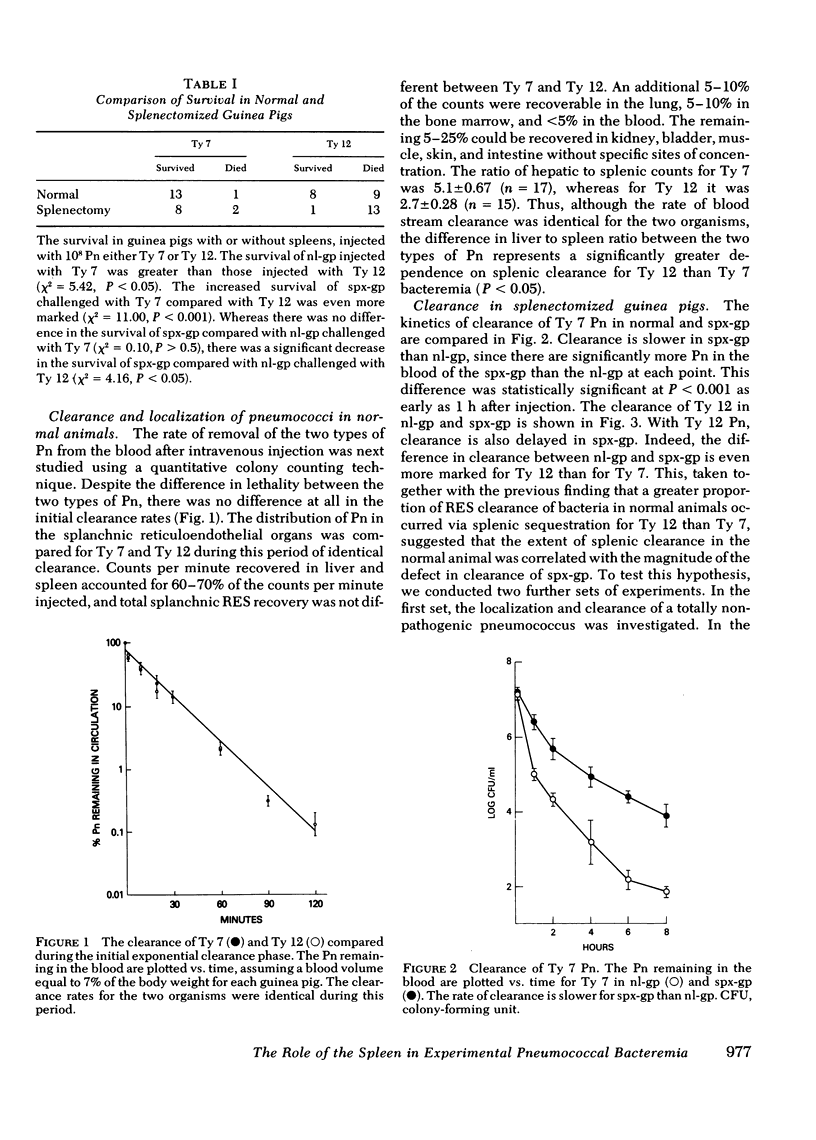
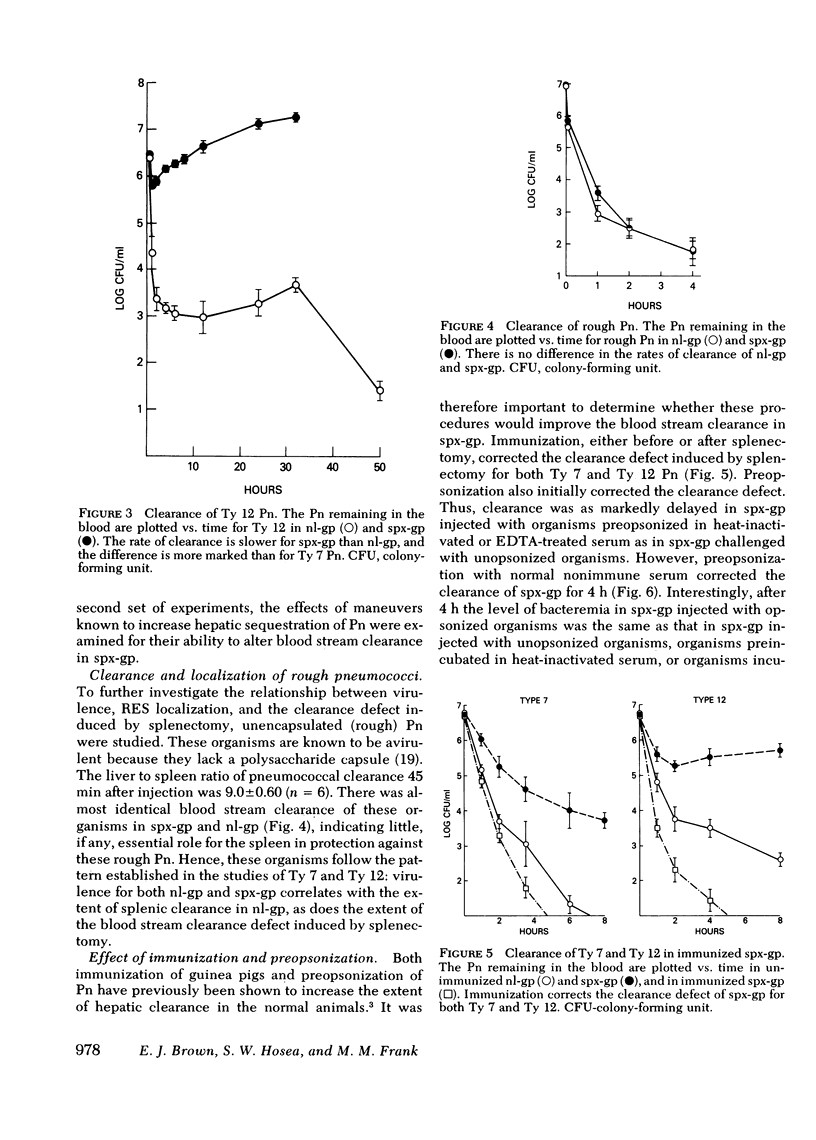
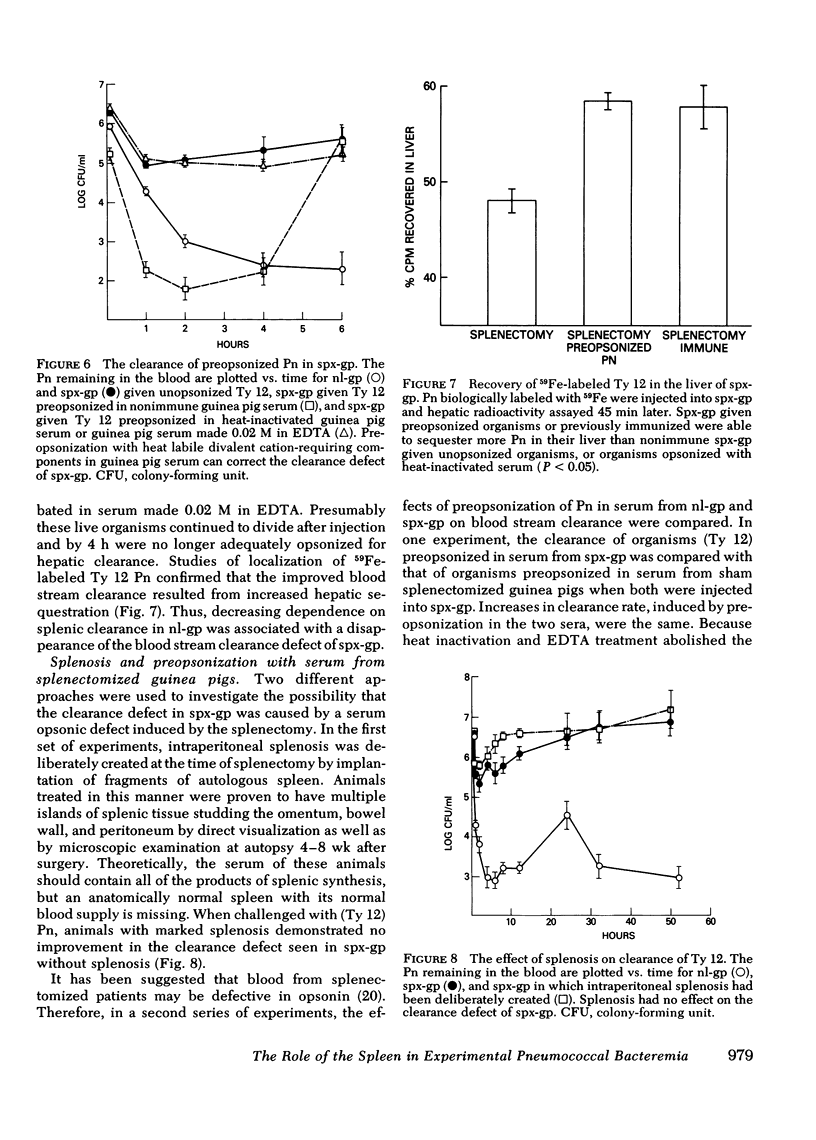
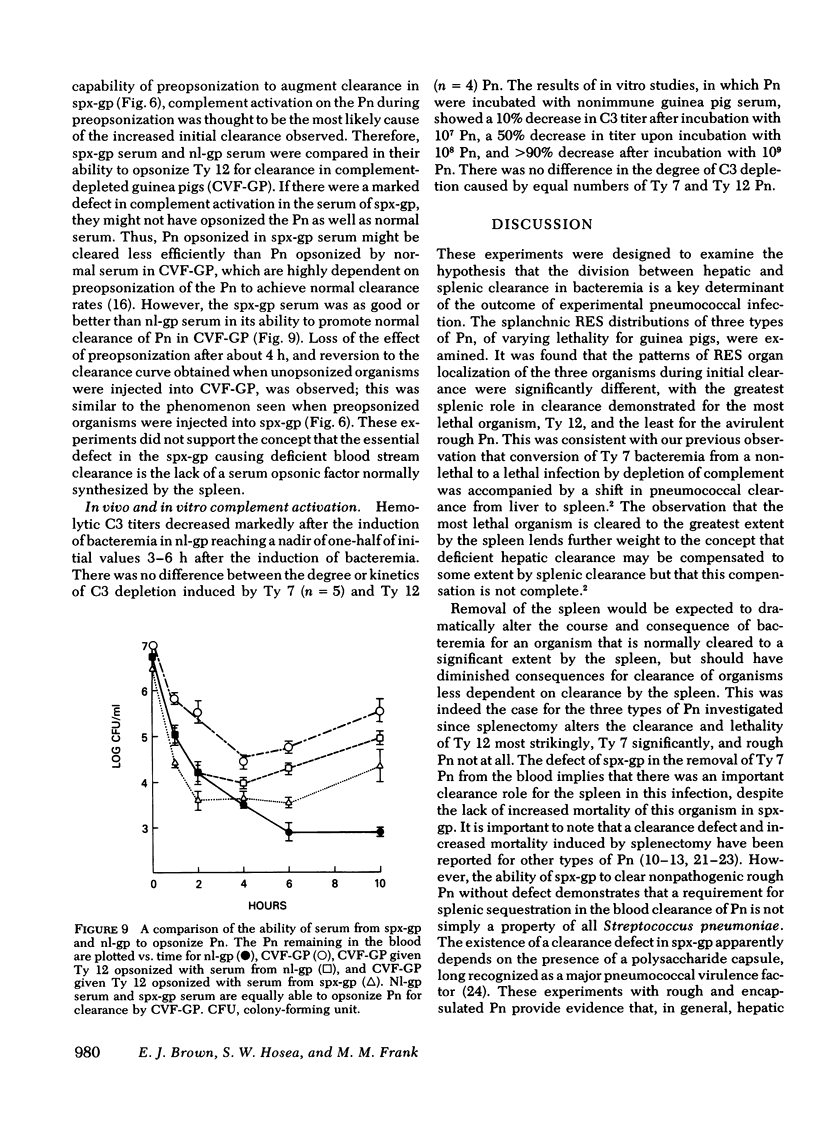
Abstract
The importance of the spleen in host defense against pneumococcal bacteremia has been suggested by a number of experimental models as well as the occurrence of the syndrome of overwhelming pneumococcal sepsis in asplenic individuals. We studied the mechanism of splenic protection against pneumococcal bacteremia using a guinea pig model. Rates of removal of pneumococci from the blood stream in normal and splenectomized guinea pigs were compared with the extent of hepatic and splenic sequestration of radiolabeled organisms for three different types of pneumococci. A relationship was found between the virulence of a pneumococcus for normal guinea pigs, the extent to which it is cleared by the spleen, and the magnitude of the defect in blood stream sterilization induced by splenectomy. The spleen plays an increasingly important role in the clearance of progressively more virulent organisms, for which hepatic clearance cannot compensate. Thus, the division between hepatic and splenic clearance of bacteremia is a key determinant of the outcome of experimental pneumococcal infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B., SEBESTYEN M. M., SCHLOSSMAN S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and Staphylococci by the reticuloendothelial system. J Exp Med. 1959 Jul 1;110(1):27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L., Freeman J. C. The syndrome of asplenia, pneumococcal sepsis, and disseminated intravascular coagulation. Ann Intern Med. 1970 Mar;72(3):389–393. doi: 10.7326/0003-4819-72-3-389. [DOI] [PubMed] [Google Scholar]
- Bogart D., Biggar W. D., Good R. A. Impaired intravascular clearance of pneumococcus type-3 following splenectomy. J Reticuloendothel Soc. 1972 Jan;11(1):77–87. [PubMed] [Google Scholar]
- Constantopoulos A., Najjar V. A., Smith J. W. Tuftsin deficiency: a new syndrome with defective phagocytosis. J Pediatr. 1972 Apr;80(4):564–572. doi: 10.1016/s0022-3476(72)80051-9. [DOI] [PubMed] [Google Scholar]
- Cooney D. R., Dearth J. C., Swanson S. E., Dewanjee M. K., Telander R. L. Relative merits of partial splenectomy, splenic reimplantation, and immunization in preventing postsplenectomy infection. Surgery. 1979 Oct;86(4):561–569. [PubMed] [Google Scholar]
- Dickerman J. D., Horner S. R., Coil J. A., Gump D. W. The protective effect of intraperitoneal splenic autotransplants in mice exposed to an aerosolized suspension of type III Streptococcus pneumoniae. Blood. 1979 Aug;54(2):354–358. [PubMed] [Google Scholar]
- Eraklis A. J., Kevy S. V., Diamond L. K., Gross R. E. Hazard of overwhelming infection after splenectomy in childhood. N Engl J Med. 1967 Jun 1;276(22):1225–1229. doi: 10.1056/NEJM196706012762203. [DOI] [PubMed] [Google Scholar]
- Gopal V., Bisno A. L. Fulminant pneumococcal infections in 'normal' asplenic hosts. Arch Intern Med. 1977 Nov;137(11):1526–1530. [PubMed] [Google Scholar]
- Hand W. L., King N. L. Serum opsonization of salmonella in sickle cell anemia. Am J Med. 1978 Mar;64(3):388–395. doi: 10.1016/0002-9343(78)90217-6. [DOI] [PubMed] [Google Scholar]
- Hosea S., Brown E., Hammer C., Frank M. Role of complement activation in a model of adult respiratory distress syndrome. J Clin Invest. 1980 Aug;66(2):375–382. doi: 10.1172/JCI109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Newman S. L., Struth A. G. An abnormality of the alternate pathway of complement activation in sickle-cell disease. N Engl J Med. 1973 Apr 19;288(16):803–808. doi: 10.1056/NEJM197304192881601. [DOI] [PubMed] [Google Scholar]
- Leung L. S., Szal G. J., Drachman R. H. Increased susceptibility of splenectomized rats to infection with Diplococcus pneumoniae. J Infect Dis. 1972 Nov;126(5):507–513. doi: 10.1093/infdis/126.5.507. [DOI] [PubMed] [Google Scholar]
- Likhite V. V. Protection against fulminant sepsis in splenectomized mice by implantation of autochthonous splenic tissue. Exp Hematol. 1978 May;6(5):433–439. [PubMed] [Google Scholar]
- Lozzio B. B., Wargon L. B. Immune competence of hereditarily asplenic mice. Immunology. 1974 Aug;27(2):167–178. [PMC free article] [PubMed] [Google Scholar]
- MARTIN S. P., KERBY G. P., HOLLAND B. C. The effect of thorotrast on the removal of bacteria in the splanchnic area of the intact animal. J Immunol. 1952 Mar;68(3):293–296. [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E. Studies on bacteriemia. I. Mechanisms relating to the persistence of bacteriemia in rabbits following the intravenous injection of staphylococci. J Exp Med. 1956 Jun 1;103(6):713–742. doi: 10.1084/jem.103.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- Rice H. M., James P. D. Ectopic splenic tissue failed to prevent fatal pneumococcal septicaemia after splenectomy for trauma. Lancet. 1980 Mar 15;1(8168 Pt 1):565–566. doi: 10.1016/s0140-6736(80)91056-9. [DOI] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., MIESCHER P. A., BENACERRAF B. STUDIES ON THE ROLE OF COMPLEMENT IN THE IMMUNE CLEARANCE OF ESCHERICHIA COLI AND RAT ERYTHROCYTES BY THE RETICULOENDOTHELIAL SYSTEM IN MICE. J Immunol. 1963 May;90:751–759. [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Weber P., Kaplan J. E. Physiologic role of cold-insoluble globulin in systemic host defense: implications of its characterization as the opsonic alpha 2-surface-binding glycoprotein. Ann N Y Acad Sci. 1978 Jun 20;312:43–55. doi: 10.1111/j.1749-6632.1978.tb16792.x. [DOI] [PubMed] [Google Scholar]
- Schulkind M. L., Ellis E. F., Smith R. T. Effect of antibody upon clearance of I-125-labelled pneumococci by the spleen and liver. Pediatr Res. 1967 May;1(3):178–184. doi: 10.1203/00006450-196705000-00004. [DOI] [PubMed] [Google Scholar]
- Schwartz A. D., Goldthorn J. F., Winkelstein J. A., Swift A. J. Lack of protective effect of autotransplanted splenic tissue to pneumococcal challenge. Blood. 1978 Mar;51(3):475–478. [PubMed] [Google Scholar]
- Shinefield H. R., Steinberg C. R., Kaye D. Effect of splenectomy on the susceptibility of mice inoculated with Diplococcus pneumoniae. J Exp Med. 1966 May 1;123(5):777–794. doi: 10.1084/jem.123.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D. B. Postsplenectomy sepsis. Perspect Pediatr Pathol. 1973;1:285–311. [PubMed] [Google Scholar]
- WARDLAW A. C., HOWARD J. G. A comparative survey of the phagocytosis of different species of bacteria by Kupffer cells; perfusion studies with the isolated rat liver. Br J Exp Pathol. 1959 Apr;40(2):113–117. [PMC free article] [PubMed] [Google Scholar]
- Whitaker A. N. The effect of previous splenectomy on the course of pneumococcal bacteriaemia in mice. J Pathol Bacteriol. 1968 Apr;95(2):357–376. doi: 10.1002/path.1700950203. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Drachman R. H. Deficiency of pneumococcal serum opsonizing activity in sickle-cell disease. N Engl J Med. 1968 Aug 29;279(9):459–466. doi: 10.1056/NEJM196808292790904. [DOI] [PubMed] [Google Scholar]



