Abstract
Adequate central nervous system noradrenergic activity enhances cognition, but excessive noradrenergic activity may have adverse effects on cognition. Previous studies have also demonstrated that noradrenergic activity is higher in older than younger adults. We aimed to determine relationships between cerebrospinal fluid (CSF) norepinephrine (NE) concentration and cognitive performance by using data from a CSF bank that includes samples from 258 cognitively normal participants aged 21–100 years. After adjusting for age, gender, education, and ethnicity, higher CSF NE levels (units of 100 pg/mL) are associated with poorer performance on tests of attention, processing speed, and executive function (Trail Making A: regression coefficient 1.5, standard error [SE] 0.77, p = 0.046; Trail Making B: regression coefficient 5.0, SE 2.2, p = 0.024; Stroop Word-Color Interference task: regression coefficient 6.1, SE 2.0, p = 0.003). Findings are consistent with the earlier literature relating excess noradrenergic activity with cognitive impairment.
Keywords: Noradrenergic system, Norepinephrine, Cognition, Aging
1. Introduction
The central noradrenergic system is a primary component of the body’s stress-response system, mediating the “fight-or-flight” response that provides optimal acute adaptation to stressful stimuli. Adaptations include peripheral sympathetic nervous system activation, promoting heart rate, blood pressure, and respiration, and central effects including enhanced arousal and alertness (Haller et al., 1997). The noradrenergic system also plays a role in a number of cognitive domains, including working memory, attention, and memory consolidation (Coull et al., 1999; McGaugh and Roozendaal, 2009; Sara, 2009).
However, noradrenergic system activity in excess may impair cognition. Animal studies have shown associations between excess noradrenergic activity and impairments in attention and working memory (Arnsten, 2011; Sara, 2009). Other studies show decreased cognitive performance in people placed under stress conditions, suggesting excess noradrenergic activity affects human cognition as well (Campbell et al., 2008; Hermans et al., 2011).
Given this association between cognitive performance and noradrenergic system activity, there remains the question of whether differences in basal levels of activity may relate to differences in cognitive performance and whether this relationship is also influenced by age. Noradrenergic system activity appears higher in older compared with younger adults, both peripherally and in the central nervous system (Featherstone et al., 1987; Lawlor et al., 1995; Supiano et al., 1990). Our group has previously demonstrated that concentrations of cerebrospinal fluid (CSF) norepinephrine (NE), the primary noradrenergic neurotransmitter, are higher in older compared with younger adults (Elrod et al., 1997; Raskind et al., 1999). Older adults tend to have poorer performance on cognitive testing than younger adults (Weintraub et al., 2009), but it is not known whether noradrenergic system age differences may be a factor in cognitive differences.
To explore this question, this study used the University of Washington’s Alzheimer’s Disease Research Center (ADRC) CSF bank, which includes CSF samples from cognitively normal participants aged 21–100 years. With this unique sample of participants who span almost the entire adult age span, we aimed to examine the relationship between performance on cognitive tasks and CSF NE concentration.
2. Methods
2.1. Study participants
We analyzed existing CSF samples and data from the University of Washington’s ADRC CSF bank. Participants were aged ≥21 years and had normal cognition, mild cognitive impairment, or Alzheimer’s disease. The CSF bank includes samples and data from multisite CSF biomarker studies (Peskind et al., 2001, 2006). The sites involved were the University of Washington, University of California, San Diego, Oregon Health and Science University, University of Pennsylvania, Indiana University, and University of California, Davis.
For the analysis described in this report, we examined samples and data only from participants with normal cognition. Criteria for normal cognition included a Mini-Mental State Examination (Folstein et al., 1975) score of 26–30 and Clinical Dementia Rating scale (Morris, 1997) score of 0 and no evidence or history of cognitive decline. All procedures were approved by the institutional review boards for participating institutions, and all participants provided written informed consent before study enrollment.
2.2. Neuropsychological testing
Based on the neuropsychologic testing battery of the Alzheimer’s Disease Centers’ Uniform Data Set, the neuropsychologic tests included in this analysis examine multiple aspects of cognition (Weintraub et al., 2009). The Mini-Mental State Examination was used as a global measure of cognition (Folstein et al., 1975). The Wechsler Memory Scale—Revised (WMS-R) Logical Memory Immediate and Delayed paragraph recall tasks measure verbal episodic memory (Wechsler and Stone, 1973). The Trail Making A task measures psychomotor speed, visuospatial function, and visual attention, and Trail Making B adds a set-shifting element and captures executive function (Armitage, 1946). Category fluency (animals) is a measure of semantic memory and language (Morris et al., 1989). Verbal fluency (letter “S”) is also a measure of language (Bolla et al., 1990). The Stroop Word-Color Interference task, which was administered at the University of Washington site only, assesses selective attention, cognitive flexibility, processing speed, and executive functioning (Stroop, 1935).
2.3. CSF collection
Lumbar punctures to obtain CSF were performed within 2 weeks of neuropsychological testing. Sample collections occurred between 9 and 11 AM with subjects fasting. Subjects were placed in the lateral decubitus position, the L3–L4 or L4–L5 interspace was infiltrated with 1% lidocaine to provide local anesthesia, and a 24-gauge Sprotte atraumatic spinal needle was used to obtain 28 mL of CSF (collected under negative pressure into sequential, 6-mL sterile polypropylene syringes). The first 3 mL of CSF was used for cell count and measurement of protein and glucose concentrations at the local hospital laboratory. The remaining 25 mL of CSF was divided into sequential 0.5 mL aliquots into polypropylene tubes, frozen immediately on dry ice at the bedside and then stored at −70 °C until assayed. Tubes assigned to the NE assay also contained the additive glutathione.
2.4. NE assay
For measurement of CSF NE concentration, 2 aliquots were combined and 0.8 mL of CSF was used. CSF was extracted using the alumina extraction method optimized by Holmes et al. (1994). Extracted samples were separated by high-pressure liquid chromatography using a reverse-phase C-18 column and measured by electrochemical detection (Coulechem II; ESA, Inc, Chelmsford, MA, USA) with 3,4-dihydroxybenzylamine as an internal standard (Szot et al., 2012). The intra-assay coefficient of variation is 7.1% (based on measurements of the internal reference standard 3,4-dihydroxybenzylamine), and the interassay coefficient of variation is 10.8% (based on measurement of pooled CSF samples repeated in each assay).
2.5. Statistical analysis
Demographics and clinical characteristics for male and female subjects were compared using 2-sample t tests for continuous variables and Pearson chi-squared test for categorical variables. Multiple linear regression models were also used to assess cross-sectional relationships between CSF NE and cognitive test performance, adjusting for age, gender, education, and ethnicity. Possible differences associated with the 6 study sites were evaluated by including a variable for site in exploratory models.
Interaction terms were added to the regression models to assess the question of whether associations between NE and cognitive functioning are similar for subjects of differing age and similar for males and females. A multiple linear regression model was used to assess the cross-sectional relationship between CSF NE and subjects’ age after controlling for gender. Interaction terms for age by gender were added to the model to assess whether the relationship between CSF NE and age differed by gender. Statistical analyses were performed using S-PLUS version 8.0.
3. Results
3.1. Study participant characteristics
The study sample included 258 individuals with normal cognition and a mean age of 56 years (standard deviation = 19, range 21–100) (Table 1). Fifty-nine percent (n = 153) of study participants were between 21 and 64 years old and 45% (n = 116) were males. Males and females were generally similar with respect to demographic characteristics, although females had slightly less formal education (p = 0.03). The number and percentage of participants from each site were as follows: University of Washington 139 (54%), University of California, San Diego 43 (17%), Oregon Health and Science University 35 (14%), Indiana University 25 (10%), University of Pennsylvania 14 (5%), and University of California, Davis 2 (1%).
Table 1.
Baseline characteristics
| Characteristics | Males | Females | All participants | p value (males vs. females)a |
|---|---|---|---|---|
| Number (%) | 116 (45%) | 142 (55%) | 258 | |
| Mean age, y (SD) | 54.4 (20.4) | 57.6 (16.8) | 56.1 (18.5) | 0.17 |
| Ethnicity, Caucasian (%) | 101 (87%) | 127 (89%) | 228 (88%) | 0.69 |
| Mean education, y (SD) | 16.6 (2.8) | 15.8 (2.6) | 16.2 (2.7) | 0.026 |
| APOE genotype, any ε4 (%) | 31 (27%) | 53 (38%) | 84 (33%) | 0.073 |
| Mean CSF NE, pg/mL (SD) | 159.9 (76.2) | 169.3 (85.4) | 165.1 (81.3) | 0.36 |
Key: APOE, apolipoprotein E; CSF, cerebrospinal fluid; NE, norepinephrine; SD, standard deviation.
Two-sample t test used to compare continuous variables and Pearson chi-squared test used to compare categorical variables.
3.2. Analysis of neuropsychological test scores and CSF NE
Higher CSF NE levels were associated with poorer performance on Trail Making A, which assesses attention and processing speed, and on Trail Making B and the Stroop Word-Color Interference task, which assess attention, processing speed, and executive functioning (Fig. 1). Adjustments were made for age, gender, education, and ethnicity (Table 2). Significant associations were not present between CSF NE and performance on memory or language tasks. Because study participants were recruited from different sites, we investigated the potential effect of site by adding site as a covariate in the statistical model. Site did not significantly alter the results (not shown).
Fig. 1.
Scatter plots with fitted line showing the association between CSF norepinephrine concentration and Trail Making A, Trail Making B, and Stroop Word-Color Interference tasks. Higher scores in these tasks indicate poorer performance. Abbreviation: CSF, cerebrospinal fluid.
Table 2.
Multivariate regression models for the effect of NE levels on cognitive performancea
| Neuropsychological test | n | Adjusted regression coefficients for NE (SE), p value |
|---|---|---|
| WMS-R Logical Memory Immediate Recall | 252 | −0.39 (0.32), 0.22 |
| WMS-R Logical Memory Delayed Recall | 252 | −0.48 (0.33), 0.15 |
| Trail Making Test Ab | 251 | 1.5 (0.77), 0.046 |
| Trail Making Test Bb | 246 | 5.0 (2.2), 0.024 |
| Stroop Word-Color Interference taskb,c | 117 | 6.1 (2.0), 0.003 |
| Category fluency: animals | 251 | −0.35 (0.46), 0.45 |
| Word production: letter “s” | 203 | −0.4 (0.51), 0.43 |
Key: WMS-R, Wechsler Memory Scale—Revised; CSF, cerebrospinal fluid; NE, norepinephrine; SE, standard error.
Adjustments for demographic features include age, gender, education, and ethnicity. Regression coefficients represent the average change in test score per 100 pg/mL increase in CSF NE.
Higher scores in these tasks indicate poorer performance; so, a positive regression coefficient indicates an association between higher CSF NE and poorer performance.
The Stroop Word-Color Interference task was administered at the University of Washington site only.
3.3. Age and the relationship between neuropsychological test scores and CSF NE
CSF NE concentrations were higher in older participants (regression coefficient = 0.89 [average difference in CSF NE concentration (picogram per milliliter) for each year difference in age], standard error (SE) 0.27, p = 0.001; Fig. 2). Notably, there was a wider range in CSF NE values in older compared with younger adults. This age-related difference in CSF NE did not differ by gender (age by gender interaction, p = 0.60).
Fig. 2.
Scatter plot with fitted regression line showing the association between age and CSF NE concentration. Abbreviation: CSF, cerebrospinal fluid.
To further investigate whether the association between CSF NE and cognitive function varied by age, we incorporated in the statistical models an interaction term between CSF NE and age. Age did not significantly modify the results (not shown).
3.4. Gender and the relationship between neuropsychological test scores and CSF NE
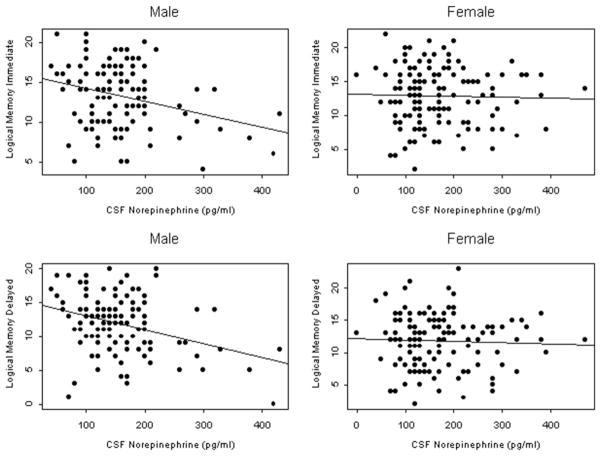
During the previously mentioned analyses, a potential difference between genders was noted. To investigate further, we added in the statistical models an interaction term between CSF NE and gender. Gender did significantly modify the relationship between CSF NE and WMS-R Logical Memory Immediate (gender by CSF NE interaction, p = 0.04) and Logical Memory Delayed Recall (gender by CSF NE interaction, p = 0.02). In an analysis with the sample stratified by gender (see Fig. 3) and adjusted for age, education, and ethnicity, higher CSF NE was associated with poorer performance in males but not females in Logical Memory Immediate Recall (regression coefficient for males: −1.3, SE 0.49, p = 0.012; regression coefficient for females: 0.14, SE 0.42, p = 0.75) and Logical Memory Delayed Recall (regression coefficient for males: −1.5, SE 0.53, p = 0.005; regression coefficient for females: 0.15, SE 0.41, p = 0.71). The relationship between CSF NE and executive function measured by Trail Making B and the Stroop Word-Color Interference task did not differ by gender (gender by CSF NE interaction, p > 0.05 for both).
Fig. 3.
Scatter plots with fitted line showing the association between CSF norepinephrine concentration and Logical Memory Immediate and Logical Memory Delayed Recall, by gender. Lower scores in these tasks reflect poorer performance. Abbreviation: CSF, cerebrospinal fluid.
4. Discussion
Our results demonstrate that higher CSF NE concentrations are associated with poorer performance on attention and executive functioning tasks and that this association remains even after adjusting for age. Results also show an association between CSF NE and poorer performance on memory tasks in male participants. These findings are the first to describe associations between CSF NE and cognition in a relatively large sample of cognitively normal adults across the adult life span.
The noradrenergic system’s role in cognition is complex. NE, as the primary noradrenergic system neurotransmitter, is produced primarily from the locus coeruleus within the brainstem. The locus coeruleus projects noradrenergic neurons to key brain areas for memory, attention, and executive functioning, including the amygdala, hippocampus, and prefrontal cortex. Animal studies have confirmed associations between noradrenergic activity and areas of cognition including attention and working memory (Arnsten, 2009; Ramos and Arnsten, 2007). It has been proposed that the relationship between cognition and the noradrenergic system is inverted and U shaped, where a moderate level of activity promotes optimal cognitive performance, but both noradrenergic activity deficiency and excess states impair cognition. Based on extensive studies in nonhuman primates, Arnsten et al have proposed that this U-shaped relationship is mediated through differential stimulation of prefrontal postsynaptic adrenergic receptor subtypes: moderate levels of noradrenergic outflow (e.g., under nonstress conditions) to the prefrontal cortex preferentially stimulate high-affinity, postsynaptic alpha-2 adrenergic receptors, which enhances cognitive processing and appropriate responses to emotionally salient stimuli. However, high levels of noradrenergic outflow to prefrontal cortex (e.g., in response to stressful stimuli) stimulate low-affinity alpha-1 adrenergic receptors, disrupting cognitive processing and impairing working memory and attention (Arnsten, 2011; Ramos and Arnsten, 2007).
In our study, the specific tests whose scores were associated with CSF NE concentration are also related to attention and executive functioning and memory in males, consistent with the literature described previously. It is also likely that the association between higher CSF NE and cognitive performance reflects the part of the U-shaped curve where there is noradrenergic activity excess. This analysis included cognitively normal healthy controls, so states of noradrenergic system deficiency would be less likely to occur in our sample. Other human studies are also consistent with our findings. Studies of cognitive performance in subjects placed under stress conditions have shown that coadministration of propranolol, a central and peripheral beta-adrenergic antagonist, lessens impairments in cognitive flexibility and test-taking performance (Alexander et al., 2007; Campbell et al., 2008; Faigel, 1991). Bemelmans et al. (2003) found that values of plasma NE collected in the afternoon were negatively correlated with performance on a word recall task, leading to the conclusion that afternoon sympathetic nervous system activity inhibits effortful processing. Also, studies of depressed patients treated with amphetamine or subjects chronically treated with the synthetic glucocorticoid, prednisone, have shown that measures of free recall were associated with CSF concentrations of NE and the metabolite 3-methoxy 4-hydroxyphenylglycol (Reus et al., 1979; Wolkowitz et al., 1993).
The finding that CSF NE levels are higher in older participants is consistent with previous findings from our laboratory and is consistent with other studies that have documented higher central nervous system noradrenergic and peripheral sympathetic nervous system activities with age. Early studies (Rowe and Troen, 1980) suggested that both basal plasma NE concentrations and plasma NE responsivity to upright posture, feeding, exercise, and mental stress are higher with age. Studies of plasma NE kinetics have demonstrated elevated plasma NE levels in older versus young individuals (Hoeldtke and Cilmi, 1985; Veith et al., 1986). Studies employing internal jugular venous sampling have demonstrated increased NE spillover from cardiac, hepatic, and mesenteric sympathetic nerves in elderly versus young men and that this elevation in NE is highly correlated with spillover of NE and 3,4-dihydroxyphenylglycol (a lipophilic metabolite formed via uptake and intraneuronal metabolism of NE) from suprabulbar subcortical brain regions, suggesting that increased sympathetic outflow reflects increased central nervous system noradrenergic drive (Esler et al., 2002). Consistent with this hypothesis, many studies have reported age-related elevations in CSF NE (Elrod et al., 1997; Raskind et al., 1988) and CSF 3-methoxy 4-hydroxyphenylglycol (an extra-neuronal metabolite of NE) (Hartikainen et al., 1991).
The gender difference in the relationship between CSF NE concentration and memory observed in this study is also interesting. In stress research, animal studies have shown gender differences in cognitive and anxiolytic responses to stress (Luine et al., 2007). In contrast with impaired cognition in males after chronic stress, female rodents show enhanced performance on the same memory tasks after the same stress. In a human study, salivary cortisol increased after exposure to a psychological stressor in a group of young healthy subjects, and higher cortisol levels were associated with poor memory function in males but not in females (Wolf et al., 2001). Estradiol alters behavioral and neurochemical responses to stress in ovariectomized rats, where estradiol treatment increases NE levels in the CA3 region of the hippocampus, decreases anxious behaviors, and enhances cognitive performance (Bowman et al., 2002). Females may also benefit more from the protective effect of brain-derived neurotrophic factor (BDNF). We previously found that the CSF BDNF concentration is on average higher in females than in males (Li et al., 2009). The complex relationships between the gonadal hormone estradiol, the brain neurotrophin BDNF, NE, and their potential effect on memory and cognition in humans require further study.
It is important to acknowledge that the processes governing cognition are complex and that multiple neurotransmitters and systems are involved. For example, the cholinergic system has been well characterized as playing a role in memory, and cholinergic deficit is a hallmark of Alzheimer’s disease with many Alzheimer’s disease treatments targeting this system (Rogers et al., 1998; Wallace et al., 2011). Any number of neurotransmitters have been implicated in attention, executive functioning, and memory, including dopamine, serotonin, histamine, glutamate, and gamma-aminobutyric acid among others (Klinkenberg et al., 2011; Robbins and Arnsten, 2009; Stormer et al., 2012; Van Ruitenbeek et al., 2010; Wallace et al., 2011; Wilkosc et al., 2010). The present study suggests that an association between NE and cognition exists, but it does not establish causality, leaving open the possibility that another process that affects both NE and cognition could explain our findings. However, an association between CSF NE and cognition is consistent with previous human studies linking elevations in plasma NE or stress with impairments in cognition, and our findings provide a unique contribution to the current understanding of NE’s role in cognition for both older and younger adults. Further research investigating the relationship between the noradrenergic system and its interactions with other brain processes is needed.
This study has a few limitations. CSF samples and neuropsychological testing were obtained on separate days but within 2 weeks, which is a time separation that limits the ability to capture acute changes in cognition or NE concentrations. However, the objective of measuring associations between basal CSF NE levels and cognitive performance was achieved by performing lumbar punctures in a standardized fashion in the morning to reduce the effects of diurnal fluctuation and limiting time separations to 2 weeks to minimize the occurrence of major life change or new medical illnesses between neuropsychological testing and lumbar puncture. The relatively large sample size for a CSF study adds to the validity of the results. The possibility of false positives from multiple statistical testing may be of concern, but the consistent nature of the associations found between CSF NE and cognitive tests that measure related domains (attention and executive function [Trail Making A and B and Stroop Word-Color Interference tasks] and memory in males [WMS-R Logical Memory Immediate and Delayed Recall]) supports the likelihood that the presence of statistically significant associations was not random.
In conclusion, this study used a unique sample of cognitively normal participants, whose ages encompassed the normal adult life span, to examine relationships between CSF NE and cognitive performance. We found associations between higher CSF NE concentrations and poorer performance in areas of attention and executive functioning and in memory for males. These associations remain significant even after adjusting for age. Our findings are consistent with the earlier literature implicating excess noradrenergic activity with cognitive impairment.
Acknowledgments
This study was supported by the VA Puget Sound Mental Illness Research, Education and Clinical Center (MIRECC), the VA Puget Sound Geriatric Research, Education, and Clinical Center, the VA Special Fellowship in Advanced Psychiatry (MIRECC), the University of Washington’s ADRC Pilot Study Award, and NIH/NIA grant P50AG005136.
Footnotes
Disclosure statement
There are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Armitage S. An analysis of certain psychological tests used in the evaluation of brain injury. Psychol Monogr. 1946;60:1–48. [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans KJ, Goekoop JG, de Rijk R, van Kempen GM. Recall performance, plasma cortisol and plasma norepinephrine in normal human subjects. Biol Psychol. 2003;62:1–15. doi: 10.1016/s0301-0511(02)00089-3. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Predictors of verbal fluency (FAS) in the healthy elderly. J Clin Psychol. 1990;46:623–628. doi: 10.1002/1097-4679(199009)46:5<623::aid-jclp2270460513>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neuro-transmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Campbell HL, Tivarus ME, Hillier A, Beversdorf DQ. Increased task difficulty results in greater impact of noradrenergic modulation of cognitive flexibility. Pharmacol Biochem Behav. 2008;88:222–229. doi: 10.1016/j.pbb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Buchel C, Friston KJ, Frith CD. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage. 1999;10:705–715. doi: 10.1006/nimg.1999.0513. [DOI] [PubMed] [Google Scholar]
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry. 1997;154:25–30. doi: 10.1176/ajp.154.1.25. [DOI] [PubMed] [Google Scholar]
- Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. 2002;282:R909–R916. doi: 10.1152/ajpregu.00335.2001. [DOI] [PubMed] [Google Scholar]
- Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr (Phila) 1991;30:441–445. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- Featherstone JA, Veith RC, Flatness D, Murburg MM, Villacres EC, Halter JB. Age and alpha-2 adrenergic regulation of plasma norepinephrine kinetics in humans. J Gerontol. 1987;42:271–276. doi: 10.1093/geronj/42.3.271. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Haller J, Makara GB, Kruk MR. Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci Biobehav Rev. 1997;22:85–97. doi: 10.1016/s0149-7634(97)00023-7. [DOI] [PubMed] [Google Scholar]
- Hartikainen P, Soininen H, Reinikainen KJ, Sirvio J, Soikkeli R, Riekkinen PJ. Neurotransmitter markers in the cerebrospinal fluid of normal subjects. Effects of aging and other confounding factors. J Neural Transm Gen Sect. 1991;84:103–117. doi: 10.1007/BF01249114. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJF, Ossewaarde L, Henckens MJAG, Qin S, van Kesteren MTR, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hoeldtke RD, Cilmi KM. Effects of aging on catecholamine metabolism. J Clin Endocrinol Metab. 1985;60:479–484. doi: 10.1210/jcem-60-3-479. [DOI] [PubMed] [Google Scholar]
- Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res. 2011;221:430–442. doi: 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Lawlor BA, Bierer LM, Ryan TM, Schmeidler J, Knott PJ, Williams LL, Mohs RC, Davis KL. Plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) and clinical symptoms in Alzheimer’s disease. Biol Psychiatry. 1995;38:185–188. doi: 10.1016/0006-3223(94)00259-6. [DOI] [PubMed] [Google Scholar]
- Li G, Peskind ER, Millard SP, Chi P, Sokal I, Yu CE, Bekris LM, Raskind MA, Galasko DR, Montine TJ. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, Farlow MR, DeCarli C, Raskind MA, Schellenberg GD, Lee VMY, Galasko DR. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56:1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46:756–765. doi: 10.1016/s0006-3223(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Veith RC, Beard JC, Gumbrecht G, Halter JB. Increased plasma and cerebrospinal fluid norepinephrine in older men: differential suppression by clonidine. J Clin Endocrinol Metab. 1988;66:438–443. doi: 10.1210/jcem-66-2-438. [DOI] [PubMed] [Google Scholar]
- Reus VI, Silberman E, Post RM, Weingartner H. d-Amphetamine: effects on memory in a depressed population. Biol Psychiatry. 1979;14:345–356. [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Troen BR. Sympathetic nervous system and aging in man. Endocr Rev. 1980;1:167–179. doi: 10.1210/edrv-1-2-167. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Stormer VS, Passow S, Biesenack J, Li SC. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: insights from molecular genetic research and implications for adult cognitive development. Dev Psychol. 2012;48:875–889. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259 (Pt 1):E422–E431. doi: 10.1152/ajpendo.1990.259.3.E422. [DOI] [PubMed] [Google Scholar]
- Szot P, Knight L, Franklin A, Sikkema C, Foster S, Wilkinson CW, White SS, Raskind MA. Lesioning noradrenergic neurons of the locus coeruleus in C57Bl/6 mice with unilateral 6-hydroxydopamine injection, to assess molecular, electrophysiological and biochemical changes in noradrenergic signaling. Neuroscience. 2012;216:143–157. doi: 10.1016/j.neuroscience.2012.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ruitenbeek P, Vermeeren A, Riedel WJ. Cognitive domains affected by histamine H(1)-antagonism in humans: a literature review. Brain Res Rev. 2010;64:263–282. doi: 10.1016/j.brainresrev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Veith RC, Featherstone JA, Linares OA, Halter JB. Age differences in plasma norepinephrine kinetics in humans. J Gerontol. 1986;41:319–324. doi: 10.1093/geronj/41.3.319. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Stone C. Manual: Wechsler Memory Scale. Psychological Corporation; New York: 1973. [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkosc M, Hauser J, Tomaszewska M, Dmitrzak-Weglarz M, Skibinska M, Szczepankiewicz A, Borkowska A. Influence of dopaminergic and serotoninergic genes on working memory in healthy subjects. Acta Neurobiol Exp. 2010;70:86–94. doi: 10.55782/ane-2010-1777. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Weingartner H, Rubinow DR, Jimerson D, Kling M, Berretini W, Thompson K, Breier A, Doran A, Reus VI. Steroid modulation of human memory: biochemical correlates. Biol Psychiatry. 1993;33:744–746. doi: 10.1016/0006-3223(93)90125-w. [DOI] [PubMed] [Google Scholar]