Abstract
The increased life expectancy of people living with HIV-1/AIDS is accompanied by increased prevalence of HIV-1 associated neurocognitive disorder (HAND). As well, these individuals are increasingly experiencing Alzheimer’s disease (AD)-like neurocognitive problems and neuropathological features such as increased deposition of amyloid beta (Aβ) protein. Findings that Aβ production occurs largely in endolysosomes, that HIV-1 transactivator protein (Tat) disrupts endolysosome function - an early pathological feature of AD - and that HIV-1 Tat can increase Aβ levels prompted us to test the hypothesis that endolysosome dysfunction is associated with HIV-1 Tat-induced increases in neuronal Aβ generation. Using primary cultured rat hippocampal neurons, we found that treatment with HIV-1 Tat caused such morphological changes as enlargement of endolysosomes as identified with LysoTracker dye and such functional changes as elevated endolysosome pH as measured ratiometrically with LysoSensor dye. The HIV-1 Tat-induced changes in endolysosome function preceded temporally HIV-1 Tat-induced increases in Aβ generation as measured by ELISA. In addition, we demonstrated that HIV-1 Tat increased endolysosome accumulation of amyloid beta precursor protein and Aβ as identified by immunostaining with 4G8 antibodies. Furthermore, we demonstrated that treatment of neurons with HIV-1 Tat increased endolysosome accumulation of beta amyloid converting enzyme (BACE-1), the rate limiting enzymatic step for Aβ production, and enhanced BACE-1 activity. Together, our findings suggest that HIV-1 Tat increases neuronal Aβ generation and thereby contributes to the development of AD-like pathology in HIV-1 infected individuals by disturbing endolysosome structure and function.
Keywords: HIV-1 Tat, amyloid beta, endolysosome, pH, BACE-1
1. Introduction
Greater than 40 million people worldwide are infected with the human immunodeficiency virus-1 (HIV-1) and pharmacotherapeutic treatment with combined antiretroviral therapeutic drugs has effectively increased the life span of people living with HIV-1/AIDS. Increased as well is the prevalence of HIV-1 associated neurocognitive disorders (HAND), a set of conditions ranging from subtle neuropsychological impairments to profoundly disabling HIV-associated dementia. Indeed, recent epidemiological studies indicate that the prevalence of HAND in the USA is greater than 50% of HIV-1 infected people (Ellis, et al., 2010, Heaton, et al., 2010).
Increasing too is the incidence of Alzheimer’s disease (AD)-like neurocognitive problems and neuropathological features of AD such as increased amyloid beta (Aβ) protein deposition in older HIV-1 infected patients (Achim, et al., 2009, Clifford, et al., 2009, Esiri, et al., 1998, Gelman and Schuenke, 2004, Green, et al., 2005, Nebuloni, et al., 2001, Pulliam, 2009, Xu and Ikezu, 2009). HIV-1 transactivator of transcription protein (Tat) has been shown to increase neuronal Aβ generation (Aksenov, et al., 2010, Giunta, et al., 2009, Rempel and Pulliam, 2005). Thus, HIV-1 Tat, an HIV-1 viral protein that continues to be implicated as a causative agent in HAND (Agrawal, et al., 2011, Buscemi, et al., 2007, Haughey, et al., 1999, King, et al., 2006b, Nath, et al., 2000, Perez, et al., 2001, Sabatier, et al., 1991, Weeks, et al., 1995), may contribute to the development of AD-like pathology in HIV-1 infected individuals, but by currently ill-defined mechanisms.
HIV-1 Tat is a nonstructural transcriptional regulator essential for the replication of HIV-1. The first exon of HIV-1 Tat encodes for the first 72 amino acids and the second exon encodes for another 14 to 32 amino acids. Others and we have shown that the neuroexcitatory and neurotoxic components of HIV-1 Tat reside within the basic region of exon 1 (Nath, et al., 1996) and that nanomolar concentrations of Tat1-72, Tat1-86, and Tat1-101 have similar efficacy and potency in terms of their neurotoxic effects (Aksenov, et al., 2009, Nath, et al., 1996). HIV-1 Tat levels in sera of HIV-1 infected patients have been reported in the nanomolar range, but these levels are almost certainly underestimated given how avidly HIV-1 Tat binds to proteins and cells (Westendorp, et al., 1995, Xiao, et al., 2000). HIV-1 Tat can be transported across the blood-brain barrier (Banks, et al., 2005, Kim, et al., 2003), can be secreted by infected macrophages and microglia, and has been detected in brain of patients with HIV-1 associated dementia (Ellis, et al., 2000, Nath, 2002, Westendorp, et al., 1995). HIV-1 Tat enters neurons rapidly via receptor-mediated endocytosis with the assistance of low-density lipoprotein receptor-related proteins (LRP-1) (Deshmane, et al., 2011, King, et al., 2006a, Liu, et al., 2000, Vendeville, et al., 2004) and is released from endolysosomes into the cytoplasm (Caron, et al., 2004, Liu, et al., 2000, Vives, et al., 1997) most likely through mechanisms involving the high H+ gradient maintained by vacuolar H+-ATPase (Vendeville, et al., 2004).
Neurons are highly polarized long-lived post-mitotic cells, and they possess an elaborate endolysosome system critical for the maintenance of neuronal function (Nixon and Cataldo, 1995, Nixon and Cataldo, 2006). Increasingly, endolysosome dysfunction, one of the earliest pathological features of AD that precedes extracellular deposition of Aβ in brain (Cataldo, et al., 2000), has been implicated in the pathogenesis of AD (Boland, et al., 2008, Cataldo, et al., 2004, Tate and Mathews, 2006) especially the generation of Aβ. Several lines of evidence indicate that Aβ is mainly generated in the endolysosome system; AβPP is internalized into endolysosomes (Chyung and Selkoe, 2003, Ferreira, et al., 1993, Perez, et al., 1999, Soriano, et al., 1999) where the rate-limiting enzyme for Aβ generation, BACE-1, is located (Rajendran, et al., 2008, Shimizu, et al., 2008, Vassar, et al., 1999).
Endolysosome dysfunction has been implicated in the pathogenesis of HAND (Gelman, et al., 2005, Spector and Zhou, 2008, Zhou and Spector, 2008) and we have shown recently that HIV-1 Tat enlarges endolysosomes, elevates endolysosome pH, and disturbs endolysosome function in neurons (Hui, et al., 2012). This prompted us to hypothesize that HIV-1 Tat increases neuronal Aβ generation by disturbing the structure and function of endolysosomes. As a starting point to test this hypothesis, here we determined the association of HIV-1 Tat-induced endolysosome dysfunction with neuronal Aβ generation. We found that endolysosome enlargement and elevation of endolysosome pH preceded HIV-1 Tat-induced increases in Aβ generation. In addition, we demonstrated that HIV-1 Tat increased endolysosome accumulation of amyloid beta precursor protein (AβPP) and Aβ. Furthermore, we demonstrated that HIV-1 Tat treatment increased endolysosome accumulation of BACE-1 and enhanced BACE-1 activity. Such findings suggest that HIV-1 Tat increases neuronal Aβ generation and thereby may contribute to the development of AD-like pathology in HIV-1 infected individuals by disturbing endolysosome structure and function.
2. Material and Methods
2.1. Primary cultures of hippocampal neurons
Primary cultures of hippocampal neurons were prepared from embryonic day 18 Sprague-Dawley rats (Harlan Laboratories Inc., WI) using a protocol approved by the University of North Dakota Animal Care and Use Committee adherent with the Guide for the Care and Use of Laboratory Animals (NIH publication number 80-23). Pregnant dams (embryonic day 17) were killed by asphyxiation with CO2. The fetuses were removed, decapitated, and meninges-free hippocampi were isolated, trypsinized, and plated onto 35-mm poly-D-lysine-coated glass-bottom tissue culture dishes. Neurons were grown in Neurobasal™ medium with L-glutamine, antibiotic/antimycotic and B27 supplement, and were maintained at 37°C and 5% CO2 for 10–14 days at which time they were used for experimentation. Typically, the purity of the neuronal cultures was greater than 95% as determined by neuronal staining with mouse anti-NeuN and goat anti-MAP2 antibodies (Millipore); astrocytes were identified using a mouse anti-GFAP antibody (Sigma). Neurons were treated either with HIV-1 Tat1-72 (a gift from Dr. Avindra Nath) at a concentration of 100 nM, mutant Tat (TatΔ31–61, 100 nM), or phosphate-buffered saline (PBS) as vehicle. The specificity of the actions of Tat1-72 was confirmed further using a Tat-specific antibody elution strategy (Buscemi, et al., 2007).
2.2. Quantification of Aβ levels by ELISA
Aβ levels were quantified using human/rat Aβ1-40 and Aβ1-42 ELISA kits as per the manufacturer’s protocol (Wako). Briefly, media from cultured neurons were collected, diluted 1:4 with standard diluent buffer, and quantified using the calorimetric sandwich ELISA method. Each sample was measured in duplicate. Protein concentrations from neurons in each dish were determined by a DC protein assay (Bio-Rad). Aβ levels were normalized to total protein content in each sample.
2.3. Live cell imaging
The morphology of endolysosomes in living neurons was determined using a LysoTracker dye. After treatment, neurons were loaded with LysoTracker Red DND-99 (50 nM, Invitrogen) for 30 min at 37°C. Fields were chosen at random and at least five images from every experimental condition were acquired by confocal microscopy (Olympus). The sizes of endolysosomes were measured with Image J software and normalized with control cells. Distribution profiles of endolysosome sizes were analyzed with non-linear regression (Gaussian fit) using Prism Graphpad software.
2.4. Measurement of endolysosome pH
Endolysosome pH was measured using a ratio-metric lysosome pH indicator dye (LysoSensor Yellow/Blue DND-160 from Invitrogen); a dual excitation dye that permits pH measurements in acidic organelles independently of dye concentration. Neurons were loaded with 2 μM LysoSensor for 5 minutes at 37°C. Light emitted a t 520 nm in response to excitation at 340 nm and 380 nm was measured every 30 seconds using a filter-based imaging system (Zeiss). The ratios of light excited at 340/380 nm and emitted at 520 nm were converted to pH using a calibration curve established using 10 μM of the H+/Na+ ionophore monensin, and 20 μM of the H+/K+ ionophore nigericin dissolved in 20 mM 2-(N-morpholino) ethane sulfonic acid (MES), 110 mM KCl, and 20 mM NaCl adjusted to pH 3.0 to 7.0 with HCl/NaOH.
2.5. Immunocytochemistry
Neurons were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100, blocked with 5% goat serum, and incubated overnight at 4°C with primary antibodies targeting early endosome antigen-1 (EEA1, 1:500, rabbit polycolonal, Santa Cruz), lysosome associated membrane protein-1 (LAMP1, 1:500, rabbit polycolonal, Santa Cruz), N-termnial AβPP (1:500, mouse monoclonal, Millipore), Aβ/AβPP (4G8, 1:500, mouse monocolonal, Signet), or BACE-1 (1: 500, mouse monoclonal, Millipore). After washing with PBS, neurons were incubated with corresponding fluorescence-conjugated secondary antibodies including Alexa 488-conjugated goat anti-mouse antibodies (Invitrogen), and Alexa 546-conjugated goat anti-rabbit antibodies (Invitrogen). Neurons were examined using an Olympus confocal microscope. Controls for specificity included staining neurons with primary antibodies without fluorescence-conjugated secondary antibodies (background controls), and staining neurons with only secondary antibodies – these controls eliminated auto-fluorescence in each channel and bleed-through (crossover) between channels. Co-localizations were analyzed with Image J software.
2.6. Immunoblotting
Neurons were lysed with RIPA buffer (Pierce) plus 10 mM NaF, 1 mM Na3VO4 and Protease Inhibitor Cocktail (Sigma). After centrifugation (14,000 x g for 10 min at 4 °C), supernatants were collected and protein concentrations were determined with a DC protein assay (Bio-Rad). Proteins (10 μg) were separated by SDS-PAGE (12% gel) and following transfer to polyvinylidene difluoride membranes (Millipore), membranes were incubated overnight at 4°C with N-terminal AβPP (1: 1000, mouse monoclonal, Millipore) and anti-BACE-1 antibody (1:1000, mouse monoclonal, Millipore). β-actin (1: 10,000, mouse monocolonal, Abcam) was used as a loading control. The immunoblots were developed with enhanced chemiluminescence, and bands were visualized and analyzed by LabWorks 4.5 software on a UVP Bioimaging System (Upland). Quantification of results was performed by densitometry and the results were analyzed as total integrated densitometric volume values (arbitrary units).
2.7. Measurement of BACE-1 enzyme activity
Activity assays of BACE-1 were determined with a BACE-1 activity kit (Calbiochem) according to the protocol provided by the manufacturer. BACE-1 activity was measured using synthetic peptide substrates containing the BACE-1 cleavage site (MCA-Glu-Val-Lys-Met-Asp-Ala-Glu-Phe-(Lys-DNP)-OH) at a 50 mM concentration in reaction buffer (50 mM acetic acid pH 4.1, 100 mM NaCl) and equal amount of proteins (10 μg) from each sample lysate. The fluorescence was measured using a fluorescent microplate reader; excitation wavelength was set at 320 nm and the emission wavelength was set at 383 nm. As a control for specificity, BACE-1 activity was tested in the absence and the presence of the BACE-1 inhibitor, H-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val-Asn-Stat-Val-Ala-Glu-Phe-OH (Calbiochem). BACE-1 activities were expressed as relative fluorescent units per 10 μg of protein. Specific activity of BACE-1 was expressed as a ratio of enzyme activity to protein levels as determined by immunoblotting.
2.8. Statistical analysis
All data were expressed as means and SEM values. Statistical significance for multiple comparisons was determined by one-way ANOVA plus a Tukey post hoc test. p < 0.05 was considered to be statistically significant.
3. Results
3.1. HIV-1 Tat increased neuronal Aβ generation
Increasingly, it has been shown that HIV-1 infected individuals on long-term combined anti-retroviral therapeutics have increased depositions of Aβ in brain as well as increased levels of intraneuronal Aβ (Achim, et al., 2009, Clifford, et al., 2009, Esiri, et al., 1998, Gelman and Schuenke, 2004, Green, et al., 2005, Nebuloni, et al., 2001, Pulliam, 2009, Xu and Ikezu, 2009). Although the underlying mechanisms remain unclear, HIV-1 Tat has been shown to increase neuronal Aβ generation (Aksenov, et al., 2010, Giunta, et al., 2009, Rempel and Pulliam, 2005). Here, we determined the extent to which HIV-1 Tat1-72 affected Aβ generation in primary cultures of rat hippocampal neurons and demonstrated that application of HIV-1 Tat1-72 (100 nM) for 2 days increased significantly (p<0.001) levels of both Aβ1-40 and Aβ1-42 (Figure 1A, B). Treatment with Tat1-72 for 1 day did not affect significantly levels of Aβ1-40 (Figure 1A) and Aβ1-42 (Figure 1B). The mutant form of HIV-1 Tat (TatΔ31–61, 100 nM) that is not directly neurotoxic (Buscemi, et al., 2007) and that was used as a control did not affect significantly levels of Aβ1-40 (Figure 1A) and Aβ1-42 (Figure 1B) even when applied to the neurons for 2 days.
Figure 1. HIV-1 Tat increased neuronal Aβ generation.
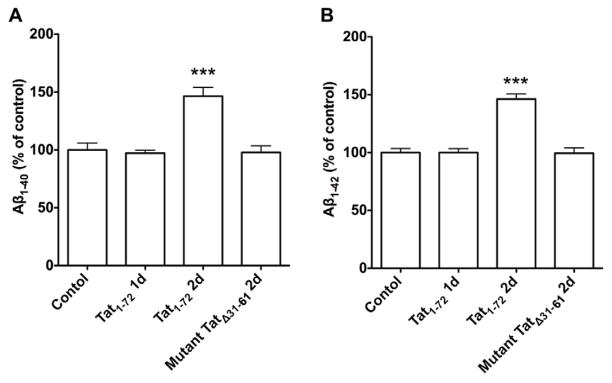
(A) HIV-1 Tat1-72 treatment (100 nM) for 2 days increased significantly levels of Aβ1-40, when compared with control groups (n=5; ***p<0.001). Neither HIV-1 Tat1-72 (100 nM) treatment for 1 day nor mutant Tat (TatΔ31–61, 100 nM) treatment for 2 days affected significantly levels of Aβ1-40. (B) HIV-1 Tat1-72 treatment (100 nM) for 2 days increased significantly levels of Aβ1-42, when compared with control groups (n=5; ***p<0.001). Neither Tat1-72 (100 nM) treatment for 1 day nor mutant Tat (TatΔ31–61, 100 nM) treatment for 2 days affected significantly levels of Aβ1-42.
3.2. HIV-1 Tat increased the accumulation of AβPP and Aβ in endolysosomes
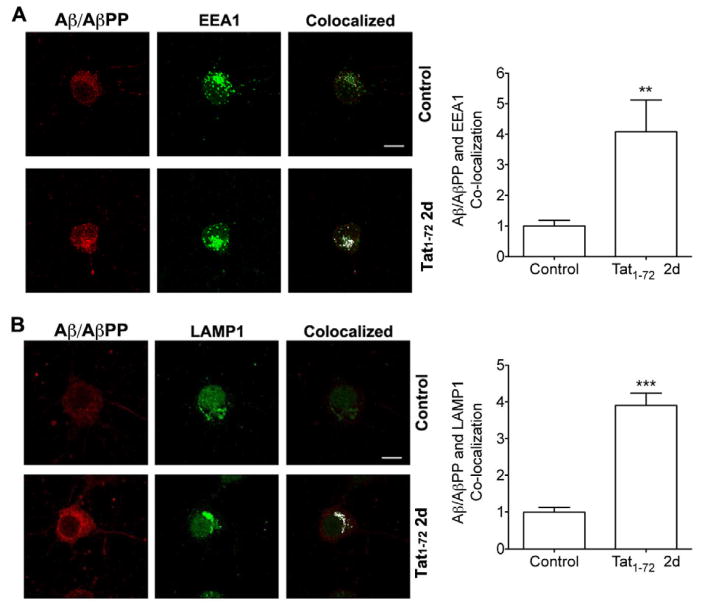
Aβ results from proteolytic cleavage of its precursor protein AβPP catalyzed by β- and γ-secretase enzymes mainly in endolysosomes (Almeida, et al., 2006, Cataldo, et al., 2004, Takahashi, et al., 2002, Van Broeck, et al., 2008). Furthermore, increased intraneuronal Aβ production, especially in endolysosomes, occurs in HIV-1 infected individuals (Achim, et al., 2009). Thus, we determined next the extent to which HIV-1 Tat affected specifically in endolysosomes the accumulation of AβPP and Aβ in primary cultured neurons using double immunofluorescent staining techniques. We found some extent expression of AβPP (Figure 2A) and Aβ (Figure 3A) in control neurons and that there was increased expression levels of AβPP (Figure 2B, C) and Aβ (Figure 3B) in neurons that were treated with HIV-1 Tat1-72 (100 nM) for 2 days. Furthermore, treatment with HIV-1 Tat1-72 (100 nM) for 2 days changed the distribution of and increased expression levels of EEA1-labeled endosomes (Figure 2A and 3A) and of LAMP1-labeled endolysosomes (Figure 2B and 3B). When HIV-1 Tat1-72 (100 nM) was applied to neurons for 2 days and colocalization studies were performed, we found that AβPP increasingly colocalized with EEA1-labeled endosomes (Figure 2A) and LAMP1-labeled endolysosomes (Figure 2B), and that Aβ increasingly (p<0.001) colocalized with EEA1-labeled endosomes (Figure 3A) and LAMP1-labeled endolysosomes (Figure 3B).
Figure 2. HIV-1 Tat increased endolysosome accumulation of AβPP.
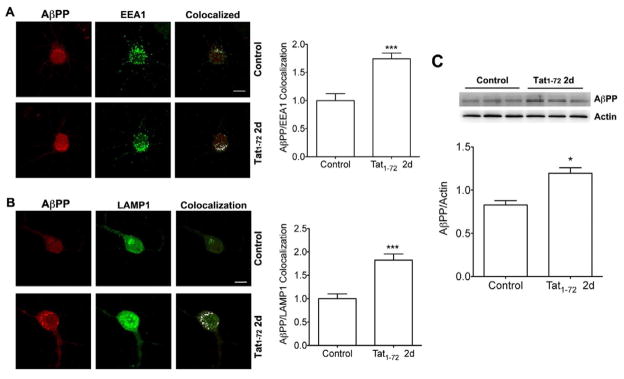
(A) Although there is some extent co-localization of AβPP with endosomes (EEA1) in control neurons, HIV-1 Tat1-72 treatment (100 nM) for 2 days increased significantly the co-localization of AβPP with endosomes (EEA1) (n = 11, ***p<0.001). Bar = 10 μm. (B) There is little co-localization of AβPP with endolysosomes (LAMP1) in control neurons, but HIV-1 Tat1-72 treatment (100 nM) for 2 days increased markedly the co-colocalization of AβPP with endolysosomes (LAMP1) (n = 9, ***p<0.001). Bar = 10 μm. (C) HIV-1 Tat1-72 treatment (100 nM) for 2 days increased significantly the protein levels of AβPP (n = 6, *p<0.05).
Figure 3. HIV-1 Tat increased endolysosome accumulation of Aβ.
(A) Although there is some extent co-localization of Aβ/AβPP (4G8) with endosomes (EEA1) in control neurons, HIV-1 Tat1-72 treatment (100 nM) for 2 days increased markedly the co-colocalization of Aβ/AβPP (4G8) with endosomes (EEA1) (n = 10, **p<0.01). Bar = 10 μm. (B) There is little co-localization of Aβ/AβPP (4G8) with endolysosomes (LAMP1) in control neurons, but HIV-1 Tat1-72 treatment (100 nM) for 2 days increased markedly the co-colocalization of Aβ/AβPP (4G8) with endolysosomes (LAMP1) (n=12, ***p<0.001). Bar = 10 μm.
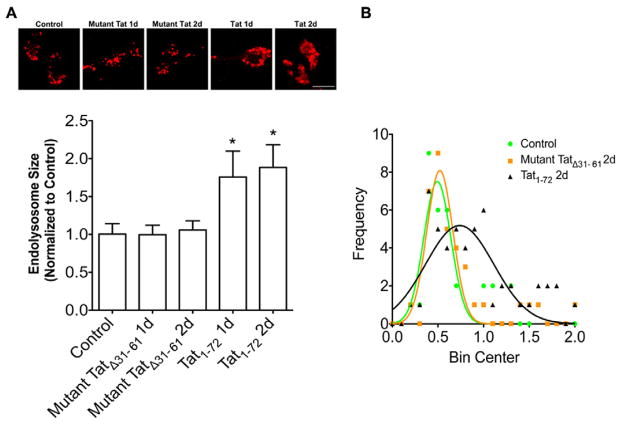
3.3. HIV-1 Tat enlarged the size of endolysosomes and elevated endolysosome pH
In neurons and other cells, HIV-1 Tat enters cells via receptor-mediated endocytosis (Deshmane, et al., 2011, King, et al., 2006a, Liu, et al., 2000, Vendeville, et al., 2004) and HIV-1 Tat accumulates first in endolysosomes. Because alterations in the structure and function of endolysosomes have been implicated in the pathogenesis of both AD and HAND, we determined next the extent to which HIV-1 Tat affected the structure and function of endolysosomes. In living neurons, we identified endolysosomes with LysoTracker, and found that treatment with HIV-1 Tat for 1 and 2 days increased significantly (p<0.05) the size of endolysosomes (Figure 4A). In contrast, treatment of neurons with mutant TatΔ31–61 for 1 or 2 days did not affect significantly endolysosome size (Figure 4A). In addition, we demonstrated that the distribution of sizes was significantly shifted to larger profiles upon HIV-1 Tat1-72 treatment for two days; the mean size of endolysosomes was 0.49 ± 0.15 (n = 40) for control neurons, 0.52 ± 0.14 (n = 36) for neurons treated with mutant TatΔ31–61, and 0.74 ± 0.37 (n = 67) for neurons treated with HIV-1 Tat1-72 (Figure 4B). The observations by others and us that HIV-1 Tat accumulates in endolysosomes and our findings described above that HIV-1 Tat alters endolysosome morphology led us to determine next the extent to which HIV-1 Tat affected endolysosome function. Because the intra-endolysosome pH is critical for endolysosome function, we determined the extent to which HIV-1 Tat affected endolysosome pH using lysoSensor dye. We found that treatment of the cultured hippocampal neurons with HIV-1 Tat for 1 or 2 days elevated significantly (p<0.001) endolysosome pH (Figure 5).
Figure 4. HIV-1 Tat disturbed the structure of neuronal endolysosomes.
(A) HIV-1 Tat1-72 treatment (100 nM) for 1 day and 2 days increased significantly the size of endolysosomes (lysoTracker) in primary cultured hippocampal neurons (*p<0.05). Mutant TatΔ31–61 treatment (100 nM) for 2 days did not affect the size of endolysosomes. Bar = 10 μm. (B) The sizes of endolysosomes from control neurons (n = 40) and neurons treated with mutant TatΔ31–61 (n = 37) or HIV-1 Tat1-72 (n = 67) for 2 days were plotted and analyzed with non-linear regression.
Figure 5. HIV-1 Tat elevated endolysosomes pH.
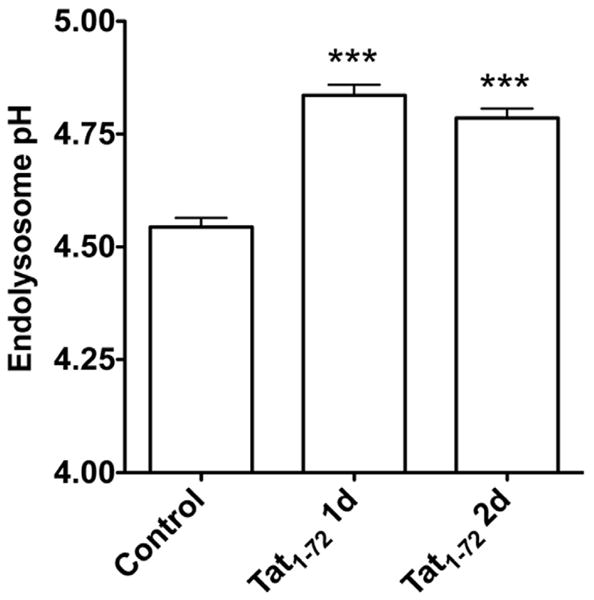
Endolysosome pH was measured ratio-metrically with a LysoSensor dye. HIV-1 Tat1-72 treatment (100 nM) for 1 day and 2 days increased significantly neuronal endolysosome pH (n=8; *** p<0.001).
3.4. HIV-1 Tat increased endolysosome accumulation of BACE-1 and enhanced BACE-1 activity
The rate-limiting step in Aβ production is catalyzed by the beta-site AβPP-cleaving enzyme 1 (BACE-1) that is present in endolysosomes and its activity is pH dependent (Rajendran, et al., 2008, Shimizu, et al., 2008, Vassar, et al., 1999). Given our observations that HIV-1 Tat increased both the size and the pH of endolysosomes we determined next the extent to which HIV-1 Tat increased Aβ production by affecting the levels of expression, enzyme activity, and intracellular distribution of BACE-1 in primary cultured neurons. We found that HIV-1 Tat treatment increased significantly (p<0.05) protein levels of BACE-1 (Figure 6A) and enhanced significantly (p<0.001) the specific activity of BACE-1, which was expressed as a ratio of BACE-1 activity to protein levels as determined by immunoblotting (Figure 6B). When intracellular distribution patterns of BACE-1 were examined in control neurons we found enhanced accumulation of BACE-1 in endosomes as identified with EEA1 antibodies (Figure 6C), but little accumulation of BACE-1 in endolysosomes as identified with LAMP1 antibodies (Figure 6D). However, HIV-1 Tat treatment for 2 days increased markedly the accumulation of BACE-1 in both endosomes (Figure 6C) and endolysosomes (Figure 6D), and increased significantly (p<0.001) the co-localization of BACE-1 with endosomes (Figure 6C) and endolysosomes (Figure 6D).
Figure 6. HIV-1 Tat increased endolysosome accumulation of BACE-1 and enhanced BACE-1 activity.
(A) HIV-1 Tat1-72 treatment (100 nM) for 2 days increased significantly protein levels of BACE-1 (n=6, *p<0.05). (B) HIV-1 Tat1-72 treatment (100 nM) for 2 days increased significantly specific activity of BACE-1 (n=8; ***p<0.001). BACE-1 activity was expressed as relative fluorescent units per 10 μg of protein. Specific activities of BACE-1 were expressed as a ratio of BACE-1 activity to protein levels as determined by immunoblotting. (C) There is some extent co-localization of BACE-1 with endosomes (EEA1) in control neurons, and HIV-1 Tat1-72 treatment (100 nM) for 2 days increased markedly the co-colocalization of BACE-1 with endosomes (EEA1) (n = 12, ***p<0.001). Bar = 10 μm. (D) There is little co-localization of BACE-1 with endolysosomes (LAMP1) in control neurons, but HIV-1 Tat1-72 treatment (100 nM) for 2 days increased markedly the co-colocalization of BACE-1 with endolysosomes (LAMP1) (n = 10, ***p<0.001). Bar = 10 μm.
4. Discussion
Increased life span of HIV-1 infected individuals has been accompanied by an increased prevalence of HAND (Ellis, et al., 2010, Heaton, et al., 2010) and an increased incidence of AD-like pathology including increased deposition of neuronal Aβ (Achim, et al., 2009). Recently, HIV-1 Tat a non-structural HIV-1 protein that continues to be implicated in the pathogenesis of HAND (Merino, et al., 2011, Nath, et al., 1996, Nuovo, et al., 1994) was reported to increase neuronal Aβ generation (Aksenov, et al., 2010, Giunta, et al., 2009, Rempel and Pulliam, 2005) by as yet unclear underlying mechanisms. Here we report that HIV-1 Tat increased neuronal Aβ generation and that this was accompanied by altered endolysosome structure and function, increased endolysosome accumulations of AβPP, BACE-1 and Aβ, and enhanced BACE-1 expression levels and specific activity. Our findings suggest that HIV-1 Tat-induced endolysosome dysfunction underlies the development of AD-like pathology in HIV-1 infected individuals.
Neurons are long-lived post mitotic cells possessing elaborate endolysosomes that when dysfunctional can cause neurodegeneration and contribute to the development of a variety of neurodegenerative diseases including AD and HAND. HIV-1 Tat enters neurons via receptor-mediated endocytosis mainly with the assistance of LRP-1 (Deshmane, et al., 2011, Liu, et al., 2000) and once inside neurons HIV-1 Tat accumulates within endolysosomes. Thus, HIV-1 Tat could by virtue of its accumulation in endolysosomes affect structural and functional features of neuronal endolysosomes. Indeed, we found that HIV-1 Tat1-72 at a concentration of 100 nM, but not mutant TatΔ31–61, enlarged markedly the size of neuronal endolysosomes and elevated endolysosome pH.
Although the underlying mechanisms are unknown, the arginine rich domain of HIV-1 Tat between amino acid residues 49 and 57 could be responsible for the HIV-1 Tat-induced elevation of endolysosome pH. Indeed, we have found that a series of other arginine rich peptides including penetratin, an amino acid domain from the Antennapedia protein (sequence 43–58) of Drosophila, a flock house virus coat peptide (sequence 35–49), and oligoarginines (R9) all have the ability to elevate endolysosome pH (unpublished observations). Others have shown that arginine rich peptides have the ability to escape endolysosomes using high proton gradient found in endolysosomes (Drin, et al., 2003, Fischer, et al., 2004, Henriques, et al., 2006, Magzoub, et al., 2005, Potocky, et al., 2003) and here we postulate that an as yet unidentified proton-dependent peptide transporter in endolysosomes might be responsible. Such a peptide transporter could transport arginine rich peptides such as HIV-1 Tat using the arginine rich domain as a signal, and during the transporting process protons would leak out and endolysosome pH would be elevated. Low pH is critical for the degradation of internalized materials, the trafficking and fusion of endolysosomes, and the formation of autophagosomes (Marshansky and Futai, 2008, Ravikumar, et al., 2010, Williamson, et al., 2010). Therefore, the elevation of endolysosome pH that we observed could result in alterations in the digestive capability of endolysosomes as evidenced by decreased specific activity of three different endolysosome enzymes, increased accumulation of internalized material, and alterations in structural and functional features of endolysosomes consistent with increased vulnerability of neurons to injury and degeneration (Wong and Cuervo, 2010).
One of the earliest pathological features of AD is altered morphological and functional feature of endolysosomes (Boland, et al., 2008, Tate and Mathews, 2006). Indeed, endosome enlargement has been noted in brains of AD patients and in non-demented patients with early signs of AD, in Down’s syndrome individuals, and in patients bearing the ApoE4 allele (Arriagada, et al., 2007, Cataldo, et al., 2004). As well, endosome enlargement was found to precede extracellular deposition of Aβ in brain (Cataldo, et al., 2000). Furthermore, pathological changes in endolysosomes may contribute to Aβ production, a pathological hallmark of AD. Specifically it has been found that AβPP and its cleavage products are present in clathrin-coated vesicles that are part of the endocytic pathway (Ferreira, et al., 1993)(Harris, Milton et al. 2010), that Aβ production is decreased in cultured cells that were stably transfected with an AβPP construct where the C-terminal endocytic targeting signal was removed (Perez, et al., 1999, Soriano, et al., 1999), that Aβ production is decreased in cells transfected with dominant negative dynamin to prevent endocytosis (Chyung and Selkoe, 2003), that BACE-1 a key enzyme for amyloidogenesis is localized in endosomes and its activity is pH dependent (Rajendran, et al., 2008, Shimizu, et al., 2008, Vassar, et al., 1999), and that Aβ is accumulated in endolysosomes of neurons from AD brain (Cataldo, et al., 2004).
Disturbed endolysosomes have been noted in brain of HIV-1 infected individuals (Gelman, et al., 2005, Spector and Zhou, 2008, Zhou and Spector, 2008) and a recent finding that increased Aβ accumulation in neuronal endolysosomes in HIV-1 infected individual (Achim, et al., 2009) suggests that endolysosome dysfunction contributes to increased Aβ generation in HIV patients. Consistent with this notion, we demonstrated that HIV-1 Tat disturbed endolysosome structure and function, promoted neuronal Aβ production, and increased endolysosome accumulation of both AβPP and Aβ. Although detailed molecular underlying mechanisms were not explicitly explored, we propose two mechanisms by which HIV-1 Tat increases neuronal Aβ generation. First, HIV-1 Tat may promote AβPP internalization. As mentioned earlier, Tat enters neuronal endolysosomes via receptor-mediated endocytosis with the assistance of LRP-1 (Deshmane, et al., 2011, Liu, et al., 2000), and it is known that LRP-1 interacts directly with AβPP (Klug, et al., 2011, Waldron, et al., 2008, Waldron, et al., 2006). Thus, the binding of HIV-1 Tat with LRP-1 and subsequent receptor-mediated endocytosis could promote AβPP internalization. Our observation that HIV-1 Tat increases endolysosome accumulation of AβPP seems to support this premise. Second, BACE-1, the rate-limiting enzyme in the production of Aβ, is present in endosomes and its activity is pH-dependent with an optimal pH around 5.0 (Rajendran, et al., 2008, Shimizu, et al., 2008, Vassar, et al., 1999). Thus, the observed elevation of endolysosome pH could be responsible for enhanced BACE-1 enzyme activity and increased Aβ production. In addition, BACE-1 has been shown to be degraded in lysosomes under more acidic conditions (pH < 4) (Koh, et al., 2005). Thus, the observed elevation of endolysosome pH could lead to decreased degradation of BACE-1 and increased accumulation of BACE-1 in endolysosomes, which also results in increased Aβ production. In addition, our observations that HIV-1 Tat-induced endolysosome dysfunction occurs prior to increased Aβ production suggest that HIV-1 Tat disrupts endolysosome function and subsequently affects Aβ production (Jin, et al., 2004). Collectively, elevated endolysosome pH could contribute to HIV-1 Tat-induced increases in neuronal Aβ generation.
Taken together, these findings suggest strongly that the altered structure and function of endolysosomes plays an important role in HIV-1 Tat-induced neuronal Aβ generation and contributes directly to the development of AD-like pathology in HIV-1 infected individuals. Our findings also suggest that blocking HIV-1 Tat endocytosis and improving endolysosome function are potential therapeutic targets against HAND.
Acknowledgments
This work was supported by P20RR0017699, P30GM103329, R01NS065957 and R21AG043338.
Footnotes
The authors have no current or potential conflicts of interest to report.
The authors verify that the data is not published or submitted elsewhere.
Animal protocol was approved by the University of North Dakota Animal Care and Use Committee adherent with the Guide for the Care and Use of Laboratory Animals (NIH publication number 80-23).
All authors have reviewed the contents of the manuscript being submitted, and all authors approve its contents and validate the accuracy of the data.
Disclosure statement
The authors have no current or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4(2):190–9. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: A model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. Attenuated neurotoxicity of the transactivation-defective HIV-1 Tat protein in hippocampal cell cultures. Exp Neurol. 2009;219(2):586–90. doi: 10.1016/j.expneurol.2009.07.005. S0014-4886(09)00271-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett. 2010;475(3):174–8. doi: 10.1016/j.neulet.2010.03.073. S0304-3940(10)00394-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida CG, Takahashi RH, Gouras GK. Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci. 2006;26(16):4277–88. doi: 10.1523/JNEUROSCI.5078-05.2006. 26/16/4277 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada C, Astorga C, Atwater I, Rojas E, Mears D, Caviedes R, Caviedes P. Endosomal abnormalities related to amyloid precursor protein in cholesterol treated cerebral cortex neuronal cells derived from trisomy 16 mice, an animal model of Down syndrome. Neurosci Lett. 2007;423(2):172–7. doi: 10.1016/j.neulet.2007.06.054. [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Nath A. Permeability of the blood-brain barrier to HIV-1 Tat. Exp Neurol. 2005;193(1):218–27. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28(27):6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26(3):661–70. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron NJ, Quenneville SP, Tremblay JP. Endosome disruption enhances the functional nuclear delivery of Tat-fusion proteins. Biochem Biophys Res Commun. 2004;319(1):12–20. doi: 10.1016/j.bbrc.2004.04.180. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging. 2004;25(10):1263–72. doi: 10.1016/j.neurobiolaging.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157(1):277–86. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyung JH, Selkoe DJ. Inhibition of receptor-mediated endocytosis demonstrates generation of amyloid beta-protein at the cell surface. J Biol Chem. 2003;278(51):51035–43. doi: 10.1074/jbc.M304989200. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JS. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73(23):1982–7. doi: 10.1212/WNL.0b013e3181c5b445. WNL.0b013e3181c5b445 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Mukerjee R, Fan S, Sawaya BE. High-Performance Capillary Electrophoresis for Determining HIV-1 Tat Protein in Neurons. PLoS One. 2011;6(1):e16148. doi: 10.1371/journal.pone.0016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278(33):31192–201. doi: 10.1074/jbc.M303938200. M303938200 [pii] [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54(4):927–36. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67(5):552–8. doi: 10.1001/archneurol.2010.76. 67/5/552 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65(1):29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993;13(7):3112–23. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Kohler K, Fotin-Mleczek M, Brock R. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J Biol Chem. 2004;279(13):12625–35. doi: 10.1074/jbc.M311461200. M311461200 [pii] [DOI] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10(2):98–108. doi: 10.1080/13550280490279816. LPWJTWVXY343NU6H [pii] [DOI] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Holzer CE, 3rd, Fabian RH, Schuenke KW, Keherly MJ, Richey FJ, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. J Acquir Immune Defic Syndr. 2005;39(4):422–5. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Giunta B, Hou H, Zhu Y, Rrapo E, Tian J, Takashi M, Commins D, Singer E, He J, Fernandez F, Tan J. HIV-1 Tat contributes to Alzheimer’s disease-like pathology in PSAPP mice. Int J Clin Exp Pathol. 2009;2(5):433–43. [PMC free article] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11. doi: 10.1097/01.aids.0000161770.06158.5c. 00002030-200503040-00006. [pii] [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73(4):1363–74. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. 75/23/2087 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques ST, Melo MN, Castanho MA. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399(1):1–7. doi: 10.1042/BJ20061100. BJ20061100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Haughey NJ, Geiger JD. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro. 2012;4(4) doi: 10.1042/AN20120017. AN20120017 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LW, Shie FS, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol. 2004;164(3):975–85. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TA, Avraham HK, Koh YH, Jiang S, Park IW, Avraham S. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J Immunol. 2003;170(5):2629–37. doi: 10.4049/jimmunol.170.5.2629. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006a;8(5):1347–57. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006b;8(5):1347–57. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Klug W, Dietl A, Simon B, Sinning I, Wild K. Phosphorylation of LRP1 regulates the interaction with Fe65. FEBS Lett. 2011;585(20):3229–35. doi: 10.1016/j.febslet.2011.09.028. S0014-5793(11)00707-1 [pii] [DOI] [PubMed] [Google Scholar]
- Koh YH, von Arnim CA, Hyman BT, Tanzi RE, Tesco G. BACE is degraded via the lysosomal pathway. J Biol Chem. 2005;280(37):32499–504. doi: 10.1074/jbc.M506199200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6(12):1380–7. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Magzoub M, Pramanik A, Graslund A. Modeling the endosomal escape of cell-penetrating peptides: transmembrane pH gradient driven translocation across phospholipid bilayers. Biochemistry. 2005;44(45):14890–7. doi: 10.1021/bi051356w. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 2008;20(4):415–26. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino JJ, Montes ML, Blanco A, Bustos MJ, Oreja-Guevara C, Bayon C, Cuadrado A, Lubrini G, Cambron I, Munoz A, Cebolla S, Gutierrez-Fernandez M, Bernardino JI, Arribas JR, Fiala M. HIV-1 neuropathogenesis: therapeutic strategies against neuronal loss induced by gp120/Tat glycoprotein in the central nervous system. Rev Neurol. 2011;52(2):101–11. rn2010185 [pii] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–8. doi: 10.1086/344528. JID020285 [pii] [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47(2):186–94. [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475–80. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebuloni M, Pellegrinelli A, Ferri A, Bonetto S, Boldorini R, Vago L, Grassi MP, Costanzi G. Beta amyloid precursor protein and patterns of HIV p24 immunohistochemistry in different brain areas of AIDS patients. AIDS. 2001;15(5):571–5. doi: 10.1097/00002030-200103300-00005. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. The endosomal-lysosomal system of neurons: new roles. Trends Neurosci. 1995;18(11):489–96. doi: 10.1016/0166-2236(95)92772-i. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2006;9(3 Suppl):277–89. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Becker J, Burk MW, Margiotta M, Fuhrer J, Steigbigel RT. In situ detection of PCR-amplified HIV-1 nucleic acids in lymph nodes and peripheral blood in patients with asymptomatic HIV-1 infection and advanced-stage AIDS. J Acquir Immune Defic Syndr. 1994;7(9):916–23. [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 Tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7(1):1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274(27):18851–6. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278(50):50188–94. doi: 10.1074/jbc.M308719200. M308719200 [pii] [DOI] [PubMed] [Google Scholar]
- Pulliam L. HIV regulation of amyloid beta production. J Neuroimmune Pharmacol. 2009;4(2):213–7. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knolker HJ, Simons K. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science. 2008;320(5875):520–3. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19(2):127–35. doi: 10.1097/00002030-200501280-00004. 00002030-200501280-00004 [pii] [DOI] [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991;65(2):961–7. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Tosaki A, Kaneko K, Hisano T, Sakurai T, Nukina N. Crystal structure of an active form of BACE1, an enzyme responsible for amyloid beta protein production. Mol Cell Biol. 2008;28(11):3663–71. doi: 10.1128/MCB.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S, Chyung AS, Chen X, Stokin GB, Lee VM, Koo EH. Expression of beta-amyloid precursor protein-CD3gamma chimeras to demonstrate the selective generation of amyloid beta(1–40) and amyloid beta(1–42) peptides within secretory and endocytic compartments. J Biol Chem. 1999;274(45):32295–300. doi: 10.1074/jbc.274.45.32295. [DOI] [PubMed] [Google Scholar]
- Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4(5):704–6. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161(5):1869–79. doi: 10.1016/s0002-9440(10)64463-x. S0002-9440(10)64463-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate BA, Mathews PM. Targeting the role of the endosome in the pathophysiology of Alzheimer’s disease: a strategy for treatment. Sci Aging Knowledge Environ. 2006;2006(10):re2. doi: 10.1126/sageke.2006.10.re2. [DOI] [PubMed] [Google Scholar]
- Van Broeck B, Vanhoutte G, Pirici D, Van Dam D, Wils H, Cuijt I, Vennekens K, Zabielski M, Michalik A, Theuns J, De Deyn PP, Van der Linden A, Van Broeckhoven C, Kumar-Singh S. Intraneuronal amyloid beta and reduced brain volume in a novel APP T714I mouse model for Alzheimer’s disease. Neurobiol Aging. 2008;29(2):241–52. doi: 10.1016/j.neurobiolaging.2006.10.016. S0197-4580(06)00358-7 [pii] [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell. 2004;15(5):2347–60. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272(25):16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol Dis. 2008;31(2):188–97. doi: 10.1016/j.nbd.2008.04.006. S0969-9961(08)00073-9 [pii] [DOI] [PubMed] [Google Scholar]
- Waldron E, Jaeger S, Pietrzik CU. Functional role of the low-density lipoprotein receptor-related protein in Alzheimer’s disease. Neurodegener Dis. 2006;3(4–5):233–8. doi: 10.1159/000095261. [DOI] [PubMed] [Google Scholar]
- Weeks BS, Lieberman DM, Johnson B, Roque E, Green M, Loewenstein P, Oldfield EH, Kleinman HK. Neurotoxicity of the human immunodeficiency virus type 1 tat transactivator to PC12 cells requires the Tat amino acid 49–58 basic domain. J Neurosci Res. 1995;42(1):34–40. doi: 10.1002/jnr.490420105. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375(6531):497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Williamson WR, Wang D, Haberman AS, Hiesinger PR. A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J Cell Biol. 2010;189(5):885–99. doi: 10.1083/jcb.201003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13(7):805–11. doi: 10.1038/nn.2575. nn.2575 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97(21):11466–71. doi: 10.1073/pnas.97.21.1146697/21/11466. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4(2):200–12. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. Aids. 2008;22(6):695–9. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]