Abstract
Background
This study reports a randomized clinical trial evaluating the efficacy of an intervention to prepare individuals to communicate BRCA1/BRCA2 results to family members.
Methods
Women aged 18 years and older, who had genetic testing, and who had adult first-degree relatives (FDRs), were randomly assigned to a communication skills-building intervention or a wellness control session. Primary outcomes were the percentage of probands sharing test results, and the level of distress associated with sharing. The ability of the Theory of Planned Behavior variables to predict the outcomes was explored.
Results
Four hundred twenty-two women were enrolled in the study, 219 (intervention) and 203 (control). Data from 137 in the intervention group and 112 in the control group were analyzed. Two hundred forty-nine probands shared test results with 838 relatives (80.1%). There were no significant differences between study groups in the primary outcomes. Combining data from both arms revealed that perceived control and specific social influence were associated with sharing. Probands were more likely to share genetic test results with their children, female relatives and relatives who they perceived had a favorable opinion about learning the results.
Conclusion
The communication skills intervention did not impact sharing of test results. The proband’s perception of her relative’s opinion of genetic testing and her sense of control in relaying this information influenced sharing. Communication of test results is selective, with male relatives and parents less likely to be informed.
Impact
Prevalent psychosocial factors play a role in the communication of genetic test results within families.
Keywords: Genetic testing, BRCA1/2, family communication, theory of planned behavior, social pressure
Introduction
There is increasing interest in the psychosocial impact of genetic testing, both on the individual who has been tested and on family members who could benefit from knowing the test results. The responsibility for informing relatives of genetic test results falls on the proband, the first family member being tested [1]. The proband must weigh the desire to protect family members from potential harm with the opportunity to provide information that may improve their health. However, this individual may have little knowledge of genetics and/or little skill in communicating complex genetic information to others.
There are several challenges associated with sharing genetic test results within families. Inconclusive and indeterminate results are associated with uncertainty and are conveyed to relatives less frequently than conclusive results [2, 3, 4]. Communicating genetic test results is more distressing for women who are carriers of deleterious BRCA1/2 gene mutations, who are the first tested among their siblings, and whose siblings prove to be non-carriers [5]. Mutation carriers have reported difficulty communicating test results [6, 7] and guilt about potentially having transmitted a mutation to their children [8]. Cancer-related emotional distress is a barrier to diffusion of test results [9]. Other factors interfering with the communication of test results are a history of depression and a highly vigilant pattern of coping with health threats, referred to as monitor status [10, 11]. There is evidence that open, positive family relationships increase the likelihood of disclosure of test results [10, 12] while emotional distance, family conflict, and loss of contact all decrease the likelihood of disclosure [8, 13, 14].
In our previous research, we found that gender and generation made independent contributions to the prediction of intention to tell genetic test results. Probands reported that they would be more likely to share genetic test results with female than male relatives, and with their children and siblings than their parents [15].
The aim of this study was to evaluate the efficacy of a communication skills-building intervention to prepare probands to explain their BRCA1/2 test results to first-degree relatives (FDRs). The primary outcomes were the percentage of probands who shared test results with their FDRs and the difficulty and distress experienced in the communication process. In addition, we were interested in knowing whether proband attitudes as well as their perceptions of personal control and social norms related to sharing genetic test results predicted the actual sharing of test results. Exploratory outcomes included proband and relative characteristics which may be associated with communication.
The communication intervention was modeled on Robert Buckman’s six-step strategy of communicating medical information [16]. Buckman’s technique was designed to help health care professionals effectively and compassionately “break bad news” to patients. We adapted his stepped process to the context of genetic counseling to give the proband the tools needed to provide accurate information in an empathic manner [17].
The Theory of Planned Behavior (TPB) was selected as the framework for the study because of its emphasis on factors that predict the performance of specific health behaviors [18, 19]. According to the theory, the immediate determinant of action is the intention to perform a behavior, such as sharing a genetic test result with relatives. Behavioral intention has three components: positive and/or negative attitudes about the behavior, perception of social influence or pressure to engage in or refrain from the behavior, and beliefs about perceived control over the behavior [20]. The TPB variables have been shown to predict several types of health behaviors, including communication about health-related decisions within families [21]. Applying this theoretical framework to communicating genetic test results, the theory predicts that an individual would engage in this behavior if he/she believed the consequence of sharing test results was valuable (attitude), knew that relatives valued this communication (social influence), and perceived that he/she had the necessary skills to communicate the results effectively (perceived control).
Materials and Methods
Sample
Participants for this study were recruited from the Risk Assessment Program (RAP) at Fox Chase Cancer Center. The RAP provides cancer risk education and individual counseling to women at risk for breast and/or ovarian cancer. Women aged 18 years and older, who completed genetic testing for BRCA1/BRCA2, and who had one or more living adult FDRs with whom she would share results were eligible. Both women with breast or ovarian cancer as well as unaffected women were eligible. The study was designed with a sample size of 110 individuals per treatment arm to test the hypothesis that the intervention group would have less distress than the control group following the sharing of their test results, as measured by the Impact of Events Scale - Revised (IES-R) intrusion score. This sample would provide 80% power to detect differences of 2.7 units or greater (one-sided hypothesis with 5% Type I); similarly, for the IES-R avoidance score, differences of at least 2.4 units would be detected.
Research Design
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Eligible individuals were randomly assigned by a computer-generated randomization table in a one-to-one ratio to either the experimental or control group. Probands were stratified by breast/ovarian cancer status (affected vs unaffected) and by number of eligible FDRs (1–3, 4, 5+). All probands were blinded to assigned study condition. The experimental group that received a communication skills building intervention was compared with a control group that received general nutrition and exercise information. The primary hypothesis was that the experimental group would be more likely to communicate genetic test results to their eligible FDRs and experience less difficulty and distress explaining their genetic test results. It was further predicted that the TPB variables would impact the effect of the intervention on the primary outcome. The association of disease status (affected vs. non affected), genetic test result, as well as psychosocial variables (depression, monitor status, and gender and generation of relative) with communication were explored.
In preparation for implementation of the study, the research staff underwent a thorough training including a review of the study protocol, the study schema and the study materials. They observed two counseling sessions to gain an understanding of the disclosure process, and were guided by the project manager through a workshop where they role played several hypothetical scenarios illustrating potential difficult issues. During the conduct of the study, a random sample of sessions was audio taped for review by the principal investigators for consistency and adherence to the protocol.
Procedure
Normal RAP procedures included an educational session covering risk factors for breast and ovarian cancer, including genetic factors, followed by an individual risk counseling session with a genetic counselor. In the individual counseling session, the counselor expanded the family tree, reviewed the hereditary nature of cancers in the family and discussed the benefits and limitations of genetic testing. For probands wishing to proceed to genetic testing, the genetic counselor described possible test results. Unambiguous test results were described as either “positive,” in which an alteration was identified that would increase cancer risk, or “true negative,” in which an alteration had been identified in the family, but was not inherited by the proband. “Indeterminate” test results were those in which a known BRCA alteration was not identified in the proband or in any other member of the family. “Inconclusive” results were those in which an alteration of uncertain significance was found. The potential importance of the test result for other family members was introduced at this session. At the subsequent disclosure session, individuals met with a RAP physician and genetic counselor for disclosure and discussion of their genetic test results. At this session, a more definitive description of the relevance of the test result for other family members was provided. The counselors were blinded to the study condition to which each proband was assigned.
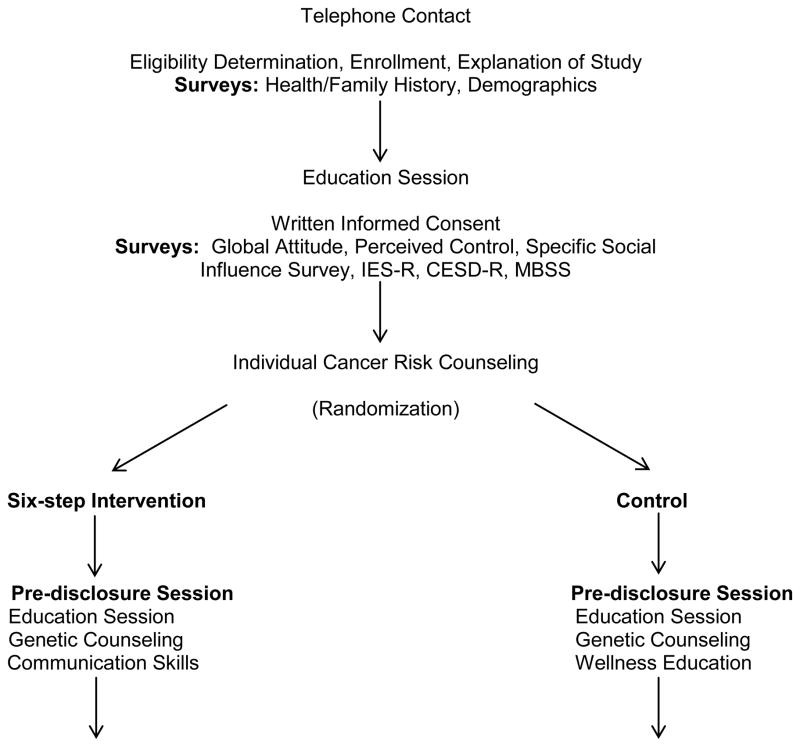
The study procedures were embedded within the normal clinic procedures. See Figure 1. If the individual calling the RAP intake line appeared to be eligible, the research study was explained and the study consent and procedures were reviewed. Individuals who expressed interest in the study gave verbal consent and were mailed a packet including a consent form, study brochure, and an extensive family history survey of all cancer diagnoses in first- and second-degree relatives.
Fig. 1.

Study Design
All persons gave their informed consent prior to their inclusion in the study. At the education session, the study consent form was reviewed and signed. A second survey packet was given to the participant to be completed prior to the individual risk counseling session. It included measures of TPB predictor variables, measures of distress related to communicating genetic test results, depressive symptoms, and monitoring coping style. Individuals who decided not to have genetic testing were withdrawn from the study, because they were no longer eligible. Those who planned to be tested were randomly assigned to receive either the intervention or control study condition. The experimental and control programs were delivered in person in two sessions by a trained health educator using a script and illustrated flipchart to standardize the intervention delivery. The script served as a checklist to ensure all key points were discussed. Three months following disclosure, a follow-up survey was sent to the probands. (See Figure 1).
Interventions
For those in the experimental arm, the first three steps of the communication strategy were delivered during the individual counseling session. Discussion points included: 1) identifying relatives who could benefit from the information; 2) choosing the communication format (phone, letter, email, etc.); and 3) assessing how much family members already knew and how much they might want to know. The next three steps of the six-step strategy were discussed after the test result disclosure session. These included: 4) sharing the actual genetic test result with family members; 5) responding to family members’ emotional reaction to the disclosure; and 6) providing genetic counseling resources for family members. A resource guide outlining cancer risk factors, including family history, the benefits and limitations of genetic testing and the points covered in the six-step communication strategy was given to each intervention participant.
The control group received a wellness education intervention before and after the disclosure of test results. Topics addressed included a review of the United States Department of Agriculture (USDA) Food Pyramid, nutritional guidelines for dietary intake of fats, a discussion of the role of antioxidants and dietary supplements, the benefits of exercise, and information about alcohol use and smoking cessation. Control group participants also received a list of web sites containing nutritional information. Probands in both the experimental and control arms were given a copy of the National Action Plan on Breast Cancer video “Genetic Testing for Breast Cancer Risk: It’s Your Choice.”
Measures
Probands completed surveys before the genetic education session, just prior to the disclosure session, and three months after the disclosure of test results. Personal characteristics were collected including age, race, ethnic background, education, occupation, marital status and cancer status to ensure comparability of the two study groups. Genetic test results were abstracted from the genetic records.
Study Variables
A Communication of Test Results survey was used to identify family members with whom the proband shared test results.
A Post Disclosure survey contained a 24-item scale completed by the proband to assess what aspects of the genetic test result were difficult and/or distressing to explain. This survey was piloted with over 125 probands who had undergone genetic testing.
The Impact of Events Scale-Revised (IES-R), a 15-item self-report questionnaire, was used to assess intrusion and avoidance, two aspects of distress related to sharing genetic test results [22].
TPB Theory-Specific Scales
The questions were developed specifically for this research to measure components of the TPB found to be associated with intention to tell genetic test results. [15]. The scales were constructed in accordance with Ajzen’s recommendations [18].
Attitude about Sharing Test Results was assessed with a 10-item scale to rate feelings about sharing genetic test results with family members. Each item contained opposite terms (e.g., comfortable-uncomfortable, wise-foolish) with degree of agreement rated on a seven-point scale. The Attitude score reflected the sum of responses for the ten items.
The Subjective Norms Scale rated two components: 1) the proband’s perception of each FDRs’ opinion about sharing genetic test results; and 2) the likelihood that the proband would be influenced by that relative.
The Perceived Behavioral Control Scale contained six items describing the participant’s confidence in her ability to share genetic test results (I will be able to explain the results; I have sufficient knowledge, etc).
Monitor status was assessed using the Miller Behavioral Style Scale (MBSS) [23] that describes four stress-evoking scenarios followed by four statements of monitoring (information-seeking) and four statements of a blunting (information-avoiding) style. Scores above the median are characterized as high monitors, scores below the median as low monitors.
Depression was measured using The Center for Epidemiologic Studies Depression Scale–Revised (CESD-R) a 20-item questionnaire that surveys depressive symptoms and mood disturbance [24].
Statistical Analyses
Analyses were performed using SAS® statistical software. Sample size for each analysis varied due to missing or incomplete data. To adjust for dropouts in the analysis, a sensitivity analysis was performed by creating a weighting factor for the probability of returning at three months based on the baseline characteristics of the retained and drop-out groups. The variables in the weighting factor included intervention group, education, monitor status, marital status, race, affected status, age, and number of family members with cancer. Results with the weighting factor were similar to the unadjusted results, so only the unadjusted findings are presented here. Differences in demographic variables for those who shared their genetic test results and those who did not were determined with Fisher’s exact test for nominal variables, Wilcoxon rank sum test for ordered variables, and t-test for continuous variables. Hypotheses related to the TPB were examined with logistic regression using the generalized estimating equation (GEE) methods to adjust for correlations arising from multiple relatives reported by each proband. This procedure used an exchangeable correlation structure to correct for the variable number of relatives associated with each proband and within family dependence in intention to convey genetic test results. Robust standard error estimates were used for statistical inference. The study was not designed nor powered to conduct the exploratory analyses which are presented, and therefore they should be considered as hypothesis generating pilot data.
Results
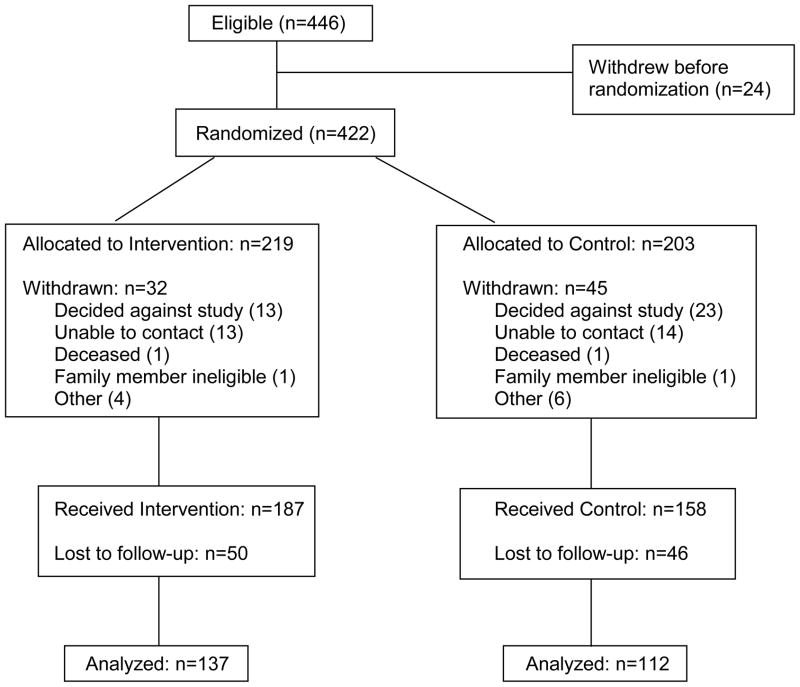
A total of 446 women were referred to the study between May 1, 2000 and August 31, 2003. Twenty four individuals declined participation or were found to be ineligible. Of the 422 women randomized to the trial, an additional 77 individuals were withdrawn for a variety of reasons. (See Figure 2). Differences in withdrawal rates by study group were marginal (intervention group, 14.6%, control group, 22.2%, P=0.06 Fisher’s exact test).
Fig. 2.
Study Flow of Participants, CONSORT Diagram
Three hundred and forty-five women enrolled in the study. The majority were Caucasian (95%), married (79%), and educated beyond high school (77%). The mean age was 48.5 years (S.D. = 11.0). Ninety-six participants (28%) did not complete questionnaires at the three-month follow-up and were considered “drop-outs” from the study. The drop-out rate was similar for both the intervention arm (27%) and the control arm (29%), p=0.63 Fisher’s exact test. The only variable associated with retention in the study was age. Older women were more likely to remain in the study (p=0.016 Fisher’s exact test). There were no differences between the arms for race, education, age, marital status or affected status. Participants in both arms listed an average of four relatives per person.
The efficacy of the intervention was evaluated using intention to treat methodology in which all analyses were conducted on the groups as they were originally assigned. Efficacy was examined using logistic regression analysis with GEE analysis to adjust for within family correlations. The primary hypothesis was that the intervention group would be more likely to share their genetic test results with FDRs, and would experience less distress sharing test results than the control group. Ninety-nine percent of probands shared their genetic test results with at least one relative, and 53% shared test results with all of their FDRs. Overall, probands shared test results with 838 of the 1046 relatives (80.1%). Contrary to our hypothesis, there was no significant difference between the intervention and control group in the percentage of probands sharing test results, or in their difficulty and distress in sharing test results. (See Table 1).
Table 1.
Primary Outcomes by Study Group
| Outcome | Intervention 137 probands |
Control 112 probands |
P valuea |
|---|---|---|---|
|
| |||
| N (percent) | N (percent) | ||
| Sharing GTR with FDRsb | |||
| Proband shared GTR with at least one relative | 136 (99.3%) | 110 (99.2%) | 0.59 |
| Proband shared test result with all relatives | 74 (54.0%) | 59 (52.7%) | 0.83 |
| Proband shared GTR with | |||
| None/Some: 0–<50% of relatives | 8 (5.8%) | 8 (7.1%) | 0.91 |
| Most: 50%–<100% of relatives | 55 (40.2%) | 45 (40.2%) | |
| All: 100% of relatives | 74 (54.0%) | 59 (52.7%) | |
| Distress reported by proband in sharing GTRc: | |||
| Any difficulty in explaining test results to any relative | 60 (43.8%) | 44 (39.3%) | 0.47 |
| Any upset in explaining test results to any relative | 41 (29.9%) | 33 (29.5%) | 0.94 |
| Mean (Median) | Mean (Median) | ||
| Depression Scale CESD-Rd | |||
| Baseline (BL), | 8.4 ( 4 ) | 9.1 ( 6 ) | 0.39 |
| 3 month Post-Disclosure (PD) | 8.1 ( 4 ) | 8.0 ( 5 ) | 0.58 |
| Change (PD minus BL) | −0.3 ( 0 ) | −1.1 ( 0 ) | 0.60 |
| Distress as assessed by IES-R (total)d | |||
| Baseline (BL) | 5.0 ( 2 ) | 6.4 ( 3 ) | 0.31 |
| 3 month Post-Disclosure (PD) | 4.6 ( 1 ) | 5.6 ( 1 ) | 0.41 |
| Change (PD minus BL) | −0.4 ( 0 ) | −0.7 ( 0 ) | 0.93 |
Abbreviations: GTR: Genetic Test Results; FDR: First Degree Relatives
P-values are two-sided, for the Chi-Square test for categorical variables and the Wilcoxon rank sum test for continuous variables
There are 1–11 relatives per proband, median is 4; median number of relatives shared GTR is 3
For the distress reported by probands, there were small numbers of missing relative responses (between 7–13 of 838 responses)
For CESD-R, and IES-R, scores, there were 16 and 12, probands excluded from the results, respectively.
Because of the null result for the primary hypothesis, the experimental and control groups were combined and further analyses were undertaken to determine which variables predicted communication of test results to relatives. Three regression models were examined to explore the impact of the TPB variables and proband and relative characteristics on the primary outcome. In a previous analysis of baseline data from this cohort, three TPB variables (attitude, social norms, and perceived control) predicted intention to tell genetic test results [15]. In regression one, this analysis was repeated in the subset of individuals who completed the study and actually shared results with at least one relative. Results were similar to our previous finding (data not shown).
Having determined that the TPB variables predicted “intention to tell,” the second regression analysis, using GEE to adjust for within family correlations, was performed with “actual sharing of test results” as the outcome variable. The probands reported intending to tell 778 of 922 relatives (84%), but actually shared genetic test results with 752 (82%). Perceived control and subjective norms were found to be associated with sharing test results. Although attitude predicted intent to share, it did not predict actual sharing of results. (See Table 2)
Table 2.
Relationship of Theory of Planned Behavior Variables with Intention and Sharing of Genetic Test Results
| Intention to Share Genetic Test Results | Shared Genetic Test Results | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Level | OR | p-value | OR | p-value |
| Perceived Control | Continuous | 1.14 | 0.0130 | 1.15 | 0.0064 |
| Specific Social Influence | Continuous | 1.05 | <0.0001 | 1.05 | <0.0001 |
| Global Attitude (GA) | Continuous | 1.04 | 0.0225 | 0.98 | 0.1645 |
Note: Data reflect 236 probands and 922 relatives. Model also includes an indicator for responses where proband did not know the relative’s opinion on sharing GTR.
A third regression was conducted with additional covariates to investigate additional variables associated with sharing genetic test results. The proposed model included perceived control, subjective norms, attitudes, monitoring status, depressive symptoms, communication-related distress, cancer status (affected vs. unaffected with breast or ovarian cancer), type of test result, and gender and generation of the relatives. (See Table 3). In this model, perceived control remained positively associated with sharing (OR = 1.13, p = .0032). The proband’s perception of the relative’s opinion of testing (subjective norms) also predicted sharing. If the proband perceived that her relative was in favor of hearing her test result, she was more likely to share her test results. (OR = 7.20, p < .0001; OR = 11.28, p <.0001, respectively). Low depressive symptoms were predictive of sharing genetic test results (OR = 3.42, p = <.0001). However, attitudes were associated with a slightly lower likelihood of sharing (OR = 0.96, p = 0.0015). Communication-related distress, monitoring coping status and affected status did not predict sharing test results.
Table 3.
Factors Associated with Sharing Genetic Test Results
| Sharing Genetic Test Result | |||
|---|---|---|---|
|
| |||
| Variable | Level | OR | p-value |
| Proband Characteristics | |||
| Perceived control | Continuous | 1.13 | 0.0032 |
| Monitor status | High vs low | 1.41 | 0.1738 |
| Depression, CESD-R | 0–15 vs 16+ | 3.42 | <0.0001 |
| Global Attitude | Continuous | 0.96 | 0.0015 |
| Distress, IES-R (total) | Continuous | 0.99 | 0.3921 |
| Affected | Yes vs no | 1.34 | 0.2496 |
| Proband perception of relative’s opinion on sharing GTR | Opposed/neutral vs don’t know rel’s opinion | 1.16 | 0.6422 |
| Somewhat in favor vs don’t know rel’s opinion | 7.20 | <0.0001 | |
| extremely in favor vs don’t know rel’s opinion | 11.28 | <0.0001 | |
| Genetic Test Results | Pos/Neg | 2.56 | 0.0102 |
| Inconclusive | 1.25 | 0.6984 | |
| Indeterminate | Referent | ||
| Relative Characteristics | |||
| Gender | F vs M | 4.81 | <0.0001 |
| Generation | 1:Child | 4.54 | 0.0004 |
| 2:Sibling | 0.73 | 0.2687 | |
| 3:Parent | Referent | ||
Note: The data reflect 220 probands with 885 relatives with complete data for the variables listed.
Unambiguous results (OR = 2.56; p = 0.0102) were more likely to be communicated than an inconclusive or indeterminate result. Gender and generation of the relative were significant predictors of sharing. Probands were more likely to tell female than male relatives (OR = 4.81, p<.0001) and were more likely to tell their children than their parents (OR = 4.54, p = .0004). Among children, daughters were more likely to be told than sons (98% vs. 88%, p=0.0013); among siblings, sisters were more likely to be told than brothers (86% vs. 57%, p<0.0001). (See Table 4).
Table 4.
Sharing of genetic test results (GTR) by relation
| Relation | Probands N |
Relatives N |
Relatives with whom GTR was shared N (percent) |
|---|---|---|---|
| Mother | 88 | 88 | 77 (87.5%) |
| Father | 94 | 94 | 64 (68.1%) |
| Sister | 164 | 284 | 243 (85.6%) |
| Brother | 160 | 259 | 153 (59.1%) |
| Daughter | 107 | 172 | 169 (98.3%) |
| Son | 94 | 149 | 132 (88.6%) |
Discussion
In this study, probands reported sharing their genetic test results with 82% of their adult FDRs, a figure that is consistent with other studies reporting rates of sharing ranging from 74% to 95% [8, 25, 26]. We predicted that probands in the intervention arm would be more likely to share their genetic test results, and have less difficulty and emotional distress in doing so than probands in the control arm. These predictions were not borne out. This may be a result of the highly motivated attitude of the women seeking genetic risk assessment, who may initiate the process already committed to providing their genetic information to their family members. This explanation is supported by the probands’ high baseline intention to share genetic test results [15]. Another factor may be the thoroughness of the predisclosure and disclosure counseling provided by the counseling team, which may be sufficiently comprehensive to prepare probands for understanding and sharing their genetic test results. This is supported by a similar study based on Buckman’s model which also failed to find a difference between the intervention and control arms in sharing test results with family members [27].
We showed in our previous paper that all three TPB variables (Attitudes, Subjective Norms, and Perceived Control) predicted intent to tell [15]. In this study, the proband’s perception of her relative’s opinion about genetic testing (Subjective Norms) was a strong predictor of sharing her test result with that relative. This is consistent with other findings that supportive family members are more likely to be included in discussions of genetic information [28], and suggests that a person’s perception of what important others think about a behavior is a powerful influence on whether to engage in the behavior. This suggests that interventions to improve family communication should consider each family member’s opinion about genetic testing, and should address concerns about the negative impact of the information on family relationships [29]. The proband’s confidence in her ability to effectively share her genetic test results (Perceived Control) also strongly predicted whether results were shared. Other studies have shown that an individual’s belief that they have the skill to communicate with family members is significantly related to actually initiating a conversation [30]. However, attitudes about sharing genetic test results with family members had a small but significant reverse association. It is possible that if the proband’s perception that her relative wanted to hear the genetic test result, and if she felt confident in her ability to relay this information, this would overcome any pre-disclosure reluctance about sharing the results. These findings also highlight what other investigators have described in the use of the TPB, i.e., its component variables perform differently in different situations. For example, attitude was the strongest determinant of intention to obtain an annual Pap smear [31], to use condoms to reduce the risk of exposure to AIDS [32], and to obtain a genetic test for a hereditary disease [33], while perceived control was most effective in predicting exercise in individuals with coronary artery disease [34]. The TPB has been shown in some studies to be culture-sensitive, and to perform differently in males than females, and in new behaviors compared to established behaviors [35] [36]. Some have argued that while the TPB model provides a useful framework for social behavior, the way each of the components of the model shape individual behaviors is to some extent dependent on individual differences [37]. The developers of the model have stated that the relative importance of attitudes, subjective norms and perceived behavioral control will vary for different behaviors and among different populations [38]. This does not negate the value of the TPB but rather suggests a need to be flexible in the weight assigned to each of its components.
In addition to the TPB variables, we found that individuals with higher depressive symptoms were less likely to share their genetic test results. Depression is often associated with pessimism, denial and avoidance tendencies which may negatively impact communicating health information [30] [40]. Probands were more likely to share definitive results (true positive or true negative), which is intuitive as these are the results which have the strongest implications for relatives. Finally, we confirmed the importance of gender and generation in predicting with which relatives the probands shared their genetic test results. The tendency to share test results more often with female relatives has been seen in other studies and likely reflects an awareness of the more immediate relevance of the information for female relatives, the traditional role of women as family caregivers, and the closer emotional ties women may have with female compared to male relatives [41, 2, 42, 43]. The probands in this study were most likely to share their genetic test results with their children, for whom they are likely to feel the strongest responsibility.
While our data do not support the efficacy of this communication skills-building intervention to improve the communication of genetic test results, we were able to elucidate the contribution of the TPB to our understanding of the factors that predict sharing of test results. This data also highlights the selective nature of the decision to share test results, with female relatives and offspring being the most likely recipients. We identified the importance of the proband’s perception of her relative’s opinion of genetic testing and the perceived ability to relay this information, in the decision to share. It also points to the role of depression as an independent factor impacting the communication process. Our findings illustrate the complex nature of intrafamilial communication, which is influenced by many personal, familial and social forces. Rather than enhancing the content of the information given, genetic counseling strategies that assess personal and familial barriers to communication may provide more targeted support and may facilitate more open sharing within the family. In line with other studies showing the importance of the extent of the communication process on the impact genetic testing information has on relatives [44], our next paper will examine those factors which are associated with actual understanding of the test results by the informed relatives.
Limitations of the Study
This study has the advantage of a randomized design grounded in a theoretic model. The sample was derived from a high-risk clinic whose participants are mainly well educated Caucasian women, and therefore not representative of the average population. However, they are representative of the group of women who to date have pursued genetic risk counseling and genetic testing. Most of them presented already knowledgeable about their familial risk and about the option to pursue testing for BRCA1/2, which could have minimized the impact of our intervention. It is not known if this intervention would have had different results for men who pursue BRCA1/2 testing. Future interventions may be more effective if they are directed specifically to communication with those relatives who are less likely to be informed of the test result, e.g. male relatives.
Acknowledgments
We are indebted to the patients who participated in the study. We thank Irene Angel, Joanne Spoltore and our Partner Hospital affiliates for their assistance in recruitment and data collection, and Lorraine Crozier and Susan Steinberg for their invaluable assistance in manuscript preparation.
Two grants from the National Cancer Institute, R01 CA81867 and P30 CA00692, provided funding for this study.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.American Society of Clinical Oncology . Policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 2.Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24:700–6. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 3.Wilson BJ, Forrest K, van Teijlingen ER, et al. Family communication about genetic risk: the little that is known. Community Genet. 2004;7:15–24. doi: 10.1159/000080300. [DOI] [PubMed] [Google Scholar]
- 4.McKinnon W, Naud S, Ashikaga T, Colletti R, Wood M. Results of an intervention for individuals and families with BRCA mutations: a model for providing medical updates and psychosocial support following genetic gesting. J Genet Couns. 2007;16:433–456. doi: 10.1007/s10897-006-9078-8. [DOI] [PubMed] [Google Scholar]
- 5.Smith KR, West JA, Croyle RT, Botkin JR. Familial context of genetic testing for cancer susceptibility: moderating effect of siblings’ test results on psychological distress one to two weeks after BRCA1 mutation testing. Cancer Epidemiol Biomarkers Prev. 1999;8:385–92. [PubMed] [Google Scholar]
- 6.Green J, Richards M, Murton F, Statham H, Hallowell N. Family communication and genetic counseling: the case of hereditary breast and ovarian cancer. Journal of Genetic Counseling. 1997;6:45–60. doi: 10.1023/A:1025611818643. [DOI] [PubMed] [Google Scholar]
- 7.Landsbergen K, Verhaak C, Kraaimaat F, Hoogerbrugge N. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4:115–9. doi: 10.1007/s10689-004-7991-2. [DOI] [PubMed] [Google Scholar]
- 8.Hughes C, Lerman C, Schwartz M, et al. All in the family: evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet. 2002;107:143–50. doi: 10.1002/ajmg.10110. [DOI] [PubMed] [Google Scholar]
- 9.Julian-Reynier C, Eisinger F, Chabal F, et al. Disclosure to the family of breast/ovarian cancer genetic test results: patient’s willingness and associated factors. Am J Med Genet. 2000;94:13–8. doi: 10.1002/1096-8628(20000904)94:1<13::aid-ajmg4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Tercyak KP, Lerman C, Peshkin BN, et al. Effects of coping style and BRCA1 and BRCA2 test results on anxiety among women participating in genetic counseling and testing for breast and ovarian cancer risk. Health Psychol. 2001;20:217–22. [PubMed] [Google Scholar]
- 11.Kodl MM, Lee JW, Matthews AK, Cummings SA, Olopade OI. Correlates of depressive symptoms among women seeking cancer genetic counseling and risk assessment at a high-risk cancer clinic. J Genet Couns. 2006;15:267–76. doi: 10.1007/s10897-006-9025-8. [DOI] [PubMed] [Google Scholar]
- 12.Koehly LM, Peterson SK, Watts BG, Kempf KK, Vernon SW, Gritz ER. A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiol Biomarkers Prev. 2003;12:304–13. [PubMed] [Google Scholar]
- 13.Peterson SK. The role of the family in genetic testing: theoretical perspectives, current knowledge, and future directions. Health Educ Behav. 2005;32:627–39. doi: 10.1177/1090198105278751. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald DJ, Sarna L, Uman GC, Grant M, Weitzel JN. Cancer screening and risk-reducing behaviors of women seeking genetic cancer risk assessment for breast and ovarian cancers. Oncol Nurs Forum. 2006;33:E27–35. doi: 10.1188/06.ONF.E27-E35. [DOI] [PubMed] [Google Scholar]
- 15.Barsevick AM, Montgomery SV, Ruth K, et al. Intention to communicate BRCA1/BRCA2 genetic test results to the family. J Fam Psychol. 2008;22:303–12. doi: 10.1037/0893-3200.22.2.303. [DOI] [PubMed] [Google Scholar]
- 16.Buckman R. How to break bad news, A guide for health care professionals. The John Hopkins University Press; Baltimore: 1992. [Google Scholar]
- 17.Daly MB, Barsevick A, Miller SM, et al. Communicating genetic test results to the family: a six-step, skills-building strategy. Fam Community Health. 2001;24:13–26. doi: 10.1097/00003727-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Ajzen A, Fishbein M. Understanding attitudes and predicting social behavior. Prentice-Hall; Englewood Cliffs: 1980. [Google Scholar]
- 19.Ajzen I. Perceived behavioral control, self-efficacy, focus of control, and the theory of planned behavior. Journal of Applied Social Psychology. 2002;32:665–83. [Google Scholar]
- 20.Lierman LM, Young HM, Kasprzyk D, Benoliel JQ. Predicting breast self-examination using the theory of reasoned action. Nurs Res. 1990;39:97–101. [PubMed] [Google Scholar]
- 21.Park HS, Smith SW. Distinctiveness and influence of subjective norms, personal descriptive and injunctive norms, and societal descriptive and injunctive norms on behavioral intent: a case of two behaviors critical to organ donation. Human Communication and Research. 2007;33:194–218. [Google Scholar]
- 22.Weiss D, Marmar C. Assessing psychological trauma and PTSD - The impact of event scale - revised. Guildford; New York: 1997. [Google Scholar]
- 23.Miller SM. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. J Pers Soc Psychol. 1987;52:345–53. doi: 10.1037//0022-3514.52.2.345. [DOI] [PubMed] [Google Scholar]
- 24.Eaton W, Smith C, Ybarra M, Muntaner C, Tien A. The use of psychological testing for treatment planning and outcomes assessment. 3. Lawrence Erlbaum Associates, Inc; Mahwah: 2004. [Google Scholar]
- 25.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12:81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGivern B, Everett J, Yager GG, Baumiller RC, Hafertepen A, Saal HM. Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med. 2004;6:503–9. doi: 10.1097/01.gim.0000144014.91237.a1. [DOI] [PubMed] [Google Scholar]
- 27.Roshanai AH, Rosenquist R, Lampic C, Nordin K. Does enhanced information at cancer genetic counseling improve counselees’ knowledge, risk percetion, satisfaction and negotiation of information to at-risk relatives? - Acta Oncologica. 2009;48:999–1009. doi: 10.1080/02841860903104137. [DOI] [PubMed] [Google Scholar]
- 28.Forrest LE, Curnow L, Delatycki MB, Skene L, Aitken MA. Health first, genetics second: exploring families’ experiences of communicating genetic information. European Jounal of Human Genetics. 2008;16:1329–1335. doi: 10.1038/ejhg.2008.104. [DOI] [PubMed] [Google Scholar]
- 29.Nycum G, Avard D, Knoppers BM. Factors influencing intrafamilial communication of hereditary breast and ovarian cancer genetic information. European Journal of Human Genetics. 2009;17(7):872–880. doi: 10.1038/ejhg.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afifi WA, Morgan SE, Stephenson MT, et al. Examining the decision to talk with family about organ donation: applying theory of motivated information management. Communication Monographs. 2006;73:188–215. [Google Scholar]
- 31.Jennings-Dozier K. Predicting intentions to obtain a pap smear among African American and Latina women: testing the theory of planned behavior. Nurs Res. 1999;48:198–205. doi: 10.1097/00006199-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Jemmott LS, Jemmott JB., 3rd Applying the theory of reasoned action to AIDS risk behavior: condom use among black women. Nurs Res. 1991;40:228–34. [PubMed] [Google Scholar]
- 33.Nordin K, Bjork J, Berglund G. Factors influencing intention to obtain a genetic test for a hereditary disease in an affected group and in the general public. Prev Med. 2004;39:1107–14. doi: 10.1016/j.ypmed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Johnston DW, Johnston M, Pollard B, Kinmonth AL, Mant D. Motivation is not enough: prediction of risk behavior following diagnosis of coronary heart disease from the theory of planned behavior. Health Psychol. 2004;23:533–8. doi: 10.1037/0278-6133.23.5.533. [DOI] [PubMed] [Google Scholar]
- 35.Tolma EL, Reininger BM, Ureda J, Evans A. Cognitive motivations associated with screening mammography in Cyprus. Prev Med. 2003;36:363–73. doi: 10.1016/s0091-7435(02)00022-1. [DOI] [PubMed] [Google Scholar]
- 36.Sheeran P, Conner M, Norman P. Can the theory of planned behavior explain patterns of health behavior change? Health Psychol. 2001;20:12–9. doi: 10.1037//0278-6133.20.1.12. [DOI] [PubMed] [Google Scholar]
- 37.Armitage CJ, Norman P, Conner M. Can the theory of planned behaviour mediate the effects of age, gender and multidimensional health locus of control? Br J Health Psychol. 2002;7:299–316. doi: 10.1348/135910702760213698. [DOI] [PubMed] [Google Scholar]
- 38.Fishbein M, Ajzen I. Theory-based behavior change interventions: comments on Hobbis and Sutton. J Health Psychol. 2005;10:27–31. doi: 10.1177/1359105305048552. discussion 7–43. [DOI] [PubMed] [Google Scholar]
- 39.Kuwabara SA, Van Voorhees BW, Gollan JK, Alexander GC. A qualitative exploration of depression in emerging adulthood: disorder, development, and social context. Gen Hosp Psychiatry. 2007;29:317–24. doi: 10.1016/j.genhosppsych.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith M, Calam R, Bolton C. Psychological factors linked to self-reported depression symptoms in late adolescence. Behav Cogn Psychother. 2009;37:73–85. doi: 10.1017/S1352465808004724. [DOI] [PubMed] [Google Scholar]
- 41.Daly MB. The Impact of social roles on the experience of men in BRCA1/2 families: Implications for counseling. J Genet Couns. 2009;18:42–8. doi: 10.1007/s10897-008-9183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung EL, Olson AD, Yu TM, Han PZ, Beattie MS. Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2211–2219. doi: 10.1158/1055-9965.EPI-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finlay E, Stopfer JE, Burlingame E. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genetic Testing. 2008;12(1):91–92. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vos J, Jansen AM, Menko F, van Asperen CJ, Stiggelbout AM, Tibben A. Family communication matters: the impact of telling relatives about unclassified variants and uninformative DNA-test results. Genet Med. 2011;13(4):333–341. doi: 10.1097/GIM.0b013e318204cfed. [DOI] [PubMed] [Google Scholar]