Abstract
Measles vaccination programs would benefit from delivery methods that decrease cost, simplify logistics, and increase safety. Conventional subcutaneous injection is limited by the need for skilled healthcare professionals to reconstitute and administer injections, and by the need for safe needle handling and disposal to reduce the risk of disease transmission through needle re-use and needlestick injury. Microneedles are micron-scale, solid needles coated with a dry formulation of vaccine that dissolves in the skin within minutes after patch application. By avoiding the use of hypodermic needles, vaccination using a microneedle patch could be carried out by minimally trained personnel with reduced risk of blood-borne disease transmission. The goal of this study was to evaluate measles vaccination using a microneedle patch to address some of the limitations of subcutaneous injection. Viability of vaccine virus dried onto a microneedle patch was stabilized by incorporation of the sugar, trehalose, and loss of viral titer was less than 1 log10(TCID50) after storage for at least 30 days at room temperature. Microneedle patches were then used to immunize cotton rats with the Edmonston-Zagreb measles vaccine strain. Vaccination using microneedles at doses equaling the standard human dose or one-fifth the human dose generated neutralizing antibody levels equivalent to those of a subcutaneous immunization at the same dose. These results show that measles vaccine can be stabilized on microneedles and that vaccine efficiently reconstitutes in vivo to generate a neutralizing antibody response equivalent to that generated by subcutaneous injection.
Keywords: Measles vaccination, Microneedle patch, Vaccine stability, Cotton rat
1. Introduction
Despite the widespread availability of an inexpensive and effective vaccine, measles virus is one of the leading causes of vaccine-preventable morbidity and mortality among children worldwide [1]. High levels of coverage are necessary for interruption of measles transmission. Measles vaccination programs have dramatically reduced the incidence of disease in both developed and developing countries [2, 3]. More than 4.5 million measles deaths have been prevented as of 2008 through implementation of the vaccination strategies developed by WHO and UNICEF. Global mortality has declined by 74% from an estimated 733,000 deaths in 2000 to 139,300 in 2010 [4]. Measles elimination, defined as the absence of endemic transmission of virus, has been achieved and sustained in the WHO Region of the Americas since 2002, and four of the five other WHO Regions, European, Eastern Mediterranean and Western Pacific, have targeted measles for elimination by 2020 or earlier [5].
The measles vaccine is currently delivered by subcutaneous injection using a needle and syringe. This delivery method creates the requirement for specifically trained healthcare personnel to administer each vaccine dose, typically at centralized locations. In contrast, the global campaign to eradicate polio has been possible, in part, because of the simplicity of delivering the oral polio vaccine, which can be administered by minimally trained personnel. Decreasing the logistical challenges associated with delivery of measles vaccine could increase vaccination coverage and reduce vaccination campaign costs.
Hypodermic injections create hazardous medical waste which must be safely destroyed. Preventing needle theft and reuse through responsible disposal methods adds significant costs to vaccination campaigns. For example, a relatively small measles vaccination campaign in the Philippines generated over 130,000 kg of sharps waste [6]. Another logistical challenge with the standard vaccination scheme is the requirement of a cold chain for vaccine storage and transport. After reconstitution, multi-dose vials must be used within 2 h or discarded [7]. This leads to vaccine wastage and increased program costs. A delivery system that eliminates the need for reconstitution and reduces or eliminates the need for cold storage and transport could enable more efficient use of measles vaccine and decrease the cost per delivered dose.
Measles vaccination using a microneedle patch may be able to address some of the limitations of conventional hypodermic injection and thereby facilitate measles mortality reduction and elimination programs. Microneedles are micron-sized needles made of metal or polymer that are designed to achieve the efficacy of hypodermic injection with the simplicity of a patch [8, 9]. Microneedles offer the possibility of eliminating or mitigating many of the logistical challenges associated with the current vaccination strategy, including reduced cost, simplified transport and storage, and increased safety. The microneedles used in this study remain on the patch after it is removed and could present a small risk for disease transmission as a sharps hazard. However, microneedles can also be fabricated from dissolving polymers in which case no potentially infectious, sharps waste would be generated [10, 11]. Microneedles require a small amount of force to penetrate the skin, and once the barrier layer has been penetrated, the vaccine is rapidly released into the skin. The microscopic wound created by the patch is superficial and heals quickly [12]. With the correct excipient conditions other vaccines have been stabilized onto a microneedle patch [13, 14]. If this high level of temperature stability could be extended to the live-attenuated measles vaccine, the cost and logistical issues associated with vaccine transport and storage could be decreased significantly. Finally, the small size of the microneedle patch would limit sharps waste following large-scale vaccination campaigns. This would decrease transport costs while also minimizing the potential for reuse.
Measles vaccine has been previously delivered to the skin using a variety of methods including the Mantoux method [15] and jet injection [16, 17]. While some studies have shown improvements after intradermal delivery [18], others found lower neutralizing antibody titers when compared with traditional delivery routes [19, 20]. The inferior serologic response to intradermal vaccination seen in these studies could result from the low dose of measles vaccine delivered (as low as 5% of the standard dose). Neither study investigated the response to a standard subcutaneous dose (at least 103 TCID50) delivered intradermally.
Stabilization of the measles vaccine in a dry state has also been previously examined. Viral infectivity loss after drying has been mitigated through both excipient selection and drying process optimization [21, 22]. Some of these dry powder vaccines were shown to be efficacious after delivery to the respiratory tract of non human primates [23–26]. However, these stabilization methods used drying processes such as spray drying and lyophilization, which are not easily compatible with microneedle fabrication and coating.
Microneedles have been used successfully as an experimental delivery system for a number of different vaccines including live virus and bacteria, inactivated virus, virus-like particles, protein sub-unit, DNA and live viral vaccines against influenza and a number of other diseases [8,10, 27–39]. However, measles vaccine has never been studied before using microneedles. In this study, we first examined the ability of excipients to stabilize the live-attenuated measles vaccine during fabrication and storage. We then compared the immune response to vaccination using a microneedle patch to conventional subcutaneous injection in the cotton rat model.
2. Materials and methods
2.1. Preparation of live-attenuated measles vaccine
The measles vaccine strain, Edmonston-Zagreb, was obtained from the collection at the Centers for Disease Control and Prevention and this strain is used in many WHO pre-qualified measles vaccines. To achieve the high titers need for coating of the microneedles, the vaccine virus was propagated in Vero cells maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY) and 2% fetal bovine serum (FBS, Gibco). Infected cells were harvested when the cytopathic effect was maximal; the cell suspension was freeze-thawed once before low-speed centrifugation to remove cellular debris [40]. The viral titer (50% tissue culture infective dose, TCID50) was measured by end-point titration. The virus was then aliquoted and stored at −70 °C until use. For the end-point dilution assay, 10-fold dilutions of the viral stock were prepared in DMEM with 2% fetal bovine serum and used to infect multiple wells of Vero cell monolayers in 24-well tissue culture plates. Plates were incubated for 7 days and scored visually for the presence or absence of viral cytopathic effect. The TCID50 was then calculated using the Karber method [41].
2.2. Vaccine stability studies
Live measles vaccine virus with an initial viral titer of 106 TCID50/mL was mixed with excipients at specific concentrations. Excipients used in this study included carboxymethylcellulose (CMC, CarboMer, San Diego, CA), trehalose (Sigma–Aldrich, St. Louis, MO), fish gelatin (Sigma–Aldrich), myoinositol (Sigma–Aldrich), and Lutrol F68 (BASF, Mt. Olive, NJ). A 2 µL drop of each resulting solution was applied to a sterile chip of stainless steel measuring 3 mm × 4 mm to simulate the surface of a stainless steel microneedle. We used this simple method of coating to screen formulations since coating actual microneedles is more time consuming. Each 2 µL drop contained a mixture of 50% measles virus solution and 50% excipient solution. The chips were allowed to dry at room temperature (22 °C) in a Class II biosafety cabinet or an incubator (37 °C). In some cases, the air was de-humidified during storage by placing the stainless steel chip inside of a 50 mL plastic tube containing desiccant (Drierite, Sigma–Aldrich) and wrapped in Parafilm (Sigma–Aldrich). After specified storage times, the vaccine coated onto the chips was reconstituted in 1 mL DMEM and viral titers were measured in Vero cells as described above.
2.3. Microneedle fabrication and coating
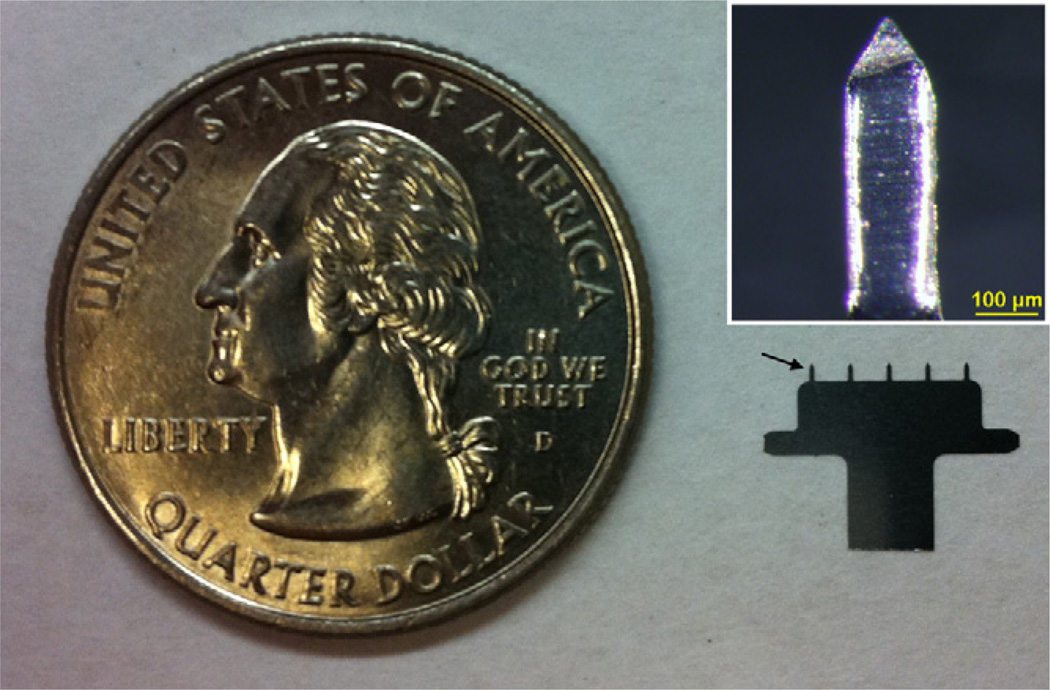
Stainless steel microneedles were fabricated by first defining the microneedle shape lithographically and then etching the microneedles in a chemical bath. This produced patches each containing a single row of five microneedles that were 750 µm long and measured 200 µm × 50 µm at the base (Fig. 1). We chose this microneedle patch design because the measles vaccine dose is sufficiently small that a full dose can be coated onto just five microneedles and because coated microneedles of similar design have been successfully used for vaccination and drug delivery in a number of published studies [9]. We chose a microneedle length of 750 µm because it matches the thickness of rat dorsal skin, which is generally reported in the range of 700–1000 µm [42, 43]. Thus, we believe vaccine coated on the microneedles was deposited along the needle track in the epidermis and dermis; it is possible that a small fraction of the vaccine was delivered to the subcutis in the case of thin skin.
Fig. 1.
A five-needle microneedle array next to a U.S. quarter coin with a diameter of 24 mm. The arrow points at one of the microneedles mounted on the holder. Inset: a single microneedle coated with measles vaccine in a trehalose-based coating formulation.
Based on a method described previously [44], the microneedles were coated with live-attenuated measles vaccine by dipping the microneedles six times into a coating a solution containing 7.5% (w/v) trehalose, 1% (w/v) CMC, 0.5% (w/v) Lutrol F68 and 105.6 TCID50/mL measles vaccine in sterile DMEM for “full-dose” microneedles. “Low-dose” microneedles used a coating solution containing 104.9 TCID50/mL measles vaccine. In this way, a five-microneedle array was coated with 1000 TCID50 after full-dose coating and with 200 TCID50 after low-dose coating, as determined by dissolving the coatings from microneedles and measuring viral titers in Vero cells. The microneedles were stored in a sterile container sealed with Parafilm wrap at room temperature in a Class II biosafety cabinet for 1 day before use.
2.4. Immunization studies
The immunogenicity of measles vaccination using microneedles was tested in cotton rats (Sigmodon hispidus). Cotton rats were divided into seven groups of 5 animals each. Groups were assigned as follows: (1) full-dose and (2) low-dose vaccination using microneedles (MN); (3) full-dose and (4) low-dose vaccination by subcutaneous injection (SC); (5) full-dose; and (6) low-dose vaccination by subcutaneous injection of vaccine eluted from microneedles (SC*); and (7) sham vaccination using sterile, uncoated microneedles.
Female, 6-week-old cotton rats were allowed at least 5 days to acclimate to the animal facility before vaccination. The day before vaccination, blood was collected from the rats via cheek bleeding. Animals were anesthetized using a ketamine/xylazine mixture during vaccination and blood collection [45]. In the microneedle vaccination groups (MN), the hair on the back of each rat was removed using electric shears followed by application of a depilatory cream (Nair, Princeton, NJ).
In the microneedle groups (MN), a microneedle array coated with the desired measles vaccine dose was pressed into the skin of the hairless region on the back of the animal. Each array was left in the skin for 10 min to ensure complete vaccine dissolution from the microneedles for delivery into the skin. Rats in the sham group were treated identically, except that no vaccine coating was applied to the microneedles. In the subcutaneous vaccination groups (SC), the stock measles vaccine was diluted using sterile phosphate-buffered saline (PBS) so that the desired dose was contained in 100 µL, which was then injected subcutaneously using a 25-gauge hypodermic needle on the back of the animal. For the reconstituted subcutaneous groups (SC*), ten microneedle arrays coated with the desired measles vaccine dose were mixed with 1 mL sterile PBS in a 10 mL centrifuge tube and vortexed for 2 min to completely dissolve the vaccine. A 25-gauge hypodermic needle was used to withdraw 100 µL of this solution and inject it SC.
At the time of vaccination, no adverse effects were noted following any of the vaccination methods. A small grid of punctures at the site of microneedle application was faintly visible in the skin when the device was removed, but no bleeding was observed. Post-vaccination, the microneedle injection sites were examined daily by animal care staff and no adverse effects were seen. The small puncture grids were no longer visible 2–3 days post vaccination and no swelling, discharge or other abnormalities were observed at any time point. The hair that had been removed began growing back within 1 week and had returned to normal in all rats by the end of the investigation.
At multiple time points after vaccination, approximately 500 µL of blood was collected from each rat by performing a cheek bleed [45]. After 200 days, the animals were anesthetized using a ketamine/xylazine mixture and euthanized by injecting 1 mL of Beuthanasia-D (Intervet, Summit, NJ) into the heart. The protocol for the cotton rat experiments was approved by the Animal Care and Use Committees of the CDC and the Georgia Institute of Technology.
2.5. Neutralizing antibody measurement
Measles neutralizing antibody titers in serum samples obtained from the cotton rats were determined by the standard plaque reduction neutralization assay [46]. For these studies, 2-fold dilutions of serum were tested beginning at a dilution of 1:4.
2.6. Statistics
All statistics were calculated using Prism software version 5.04 (Graphpad, La Jolla, CA). All listed averages other than Tmax represent the geometric mean of the tested samples. Comparisons between individual samples were done using an unpaired t-test with a significance cutoff of p < 0.05. For comparisons between 3 or more samples, a two-way ANOVA with a Bonferroni post-test was used.
3. Results
3.1. Vaccine stabilization
One of the advantages of vaccination using a microneedle patch is that the vaccine is stored in a dry state and is administered to the patient without reconstitution. When coating microneedles, the thin coating film dries within seconds, leaving no time for transfer to a lyophilization chamber. Therefore, it was necessary to optimize formulation during this rapid drying step to maintain vaccine viability during patch fabrication.
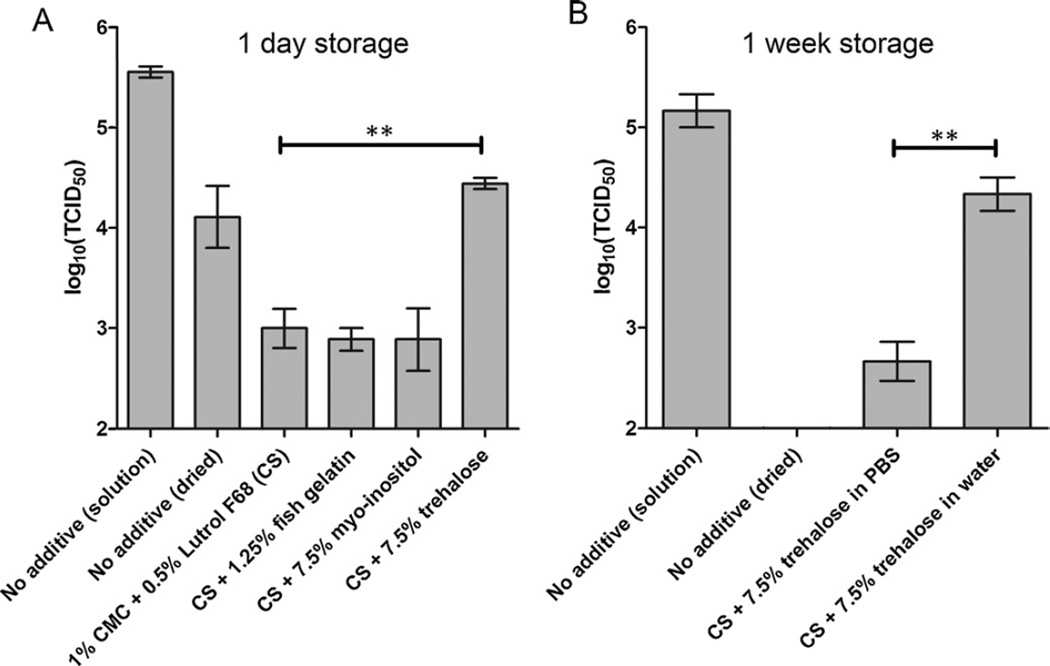
In a first assessment of virus stability during drying, vaccine stock solution with an initial titer of 105.6 TCID50/mL infectivity was dried onto microneedles without additives; this resulted in a greater than 10-fold reduction in virus titer (p < 0.02; Fig. 2A). Then, excipients were added to the solution to make thick, uniform coatings on the microneedles. Carboxymethylcellulose (CMC) was used to increase the solution’s viscosity and surfactant (Lutrol F68) to lower surface tension. These additives (CMC and Lutrol F68) in the coating solution destabilized the measles virus even further and reduced the TCID50 of eluted virus by more than 100-fold compared to the stock solution (p < 0.001; Fig. 2A).
Fig. 2.
Effect of coating formulation on measles virus infectivity after drying onto microneedle surfaces. Coatings were dried and then stored at room temperature (~22 °C) and relative humidity (~50%) for (A) 24 h or (B) 1 week. The coating solution (CS) contained 2% CMC and 1% Lutrol F68. In (A), all coating solutions were prepared using phosphate-buffered saline (PBS). In (B), coating solutions were prepared with and without PBS, as indicated on the graph. Asterisk (**) indicates a significant difference (p < 0.005). Data points represent the average ± standard error of the mean (SEM) from n = 3 independently tested samples.
To minimize loss of viral infectivity, a collection of excipients previously shown to stabilize lyophilized measles vaccines and approved for use in humans were evaluated [26]. Fish gelatin and the sugar, myo-inositol, provided no significant improvement of infectivity compared to the use of coating solution without these additives (p > 0.5; Fig. 2A). Addition of the sugar trehalose at a concentration of 7.5%, however, significantly reduced loss of infectivity compared to the coating solution without additives (p < 0.005) and the titer of the eluted vaccine was within approximately 1 log10(TCID50) of the vaccine stock solution (Fig. 2A). Unfortunately, after 1 week of storage at room temperature using this formulation (coating solution and 7.5% trehalose in PBS), virus infectivity decreased by more than 2 log10(TCID50) (p < 0.03; Fig. 2B).
The coating solutions used so far were all prepared in PBS. To address possible osmotic effects, a coating solution was prepared with trehalose but without PBS. Use of this coating solution significantly increased stability relative to the saline-containing solution (p < 0.005) and resulted in a loss of just 0.8 log10(TCID50) after drying and storage at room temperature and ambient humidity for 1 week (Fig. 2B). This formulation containing trehalose and lacking PBS was used for all remaining experiments in this study.
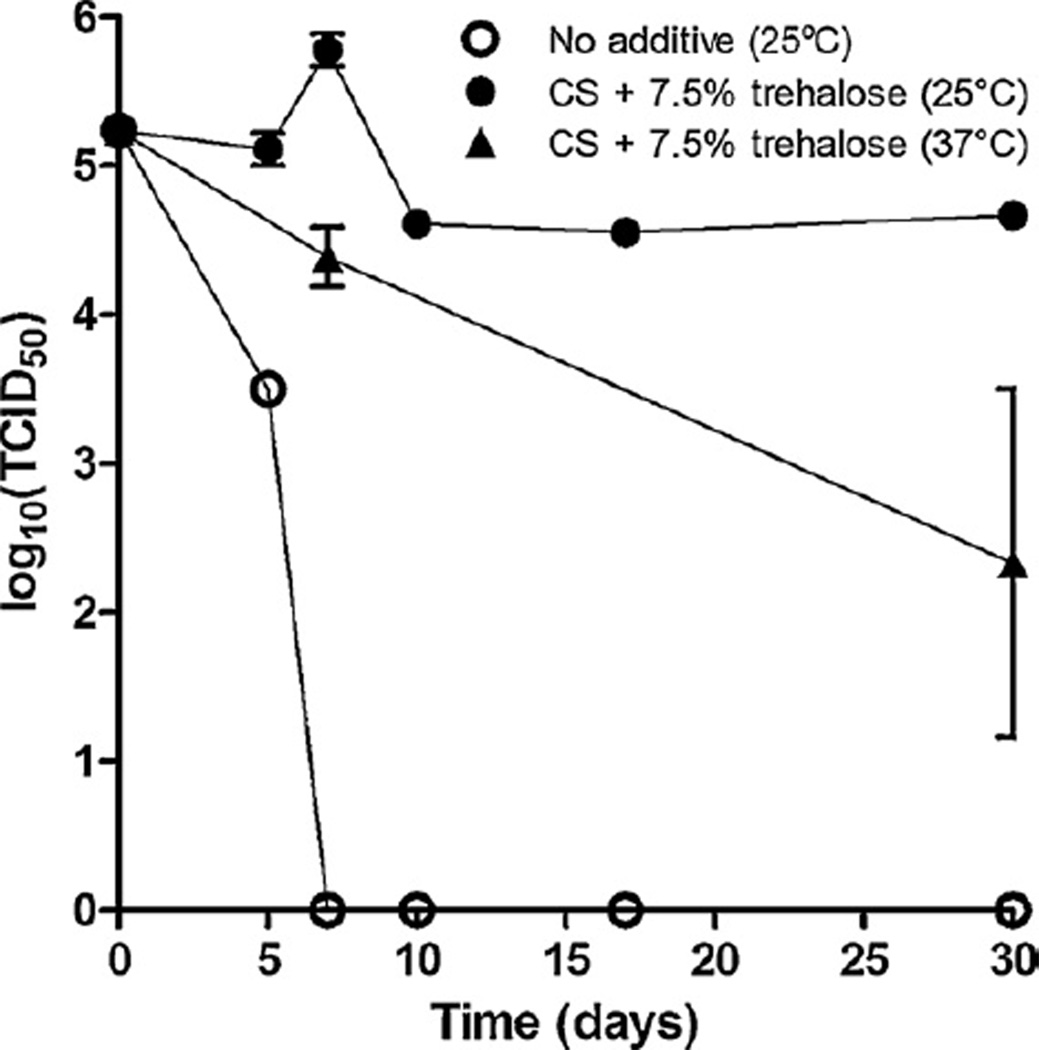
The current WHO standard for stability of lyophilized measles vaccine is less than 1 log10(TCID50) unit of infectivity loss after 30 days at 25 °C or 1 week at 37 °C [47]. The next set of experiments was designed to determine if measles virus coated onto microneedles could meet the WHO standard. These tests were performed with the addition of desiccant to control for humidity. First, a control sample of virus diluted in standard DMEM was seen to rapidly lose activity at 25 °C and no infectivity was reported by the 1 week time point. Following the addition of the stabilizing solution, samples dried at room temperature exhibited a viral titer loss of 0.57 log10(TCID50) units of infectivity after 30 days of drying at 25 °C. At 37 °C, the loss in viability after 1 week was 0.85 log10(TCID50), although longer exposure resulted in additional loss of viability up to 2.91 log10(TCID50) (Fig. 3). Therefore, the optimized coating formulation developed in this study was sufficient to meet one of the WHO standards for measles vaccine stability, although further improvements in stability would be desirable and are currently being evaluated.
Fig. 3.
Loss of measles virus infectivity over time as a function of formulation and storage temperature. Coatings were dried at room temperature and humidity and then stored in sealed tubes with desiccant for 30 days at 25 °C or 37 °C. Formulations included no additives (i.e., no coating solution or trehalose) and coating solution (CS) with 7.5% trehalose. Data points represent the average ± SEM (n = 3). The data points for the 10, 17 and 30 day time points for the 25 °C samples had a SEM of 0 because all replicates had the same values.
3.2. Immunization studies
The immunogenicity of measles vaccination using a microneedle-based vaccine was evaluated in the cotton rat. This animal model was chosen because it is a well-studied, small-animal model for measles viral infection and is commonly used in measles vaccination experiments [48]. The goal of the study was to compare the immunogenicity of measles vaccination using a microneedle patch with the immunogenicity of the same vaccine dose delivered by subcutaneous injection. One group (n = 5) received subcutaneous injection (SC) as a positive control to represent the current approach used in human vaccination. A second subcutaneous group received an injection containing measles vaccine that had been dried on a microneedle patch and reconstituted in PBS prior to subcutaneous injection (SC*). This group was included to account for the possible loss of immunogenicity of the measles vaccine during fabrication of the microneedle patches independent of the route of administration. The microneedle group (MN) received a single microneedle patch applied to the skin of the back. Groups were immunized either with a standard human dose of measles vaccine (1000 TCID50) or a reduced dose (20% of standard dose, 200 TCID50) to investigate possible dose-sparing associated with vaccination in the skin.
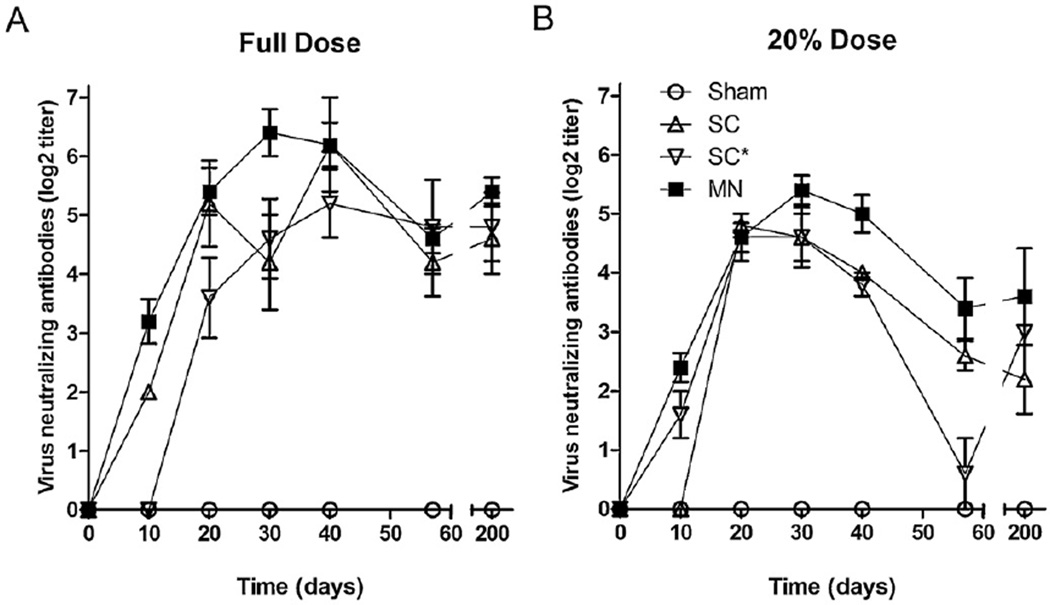
All vaccinated animals demonstrated a detectable antibody response after day 20. Additionally, in all vaccinated groups (MN, SC, SC*) at both doses, the time course of the neutralizing antibody response was similar (Fig. 4). At the day 10 time point, both MN groups had statistically higher titers than the subcutaneous controls at the same dose (p < 0.005). This suggests that microneedle delivery of the measles vaccine may generate a more rapid antibody response than subcutaneous injection, but this observation needs to be tested further. Peak neutralizing antibody titers occurred in all groups at approximately 30 days post vaccination with no statistically significant differences in titer observed (Table 1, p > 0.05). The peak titers achieved in all vaccinated groups were statistically indistinguishable among the standard dose groups (Table 1, Fig. 4A, p > 0.05) and among the reduced dose groups (Table 1, Fig. 4B, p > 0.05). All vaccinated groups achieved peak titers significantly greater than the sham control group (p < 0.005), which had no detectable neutralization activity.
Fig. 4.
Neutralizing antibody responses after vaccination using microneedles compared to subcutaneous injection. Cotton rats were vaccinated with (A) a full human dose (1000 TCID50) or (B) 20% of a full human dose (200 TCID50). Vaccination was performed with microneedles (MN), subcutaneous injection of unprocessed vaccine (SC), subcutaneous injection of vaccine coated onto microneedles and reconstituted before injection (SC*) or as a sham vaccination using microneedles with a vaccine-free coating. Antibody titers were determined by plaque neutralization. Blood was collected from each animal at the given time points and tested independently. Data points represent the average ± SEM (n = 5).
Table 1.
Immune response characteristics of measles vaccination using microneedles.a
| Groupb |
TMAX ± SEM (days)c |
Peak titer ± SEM (log2 antibody titer) |
Day 200 Titer ± SEM (log2 antibody titer) |
|---|---|---|---|
| SC (Full) | 32 ± 4.9 | 6.8 ± 0.2 | 4.6 ± 0.6 |
| SC* (Full) | 32 ± 3.7 | 6.0 ± 0.3 | 4.8 ± 0.6 |
| MN (Full) | 28 ± 2.0 | 6.8 ± 0.2 | 5.4 ± 0.2 |
| SC (20%) | 24 ± 2.4 | 5.2 ± 0.2 | 2.2 ± 0.6 |
| SC* (20%) | 20 ± 0.0 | 4.6 ± 0.4 | 3.0 ± 0.0 |
| MN (20%) | 26 ± 2.4 | 5.6 ± 0.2 | 3.6 ± 0.8 |
Reported values were determined from antibody time course data for each animal and then averaged. The corresponding average antibody time course data are shown in Fig. 4.
Vaccination was performed by subcutaneous injection of unprocessed vaccine (SC), subcutaneous injection of vaccine coated onto microneedles and reconstituted before injection (SC*) or microneedles (MN) using the full human dose (Full) or 20% of the human dose (20%).
TMAX is the average time at which antibody titers peaked.
Blood was also collected 200 days after vaccination to examine long-term antibody responses. Both the MN (full dose) and SC (full dose) groups showed a statistically significant decrease in titer from the peak over time (p < 0.05). It is notable; however, that neutralizing antibodies were detected in all vaccinated groups more than 6 months after immunization. Peak titers among the MN and SC groups vaccinated with the standard dose were approximately 3-fold greater than those among MN, SC and SC* groups vaccinated with the reduced dose (Table 1, p < 0.005).
4. Discussion
The goal of this study was to evaluate a microneedle patch for delivering measles vaccine. The microneedles were designed to be applied as a skin patch without the need for reconstitution. In contrast to conventional subcutaneous injection, administration of the microneedle patch should require minimal training, and therefore, reduce the need for injections by highly trained healthcare professionals. This simple delivery method could reduce the cost of vaccination, and facilitate mass vaccination campaigns aimed at achieving regional measles elimination and future eradication.
The microneedle patch was also designed for cost-effective manufacturing. The vaccine-free microneedle patches can be mass produced at a cost that should be similar to or even less than the cost of a needle and syringe. Coating vaccine onto microneedle patches was carried out as a simple, automated, dip-coating process that can readily be scaled up for low-cost mass production as well. Indeed, microneedle patches coated with parathyroid hormone have already been manufactured commercially and used in Phase I and Phase II human clinical trials [49]. For these reasons, we anticipate that mass-produced microneedle vaccine patches may be manufactured at cost similar to conventional lyophilized measles vaccine. However, as a single-dose presentation, microneedle patches should reduce the extensive wastage currently associated with measles vaccine in multi-dose vials [50].
In this study, immunogenicity after measles vaccination using microneedles was statistically indistinguishable from vaccination by the traditional subcutaneous route in the cotton rat model, including the time course of the immune response, peak titers and titers measured >6 months after vaccination. This shows that vaccination using a microneedle patch can induce an antibody response to measles virus that is equivalent to the response following standard subcutaneous injection. Though the optimal animal model for evaluating measles vaccines is the rhesus macaque [24], cotton rats were chosen for this study because they provided a low cost, small animal model for evaluating the ability of the microneedle vaccines to reconstitute in vivo and generate a neutralizing antibody response. Cottons rats are an accepted small animal model for measles, and this model has been used in many research projects. Both vaccine and wild-type strains of measles have been shown to replicate in cotton rats [48].
In this study, a vaccine coating formulation was developed that enabled vaccine-coated microneedles to meet the accelerated stability criterion of the WHO, i.e., storage for 1 week at 37 °C while retaining at least 10% virus viability [47]. Removal of the salts in PBS, which probably reduced osmotic stresses during drying, and addition of the sugar trehalose, which is believed to stabilize the vaccine antigen structure, maintained vaccine virus viability for 1 month at 25 °C in the presence of desiccant.
The use of trehalose in the coating formulation was important to maintaining stability of the measles virus. This disaccharide has been widely used as a stabilizer in many different biological systems [51–53]. It is thought that trehalose replaces the water around hydrophilic protein regions during drying, thereby preventing protein denaturation [54]. Other stabilizers tested in this study were not effective. Fish gelatin and myo-inositol were chosen because they are included in the formulations of other currently available vaccines and they have been shown to have stabilizing effects on measles virus in the literature [26].
A dose-sparing effect has sometimes been seen when using microneedles with other vaccines [55, 56], but dose sparing was not seen in this study with the measles vaccine at the doses used. As the mechanism of dose sparing in the skin is still under investigation [57], the reasons why dose sparing was not seen in this study are not clear.
5. Conclusion
This study compared administration of live-attenuated measles vaccine using a microneedle patch to conventional subcutaneous injection for the first time. We showed that the measles virus can be coated and dried onto metal microneedles with acceptable stability during storage. Vaccination of cotton rats showed that microneedle vaccination produced antibody titers similar to vaccine delivered using a conventional subcutaneous injection. However, unlike subcutaneous injection, measles vaccination with a microneedle patch is rapid and simple to administer, which could dramatically decrease the training required for measles elimination campaigns [58]. The patches themselves are small and lightweight, easy to dispose of, and expected to require low-cost manufacturing in mass production. We conclude that delivery of measles vaccine with a microneedle patch can be efficacious and could provide a means to significantly increase vaccine coverage as many regions advance towards measles elimination.
Acknowledgements
The authors would like to thank Vladimir Zarnitsyn for designing and building the system used to coat the needles and giving support during its use; Samir Patel and Mark Papania for helpful discussions, James Norman for help with statistical analysis and Donna Bondy for administrative support. This work was supported in part by grants from the Georgia Research Alliance and the National Institutes of Health. Mark Prausnitz is an inventor on patents and has a significant financial interest in a company that is developing microneedle-based products. This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
References
- 1.Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis. 2004;189(Suppl. 1):S27–S35. doi: 10.1086/381592. [DOI] [PubMed] [Google Scholar]
- 2.Bishai D, Johns B, Nair D, Nabyonga-Orem J, Fiona-Makmot B, Simons E, et al. The cost-effectiveness of supplementary immunization activities for measles: a stochastic model for Uganda. J Infect Dis. 2011;204(Suppl. 1):S107–S115. doi: 10.1093/infdis/jir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin A, Burgess C, Garrison LP, Jr, Bauch C, Babigumira J, Simons E, et al. Global eradication of measles: an epidemiologic and economic evaluation. J Infect Dis. 2011;204(Suppl. 1):S98–S106. doi: 10.1093/infdis/jir096. [DOI] [PubMed] [Google Scholar]
- 4.Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012;379(9832):2173–2178. doi: 10.1016/S0140-6736(12)60522-4. [DOI] [PubMed] [Google Scholar]
- 5.Strebel PM, Cochi SL, Hoekstra E, Rota PA, Featherstone D, Bellini WJ, et al. A world without measles. J Infect Dis. 2011;204(Suppl. 1):S1–S3. doi: 10.1093/infdis/jir111. [DOI] [PubMed] [Google Scholar]
- 6.Emmanuel J, Ferrer M, Ferrer F. Waste management and disposal during the Philippine follow-up measles campaign. Health Care Without Harm and The Health Department of The Philippines. 2004 [Google Scholar]
- 7.Klamm H, Pollex G, Henning U. Thermal inactivation of different measles virus strains. Acta Virol. 1991;35(2):200–202. [PubMed] [Google Scholar]
- 8.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2012.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito S, Ito Y, Kiyohara T, Kataoka M, Ochiai M, Takada K. Antigen-loaded dissolving microneedle array as a novel tool for percutaneous vaccination. Vaccine. 2012;30(6):1191–1197. doi: 10.1016/j.vaccine.2011.11.111. [DOI] [PubMed] [Google Scholar]
- 12.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152(3):349–355. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res. 2011;28(1):135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comparative trial of live attenuated measles vaccine in Hong Kong by intramuscular and intradermal injection. Bull World Health Org. 1967;36(3):375–384. [PMC free article] [PubMed] [Google Scholar]
- 16.Kok PW, Kenya PR, Ensering H. Measles immunization with further attenuated heat-stable measles vaccine using five different methods of administration. Trans R Soc Trop Med Hyg. 1983;77(2):171–176. doi: 10.1016/0035-9203(83)90059-7. [DOI] [PubMed] [Google Scholar]
- 17.Wood PB, Soheranda KS, Bracken PM, Houser NE. Measles vaccination in Zaire—when and how? Trans R Soc Trop Med Hyg. 1980;74(3):381–382. doi: 10.1016/0035-9203(80)90105-4. [DOI] [PubMed] [Google Scholar]
- 18.Cutts FT, Clements CJ, Bennett JV. Alternative routes of measles immunization: a review. Biologicals. 1997;25(3):323–338. doi: 10.1006/biol.1997.0103. [DOI] [PubMed] [Google Scholar]
- 19.Etchart N, Hennino A, Friede M, Dahel K, Dupouy M, Goujon-Henry C, et al. Safety and efficacy of transcutaneous vaccination using a patch with the live-attenuated measles vaccine in humans. Vaccine. 2007;25(39–40):6891–6899. doi: 10.1016/j.vaccine.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.de Moraes JC, Leon ME, Souza VA, Pannuti C, Travisanello C, Halsey NA, et al. Intradermal administration of measles vaccines. Bull Pan Am Health Org. 1994;28(3):250–255. [PubMed] [Google Scholar]
- 21.Kissmann J, Ausar SF, Rudolph A, Braun C, Cape SP, Sievers RE, et al. Stabilization of measles virus for vaccine formulation. Hum Vaccin. 2008;4(5):350–359. doi: 10.4161/hv.4.5.5863. [DOI] [PubMed] [Google Scholar]
- 22.Ohtake S, Martin RA, Yee L, Chen D, Kristensen DD, Lechuga-Ballesteros D, et al. Heat-stable measles vaccine produced by spray drying. Vaccine. 2010;28(5):1275–1284. doi: 10.1016/j.vaccine.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Kisich KO, Higgins MP, Park I, Cape SP, Lindsay L, Bennett DJ, et al. Dry powder measles vaccine: particle deposition, virus replication, and immune response in cotton rats following inhalation. Vaccine. 2011;29(5):905–912. doi: 10.1016/j.vaccine.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 24.de Swart RL. Measles studies in the macaque model. Curr Top Microbiol Immunol. 2009;330:55–72. doi: 10.1007/978-3-540-70617-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin WH, Griffin DE, Rota PA, Papania M, Cape SP, Bennett D, et al. Successful respiratory immunization with dry powder live-attenuated measles virus vaccine in rhesus macaques. Proc Natl Acad Sci USA. 2011;108(7):2987–2992. doi: 10.1073/pnas.1017334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burger JL, Cape SP, Braun CS, McAdams DH, Best JA, Bhagwat P, et al. Stabilizing formulations for inhalable powders of live-attenuated measles virus vaccine. J Aerosol Med Pulm Drug Deliv. 2008;21(1):25–34. doi: 10.1089/jamp.2007.0658. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106(19):7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikszta JA, Dekker JP, 3rd, Harvey NG, Dean CH, Brittingham JM, Huang J, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74(12):6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bal SM, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations. Pharm Res. 2010;27(9):1837–1847. doi: 10.1007/s11095-010-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17(6):811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrdoljak A, McGrath MG, Carey JB, Draper SJ, Hill AV, O’Mahony C, et al. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release. 2012;159(1):34–42. doi: 10.1016/j.jconrel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weldon WC, Martin MP, Zarnitsyn V, Wang B, Koutsonanos D, Skountzou I, et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol. 2011;18(4):647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–577. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, et al. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA. 2009;106(45):18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5(10):e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiraishi Y, Nandakumar S, Choi SO, Lee JW, Kim YC, Posey JE, et al. Bacillus Calmette–Guerin vaccination using a microneedle patch. Vaccine. 2011;29(14):2626–2636. doi: 10.1016/j.vaccine.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearton M, Kang SM, Song JM, Anstey AV, Ivory M, Compans RW, et al. Changes in human Langerhans cells following intradermal injection of influenza virus-like particle vaccines. PLoS One. 2010;5(8):e12410. doi: 10.1371/journal.pone.0012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prow TW, Chen X, Prow NA, Fernando GJ, Tan CS, Raphael AP, et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6(16):1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 39.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201(2):190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Udem SA. Measles virus: conditions for the propagation and purification of infectious virus in high yield. J Virol Methods. 1984;8(1–2):123–136. doi: 10.1016/0166-0934(84)90046-6. [DOI] [PubMed] [Google Scholar]
- 41.Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. N-S Arch Pharmacol. 1931;162(4):480–483. [Google Scholar]
- 42.Ikawa A, Ishii Y, Suzuki K, Yasoshima A, Suzuki N, Nakayama H, et al. Age-related changes in the dorsal skin histology in Mini and Wistar rats. Histol Histopathol. 2002;17(2):419–426. doi: 10.14670/HH-17.419. [DOI] [PubMed] [Google Scholar]
- 43.Bronaugh RL, Stewart RF, Congdon ER. Methods for in vitro percutaneous absorption studies. II. Animal models for human skin. Toxicol Appl Pharmacol. 1982;62(3):481–488. doi: 10.1016/0041-008x(82)90149-1. [DOI] [PubMed] [Google Scholar]
- 44.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayers JD, Rota PA, Collins ML, Drew CP. Alternatives to retroorbital blood collection in hispid cotton rats (Sigmodon hispidus) J Am Assoc Lab Anim Sci. 2012;51(2):239–245. [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26(1):59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 47.Expanded programme on immunization. Stability of vaccines. Wkly Epidemiol Rec. 1990;65(30):233–235. [PubMed] [Google Scholar]
- 48.Niewiesk S. Current animal models: cotton rat animal model. Curr Top Microbiol Immunol. 2009;330:89–110. doi: 10.1007/978-3-540-70617-5_5. [DOI] [PubMed] [Google Scholar]
- 49.Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1–34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011;28(1):159–165. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 50.Lee BY, Norman BA, Assi TM, Chen SI, Bailey RR, Rajgopal J, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010;28(32):5292–5300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang MS, Jang H, Kim MC, Kim MJ, Joh SJ, Kwon JH, et al. Development of a stabilizer for lyophilization of an attenuated duck viral hepatitis vaccine. Poult Sci. 2010;89(6):1167–1170. doi: 10.3382/ps.2009-00620. [DOI] [PubMed] [Google Scholar]
- 52.Yang JL, Mu H, Lu ZR, Yin SJ, Si YX, Zhou SM, et al. Trehalose has a protective effect on human brain-type creatine kinase during thermal denaturation. Appl Biochem Biotechnol. 2011;165(2):476–484. doi: 10.1007/s12010-011-9266-3. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Liu L, Qian Y, Chen Y. The effects of cryoprotectants on the freeze-drying of ibuprofen-loaded solid lipid microparticles (SLM) Eur J Pharm Biopharm. 2008;69(2):750–759. doi: 10.1016/j.ejpb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Koster KL, Lei YP, Anderson M, Martin S, Bryant G. Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys J. 2000;78(4):1932–1946. doi: 10.1016/S0006-3495(00)76741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernando GJ, Chen X, Prow TW, Crichton ML, Fairmaid EJ, Roberts MS, et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One. 2010;5(4):e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147(3):326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Combadiere B, Liard C. Transcutaneous and intradermal vaccination. Hum Vaccin. 2011;7(8):811–827. doi: 10.4161/hv.7.8.16274. [DOI] [PubMed] [Google Scholar]
- 58.Measles Initiative [Internet] [cited 2012 June 16];The American National Red Cross; c2012. Available from: http://measlesinitiative.org/.