.05; Fig. 4d).
Fig. 4.
Effects of pipetting on intracellular reactive oxygen species (ROS) levels in epididymal rat spermatozoa. (a) To evaluate the effect of pipetting on intracellular ROS in epididymal rat spermatozoa, DCF mean fluorescence intensity (MFI) was measured under basal (without tert-butyl hydroperoxide [TBHP] treatment) and stimulated (+TBHP) conditions in total and viable sperm populations that were subjected to pipetting. (b) In addition, the percentage of viable spermatozoa was evaluated under basal and stimulated conditions after pipetting of sperm. (c, d) To determine susceptibility to the ROS-stimulating product, fold increases in DCFMFI (c) and decreases in viability (d) following TBHP treatment were calculated. C, control; P, pipetting. Data are the mean ± s.e.m. (n = 8). *P < 0.05 compared with control. †P < 0.05 compared with basal ROS production.
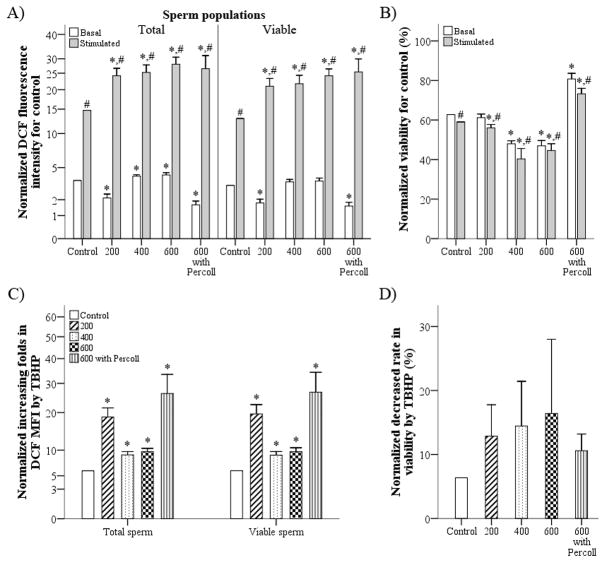
Minimal centrifugation at 200g decreased basal intracellular ROS in total and viable sperm populations, whereas centrifugation at 400g and 600g increased basal intracellular ROS in the total sperm population (P < 0.005, Fig. 5a). Percoll gradient separation at 600g decreased basal intracellular ROS in total and viable sperm populations (P < 0.001; Fig. 5a). However, all centrifugation conditions resulted in an increase in stimulated intracellular ROS compared with the control group in all sperm populations (P < 0.005; Fig. 5a). All rat spermatozoa that were subjected to different centrifugation conditions and the control group were susceptible to TBHP treatment, as evidenced by an increase in DCFMFI (P < 0.05; Fig. 5a and a decrease in the percentage of viable spermatozoa (P < 0.05; Fig. 5b) after TBHP treatment compared with untreated samples. Moreover, spermatozoa that were subjected to different centrifugation conditions were more susceptible to intracellular formation of ROS in response to TBHP compared with the control group (P < 0.005; Fig. 5c). However, there were no significant differences in the decreased viability after TBHP treatment between the centrifugation groups and control (P > 0.05; Fig. 5d).
Fig. 5.
Effects of different centrifugation factors on intracellular reactive oxygen species (ROS) levels in epididymal rat spermatozoa. (a) The mean fluorescence intensity (MFI) of DCF per total and viable sperm populations and (b) the percentage of viable spermatozoa were determined under basal and stimulated conditions after centrifugation of spermatozoa at different speeds. (c, d) In addition, fold increases in DCFMFI (c) and decreased viability (d) after tert-butyl hydroperoxide (TBHP) treatment were calculated. Data are the mean ± s.e.m. (n = 10 for 200g; n = 4 for 400g and 600g; n = 8 for 600g with Percoll gradient medium). *P < 0.05 compared with control. †P < 0.05 compared with basal ROS production.
Based on evaluations of samples treated by all mechanical stress factors, correlations among sperm parameters are presented in Table 2. There were strong positive correlations among total motility, PMI and MMP (P < 0.001), whereas intracellular ROS showed no or negatively weak correlations with total motility, PMI and MMP (P < 0.05). Basal ROS showed no correlations with other parameters (P > 0.05) and stimulated ROS and the rate of ROS generation showed weak negative correlations with total motility (P < 0.05).
Table 2.
Correlation analysis for epididymal rat spermatozoa subjected to mechanical stress
| Intact plasma membrane (%) | MMP (%) | Intracellular ROS in viable spermatozoa | ||||
|---|---|---|---|---|---|---|
| Total spermatozoa with high MMP | Viable spermatozoa with high MMP | Basal ROS | Stimulated ROS | Rate of ROS generation | ||
| Total motility (%) | 0.829** | 0.809** | 0.808** | 0.118 | −0.360* | −0.340* |
| Intact plasma membrane (%) | 0.809** | 0.810** | −0.044 | −0.308* | −0.227 | |
| Total spermatozoa with high MMP (%) | 0.992** | −0.162 | −0.133 | 0.123 | ||
| Viable spermatozoa with high MMP (%) | −0.151 | −0.099 | 0.144 | |||
| Basal ROS/viable spermatozoa | 0.316* | −0.333* | ||||
| Stimulated ROS/viable spermatozoa | 0.726** | |||||
Data are either Pearson or Spearman’s rho correlations, depending on whether the data were normally distributed. The r-values indicate the strength and direction (+/−) of the correlation.
Correlation is significant at the 0.05 level (two-tailed);
correlation is significant at the 0.005 level (two-tailed).
MMP, mitochondrial membrane potential; ROS, reactive oxygen species
Effect of osmotic stresses on rat sperm function
The effects of anisotonic treatments on rat sperm motility, PMI and MMP are given in Table 3. Anisotonic conditions (150 and 450 mOsmol) decreased the motility, PMI and MMP of rat epididymal spermatozoa compared with isotonic conditions (P < 0.05). In particular, the hypotonic condition caused a higher loss of motility, PMI and MMP than the hypertonic condition (P < 0.05). Almost all the spermatozoa exhibited a loss of motility, PMI and MMP after exposure to the hypotonic condition. In addition, >90% of viable spermatozoa maintained bright Jagg+ in the isotonic condition, whereas there was a significant decrease in this sperm population in anisotonic media, with the lowest percentage observed in the hypotonic condition (P < 0.05). Similarly, low JaggMFI per viable spermatozoon was observed in rat spermatozoa that had been exposed to the hypotonic condition (P < 0.005).
Table 3.
Effect of osmotic stress on motility, plasma membrane integrity and mitochondrial membrane potential in epididymal rat spermatozoa
| Sperm functional parameters | Isotonic | Hypotonic | Hypertonic |
|---|---|---|---|
| Motility characteristics | |||
| Total motility (%) | 78.12 ± 2.04a | 9.56 ± 2.11b | 50.27 ± 4.50c |
| Progressive motility (%) | 21.63 ± 0.89a | 2.54 ± 0.89b | 9.41 ± 1.96c |
| Average path velocity (μm s−1) | 169.58 ± 7.43a | 27.34 ± 4.99b | 83.96 ± 9.03c |
| Intact plasma membrane (%) | 65.20 ± 1.36a | 3.17 ± 0.61b | 48.63 ± 4.98c |
| High MMP (intensity of orange JC-1 staining) | |||
| Bright Jagg+ (%) | 61.36 ± 2.30a | 2.01 ± 0.29b | 37.85 ± 5.67c |
| Bright Jagg+/PI− (%) | 60.19 ± 2.24a | 1.80 ± 0.27b | 37.13 ± 5.67c |
| Ratio of bright Jagg+ to viable spermatozoa (%) | 93.38 ± 0.85a | 23.77 ± 5.76b | 76.85 ± 7.06c |
| JaggMFI/total spermatozoa | 702.23 ± 53.05a | 217.02 ± 18.84b | 429.54 ± 60.17c |
| JaggMFI/viable spermatozoa | 1280.53 ± 105.45a | 371.99 ± 28.54b | 1084.21 ± 137.45a |
Data are the mean ± s.e.m. (n = 8). Within rows, different superscript letters indicate significant differences (P < 0.05).
MMP, mitochondrial membrane potential; Jagg, formation of an aggregate by JC-1 that fluoresces orange; MFI, mean fluorescence intensity; PI, propidium iodide
The hypotonic condition resulted in higher basal ROS in both the total and viable sperm populations (P < 0.05), as well as higher stimulated ROS in the total sperm population compared with the isotonic condition (P < 0.05; Fig. 6a). However, there were no significant differences in basal and stimulated ROS between the isotonic and hypertonic conditions (P > 0.05). TBHP treatment increased ROS in all experimental groups (P < 0.05; Fig. 6a) and decreased the viability of spermatozoa that were exposed to isotonic and hypertonic conditions (P < 0.05; Fig. 6b). However, the susceptibility of spermatozoa to the exogenous ROS source did not differ between anisotonic and isotonic conditions, with no significant differences in ROS generating rate and decreased viability after TBHP treatment among any of the experimental groups (P > 0.05; Fig. 6c, d).
Fig. 6.
Effects of anisotonic conditions on intracellular reactive oxygen species (ROS) levels in epididymal rat spermatozoa. (a) The mean fluorescence intensity (MFI) of DCF per total and viable sperm populations and (b) the percentage of viable spermatozoa were determined under basal and stimulated conditions after exposing spermatozoa to different osmotic (isotonic, hypotonic and hypertonic) conditions. (c, d) In addition, fold increases in DCFMFI (c) and decreased viability (d) after exposure of spermatozoa to each of the osmotic conditions. Data are the mean ± s.e.m. (n = 8). *P < 0.05 compared with basal. Different letters within same sperm population and tert-butyl hydroperoxide (TBHP) treatment indicate significant differences among osmotic conditions (P < 0.05).
Table 4 lists the correlations among sperm parameters based on evaluations of samples exposed to different osmotic conditions. There were strong positive correlations among total motility, PMI and MMP (P ≤ 0.005), whereas intracellular ROS showed no or negatively moderate correlations with the sperm parameters (P < 0.05). Basal ROS showed moderate negative correlations with total motility, PMI and MMP (P < 0.05), and stimulated ROS and the rate of ROS generation showed no correlations with the other sperm parameters (P > 0.05).
Table 4.
Correlation analysis for epididymal rat spermatozoa subjected to different osmotic conditions
| Intact plasma membrane (%) | MMP (%) | Intracellular ROS in viable spermatozoa | ||||
|---|---|---|---|---|---|---|
| Total spermatozoa with high MMP | Viable spermatozoa with high MMP | Basal ROS | Stimulated ROS | Rate of ROS generation | ||
| Total motility (%) | 0.847** | 0.886** | 0.882** | −0.417* | −0.049 | 0.091 |
| Intact plasma membrane (%) | 0.977** | 0.974** | −0.537* | −0.157 | 0.062 | |
| Total spermatozoa with high MMP (%) | 0.990** | −0.485* | −0.115 | 0.099 | ||
| Viable spermatozoa with high MMP (%) | −0.455* | −0.074 | 0.124 | |||
| Basal ROS/viable spermatozoa | 0.425* | 0.207 | ||||
| Stimulated ROS/viable spermatozoa | 0.940** | |||||
Data show Spearman’s rho correlations, with the r-values indicating the strength and direction (+/−) of the correlation.
Correlation is significant at the 0.05 level (two-tailed);
correlation is significant at the 0.005 level (two-tailed).
MMP, mitochondrial membrane potential; ROS, reactive oxygen species
Discussion
The collection of epididymal rat spermatozoa and their subsequent pipetting, centrifugation and gradient centrifugation are some of the most commonly used laboratory practices in basic reproductive biology and toxicology, as well as in genome resource centres. In addition, the cryopreservation process introduces an osmotic volume excursion that may cause major physical stress to the spermatozoa. The present study was designed to evaluate the effects of these physical interventions primarily at the level of mitochondrial function and intracellular ROS production.
Similar to our previous report (Varisli et al. 2009a), pipetting decreased total motility and PMI of rat spermatozoa, whereas centrifugation with Percoll treatment increased total motility and PMI. The effect of centrifugation on sperm functional parameters can be influenced by the force and time of centrifugation (Makler and Jakobi 1981; Katkov and Mazur 1998; Nakatsukasa et al. 2001; Varisli et al. 2009a). The centrifugation force of 200g (10 min) used in the present study is considered a minimal force and, indeed, it did not affect rat sperm motility and PMI. Conversely, increasing the centrifugation force to 400g and 600g had a detrimental effect on motility and PMI of rat spermatozoa.
The osmotic pressure of cauda epididymal fluid is relatively higher than that of the seminal plasma and fluids in the female reproductive tract. Thus, cauda epididymal spermatozoa may encounter subtle osmotic stress when they are exposed to isotonic media during sperm recovery. It was previously documented that epididymal bull spermatozoa recovered in a medium with cauda epididymal plasma (360 mOsmol kg−1) exhibited better viability and volume regulatory ability than spermatozoa recovered in a medium isotonic with seminal plasma (300 mOsmol kg−1) medium (Sahin et al. 2009). This study is in agreement with a previous study in which larger loss of rat sperm motility and PMI were seen under hypotonic (150 mOsmol) than hypertonic (450 mOsmol) conditions (Si et al. 2006).
In the present study, three different staining patterns (bright, faint and no orange fluorescence) were evaluated by flow cytometric analysis as indicators of MMP. It has been suggested that mitochondria of highly motile spermatozoa appear bright orange, some of those of non-motile and slightly motile spermatozoa appear faint orange and those of non-motile spermatozoa appear green (Gravance et al. 2000, 2003). Spermatozoa that stained faint orange were clearly compromised in terms of both MMP and motility (Gravance et al. 2003). Therefore, bright Jagg+/PI− spermatozoa are considered to be highly functional spermatozoa based on MMP, viability and vigorous motility. In this respect, pipetting and centrifugation at 400g and 600g resulted in the loss of mitochondrial function, whereas centrifugation at 200g did not affect mitochondrial function. Percoll gradient separation increased mitochondrial function. However, centrifugation at 200g, 400g or 600g had no effect on the ratio of bright Jagg+ to viable spermatozoa or JaggMFI per viable spermatozoon. These results suggest that viable spermatozoa subjected to centrifugation maintain mitochondrial function without changes in MMP. Anisotonic conditions also decreased MMP and, in particular, viable spermatozoa exposed to the hypotonic condition did not maintain their MMP.
Percoll is no longer used in human infertility clinics owing to a risk of endotoxin contamination and potential deleterious effect on sperm PMI (Strehler et al. 1998). However, Percoll is still used extensively in many research laboratories (Phillips et al. 2012). In the present study, Percoll gradient separation improved sperm motility, PMI and MMP, but the recovery rate was low (20%). Although Percoll gradient separation enhanced sperm functions, this procedure has the disadvantage of producing a low final yield of spermatozoa, similar to results reported for feline and canine spermatozoa (Filliers et al. 2008; Phillips et al. 2012). Further studies are needed to determine other density gradient centrifugation media for rat spermatozoa.
Previous studies have demonstrated that mechanical and osmotic stresses increase ROS in spermatozoa (Agarwal et al. 1994; Aitken and Vernet 1998; Aitken et al. 2010; McCarthy et al. 2010). In these studies, centrifugation, resuspension and vortexing caused excessive generation of ROS in the motile human sperm population (Agarwal et al. 1994), and Percoll centrifugation increased the formation of 8OHdG (a marker of oxidative DNA damage) in viable human spermatozoa (Aitken et al. 2010). Recently, McCarthy et al. (2010) reported that, in the case of rhesus macaque spermatozoa, hypotonic and hypertonic conditions significantly increased ·O2− generation and lipid peroxidation, but not H2O2 production. In the present study, basal intracellular ROS was decreased by pipetting, centrifugation at 200g and Percoll gradient separation. With regard to osmotic challenges, although basal intracellular ROS was increased under the hypotonic condition, it was not changed in the hypertonic condition in total and viable spermatozoa. It is unclear why basal intracellular ROS was low in rat spermatozoa after mechanical disruption. It is possible that antioxidant defence mechanisms may be activated in spermatozoa subjected to the mechanical stress. Alternatively, it has been documented that Percoll gradient separation may decrease ROS levels by removing dead spermatozoa that generate high ROS (Matás et al. 2011). Although the mechanism that triggers ROS production under conditions of hypotonic stress is not known, ROS production in NIH3T3 cells following exposure to hypotonic conditions was reported to involve NADPH-dependent reduction of oxygen via NAD(P)H oxidase (Lambert 2003). It was suggested that the ROS-producing step following exposure to hypotonic conditions is mediated by an NADPH oxidase downstream to phospholipase (PLA2) activation (Lambert 2003; Lambert et al. 2006). It has reported that PLA2 is an upstream, initial element of the swelling-induced signalling cascade (Lehtonen and Kinnunen 1995). Activation of PLA2 may then lead to an increase in arachidonic acid, which subsequently activates the NADPH oxidase complex, resulting in increased ROS production (Lambert 2003; Lambert et al. 2006; McCarthy et al. 2010).
Freezing extenders that are used for rat sperm cryopreservation are typically around 450 mOsmol. In the present study, rapid addition and dilution of rat spermatozoa in 450 mOsmol sucrose solution did not increase ROS production. This could imply that osmotic stress may not be the reason for increased ROS production during the addition and removal of the freezing extender. However, ROS production may be induced during freezing process because ice nucleation of extracellular water would markedly change the osmolality of the unfrozen water faction, causing hypertonic conditions (Mazur 1984). In fact, hypertonic conditions of >500 mOsmol have been reported to increase ·O2− and lipid peroxidation in rhesus macaque spermatozoa (McCarthy et al. 2010).
Centrifugation may be critical for the increase in ROS production in spermatozoa. One report suggested that the duration of centrifugation was important for the induction of ROS formation in human semen and recommended a shorter centrifugation period during the preparation of spermatozoa for assisted reproductive techniques (Shekarriz et al. 1995). In the present study, the force of centrifugation played an important role in inducing ROS formation in rat spermatozoa. Specifically, ROS levels in total spermatozoa began to increase above basal levels at >400g force. However, stimulated ROS, including the rate of ROS generation, was increased by all centrifugation forces used. We think that ROS production under conditions without external ROS stimuli may be related to the centrifugation force, whereas ROS production under conditions with external ROS stimuli may be highly susceptible regardless of centrifugation force.
Spermatozoa are highly susceptible to ROS damage because they have a small amount of cytoplasm containing scavenging enzymes (Henkel 2005; Bansal and Bilaspuri 2008). Oxidative stress occurs in spermatozoa when ROS levels exceed the available total antioxidant capacity (Mahfouz et al. 2010). In fact, TBHP treatment significantly increased ROS levels in spermatozoa and significantly decreased sperm viability in all experimental groups, except for the hypotonic group. It is considered that all spermatozoa are highly susceptible to inducers of ROS production and the antioxidant capacity is very low in rat spermatozoa. Interestingly, responses to pipetting, centrifugation and Percoll gradient separation were more sensitive following stimulation with TBHP than in the control group. After TBHP treatment, ROS production was significantly greater in spermatozoa treated with mechanical stress factors than in the control group. Similarly, a recent study reported that Percoll separated canine spermatozoa exhibited higher susceptibility to external sources of ROS (Kim et al. 2010). In a previous study, we found that pipetting markedly reduced rat sperm motility, although it did not cause significant loss of motility of boar, bull and ram spermatozoa, indicating a higher sensitivity of rat spermatozoa to pipetting (Varisli et al. 2009a). Therefore, the increase in susceptibility to TBHP after pipetting may be a unique property of rat spermatozoa.
In contrast with our expectations, rat spermatozoa subjected to mechanical stresses did not exhibit a significant reduction in viability following TBHP treatment, despite higher ROS generation. A previous study conducted on macaque spermatozoa reported that the effects of ROS generated by the xanthine/xanthine oxidase system were dose dependent; specifically, as the concentration of ROS increased, motility decreased and lipid peroxidation increased, although there was no effect on viability (McCarthy et al. 2010). Although we did not measure motility after TBHP treatment in the present study, the high level of ROS generated by TBHP may have a more adverse effect on motility than viability in groups subjected to mechanical stress compared with control.
Mitochondria are the major site of intracellular ROS formation, which may be due to disruption or leakage of electrons during electron transport. The coupling of electron transport to oxidative phosphorylation maintains a high MMP required for mitochondrial ATP production. The coupling of electron transport with oxidative phosphorylation in spermatozoa could be disrupted by ROS formation and result in a decrease in the number of spermatozoa that maintain the high MMP required for mitochondrial ATP production and sperm motility (Guthrie and Welch 2006). Therefore, spermatozoa subjected to mechanical stresses may exhibit decreased ATP production and subsequent loss of motility due to decreased MMP during the course of laboratory manipulations, such as exposure to ROS-producing conditions.
Previous studies on boar (Gaczarzewicz et al. 2010) and rat (Gravance et al. 2000) spermatozoa reported that PMI is positively correlated with motility and the percentage of spermatozoa with high MMP. These results are consistent with those of the present study in that we also found that total motility, PMI and high mitochondrial function were highly correlated with each other. However, no or only weak or moderate correlations were found between ROS and these parameters. Therefore, it may be difficult to predict the quality of rat spermatozoa on the basis of ROS levels, because ROS may be independent of motility, PMI and mitochondrial function.
In conclusion, pipetting decreased total motility, PMI and MMP. Centrifugation at 200g did not affect motility, PMI or MMP, whereas centrifugation at 400g and 600g resulted in a loss of these sperm functions, indicating that centrifugation force has an effect on sperm function. Conversely, Percoll gradient separation increased total motility, PMI and MMP. Spermatozoa treated with the mechanical stresses had lower basal ROS levels, although they were more susceptible to the external source of ROS. The mechanical stresses may affect sperm quality under ROS-producing conditions owing to the high sensitivity to external ROS. Moreover, anisotonic stress decreased total motility, PMI and MMP, and hypotonic stress may cause oxidative stress by increasing basal ROS. Therefore, careful consideration is required before applying these laboratory techniques to spermatozoa, particularly under ROS-producing conditions, and unnecessary handling should be limited for the preparation of epididymal rat spermatozoa. These results will contribute to the selection of proper handling techniques and conditions for the use of rat spermatozoa in biomedical research.
Effects of physical stress on rat sperm function.
Laboratory rats are commonly used experimental animal models in many areas of biomedical research to gain a better understanding of the genetic basis of human diseases. In contrast with spermatozoa from other mammalian species, rat spermatozoa are known for their hypersensitivity to physical interventions, such as handling, centrifugation, gradient separation, hypothermia and freezing. The present study provides fundamental information on the detrimental effects of reactive oxygen species and mitochondrial dysfunction on rat sperm motility after the application of physical stress.
Acknowledgments
This work was supported by National Institutes of Health National Center for Research Resources grants R21 RR025913-02 and P40 RR16939.
References
- Agarwal A, Ikemoto I, Loughlin KR. Effect of sperm washing on levels of reactive oxygen species in semen. Arch Androl. 1994;33:157–162. doi: 10.3109/01485019408987819. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Agca Y, Gilmore J, Byers M, Woods EJ, Liu J, Critser JK. Osmotic characteristics of mouse spermatozoa in the presence of extenders and sugars. Biol Reprod. 2002;67:1493–1501. doi: 10.1095/biolreprod.102.005579. [DOI] [PubMed] [Google Scholar]
- Agca Y, Mullen S, Liu J, Johnson-Ward J, Gould K, Chan A, Critser J. Osmotic tolerance and membrane permeability characteristics of rhesus monkey (Macaca mulatta) spermatozoa. Cryobiology. 2005;51:1–14. doi: 10.1016/j.cryobiol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988;9:367–376. doi: 10.1002/j.1939-4640.1988.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Vernet P. Maturation of redox regulatory mechanisms in the epididymis. J Reprod Fertil Suppl. 1998;53:109–118. [PubMed] [Google Scholar]
- Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25:2415–2426. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Lasso JL, Blasco L, Nunez RC, Heyner S, Caballero PP, Storey BT. Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim-up procedure without centrifugation extends their motile lifetime. Hum Reprod. 1993;8:1087–1092. doi: 10.1093/oxfordjournals.humrep.a138198. [DOI] [PubMed] [Google Scholar]
- Arcidiacono A, Walt H, Campana A, Balerna M. The use of Percoll gradients for the preparation of subpopulations of human spermatozoa. Int J Androl. 1983;6:433–445. doi: 10.1111/j.1365-2605.1983.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Bilaspuri GS. Oxidative stress alters membrane sulfhydryl status, lipid and phospholipid contents of crossbred cattle bull spermatozoa. Anim Reprod Sci. 2008;104:398–404. doi: 10.1016/j.anireprosci.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod. 1983;28:235–247. doi: 10.1095/biolreprod28.1.235. [DOI] [PubMed] [Google Scholar]
- Benau DA, Storey BT. Characterization of the mouse sperm plasma membrane zona-binding site sensitive to trypsin inhibitors. Biol Reprod. 1987;36:282–292. doi: 10.1095/biolreprod36.2.282. [DOI] [PubMed] [Google Scholar]
- Correa LM, Thomas A, Meyers SA. The macaque sperm actin cytoskeleton reorganizes in response to osmotic stress and contributes to morphological defects and decreased motility. Biol Reprod. 2007;77:942–953. doi: 10.1095/biolreprod.107.060533. [DOI] [PubMed] [Google Scholar]
- Ericsson SA, Garner DL, Thomas CA, Downing TW, Marshall CE. Interrelationships among fluorometric analyses of spermatozoal function, classical semen quality parameters and the fertility of frozen–thawed bovine spermatozoa. Theriogenology. 1993;39:1009–1024. doi: 10.1016/0093-691X(93)90002-M. [DOI] [PubMed] [Google Scholar]
- Filliers M, Rijsselaere T, Bossaert P, De Causmaecker V, Dewulf J, Pope CE, Van Soom A. Computer-assisted sperm analysis of fresh epididymal cat spermatozoa and the impact of cool storage (4 degrees C) on sperm quality. Theriogenology. 2008;70:1550–1559. doi: 10.1016/j.theriogenology.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Gaczarzewicz D, Piasecka M, Udala J, Blaszczyk B, Stankiewicz T, Laszczynska M. Plasma membrane changes during the liquid storage of boar spermatozoa: a comparison of methods. Acta Vet Hung. 2010;58:105–116. doi: 10.1556/AVet.58.2010.1.11. [DOI] [PubMed] [Google Scholar]
- Gravance CG, Garner DL, Miller MG, Berger T. Fluorescent probes and flow cytometry to assess rat sperm integrity and mitochondrial function. Reprod Toxicol. 2000;15:5–10. doi: 10.1016/S0890-6238(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Gravance CG, Garner DL, Miller MG, Berger T. Flow cytometric assessment of changes in rat sperm mitochondrial function after treatment with pentachlorophenol. Toxicol In Vitro. 2003;17:253–257. doi: 10.1016/S0887-2333(03)00039-0. [DOI] [PubMed] [Google Scholar]
- Guthrie HD, Welch GR. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J Anim Sci. 2006;84:2089–2100. doi: 10.2527/jas.2005-766. [DOI] [PubMed] [Google Scholar]
- Henkel R. The impact of oxidants on sperm function. Andrologia. 2005;37:205–206. doi: 10.1111/j.1439-0272.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;1:108. doi: 10.1186/1477-7827-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katkov II, Mazur P. Influence of centrifugation regimes on motility, yield, and cell associations of mouse spermatozoa. J Androl. 1998;19:232–241. [PubMed] [Google Scholar]
- Katkov II, Mazur P. Factors affecting yield and survival of cells when suspensions are subjected to centrifugation. Influence of centrifugal acceleration, time of centrifugation, and length of the suspension column in quasi-homogeneous centrifugal fields. Cell Biochem Biophys. 1999;31:231–245. doi: 10.1007/BF02738241. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yu DH, Kim YJ. Apoptosis-like change, ROS, and DNA status in cryopreserved canine sperm recovered by glass wool filtration and Percoll gradient centrifugation techniques. Anim Reprod Sci. 2010;119:106–114. doi: 10.1016/j.anireprosci.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Koshimoto C, Gamliel E, Mazur P. Effect of osmolality and oxygen tension on the survival of mouse sperm frozen to various temperatures in various concentrations of glycerol and raffinose. Cryobiology. 2000;41:204–231. doi: 10.1006/cryo.2000.2281. [DOI] [PubMed] [Google Scholar]
- Lambert IH. Reactive oxygen species regulate swelling-induced taurine efflux in NIH3T3 mouse fibroblasts. J Membr Biol. 2003;192:19–32. doi: 10.1007/s00232-002-1061-1. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol. 2006;187:75–85. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- Lazar J, Moreno C, Jacob HJ, Kwitek AE. Impact of genomics on research in the rat. Genome Res. 2005;15:1717–1728. doi: 10.1101/gr.3744005. [DOI] [PubMed] [Google Scholar]
- Lee JA, Spidlen J, Boyce K, Cai J, Crosbie N, Dalphin M, Furlong J, Gasparetto M, Goldberg M, Goralczyk EM, et al. 2008MIFlowCyt: the minimum information about a flow cytometry experiment Cytometry A73A926–930. 10.1002/cyto.a.20623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys J. 1995;68:1888–1894. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack SR, Zaneveld LJ. Acrosomal enzymes and ultrastructure of unfrozen and cryotreated human spermatozoa. Gamete Res. 1987;18:375–383. doi: 10.1002/mrd.1120180411. [DOI] [PubMed] [Google Scholar]
- Mahfouz RZ, du Plessis SS, Aziz N, Sharma R, Sabanegh E, Agarwal A. Sperm viability, apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. Fertil Steril. 2010;93:814–821. doi: 10.1016/j.fertnstert.2008.10.068. [DOI] [PubMed] [Google Scholar]
- Makler A, Jakobi P. Effects of shaking and centrifugation on human sperm motility. Arch Androl. 1981;7:21–26. doi: 10.3109/01485018109009371. [DOI] [PubMed] [Google Scholar]
- Matás C, Vieira L, García-Vázquez FA, Avilés-López K, López-Úbeda R, Carvajal JA, Gadea J. Effects of centrifugation through three different discontinuous Percoll gradients on boar sperm function. Anim Reprod Sci. 2011;127:62–72. doi: 10.1016/j.anireprosci.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Baumber J, Kass PH, Meyers SA. Osmotic stress induces oxidative cell damage to rhesus macaque spermatozoa. Biol Reprod. 2010;82:644–651. doi: 10.1095/biolreprod.109.080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers SA. Spermatozoal response to osmotic stress. Anim Reprod Sci. 2005;89:57–64. doi: 10.1016/j.anireprosci.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa E, Inomata T, Ikeda T, Shino M, Kashiwazaki N. Generation of live rat offspring by intrauterine insemination with epididymal spermatozoa cryopreserved at −196 degrees C. Reproduction. 2001;122:463–467. doi: 10.1530/rep.0.1220463. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa E, Kashiwazaki N, Takizawa A, Shino M, Kitada K, Serikawa T, Hakamata Y, Kobayashi E, Takahashi R, Ueda M, Nakashima T, Nakagata N. Cryopreservation of spermatozoa from closed colonies, and inbred, spontaneous mutant, and transgenic strains of rats. Comp Med. 2003;53:639–641. [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41:528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995;44:859–869. doi: 10.1016/0093-691X(95)00271-9. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Harrison RA. Mathematical analysis of mis-estimation of cell subsets in flow cytometry: viability staining revisited. J Immunol Methods. 2011;368:71–79. doi: 10.1016/j.jim.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Waberski D, Bollwein H, Sieme H. Identifying non-sperm particles during flow cytometric physiological assessment: a simple approach. Theriogenology. 2010;73:995–1000. doi: 10.1016/j.theriogenology.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Phillips TC, Dhaliwal GK, Verstegen-Onclin KM, Verstegen JP. Efficacy of four density gradient separation media to remove erythrocytes and nonviable sperm from canine semen. Theriogenology. 2012;77:39–45. doi: 10.1016/j.theriogenology.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Sahin E, Petrunkina AM, Waberski D, Harrison RA, Topfer-Petersen E. Control of bull sperm cell volume during epididymal maturation. Reprod Fertil Dev. 2009;21:469–478. doi: 10.1071/RD08162. [DOI] [PubMed] [Google Scholar]
- Shekarriz M, DeWire DM, Thomas AJ, Jr, Agarwal A. A method of human semen centrifugation to minimize the iatrogenic sperm injuries caused by reactive oxygen species. Eur Urol. 1995;28:31–35. doi: 10.1159/000475016. [DOI] [PubMed] [Google Scholar]
- Si W, Benson JD, Men H, Critser JK. Osmotic tolerance limits and effects of cryoprotectants on the motility, plasma membrane integrity and acrosomal integrity of rat sperm. Cryobiology. 2006;53:336–348. doi: 10.1016/j.cryobiol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Leibo SP. Cryopreservation of mouse spermatozoa. II. Relationship between survival after cryopreservation and osmotic tolerance of spermatozoa from three strains of mice. Cryobiology. 1997;35:255–269. doi: 10.1006/cryo.1997.2047. [DOI] [PubMed] [Google Scholar]
- Strehler E, Baccetti B, Sterzik K, Capitani S, Collodel G, De Santo M, Gambera L, Piomboni P. Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa (Notulae seminologicae 13) Hum Reprod. 1998;13:120–123. doi: 10.1093/humrep/13.1.120. [DOI] [PubMed] [Google Scholar]
- Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33:22–32. doi: 10.1111/j.1365-2605.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- Tanphaichitr N, Millette CF, Agulnick A, Fitzgerald LM. Egg-penetration ability and structural properties of human sperm prepared by Percoll-gradient centrifugation. Gamete Res. 1988;20:67–81. doi: 10.1002/mrd.1120200107. [DOI] [PubMed] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- Varisli O, Uguz C, Agca C, Agca Y. Various physical stress factors on rat sperm motility, integrity of acrosome, and plasma membrane. J Androl. 2009a;30:75–86. doi: 10.2164/jandrol.107.004333. [DOI] [PubMed] [Google Scholar]
- Varisli O, Uguz C, Agca C, Agca Y. Effect of chilling on the motility and acrosomal integrity of rat sperm in the presence of various extenders. J Am Assoc Lab Anim Sci. 2009b;48:499–505. [PMC free article] [PubMed] [Google Scholar]
- Walters EM, Men H, Agca Y, Mullen SF, Critser ES, Critser JK. Osmotic tolerance of mouse spermatozoa from various genetic backgrounds: acrosome integrity, membrane integrity, and maintenance of motility. Cryobiology. 2005;50:193–205. doi: 10.1016/j.cryobiol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Willoughby CE, Mazur P, Peter AT, Critser JK. Osmotic tolerance limits and properties of murine spermatozoa. Biol Reprod. 1996;55:715–727. doi: 10.1095/biolreprod55.3.715. [DOI] [PubMed] [Google Scholar]