Abstract
Skeletal muscle atrophy is a very common clinical challenge in many disuse conditions. Maintenance of muscle mass is crucial to combat debilitating functional consequences evoked from these clinical conditions. In contrast, hibernation represents a physiological state in which there is natural protection against disuse atrophy despite prolonged periods of immobilization and lack of nutrient intake.
Even though peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1-α (PGC-1α) is a central mediator in muscle remodeling pathways, its role in the preservation of skeletal muscle mass during hibernation remains unclear. Since PGC-1α regulates muscle fiber type formation and mitochondrial biogenesis, we analyzed muscles of 13-lined ground squirrels. We find that animals in torpor exhibit a shift to slow-twitch Type I muscle fibers. This switch is accompanied by activation of the PGC-1α-mediated endurance exercise pathway. In addition, we observe increased antioxidant capacity without evidence of oxidative stress, a marked decline in apoptotic susceptibility, and enhanced mitochondrial abundance and metabolism.
These results show that activation of the endurance exercise pathway can be achieved in vivo despite prolonged periods of immobilization, and therefore might be an important mechanism for skeletal muscle preservation during hibernation. This PGC-1α regulated pathway may be a potential therapeutic target promoting skeletal muscle homeostasis and oxidative balance to prevent muscle loss in a variety of inherited and acquired neuromuscular disease conditions.
Keywords: endurance exercise, peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1-α (PGC-1α), muscle fiber type, muscle atrophy, oxidative balance, hibernation
Introduction
Significant loss of muscle mass can occur as a result of several disuse conditions such as limb immobilization, bed rest, denervation, or microgravity. Aging, cachexia and many disease states can also lead to atrophy of the muscle fibers (Degens and Alway, 2006, di Prampero and Narici, 2003, Hornberger, et al., 2001, Jackman and Kandarian, 2004). In contrast, hibernation is a physiological state in which certain animal species overcome the challenges that arise from both prolonged immobilization and absence of feeding (Carey, et al., 2003). Despite facing several extreme conditions including long-term-immobilization, hypometabolism, hypoxia, and lack of food intake, these hibernators are capable of a remarkable preservation of skeletal muscle mass (Andres-Mateos, et al., 2012, Lee, et al., 2008).
Muscle remodeling in the absence of injury occurs as a response to environmental demands such as low caloric input or exercise (Bassel-Duby and Olson, 2006, Fluck and Hoppeler, 2003). Endurance exercise adaptations are mediated by the peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1-α (PGC-1α), which is a key regulator of mitochondrial biogenesis and fuel homeostasis in skeletal muscle (Calvo, et al., 2008, Lin, et al., 2005). PGC-1α has been shown to regulate the formation of Type I, oxidative slow-twitch fibers which are a hallmark of high endurance exercise (Lin, et al., 2002).
The PGC-1α-mediated signaling cascade includes AMP-activated Protein Kinase (AMPK) that acts as a sensor of the energy status of the cell and activates PGC-1α by phosphorylation (Greer, et al., 2007, Jager, et al., 2007, Jorgensen, et al., 2005). Members of the MAPK family such as p38 MAPK also increase PGC-1α activity in response to physical exercise (Akimoto, et al., 2005, Yu, et al., 2003). The Nuclear Respiratory Factors 1 and 2 (Nrf-1 and Nrf-2), key nuclear encoded proteins involved in mitochondrial respiration and function, are downstream targets of PGC-1α (Scarpulla, 2002, Wu, et al., 1999). By activating Nrf-1 and MEF-2A, PGC-1α coordinates the increase of GLUT4, which is associated with enhanced insulin-stimulated glucose transport (Baar, et al., 2003, Wende, et al., 2007).
PGC-1α also plays a vital role in increasing mitochondrial volume and function, and stimulating the antioxidant defense machinery by regulating numerous antioxidant proteins including the reactive oxygen species (ROS)-detoxifying enzymes Manganese superoxide dismutase (MnSOD), catalase, and uncoupling proteins (Lin, et al., 2005, St-Pierre, et al., 2006, St-Pierre, et al., 2003). PGC-1α can tune mitochondrial apoptotic susceptibility by modulating pro- and anti-apoptotic proteins (Adhihetty, et al., 2009). Overall, these PGC1-α mediated muscle adaptations are not only protective against muscle wasting, but also against metabolic imbalance (Koves, et al., 2005, Minnaard, et al., 2005, Wang, et al., 2003).
Despite PGC-1α being critical for understanding the mechanisms of adaptive plasticity in skeletal muscle, its role in mediating the protection of skeletal muscles during hibernation is not known. In this study, we performed analyses of muscles before and during hibernation of the 13-lined ground squirrel, a natural hibernator that is able to survive prolonged periods of immobilization without significant loss of muscle mass.
Material and Methods
13-lined Ground Squirrels (Ictidomys tridecemlineatus)
All experimental procedures with 13-lined ground squirrels conformed to federal welfare guidelines and were pre-approved by the Institutional Animal Care and Use Committee (IACUC) of Johns Hopkins University School of Medicine. Hibernation-naïve euthermic 13-lined ground squirrels of both sexes were obtained from the captive breeding colony at the University of Wisconsin Oshkosh. Squirrels were supplied with food and water ad libitum during the summer period and after emerging from hibernation. When the squirrels evidenced periods of torpor, they were moved into 4°C and dark hibernaculum, and food and water was removed after several weeks without consumption (Vaughan, et al., 2006). During hibernation, the animals nest in shredded paper material, assume a fetal position, and lower their body temperature to ambient levels, often near freezing (Vaughan, et al., 2006). In addition, the hibernation period is characterized by a decline in heart rate from 300 b.p.m. to 5–10 b.p.m., and a concomitant decrease in ventilation rate and activity. The animals go through periodic interbout arousals every 3 weeks for a few hours, where shivering thermogenesis returns body temperature to normal, however, the animals do not show signs of food or water intake. (Van Breukelen and Martin, 2002).
As obligate hibernators, 13-lined ground squirrels enter hibernation in November/December and emerge in April/May. For the experimental hibernating group (n=10), squirrel muscle was collected 4–5 months after first immergence into torpor, while they were in full torpid, hypothermic state. When the squirrels emerged from hibernation, they were returned into a warm room and food and water was reinstated. 2–3 months hereafter, the squirrels were sacrificed and these comprised the control, non-hibernating group (n=6). A total of 16 squirrels went through hibernation, all of them survived and were healthy when sacrificed or emerged from hibernation. Animals were killed by decapitation after isoflurane anesthesia and the quadriceps muscle was quickly dissected from both hindlimbs and flash frozen.
Histology and Immunofluorescence
Skeletal quadriceps muscle was mounted in Tissue-Tek O.C.T Compound (Sakura Finetek) and flash frozen in cool isopentane. 10 μm sections of the tissue were cut with a cryostat. Sections were stained with hematoxylin and eosin following standard protocols. For immunofluorescence staining, sections were blocked with 3% goat serum/5% bovine serum albumin at room temperature and incubated with the following primary antibodies overnight at 4°C: BA-D5 myosin heavy chain I, BF-F3 myosin heavy chain IIB, Sc71 myosin heavy chain IIA (Developmental Studies Hybridoma Bank), followed by Alexa Fluor conjugated antibodies 350, 488 and 594 (Invitrogen) for 1 hour at room temperature. Sections were mounted with Fluoromount-G (SouthernBiotech). All images were acquired with an Eclipse i80 microscope (Nikon).
For mitochondrial staining, quadriceps sections were incubated with MitoTracker Green FM (Molecular Probes) 100–200 nM at 37°C for 15 min, washed with PBS and mounted with DAPI Hard media (Vector Laboratories). The LSM510 confocal laser-scanning microscope (Zeiss) was used for confocal microscopy with a 63X lens objective. Focal series of 0.9 μm horizontal planes (Z-scan) spaced at 1 μm were registered.
Morphometry
The distribution percentage of Type I, Type IIA and Type IIB fibers was calculated by using Nikon NS elements BR 3.0 software (Laboratory Imaging, Nikon). A minimum of 1,500 muscle fibers per animal was analyzed.
Western Blot and Density Analyses
Quadriceps samples were homogenized in ice-cold lysis buffer (NP-40 1%, Glycerol 10%, NaCl 137 mM, TrisHCl 20 mM at pH=7.5) with the addition of protease (Complete Mini, Roche) and phosphatase (PhosSTOP, Roche) cocktail inhibitors and centrifuged at 14,000 rpm for 15 min at 4 °C. Protein concentrations were determined with the Pierce BCA Protein Assay Kit (Thermo Scientific). 20 μg of protein were electrophoresed using a Bis-Tris or Tris-Glycine Gel (Invitrogen) and transferred onto nitrocellulose membranes. Membranes were incubated overnight at 4°C with the following primary antibodies diluted in blocking solution (5% milk/PBST): Catalase, Mfn-2, MnSOD, Nrf-1, UCP-2, UCP-3, VDAC-1/Porin (Abcam); Bcl-2 (BD Transduction Laboratories); Phospho-AMPKα (Thr172), AMPKα, Cytochrome C, Phospho-p38 (Thr180/Tyr182), p38, SIRT3 (Cell Signaling); Fis1 (Enzo Life Sciences); tFAM (GenWay Biotech); ATP Synthase (Invitrogen); SIRT1 (Millipore); PGC-1α (Millipore and Novus Biologicals); Nrf-2 (R&D Systems); GAPDH, GLUT4, Mfn-1 (Santa Cruz). Horseradish Peroxidase–linked secondary antibodies (Amersham) were used to detect and SuperSignal West Dura or Femto Stable Peroxide Buffer (Thermo Scientific) to visualize bands. Quantification of all immunoblots was performed using ImageJ (National Institutes of Health). Fold changes were calculated against GAPDH for whole cell lysates and against VDAC-1 for mitochondrial fraction lysates.
Mitochondrial Fractionation
Mitochondrial proteins from skeletal muscle were isolated using a standard protocol (Frezza, et al., 2007). Briefly, quadriceps muscle (50–100 mg) was minced with scissors in 5 ml ice-cold dissection buffer (10 mM EDTA in PBS). Tissue was homogenized and resuspended in 5 ml digestion buffer (10 mM EDTA, 0.05% trypsin in PBS) for 30 min at 37°C, then centrifuged at 200 g for 5 min. The pellet was resuspended in ice-cold IBm1 (67mM sucrose, 50mM Tris/Hcl, 50mM KCl, 10mM EDTA, 0.5% BSA at pH 7.4) and then homogenized using a Teflon pestle in precooled glassware. The resulting homogenate was centrifuged at 1600 g for 10 min at 4 °C. The supernatant was again centrifuged at 8000 g for 10 min at 4°C. The pellet was resuspended in 5 ml ice-cold IBm2 (250 mM sucrose, 3mM EGTA/Tris, 10mM Tris/HCl at pH 7.4) and centrifuged at 8000 g for 10 min at 4°C. Finally, the resulting pellet of mitochondria was resuspended in the IBm2 buffer.
Oxyblot
Oxyblots were performed using the OxyBlot Protein Oxidation Kit (Millipore), according to the manufacturer’s recommendations. 50 mM DTT was added to the mitochondrial protein samples. Carbonyl groups were derivatized with 1X 2,4-dinitrophenylhydrazine DNPH solution. Immunoblot analysis was performed with an antibody against DNP and VDAC-1/Porin (Abcam). Quantification of oxidized proteins was then performed using ImageJ (National Institutes of Health).
Real-Time PCR
Total RNA isolation from ground squirrel quadriceps muscle was performed with TRIzol (Invitrogen) and treated with a Turbo DNA free Kit (Ambion). Purified RNA was then used to synthesize cDNA by reverse transcription using the TaqMan RT reaction (Applied Biosystems). PCR amplification was performed with an ABI PRISM 7900HT Sequence System (Applied Biosystems) using SYBR Green PCR (Applied Biosystems). Transcript expression was normalized by GAPDH. The primers used were: GAPDH forward: 5′caccatcttccaggagcgag3′, reverse: 5′ccttctccatggtggtgaagac3′; PGC-1α forward: 5′ccaaatgaccccaagggttc3′, reverse: 5′tatgaggaggagtggtgggtg3′.
Statistical analysis
All values are expressed as mean ± SEM. Significance between two groups was determined by the unpaired Student’s t-test with a p-value ≤0.05 considered to be statistically significant.
Results
Muscle Fiber Switch to Slow Type I Fibers and Activation of the PGC-1α-mediated Endurance Pathway during Hibernation
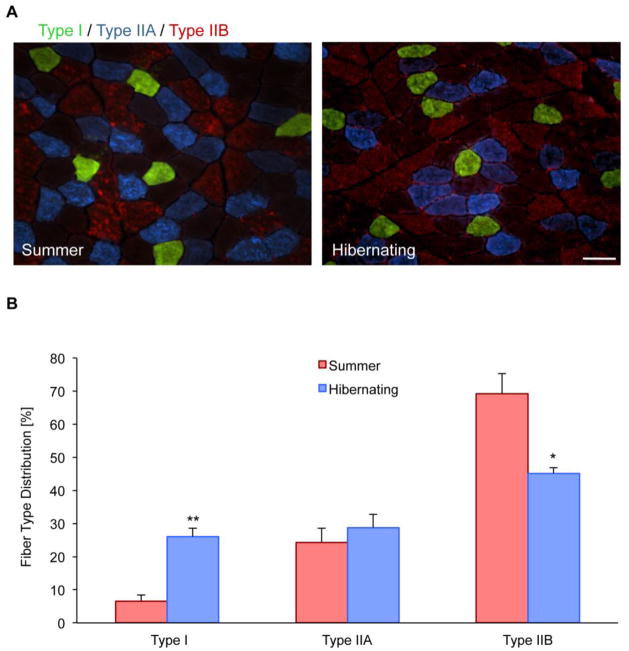
Despite long periods of immobilization and caloric restriction, muscle morphology remains unchanged during hibernation (Andres-Mateos, et al., 2012). To further expand these observations, we performed fiber type staining for Type I, IIa and IIb muscle fibers in quadriceps muscles from both hibernating and non-hibernating squirrels. Non-hibernating muscle was composed of 69.2 ± 10.7% (mean ± SD) fast-twitch Type IIb fibers and 6.6 ± 3.1% slow-twitch Type I fibers. During the hibernating period however, there was a significant decrease of Type IIb fibers to 45.2 ± 2.9% and an increase of Type I fibers to 26.1 ± 4.3% (Fig. 1A and 1B), leading to a more oxidative phenotype.
Figure 1. Slow-type muscle fiber switch during hibernation.
A, Immunofluorescent staining of squirrel quadriceps muscle reveals the relative abundance of Type I, IIA, and IIB fiber types. Scale bar 100 μm. B, Comparison of muscle fiber type percentages between muscle from non-hibernating and hibernating squirrels. During hibernation there is a significant increase in slow-twitch, Type I muscle fibers and a decrease in fast-twitch, Type IIB fibers. (*P<0.05; **P<0.01)
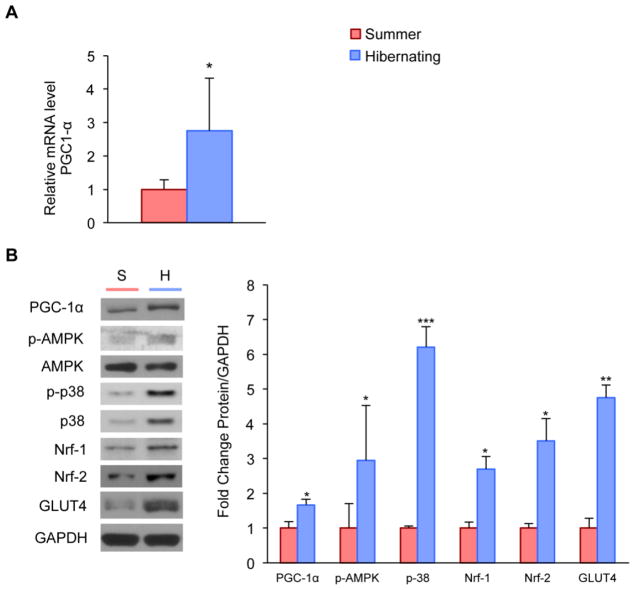
Because PGC-1α has been shown to be a key regulator of mitochondrial biogenesis and muscle fiber type switching (Calvo, et al., 2008, Lin, et al., 2005, Lin, et al., 2002), we examined PGC-1α mRNA and protein in the quadriceps muscle and found significantly increased expression of PGC-1α and its protein level during hibernation (Fig. 2A and B). PGC-1α can be activated through phosphorylation by both AMP-activated Protein Kinase (AMPK) and members of the MAPK family such as p38 (Akimoto, et al., 2005, Jager, et al., 2007, Yu, et al., 2003). We found that hibernating squirrels showed a significant increase in the phosphorylated, active form of AMPK (Fig. 2B). Protein levels of total p38 MAPK were increased as well (Fig. 2B). Downstream targets of PGC-1α, including the Nuclear Respiratory Factors 1 and 2 (Nrf-1 and Nrf-2), also showed significant increases during hibernation (Fig. 2B) (Scarpulla, 2002, Wu, et al., 1999). By activating Nrf-1, PGC-1α also coordinates the increase of the GLUT4 isoform of the glucose transporter, which allows rapid glucose uptake into the muscle cell (Baar, et al., 2003, Ramachandran, et al., 2008, Wende, et al., 2007). We found that relative abundance of GLUT4 protein was enhanced during hibernation (Fig. 2B).
Figure 2. Activation of the PGC-1α-mediated endurance pathway in skeletal muscle (whole cell lysate) during hibernation.
A, Real-time PCR of PGC-1α in whole muscle lysate reveals significantly enhanced mRNA levels during hibernation. B, Western blot and density analyses of PGC-1α and its down- and upstream targets: protein levels of p-AMPK, p-38, Nuclear Respiratory Factors 1 and 2 (Nrf-1 and Nrf-2), and GLUT4 are significantly upregulated in hibernating quadriceps muscle. (*P<0.05; **P<0.01; ***P<0.001)
These results suggest that hibernating squirrels are able to activate the PGC-1α-mediated signaling cascade despite prolonged immobilization and absence of feeding.
Increased Mitochondrial Antioxidant Stress Response during Periods of Hibernation
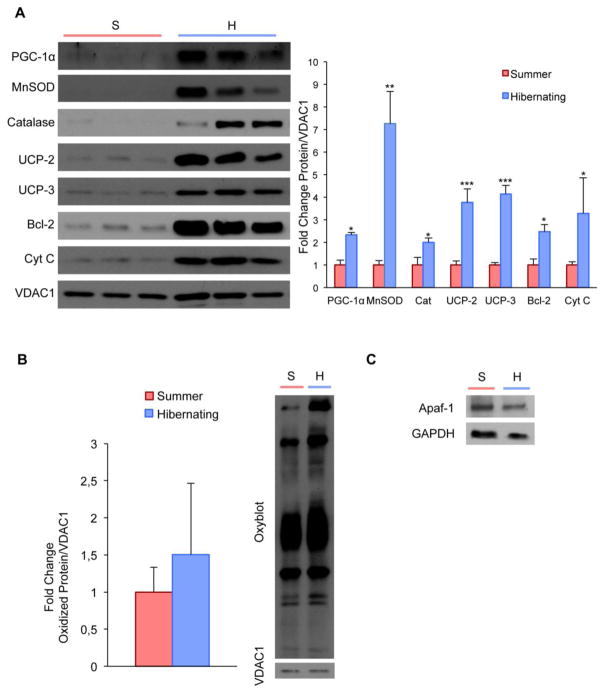
PGC-1α activation is associated with antioxidant stress response and anti-apoptotic signaling. Mitochondrial proteins including the antioxidant enzymes Manganese superoxide dismutase (MnSOD) and catalase as well as uncoupling proteins UCP-2 and UCP-3 are regulated by PGC-1α upon oxidative stress (Puigserver, et al., 1998, St-Pierre, et al., 2006, St-Pierre, et al., 2003). In order to examine mitochondrial changes, we isolated mitochondrial fractions from quadriceps muscle of hibernating and non-hibernating squirrels. We found that protein levels of PGC-1α, MnSOD, catalase, UCP-2 and UCP-3 were significantly increased in the mitochondrial fractions during hibernation (Fig. 3A). We also quantified oxidized proteins in the quadriceps mitochondrial fractions, yet found no signs of oxidative stress in hibernating squirrels (Fig. 3B). These results suggest that the 13-lined ground squirrels have evolved an efficient way to overcome the oxidative challenge of hibernation by overexpressing the mitochondrial antioxidant machinery.
Figure 3. Upregulation of antioxidant, uncoupling, and antiapoptotic proteins in mitochondrial fractions from squirrel quadriceps muscle during hibernation without detection of oxidative stress.
A, Western blot and density analyses in mitochondrial fractions from squirrel quadriceps muscle shows significant upregulation of PGC-1α, Manganese superoxide dismutase (MnSOD), catalase (Cat), uncoupling proteins 2 and 3 (UCP-2 and UCP-3), Bcl-2, and Cytochrome C (Cyt C). B, Despite increased oxidative stress proteins, OxyBlot analyses reveal unaltered oxidized protein levels of DNP-derivatized quadriceps muscle mitochondria. Quantification is normalized to the protein levels of VDAC1/Porin. C, In concomitance to increased antiapoptotic proteins during hibernation, Western blot shows a trend in decreased protein levels of the pro-apoptotic regulator Apaf-1 in the hibernating group. (*P<0.05; **P<0.01, ***P<0.001)
Previous evidence suggests that PGC-1α can decrease mitochondrial apoptotic susceptibility as part of the cellular response to endurance exercise (Adhihetty, et al., 2007, Adhihetty, et al., 2009). We examined mitochondrial protein levels of apoptotic regulators including cytochrome C and Bcl-2, which has also been implicated as an antioxidant regulator (Hockenbery, et al., 1993, Kowaltowski, et al., 2004). We found significantly increased mitochondrial protein levels of cytochrome C and Bcl-2 (Fig. 3A) and, concurrently, a trend in decreased protein level of the pro-apoptotic regulator Apaf-1 (Fig. 3C). These findings are consistent with a marked decline in the apoptotic susceptibility of skeletal muscle during hibernation.
Enhanced Mitochondrial Abundance and Metabolism during Hibernation
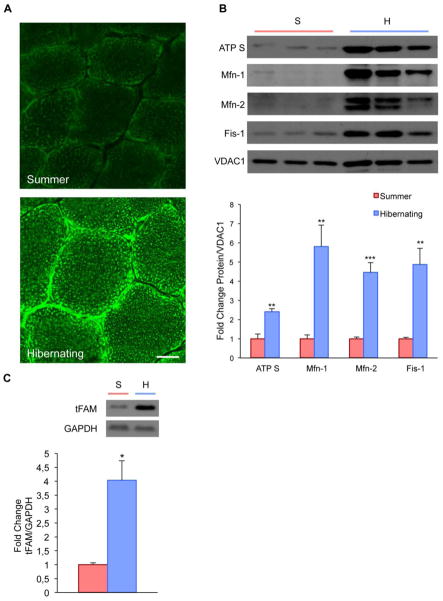
Since we found increased mitochondrial oxidative capacity in skeletal muscle during hibernation, we used MitoTracker staining to explore mitochondrial abundance. The staining showed an increase of both cytoplasmic and subsarcolemmal mitochondria in the quadriceps muscle of hibernating animals (Fig. 4A). This increase was accompanied by higher protein levels of ATP Synthase and the mitochondrial transcription factor A (tFAM) (Fig. 4B and C), suggesting increased oxidative metabolism in the muscle of hibernating animals (Scarpulla, 2002).
Figure 4. Mitochondrial biogenesis and dynamics during hibernation.
A, MitoTracker staining shows accumulation of subsarcolemmal and cytoplasmic mitochondria in quadriceps muscle of hibernating squirrels. Scale bar, 10 μm. B, Western blot and density analyses of mitochondrial fractions shows significantly increased protein levels of ATP Synthase, Mitofusin-1 (Mfn-1), Mitofusin-2 (Mfn-2), and Fis-1, suggesting enhanced mitochondrial biogenesis during periods of hibernation. C, Relative increase of mitochondrial transcription factor A protein during hibernation. (**P<0.01, ***P<0.001)
Increased mitochondrial volume and function are associated with mitochondrial network dynamics and continuous remodeling – it is thought to require an elaborate equilibrium of the fusion and fission machinery (Chen and Chan, 2005, Rube and van der Bliek, 2004). These fusion and fission dynamics are important for the maintenance and integrity of functional mitochondria. Previous data have also demonstrated increased both fusion and fission protein levels in skeletal muscle after exercise (Cartoni, et al., 2005, Ding, et al., 2010). We tested the mitochondrial protein levels of Fis-1, Mfn-1 and Mfn-2, which are key regulators involved in mitochondrial fission and fusion. We found significantly increased relative abundance of these proteins in the mitochondrial fraction of quadriceps muscle during hibernation (Fig. 4B).
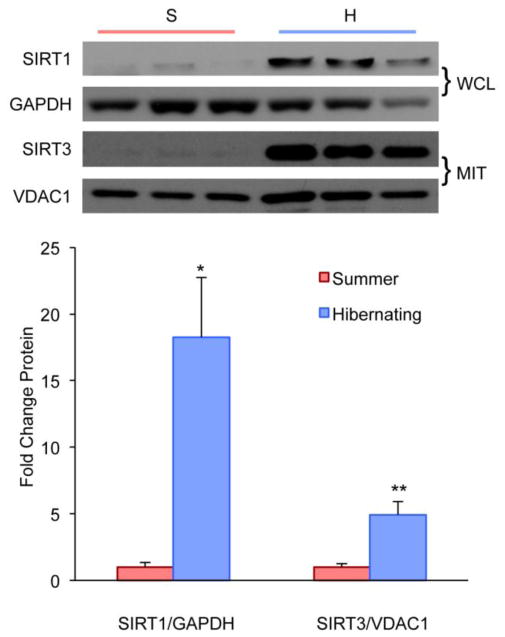
Increased Sirtuin Protein Levels during Hibernation
NAD+-dependent protein deacetylases sirtuins are functional regulators of PGC-1α-associated mitochondrial activity (Gerhart-Hines, et al., 2007, Kong, et al., 2010) As metabolic sensors they are thought to link mitochondrial biogenesis with caloric restriction and cold exposure (D’Antona, et al., 2010, Haigis and Sinclair, 2010). Sirtuins have also been implicated in the exercise response: SIRT1 activates PGC-1α via deacetylation and is highly expressed after endurance exercise (Canto, et al., 2010, Gerhart-Hines, et al., 2007, Gurd, et al., 2010, Suwa, et al., 2008). Remarkably, we observed a 18-fold increase of SIRT1 protein levels in whole muscle lysates during hibernation (Fig. 5). SIRT3, which is localized to the mitochondria, has been shown to mediate PGC-1α effects on cellular ROS production and mitochondrial biogenesis (Kelly, 2010, Kong, et al., 2010). We also found a significant increase in SIRT3 in the mitochondrial fraction of quadriceps from hibernating squirrels when compared to the non-hibernating animals (Fig. 5). Our data support increased mitochondrial protein abundance with enhanced metabolic and antioxidant capacity during hibernation.
Figure 5. Increased sirtuin protein levels during hibernation.
Western blot and density analyses of SIRT 1 in whole cell lysate (WCL) of quadriceps muscle shows an 18-fold upregulation during hibernation. SIRT3 is significantly increased in mitochondrial lysate fractions (MIT) of quadriceps muscle. (*P<0.05; **P<0.01)
Discussion
Extended periods of immobilization comprise a common and challenging clinical issue, ultimately having a deleterious impact on muscle function (Degens and Alway, 2006, di Prampero and Narici, 2003, Hornberger, et al., 2001, Jackman and Kandarian, 2004). Muscle inactivity not only results in atrophy and a decrease in muscle weight and strength, but also in mitochondrial dysbalance (Hortobagyi, et al., 2000, Nicks, et al., 1989). It is associated with mitochondrial loss, morphological changes, and impaired mitochondrial function (Powers, et al., 2012). However, mammalian hibernators are capable of preserving their muscle mass despite no caloric intake and long periods of immobilization (Andres-Mateos, et al., 2012). We have demonstrated that during hibernation the 13-lined ground squirrel activates PGC-1α signaling in ways that are very similar to endurance exercise. Previous studies in rats have shown that hindlimb immobilization or muscle unloading is associated with a slow-to-fast fiber type transformation, resulting in a muscle profile more susceptible to fatigue (Fitts, et al., 2001, Stevenson, et al., 2003, Thorlund, et al., 2011). In contrast, we demonstrate that hibernating skeletal muscle exhibits a fiber type switch towards slow Type I muscle fibers and a concomitant decrease in fast Type IIB fibers. The hibernating squirrels show not only a marked resilience to the morphological consequences of disuse atrophy but also a switch in muscle fiber type composition favoring a more oxidative, fatigue-resistant phenotypic profile.
PGC-1α has a pivotal role in regulating fiber type conversion and exercise training-induced skeletal muscle adaptations (Handschin, et al., 2007, Lin, et al., 2002). In the hibernating squirrel we observe the activation of the PGC-1α-mediated signaling cascade (Fig. 6): Not only do we detect increased levels of PGC-1α mRNA and protein itself, but we also show increased protein levels of its upstream activators p38 and pAMPK and its downstream targets Nrf-1, Nrf-2 and GLUT4 (Baar, et al., 2002, Daugaard, et al., 2000, Eddy, et al., 2005). Our findings reveal that the PGC-1α-mediated endurance exercise pathway can be activated in vivo despite the hibernators’ extreme state of inactivity. PGC-1α also impacts mitochondrial homeostasis through enhanced antioxidant status, reduced ROS generation and altered pro- and antiapoptotic protein abundance. Specifically, recent studies showed a rise in oxidative stress enzymes during and after endurance exercise (Jiang, et al., 2009, Khassaf, et al., 2001). In addition, Bcl-2 protein levels are increased in exercised rodents and cytochrome C upregulation has been reported in both endurance exercise and high-intensity interval training (Leick, et al., 2010, Wright, et al., 2007). Both of these proteins are proposed to have a protective effect on the muscle against apoptosis. Our observations of muscle mitochondria in hibernating squirrels correspond with previously observed responses to exercise (Jiang, et al., 2009, Khassaf, et al., 2001, Leick, et al., 2010, Wright, et al., 2007). This is reflected by increased protein levels of the antioxidant proteins MnSOD, catalase and uncoupling proteins 2 and 3 as well as elevation of the apoptotic regulator proteins Bcl-2 and cytochrome C. Previous studies on hibernating ground squirrels have also reported enhanced antioxidant gene expression and protein abundance during hibernation (Allan and Storey, 2012, Morin, et al., 2008, Morin and Storey, 2007).
Figure 6. Mitochondrial signaling networks in hibernation.
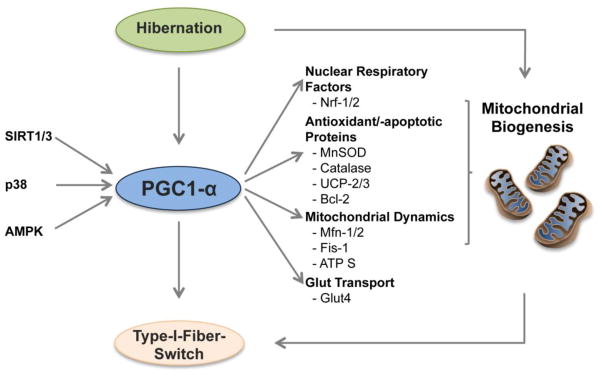
Activation of the PGC1α-mediated endurance pathway contributes to the enhanced mitochondrial biogenesis and Type I fiber switch seen during hibernation.
Since mitochondrial exercise adaptations are observed in hibernating squirrel tissue, the concern raises whether endurance exercise damages occur as well during hibernation, such as oxidative stress formation promoted through enhanced ROS formation (Powers and Jackson, 2008). In addition, long-term immobilization has been also associated with increased mitochondrial ROS production (Powers, et al., 2012). Unexpectedly, we did not find signs of increased oxidative stress formation during hibernation as revealed by unchanged oxidized mitochondrial protein levels. Thus, given these increased basal protein levels of antioxidant enzymes during hibernation, hibernating skeletal muscle may ultimately be protected from oxidative stress and apoptosis.
During torpor/arousal periods, euthermic rewarming from the hypometabolic state occurs and requires an augmented capacity for shivering and nonshivering thermogenesis. In addition to their antioxidant property, uncoupling proteins have been proposed to play a role in thermal homeostasis and energy balance during hibernation (Boyer, et al., 1998). The activation of uncoupling proteins may play a dual role in atrophy-protective and thermoregulative mechanisms during the hibernating season.
It is well-established that endurance exercise increases the per-cell abundance of mitochondria and respiratory enzymes (Booth, 1977, Holloszy, 1967). Previous data have also shown that mRNA levels of mitochondrial fusion proteins Mfn-1/2 and fission protein Fis-1 are elevated significantly post-exercise (Cartoni, et al., 2005, Ding, et al., 2010). These fusion and fission proteins are highly involved in electrical and biochemical connectivity and protection of mitochondrial DNA (Berman, et al., 2008). Keeping a proper balance of mitochondrial network dynamics is essential to maintain functional mitochondria (Chen and Chan, 2005, Rube and van der Bliek, 2004). Disruption of dynamic remodeling regulated by proteins involved in mitochondrial fusion and fission can also lead to muscle atrophy (Romanello, et al., 2010). Our findings show increased abundance of mitochondria and enhanced protein levels of the respiratory enzymes ATP Synthase and cytochrome C in hibernating quadriceps muscle. Furthermore, we find increased protein levels of Mfn-1/2 and Fis-1 during hibernation. These results suggest that during hibernation the increase in mitochondrial number, as well as fusion and fission proteins, may enhance the capacity for ATP generation. This promotes metabolic protection to maintain functional and balanced mitochondrial dynamics.
It has been shown that the NAD+-dependent protein deacetylases sirtuins are functional regulators of PGC-1α-associated mitochondrial biogenesis (Gerhart-Hines, et al., 2007, Kong, et al., 2010) Sirtuins are metabolic sensors that may link caloric restriction, cold exposure, and induction of mitochondrial metabolism (D’Antona, et al., 2010). Consistent with this hypothesis, we show that both SIRT1 and SIRT3 are significantly increased during hibernation. This finding not only substantiates the observed activation of the PGC-1α-mediated endurance pathway, it also highlights its clinical role in disuse atrophy: activators of SIRT1, such as Resveratrol, have been utilized as exercise mimetic in rodents to improve mitochondrial biogenesis and increase endurance capacity (Lagouge, et al., 2006, Momken, et al., 2011).
Besides the impact of endurance exercise on skeletal muscle, resistance exercise encompasses myofiber hypertrophy, increased protein and RNA content, and enhanced tension output (Adams, et al., 2004, Wong and Booth, 1988). The Akt/mTOR pathway is a central mediator of myofiber hypertrophy by regulating muscle cell growth and protein synthesis (Baar and Esser, 1999, Haddad and Adams, 2002, Kubica, et al., 2005). Previous work has shown that numerous components of this resistance exercise pathway also become activated during hibernation (Andres-Mateos, et al., 2012). The current paradigm suggests that the endurance and resistance pathways are mutually exclusive due to the so-called “AMPK-PKB” switch, which implies that phosphorylated AMPK inhibits the activity of mTOR and its downstream targets (Atherton, et al., 2005, Nader, 2006). In contrast to this model, we find a synchronization of both resistance and endurance pathways in the hibernating squirrel and demonstrate that a parallel activation of both pathways can occur in vivo. It is therefore reasonable to suggest that crosstalk of these two pathways might be more beneficial than activating solely one of them.
The co-existence of these two pathways might at first appear paradoxical. However, it is possible that the seemingly paradoxical activation of converse pathways may ultimately result in the same active change to an oxidative phenotype. In other adaptive instances, such as opposite changes in mechanical stress in the heart, an induction of similar patterns of gene expression has also been observed, namely the return to a “fetal gene program” (Depre, et al., 1998, Razeghi, et al., 2002). Therefore, these opposite pathways during hibernation may lead to the same direction of change – a fiber switch towards slow Type I muscle fibers.
Skeletal muscle is the largest physiological organ undergoing significant remodeling during exercise. The adaptations elicited by endurance exercise are complex and involve mitochondrial bioenergetics, redox homeostasis, and protein metabolism. These adaptations, such as enrichment of oxidative slow-twitch Type I fibers, are critical for maintaining skeletal muscle homeostasis when challenged with muscle wasting conditions (Minnaard, et al., 2005, Wenz, et al., 2009). Our results show that hibernating ground squirrels exhibit a remarkable plasticity in skeletal muscle that resembles both the myofiber transitions and protein level alterations that occur in the mammalian response to endurance exercise training. The mitochondrial homeostasis of antioxidant enzymes, apoptotic proteins, and the fusion and fission machinery may aid in the remodeling of muscle mass and combating the oxidative challenge during hibernation.
Many clinical conditions including inherited myopathies, disuse conditions (immobilization, bed rest, denervation, or microgravity), aging, and cachexia result in significant loss of muscle mass. In these circumstances, maintenance of muscle mass is crucial in combating debilitating functional consequences. However, there are no effective and safe treatment strategies available to prevent muscle atrophy (Glass, 2003, Narici and de Boer, 2011, Wagner, 2008). The 13-lined ground squirrel animal model is physiologically protected against disuse atrophy. This enables us to study underlying molecular pathways and potential pharmacological targets that effectively trigger skeletal muscle remodeling, regulate oxidative balance, and susceptibility to atrophy. Importantly, we have shown that activation of the endurance pathway can be achieved in vivo despite prolonged periods of immobilization. Future studies will be aimed at finding potential molecular targets for muscle wasting conditions that are so severe that they cannot withstand physical activity.
Highlights.
Hibernating 13-lined ground squirrels exhibit a shift to slow-twitch Type I muscle fibers.
The muscle fiber switch is accompanied by an activation of the PGC-1α-mediated endurance exercise pathway.
Increased antioxidant capacity without evidence of oxidative stress is seen in hibernating skeletal muscle.
We observe a marked decline in apoptotic susceptibility, and enhanced mitochondrial metabolism.
An activation of the PGC-1α-mediated endurance exercise pathway can be achieved in vivo despite prolonged periods of immobilization during hibernation.
Acknowledgments
LAL is supported by NIH R01 GM29090. RDC is supported by an NIH Director’s New Innovator Award DP2 OD004515, an NIH award 5K08NS055879, an MDA award #101938, and the Dana and Albert R. Broccoli Charitable Foundation for Research. RHAF is supported by grant 13N7871 of the German Federal Ministry of Education and Research and a grant from the Ministry of Science, Research and the Arts of Baden-Wuerttemberg, Germany.
Footnotes
Author Contributions
Conceived and designed the experiments: RDC EAM RX RM. Performed the experiments: RX EAM RM. Analyzed the data: RX EAM RM. Contributed reagents/materials/analysis tools: LAL DKM RHAF. Wrote the manuscript: RX EAM RDC EMM RM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams GR, Cheng DC, Haddad F, Baldwin KM. Skeletal muscle hypertrophy in response to isometric, lengthening, and shortening training bouts of equivalent duration. Journal of applied physiology. 2004;96:1613–1618. doi: 10.1152/japplphysiol.01162.2003. [DOI] [PubMed] [Google Scholar]
- 2.Adhihetty PJ, O’Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. Journal of applied physiology. 2007;102:1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- 3.Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. American journal of physiology. Cell physiology. 2009;297:C217–225. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- 4.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. The Journal of biological chemistry. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 5.Allan ME, Storey KB. Expression of NF-kappaB and downstream antioxidant genes in skeletal muscle of hibernating ground squirrels, Spermophilus tridecemlineatus. Cell biochemistry and function. 2012;30:166–174. doi: 10.1002/cbf.1832. [DOI] [PubMed] [Google Scholar]
- 6.Andres-Mateos E, Brinkmeier H, Burks TN, Mejias R, Files DC, Steinberger M, Soleimani A, Marx R, Simmers JL, Lin B, Finanger Hedderick E, Marr TG, Lin BM, Hourde C, Leinwand LA, Kuhl D, Foller M, Vogelsang S, Hernandez-Diaz I, Vaughan DK, Alvarez de la Rosa D, Lang F, Cohn RD. Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO molecular medicine. 2012 doi: 10.1002/emmm.201201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- 8.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. The American journal of physiology. 1999;276:C120–127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 9.Baar K, Song Z, Semenkovich CF, Jones TE, Han DH, Nolte LA, Ojuka EO, Chen M, Holloszy JO. Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17:1666–1673. doi: 10.1096/fj.03-0049com. [DOI] [PubMed] [Google Scholar]
- 10.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 11.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annual review of biochemistry. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 12.Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell death and differentiation. 2008;15:1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth F. Effects of endurance exercise on cytochrome C turnover in skeletal muscle. Annals of the New York Academy of Sciences. 1977;301:431–439. doi: 10.1111/j.1749-6632.1977.tb38219.x. [DOI] [PubMed] [Google Scholar]
- 14.Boyer BB, Barnes BM, Lowell BB, Grujic D. Differential regulation of uncoupling protein gene homologues in multiple tissues of hibernating ground squirrels. The American journal of physiology. 1998;275:R1232–1238. doi: 10.1152/ajpregu.1998.275.4.R1232. [DOI] [PubMed] [Google Scholar]
- 15.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. Journal of applied physiology. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 16.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell metabolism. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiological reviews. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 18.Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. The Journal of physiology. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Human molecular genetics. 2005;14(Spec No 2):R283–289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 20.D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell metabolism. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000;49:1092–1095. doi: 10.2337/diabetes.49.7.1092. [DOI] [PubMed] [Google Scholar]
- 22.Degens H, Alway SE. Control of muscle size during disuse, disease, and aging. Int J Sports Med. 2006;27:94–99. doi: 10.1055/s-2005-837571. [DOI] [PubMed] [Google Scholar]
- 23.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nature medicine. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 24.di Prampero PE, Narici MV. Muscles in microgravity: from fibres to human motion. Journal of biomechanics. 2003;36:403–412. doi: 10.1016/s0021-9290(02)00418-9. [DOI] [PubMed] [Google Scholar]
- 25.Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, Wen L, Liu S, Ji LL, Zhang Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochimica et biophysica acta. 2010;1800:250–256. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Eddy SF, Morin P, Jr, Storey KB. Cloning and expression of PPAR-gamma and PGC-1alpha from the hibernating ground squirrel, Spermophilus tridecemlineatus. Molecular and cellular biochemistry. 2005;269:175–182. doi: 10.1007/s11010-005-3459-4. [DOI] [PubMed] [Google Scholar]
- 27.Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. The Journal of experimental biology. 2001;204:3201–3208. doi: 10.1242/jeb.204.18.3201. [DOI] [PubMed] [Google Scholar]
- 28.Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Reviews of physiology, biochemistry and pharmacology. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 29.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nature protocols. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 30.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. The EMBO journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nature cell biology. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 32.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. The Journal of biological chemistry. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 33.Gurd BJ, Perry CG, Heigenhauser GJ, Spriet LL, Bonen A. High-intensity interval training increases SIRT1 activity in human skeletal muscle. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35:350–357. doi: 10.1139/H10-030. [DOI] [PubMed] [Google Scholar]
- 34.Haddad F, Adams GR. Selected contribution: acute cellular and molecular responses to resistance exercise. Journal of applied physiology. 2002;93:394–403. doi: 10.1152/japplphysiol.01153.2001. [DOI] [PubMed] [Google Scholar]
- 35.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annual review of pathology. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. The Journal of biological chemistry. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 37.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 38.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. The Journal of biological chemistry. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 39.Hornberger TA, Hunter RB, Kandarian SC, Esser KA. Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am J Physiol Cell Physiol. 2001;281:C179–187. doi: 10.1152/ajpcell.2001.281.1.C179. [DOI] [PubMed] [Google Scholar]
- 40.Hortobagyi T, Dempsey L, Fraser D, Zheng D, Hamilton G, Lambert J, Dohm L. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. The Journal of physiology. 2000;524(Pt 1):293–304. doi: 10.1111/j.1469-7793.2000.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 42.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang N, Zhang G, Bo H, Qu J, Ma G, Cao D, Wen L, Liu S, Ji LL, Zhang Y. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: a potential antioxidant function. Free radical biology & medicine. 2009;46:138–145. doi: 10.1016/j.freeradbiomed.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 45.Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Alternative medicine review: a journal of clinical therapeutic. 2010;15:245–263. [PubMed] [Google Scholar]
- 46.Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. Journal of applied physiology. 2001;90:1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- 47.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PloS one. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. The Journal of biological chemistry. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 49.Kowaltowski AJ, Fenton RG, Fiskum G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free radical biology & medicine. 2004;37:1845–1853. doi: 10.1016/j.freeradbiomed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. The Journal of biological chemistry. 2005;280:7570–7580. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- 51.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Lee K, Park JY, Yoo W, Gwag T, Lee JW, Byun MW, Choi I. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy: proteomic and molecular assessment. Journal of cellular biochemistry. 2008;104:642–656. doi: 10.1002/jcb.21653. [DOI] [PubMed] [Google Scholar]
- 53.Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Experimental gerontology. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 56.Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK. Skeletal Muscle wasting and contractile performance in septic rats. Muscle & nerve. 2005;31:339–348. doi: 10.1002/mus.20268. [DOI] [PubMed] [Google Scholar]
- 57.Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, Zahariev A, Zahn S, Stein TP, Sebedio JL, Pujos-Guillot E, Falempin M, Simon C, Coxam V, Andrianjafiniony T, Gauquelin-Koch G, Picquet F, Blanc S. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 58.Morin P, Jr, Ni Z, McMullen DC, Storey KB. Expression of Nrf2 and its downstream gene targets in hibernating 13-lined ground squirrels, Spermophilus tridecemlineatus. Molecular and cellular biochemistry. 2008;312:121–129. doi: 10.1007/s11010-008-9727-3. [DOI] [PubMed] [Google Scholar]
- 59.Morin P, Jr, Storey KB. Antioxidant defense in hibernation: cloning and expression of peroxiredoxins from hibernating ground squirrels, Spermophilus tridecemlineatus. Archives of biochemistry and biophysics. 2007;461:59–65. doi: 10.1016/j.abb.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 60.Nader GA. Concurrent strength and endurance training: from molecules to man. Medicine and science in sports and exercise. 2006;38:1965–1970. doi: 10.1249/01.mss.0000233795.39282.33. [DOI] [PubMed] [Google Scholar]
- 61.Narici MV, de Boer MD. Disuse of the musculo-skeletal system in space and on earth. European journal of applied physiology. 2011;111:403–420. doi: 10.1007/s00421-010-1556-x. [DOI] [PubMed] [Google Scholar]
- 62.Nicks DK, Beneke WM, Key RM, Timson BF. Muscle fibre size and number following immobilisation atrophy. Journal of anatomy. 1989;163:1–5. [PMC free article] [PubMed] [Google Scholar]
- 63.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological reviews. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. American journal of physiology. Endocrinology and metabolism. 2012;303:E31–39. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 66.Ramachandran B, Yu G, Gulick T. Nuclear respiratory factor 1 controls myocyte enhancer factor 2A transcription to provide a mechanism for coordinate expression of respiratory chain subunits. The Journal of biological chemistry. 2008;283:11935–11946. doi: 10.1074/jbc.M707389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Razeghi P, Myers TJ, Frazier OH, Taegtmeyer H. Reverse remodeling of the failing human heart with mechanical unloading. Emerging concepts and unanswered questions. Cardiology. 2002;98:167–174. doi: 10.1159/000067313. [DOI] [PubMed] [Google Scholar]
- 68.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. The EMBO journal. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rube DA, van der Bliek AM. Mitochondrial morphology is dynamic and varied. Molecular and cellular biochemistry. 2004;256–257:331–339. doi: 10.1023/b:mcbi.0000009879.01256.f6. [DOI] [PubMed] [Google Scholar]
- 70.Scarpulla RC. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene. 2002;286:81–89. doi: 10.1016/s0378-1119(01)00809-5. [DOI] [PubMed] [Google Scholar]
- 71.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 72.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. The Journal of biological chemistry. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 73.Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. The Journal of physiology. 2003;551:33–48. doi: 10.1113/jphysiol.2003.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism: clinical and experimental. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Thorlund JB, Jakobsen O, Madsen T, Christensen PA, Nedergaard A, Andersen JL, Suetta C, Aagaard P. Changes in muscle strength and morphology after muscle unloading in Special Forces missions. Scandinavian journal of medicine & science in sports. 2011;21:e56–63. doi: 10.1111/j.1600-0838.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 76.Van Breukelen F, Martin SL. Invited review: molecular adaptations in mammalian hibernators: unique adaptations or generalized responses? J Appl Physiol. 2002;92:2640–2647. doi: 10.1152/japplphysiol.01007.2001. [DOI] [PubMed] [Google Scholar]
- 77.Vaughan DK, Gruber AR, Michalski ML, Seidling J, Schlink S. Capture, care, and captive breeding of 13-lined ground squirrels, Spermophilus tridecemlineatus. Lab Anim (NY) 2006;35:33–40. doi: 10.1038/laban0406-33. [DOI] [PubMed] [Google Scholar]
- 78.Wagner KR. Approaching a new age in Duchenne muscular dystrophy treatment. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2008;5:583–591. doi: 10.1016/j.nurt.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 80.Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. The Journal of biological chemistry. 2007;282:36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- 81.Wenz T, Diaz F, Hernandez D, Moraes CT. Endurance exercise is protective for mice with mitochondrial myopathy. Journal of applied physiology. 2009;106:1712–1719. doi: 10.1152/japplphysiol.91571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Wong TS, Booth FW. Skeletal muscle enlargement with weight-lifting exercise by rats. Journal of applied physiology. 1988;65:950–954. doi: 10.1152/jappl.1988.65.2.950. [DOI] [PubMed] [Google Scholar]
- 83.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. The Journal of biological chemistry. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 84.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 85.Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. The Journal of physiology. 2003;546:327–335. doi: 10.1113/jphysiol.2002.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]