Abstract
An inflammatory microenvironment may cause organ degenerative diseases and malignant tumors. However, the precise mechanisms of inflammation-induced diseases are not fully understood. Here we show that the proinflammatory cytokines interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) synergistically impair self-renewal and differentiation of mesenchymal stem cells (MSCs) via nuclear factor κB (NFκB)–mediated activation of Mothers against decapentaplegic homolog 7 (SMAD7) in ovariectomized (OVX) mice. More interestingly, a long-term elevated levels of IFN-γ and TNF-α result in significantly increased susceptibility to malignant transformation in MSCs through NFκB–mediated upregulation of the oncogenes c-Fos and c-Myc. Depletion of either IFN-γ or TNF-α in OVX mice abolishes MSC impairment and the tendency toward malignant transformation with no NFκB–mediated oncogene activation. Systemic administration of aspirin, which significantly reduces the levels of IFN-γ and TNF-α, results in blockage of MSC deficiency and tumorigenesis by inhibition of NF-κB/SMAD7 and NFκB/c-FOS and c-MYC pathways in OVX mice. In summary, this study reveals that inflammation factors, such as IFN-γ and TNF-α, synergistically induce MSC deficiency via NFκB/SMAD7 signaling and tumorigenesis via NFκB–mediated oncogene activation.
Keywords: Mesenchymal stem cells, Stem cell-microenvironment interactions, Differentiation, Cancer, Cytokines
INTRODUCTION
Degenerative diseases, such as osteoporosis, usually show elevated inflammatory activity [1, 2]. Postmenopausal osteoporosis is the most common form of osteoporosis, in which estrogen deficiency-induced T cell activation promotes osteoclastogenesis to reduce bone mineral density (BMD) [3–5]. Although antiresorptive therapies have been utilized in clinical treatment, concerns have been raised by the emergence of side effects, such as bisphosphonate-related osteonecrosis of the jaw (BRONJ) [6, 7]. Thus, it is critical to further understand the underlying mechanisms of osteoporosis and develop novel therapeutic strategies accordingly.
Mesenchymal stem cells (MSCs) are a population of stromal progenitor cells with self-renewal and multipotent differentiation capabilities, possessing the potential to replace damaged and diseased tissues [8, 9]. Accumulating evidence shows that deficiency in MSC/osteoblast lineage consistently contributes to bone degenerative phenotypes in osteoporosis in both mice and humans [2, 10–13]. However, it remains unclear how MSCs are impaired in these immune disorder–associated diseases. If left untreated, degenerative diseases may become a risk factor for cancer occurrence [14, 15], in which chronic infections may play an important role [16]. Moreover, anti-inflammatory treatments show effectiveness in reducing the risk of cancer metastasis [17]. It was speculated that either osteosarcoma or Ewing's sarcoma may directly originate from spontaneous mutation of bone marrow-derived MSCs [18–21]. Therefore, it is possible that a long-term inflammatory environment may eventually induce MSC tumorigenesis.
It has been proved that MSCs closely interact with the host immune system. MSCs are able to inhibit proinflammatory cytokines by targeting several subsets of immune cells, including T cells, B cells, dendritic cells and natural killer cells [22–24], and such immunoregulatory property of MSCs provides the foundation for clinical applications in treating several autoimmune diseases [2, 25]. Conversely, immune components target MSCs. For example, activated T-cells can induce bone marrow MSC apoptosis via the TNF receptor superfamily member 6 (FAS)/FASL and CD40/CD40L pathways, respectively [10, 26]. Lymphocytes can impair survival of implanted MSCs by secreting the proinflammatory cytokines IFN-γ and TNF-α, thereby negatively affecting MSC-mediated tissue regeneration [27, 28]. However, it remains unknown whether specific inflammatory factors, such as IFN-γ and TNF-α, affect MSC properties in an immune disorder-associated model, including OVX-induced osteoporotic mice. Among many inflammatory cytokines, IFN-γ and TNF-α have been proved to play crucial roles in estrogen–deficient osteoporosis [29, 30]. Previous studies have shown that OVX enhances production of TNF-α by T cells, which in turn, augments macrophage colony-stimulating factor (M-CSF)– and receptor activator of NF-κB ligand (RANKL)–induced osteoclastogenesis [29]. Furthermore, it has been demonstrated that OVX upregulates IFN-γ–induced class II transactivator, resulting in enhanced T cell activation and prolonged lifespan of activated T cells [30].
Mothers against decapentaplegic homolog 7, or SMAD7, is an important inhibitory factor of the TGFβ/SMAD pathway, which is one of important signaling pathways regulating osteogenic differentiation. The relationship between SMAD7 and NF-κB is not clear. Although it was reported that SMAD7 inhibits nuclear translocation and transcriptional activity of the cell survival factor NF-κB [31, 32], other studies demonstrated that proinflammatory cytokines, such as interleukin-1β (IL-1β) and TNF-α, upregulated SMAD7 through the activation of the NF-κB pathway in fibroblasts and chondrocytes [33, 34]. Therefore, IFN-γ and TNF-α may upregulate SMAD7 via the activation of the NF-κB pathway, thereby inducing the deficiency of osteogenic differentiation of MSCs. In this study, we determine that IFN-γ and TNF-α, as critical inflammatory factors, synergistically induce MSC deficiency via NFκB/SMAD7 signaling, whereas their long-term effect can result in the induction of MSC tumorigenesis by NFκB–mediated oncogene activation.
MATERIALS AND METHODS
Animals
Female C3H/HeJ, C57BL/6, TNF-α knockout mice (B6;129S-Tnftm1Gkl/J) and IFN-γ knockout mice (B6.129S7-Ifngtm1Ts/J) were purchased from Jackson Lab (Bar Harbor, ME). Generation of ovariectomized (OVX) mice was performed as described previously [35] and age–matched mice receiving sham operation served as control. Female immunocompromised mice (Beige nude/nude XIDIII) were purchased from Harlan (Indianapolis, IN). These animal experiments were performed under institutionally approved protocols for the use of animal research (University of Southern California # 10941, 11141 and 11327).
Antibodies
Anti-mouse TNF-α, SMAD7, alkaline phosphatase (ALP), TNF receptor-1 (TNFR1), TNFR2, IFN-γ receptor α (IFNGRα), Vimentin (VIM), pan-Cytokeratin (pan-CK), proliferating cell nuclear antigen (PCNA), c-FOS and c-MYC antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA); IFN-γ antibody was purchased from Abbiotec (San Diego, CA); phosphorylated nuclear factor κB (p-NFκB), NFκBp65, p-NFκBp50, NFκBp50, phosphorylated nuclear factor κ B inhibitor (p-IκB), IκBα, p-SMAD1, SMAD1, runt-related transcription factor 2 (RUNX2) and Signal Transducers and Activators of Transcription factor 1 (STAT-1) were purchased from Cell Signaling (Danvers, MA). Anti-β-Actin antibody was purchased from Sigma-Aldrich (St. Louis, MO).
Measurement of TNF-α and IFN-γ in blood serum, bone marrow, derma and fat
Peripheral blood was collected from retro-orbital venous plexus, and IFN-γ and TNF-α ELISA (BioLegend, San Diego, CA) was performed to detect in blood serum according to their manufactures' instructions. Total protein from bone marrow, derma and subcutaneous fat was extracted using M-PER mammalian protein extraction reagent (Thermo, Rockford, IL), and IFN-γ and TNF-α were measured by ELISA, respectively.
Isolation of mouse MSCs
For isolation of bone marrow MSCs, bone marrow cells were flushed out from bone cavity of femurs and tibias of mice with 2% heat-inactivated fetal bovine serum (FBS; Equitech-Bio, Kerrville, TX) in PBS. For isolation of dermal MSCs and subcutaneous fat MSCs, dermis and subcutaneous fat were carefully separated from the full skin under a dissecting microscope, cut into small pieces and digested with 1.5 mg/mL collagenase type I (Worthington, Lakewood, NJ) and Dispase (Roche, Indianapolis, IN) in PBS 2 mg/mL for 1 hour at 37 °C to release the individual cells. A single-cell suspension of all nucleated cells was obtained by passing all bone marrow cells through a 70-μm cell strainer (BD Bioscience, San Jose, CA), respectively. All the single cells were seeded into 100-mm culture dishes (Corning, Corning, NY) and initially incubated for 48 h at 37 °C and 5% CO2. To eliminate the nonadherent cells, the cultures were washed with PBS twice on the second day. The attached cells were cultured for 16 d with alpha minimum essential medium (α-MEM, Invitrogen, Grand Island, NY) supplemented with 20% FBS, 2 mM L-glutamine (Invitrogen), 55 μM 2-mercaptoethanol (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). To confirm mesenchymal stem cell character, flow cytometric analysis was used to show that these MSCs were positive for CD73, CD90, Sca-1 and SSEA-4 and negative for CD11b, CD31, CD34 and CD45.
Population-doubling assay
Population doublings (PD) for each passage was determined by using the formula PD≈log2[Nc/No], where No is the original cell population and Nc is the number of cells at confluence, and by adding the PD from each passage together to obtain the PD values. The PD assay for mouse MSCs began with passage 1.
Osteogenic differentiation of MSCs
For osteogenic induction in vitro, mouse MSCs were seeded on 35-mm dishes (Corning) and cultured in the growth medium until the cells reached confluence. To induce osteogenic differentiation, the medium was changed to an osteogenic medium consisting of L-ascorbic acid phosphate and β-glycerophosphate. Two weeks after osteogenic induction, total protein was extracted and analyzed for osteogenic markers by Western blot. After 4–6 weeks, calcium deposits formed by osteoblasts on the dishes were detected by staining with 2% Alizarin Red (Sigma-Aldrich).
Histological and histomorphometric analysis
Femurs and skins were fixed with 4% paraformaldehyde, followed by decalcification with 10% EDTA (pH 8.0) for femurs, and embedded in paraffin. For histological and histomorphometric analysis, sections were deparaffinized and stained with H&E, followed by trabecular percentage and derma thickness calculation using ImageJ software.
Immunohistochemistry staining
For detection of TNF-α, IFN-γ, VIM, pan-CK, PCNA, p-NFκBp65, c-FOS and c-MYC, the sections were blocked with serum matched to secondary antibodies, incubated with the first antibodies for 30min at room temperature, and stained using VECTASTAIN Elite ABC Kit (Vector, Burlingame, CA) and DAB Kit (Invitrogen), according to the manufacturers' instructions.
Administration of IFN-γ and TNF-α neutralizing antibodies in OVX mice
To chronically deplete IFN-γ and TNF-α, at 1 day before OVX, 12-week-old female C3H mice were injected intraperitoneally (IP) with 250 μg of IFN-γ and TNF-α neutralizing antibodies (BioLegend) in 0.5 ml sterile saline every 3 days until 21 days post-OVX, respectively. In control groups, 250 μg isotype control antibodies in 0.5 ml sterile saline were identically administered.
Administration of IFN-γ and TNF-α in nude mice
To mimic IFN-γ- and TNF-α-induced MSC deficiency an in vivo model, 8-week-old female nude mice were injected with 4 μg recombinant mouse IFN-γ (BioLegend) and 1 μg recombinant mouse TNF-α (BioLegend), either alone or in combination, in 0.2 ml sterile saline via tail vein every 3 days for 21 days, respectively.
Inhibition of osteogenic differentiation by IFN-γ and TNF-α treatment
Half a million MSCs were seeded onto each 6-well culture dish (Corning) with or without treatment using IκB kinase α (IKKα), SMAD7, TNFR1 or IFNGRα siRNA (Santa Cruz) according to the manufacturer's instruction. IFN-γ and TNF-α, either alone or incombination, were used to treat the MSCs with normal growth media or osteogenic media for 14 days. Total protein was extracted for detection of NFκB pathway and osteogenic markers using Western blot. For some experiments, the culture plates were stained with 2% toluidine blue O and 2% paraformaldehyde to evaluate cell survival.
Western blot analysis
Total protein was extracted using M-PER mammalian protein extraction reagent (Thermo). Nuclear protein was obtained using NE-PER nuclear and cytoplasmic extraction reagent (Thermo). Protein was applied and separated on 4–12% NuPAGE gel (Invitrogen) and transferred to ImmobilonTM-P membranes (EMD Millipore, Billerica, MA). After blocking with 5% non-fat dry milk for 1 hour, the membranes were incubated with the primary antibodies (1:200–1000 dilution) at 4 °C overnight. Horseradish peroxidase-conjugated IgG (Santa Cruz) was used to treat the membranes for 1 hour and subsequently treated with a chemiluminescent substrate (Thermo). The bands were detected on BIOMAX MR films (Kodak). Each membrane was also stripped using a stripping buffer (Thermo) and re-probed with anti-β-Actin to determine the loading amount.
Sarcomagenesis of BMMSCs in MCA-treated Mice
In order to determine the susceptibility to tumorigenesis of BMMSCs in vivo, we locally applied 3-methycholanthrene (MCA) to the bone marrow of tibias as a carcinogen to induce osteosarcoma according to the method described in previous studies with modifications [36, 37]. One month after OVX or sham operation, a small hole was created in the metaphysis of right tibia using a dental bur after anesthesia, and a single dose of 0.5 mg MCA in 10 ul of dimethyl sulfoxide (DMSO) was injected into the bone marrow of tibia, followed by sealing using bone wax. Control mice received identical procedures except that DMSO without MCA was implanted into tibias. MCA-treated mice were closely observed for tumor occurrence for up to 4 months. MCA-treated tibias were harvested, demineralized with 10% EDTA, and embedded for hematoxylin and eosin (H&E), trichrome staining and immunostainings for VIM, pan-CK and PCNA.
MSC spontaneous malignant transformation assay
To evaluate the effects of inflammatory microenvironment to MSC malignant transformation in vitro, we treated mouse BMMSCs with long-term IFN-γ (50 ng/ml) and TNF-α (5 ng/ml), with or without treatment of NFκB inhibitor BAY 11-7082 (1 μM, Sigma-Aldrich), and continuously passaged to gradually acquire unlimited population doublings as previously described [18]. Giemsa staining was performed to monitor the cell shape.
Transplantation and injection of BMMSCs
To assess tumorigenesis and migration potential, 2×106 and 1×106 of BMMSCs were injected into 8-week-old nude mice subcutaneously and intravenously through tail vein, respectively. Transplants and lungs were harvested 8 weeks after transplantation, and the paraffin-embedded sections were stained with H&E, Trichrome, or incubated with VIM, pan-CK and PCNA antibodies (Santa Cruz) for immunohistochemistry analysis.
Semiquantitative real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from the cultures using SV total RNA isolation kit (Promega, Madison, WI) following the manufacturer's protocols. The cDNA was synthesized from 100 ng of total RNA using Superscript III (Invitrogen). PCR was performed on a CFX 96 real-time PCR thermocycler (BioRad, Hercules, CA) using gene-specific primers and Cybergreen supermix (BioRad, Hercules, CA). Primer pairs are as follows: mouse c-Fos, forward primer (AAACCGCATGGAGTGTGTTGTTCC) and reverse primer (TCAGACCACCTCGACAATGCATGA); mouse c-Myc, forward primer (GCCACGTCTCCACACATCAG) and reverse primer (TCTTGGCAGCAGGATAGTCCTT). The conditions for amplification were as follows: 94 °C for 5 min, followed by 40 cycles of 94 °C for 20 s; 60 °C for 20 s; and 72 °C for 25 s. Data from the real-time PCR reactions were analyzed using CFX Manager™ Software, Version 2 (Bio-Rad Laboratories). Relative changes in mRNA expression were analyzed using the 2-ΔΔCt method, with Gapdh serving as an internal reference to correct for differences in RNA extraction or reverse transcription efficiencies. These standardized data were used to calculate fold-differences in gene expression. All real-time PCR amplifications were performed in triplicate.
Aspirin administration in OVX mice
To determine the effects of aspirin to prevent MSC tumorigenesis and MSC deficiency in vivo, mice were fed with aspirin (1 mg/ml, Sigma-Aldrich) dissolved in water or placebo for 2 months in OVX mice and 4 months in MCA-treated OVX mice. BMMSCs and DMSCs were harvested for PD and osteogenic differentiation analysis.
Statistics
SPSS 13.0 was used to perform statistical analysis. Significance was assessed by independent two-tailed Student's t-test or analysis of variance (ANOVA). The P values less than 0.05 were considered significant.
RESULTS
Elevated levels of IFN-γ and TNF-α synergistically impair MSCs in OVX mice
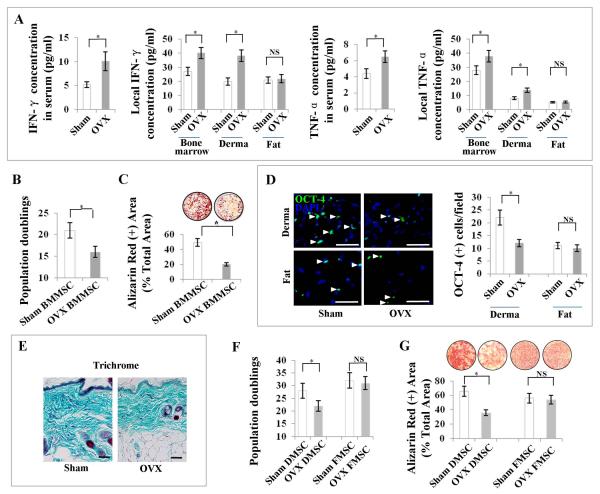
Of many immune disorder–associated degenerative diseases, postmenopausal osteoporosis is an important example. It features overactivation of proinflammatory cytokine-producing T cells and decreased osteogenic differentiation capabilities in bone marrow MSCs (BMMSCs), resulting in reduced bone mineral density (BMD) [2–5, 10]. IFN-γ and TNF-α have been proved to play crucial roles in estrogen–deficient osteoporosis [29, 30]. Blockage of either IFN-γ or TNF-α can abolish OVX-induced osteoporotic phenotype [3, 5]. Moreover, OVX enhances T cell activation and prolonged lifespan of active T cells through IFN-γ-induced class II transactivator [30]. Our recent study also showed that IFN-γ and TNF-α synergistically impaired implanted MSCs in vivo [28]. Based on these observations, we asked whether IFN-γ and TNF-α would affect MSC properties in OVX-induced osteoporotic mice. We hypothesized that IFN-γ and TNF-α, as two key representatives in the inflammatory microenvironment, might synergistically play an important role in inducing MSC deficiency in OVX-induced osteoporotic mice. Concentrations of both IFN-γ and TNF-α in serum, bone marrow and derma, but not in subcutaneous fat, were markedly increased in OVX mice when compared with sham-operated mice by ELISA and immunohistochemical staining analysis (Fig. 1A; Supporting Information Fig. S1). BMMSCs derived from OVX mice showed markedly decreased population doublings when compared to sham mouse-derived BMMSCs, indicating that BMMSC self-renewal capability was impaired in OVX mice (Fig. 1B). Importantly, osteogenic differentiation of BMMSCs was also impaired in OVX mice, as indicated by Alizarin red staining to show markedly decreased mineralized nodule formation after induction of osteogenic media (Fig. 1C). Because derma and subcutaneous fat, as neighboring tissues, showed different alteration in the levels of IFN-γ and TNF-α following OVX procedure (Fig. 1A), we speculate that OVX may affect derma and subcutaneous fat MSCs differently from bone marrow MSCs. Although OCT-4 is not a specific marker for MSCs, it has been proved to be an important marker to indicate that MSCs possess self-renewal capacity and can retain their undifferentiated status [38]. Therefore, we used OCT-4 as a marker of functional stem cell assurance for MSCs. We found that the derma, but not the fat, from OVX mice harbored markedly less OCT-4–positive MSCs (Fig. 1D), contributing to a markedly decreased collagen deposition in the derma as shown by Trichrome staining, when compared with sham-operated mice (Fig. 1E). To further confirm decreased MSC number in OVX mice, we performed histoimmunostaining to show markedly reduced number of dermal MSCs, which express the MSC markers CD105, CD44 and CD73 in OVX mice (Supporting Information Fig. S2). Moreover, OVX-derived dermal MSCs (DMSCs), but not fat MSCs (FMSCs), showed markedly decreased population doublings (Fig. 1F) and osteogenic differentiation (Fig. 1G), which served as a representative to determine the differentiation capabilities of MSCs in the experiments thereafter. Consequently, OVX mice showed markedly deteriorated trabecular bone structures and reduced dermal thickness when compared with sham-operated mice (Supporting Information Fig. S3 A, S3 B).
Figure 1. Elevated levels of IFN-γ and TNF-α are associated with MSC deficiency in OVX mice.
(A) ELISA analysis showed that IFN-γ and TNF-α levels were elevated in serum, bone marrow and derma, but not in fat, in OVX mice. (B) OVX-derived bone marrow MSCs (BMMSCs) showed markedly decreased population doublings, as compared with sham-derived BMMSCs. (C) Alizarin red staining showed that OVX-derived BMMSCs had a lower capacity to form mineralized nodules than those derived from sham mice when cultured under osteogenic inductive conditions. (D) OCT-4 immunostaining showed that derma, but not fat, from OVX mice harbored markedly less MSCs when compared with sham-operated mice. (E) Trichrome staining showed that derma from OVX mice contained less collagen deposition than sham-operated mice. (F) OVX-derived dermal MSCs (DMSCs), but not fat MSCs (FMSCs), showed markedly decreased population doublings as compared with sham-derived DMSCs. (G) Alizarin red staining showed that OVX-derived DMSCs, but not FMSCs, had a lower capacity to form mineralized nodules than those from sham mice. Error bars represent mean ± SEM, n = 8 animals per group. *, P < 0.05. Scale bars, 100 μm.
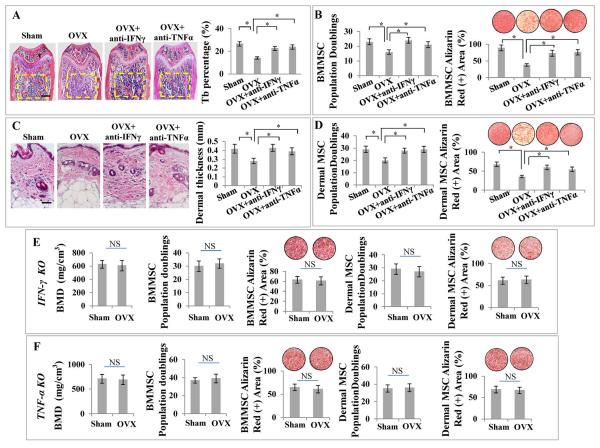
We next asked whether elevated levels of IFN-γ and TNF-α would synergistically cause MSC deficiency. To clarify this, we first systemically administered either IFN-γ or TNF-α neutralizing antibody to OVX mice to test whether depletion of IFN-γ or TNF-α could prevent OVX-induced MSC deficiency. Indeed, administration of IFN-γ neutralizing antibody was able to markedly reduce IFN-γ level in the serum of OVX mice, without affecting TNF-α level; similarly, administration of TNF-α neutralizing antibody could markedly reduce the serum TNF-α level in OVX mice, without changing IFN-γ level (data not shown). Interestingly, histological analysis showed that either IFN-γ or TNF-α neutralizing antibody was able to markedly inhibit bone loss in OVX mice (Fig. 2A). MSC characterization showed that either IFN-γ or TNF-α neutralizing antibody treatment in vivo markedly improved population doublings and osteogenic differentiation in OVX-derived BMMSCs (Fig. 2B). Also, either IFN-γ or TNF-α neutralizing antibody was able to prevent derma atrophy in OVX mice (Fig. 2C) and improve population doublings and differentiation capabilities in OVX-derived DMSCs (Fig. 2D). In order to further dissect whether IFN-γ and TNF-α synergistically cause MSC deficiency in OVX mice, we confirmed that OVX was not able to induce BMD loss in either IFN-γ knockout or TNF-α knockout mice (Fig. 2E, 2F). Importantly, population doublings and differentiation capabilities were not decreased in BMMSCs or DMSCs in IFN-γ knockout or TNF-α knockout mice after OVX (Fig. 2E, 2F). To further confirm the synergistic effect of IFN-γ and TNF-α in vivo, we systemically infused IFN-γ and TNF-α, either alone or in combination, to immunocompromised mice via tail vein. Interestingly, infusion of IFN-γ and TNF-α in combination, but not separately, caused a dramatic reduction in population doublings and differentiation capabilities of both BMMSCs and DMSCs (Fig. 3A–3D), resulting in both osteopenia phenotype and dermal atrophy with poor collagen deposition as shown by Trichrome staining (Fig. 3E–3G; Supporting Information Fig. S4 A, S4 B). Furthermore, when IFN-γ and TNF-α, either alone or in combination, were subcutaneously transplanted with hydrogel as a controlled release system, DMSC population doublings and differentiation capabilities were reduced by IFN-γ and TNF-α in combination, but not separately (Supporting Information Fig. S5 A, S5 B), thereby causing derma atrophy (Supporting Information Fig. S5 C). These in vivo data suggest that elevated levels of IFN-γ and TNF-α synergistically, but not independently, cause MSC deficiency.
Figure 2. Depletion of either IFN-γ or TNF-α is sufficient to rescue MSC deficiency in OVX mice.
(A) H&E staining showed that either IFN-γ or TNF-α neutralizing antibody administration markedly inhibited bone loss in OVX mice, respectively. (B) Either IFN-γ or TNF-α neutralizing antibody in vivo treatment markedly improved population doublings and mineralized nodule formation in OVX-derived BMMSCs. (C) H&E staining showed that either IFN-γ or TNF-α neutralizing antibody was able to prevent OVX-induced derma atrophy. (D) Either IFN-γ or TNF-α neutralizing antibody in vivo treatment markedly improved population doublings and differentiation of OVX-derived DMSCs. (E, F) BMD in femurs was not significantly changed at 1 month post OVX when compared with BMD of sham-operated femurs in IFN-γ and TNF-α knockout mice, respectively. Population doublings and osteogenic differentiation of BMMSCs and DMSCs were not significantly altered in OVX IFN-γ and TNF-α knockout mice. Error bars represent mean ± SEM, n = 5 animals per group. *, P < 0.05. Scale bars, 100 μm.
Figure 3. IFN-γ and TNF-α synergistically induce MSC deficiency in nude mice.
(A–D) Infusion of IFN-γ and TNF-α in combination, but not separately, caused a dramatic reduced population doublings and osteogenic differentiation of BMMSCs and DMSCs, as indicated by Alizarin red staining. (E) Histological quantification demonstrated that only the group infused with both IFN-γ and TNF-α showed significant decrease in trabecular bone thickness. (F–G) Trichrome staining showed that IFN-γ/TNF-α treatment group had poor collagen deposition and derma atrophy. Error bars represent mean ± SEM, n = 5 animals per group. *, P < 0.05. Scale bars, 100 μm.
IFN-γ and TNF-α synergistically induce NFκB/SMAD7 pathway activation, causing differentiation deficiency in MSCs
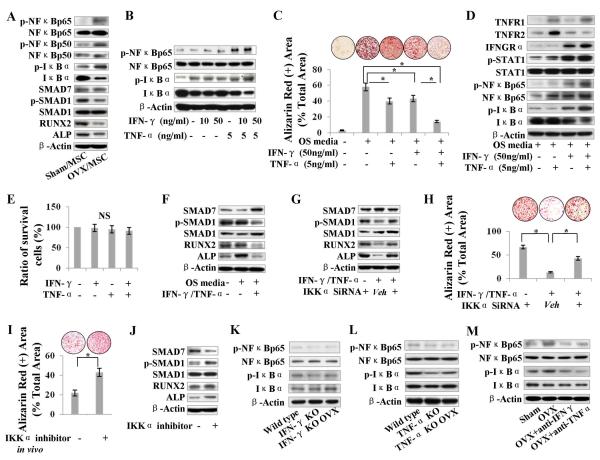
In order to examine the mechanism by which IFN-γ and TNF-α synergistically induce impairment of MSCs, we demonstrated that BMMSCs and DMSCs derived from OVX mice showed the upregulation of p-NFκB, p-IκB and inhibitory SMAD7, but the downregulation of osteogenic genes, including phosphorylated SMAD1 (p-SMAD1), RUNX2 and ALP (Fig. 4A; Supporting Information Fig. S6 A). To mimic in vivo microenvironment in OVX bone marrow, we treated BMMSCs with IFN-γ and TNF-α in different concentrations, either alone or in combination, and found that TNF-α-induced NFκBp65 activation in a dose-dependent manner with a maximal activation at concentrations of 5 and 20 ng/ml (Supporting Information Fig. S6 B), whereas the combination of IFN-γ (50 ng/ml) and TNF-α (5 ng/ml) induced more significant upregulation of NFκBp65 signaling (Fig. 4B). Interestingly, IFN-γ and TNF-α in combination, but not separately, caused a significant reduction of mineralized nodule formation in BMMSCs (Fig. 4C) and DMSCs cultured in osteogenic media (Supporting Information Fig. S6 C, S6 D), without dramatically affecting MSC survival (Fig. 4E). Furthermore, Western blot showed that IFN-γ/TNF-α–induced NFκB activation upregulated inhibitory SMAD7, but downregulated osteogenic genes, including p-SMAD1, RUNX2, and ALP (Fig. 4F). When NFκB expression was knocked down by IKKα siRNA, IFN-γ/TNF-α–induced upregulation of SMAD7 and downregulation of p-SMAD1, RUNX2, and ALP were abolished, leading to the rescue of IFN-γ/TNF-α–induced reduction of mineralized nodule formation in BMMSCs (Fig. 4G, 4H) and DMSCs (Supporting Information Fig. S6 E, S6 F). Under osteogenic induction, IFN-γ and TNF-α treatment in combination was able to dramatically upregulate IFN-γ receptor IFNGRα and TNF-α receptor TNFR1, but not TNFR2, when compared with IFN-γ and TNF-α treatment alone (Fig. 4D). Combination knockdown of IFNGRα and TNFR1 resulted in marked inhibition of IFN-γ–/TNF-α–induced activation of NFκB pathway when compared with knockdown of TNFR1 alone, suggesting that IFN-γ activates TNF-α–induced NFκB activation via IFNGRα (Supporting Information Fig. S7 A). Moreover, IFN-γ, either alone or in combination with TNF-α, was able to markedly upregulate p-STAT-1 (Fig. 4D), whereas knockdown of STAT1 dramatically reduced the TNF-α–/IFN-γ–induced activation of NFκB pathway, suggesting that IFN-γ/IFNGRα pathway enhances TNF-α–triggered NFκB activation via STAT-1 activation (Supporting Information Fig. S7 B). We confirmed the efficiency of IKKα, IFNGRα, TNFR1, STAT-1 and SMAD7 knockdown by their specific siRNAs using Western blot analysis (Supporting Information Fig. S7 C, S7 D). Additionally, knockdown of SMAD7 expression by siRNA assay resulted in rescuing TNF-α–/IFN-γ–induced reduction of mineralized nodule formation and downregulation of p-SMAD1, RUNX2, and ALP in BMMSCs and DMSCs, without affecting p-IκB and p-NFκB (Supporting Information Fig. S8 A–S8 D). These data suggest that SMAD7 acts as a downstream target of NFκB signaling in TNF-α–/IFN-γ–induced MSC osteogenic deficiency.
Figure 4. IFN-γ and TNF-α synergistically activate NFκB/SMAD7 pathway, resulting in BMMSC deficiency.
(A) Western blot showed upregulation of p-NFκBp65, p-IκB, SMAD7, but downregulation of p-SMAD1, RUNX2 and ALP in OVX-derived BMMSCs. (B) When BMMSCs were treated with IFN-γ and TNF-α, combination of IFN-γ (50 ng/ml) and TNF-α (5 ng/ml) treatment caused significant upregulation of NFκBp65 and p-IκB, as assessed by Western blot analysis. (C) Alizarin red staining showed that IFN-γ and TNF-α in combination, but not independently, caused a markedly reduced mineralized nodule formation in BMMSCs. (D) Western blot showed that IFN-γ and TNF-α treatment in combination was able to dramatically upregulate IFNGRα and TNFR1, but not TNFR2, under osteogenic induction. IFN-γ, either alone or in combination with TNF-α, was able to markedly upregulate p-STAT-1. (E) Toluidine blue staining showed that neither IFN-γ nor TNF-α treatment affected MSC survival. (F) Western blot showed that TNF-α–/IFN-γ –induced NFκB activation upregulated SMAD7, but downregulated the osteogenic markers p-SMAD1, RUNX2 and ALP. (G–H) With knockdown of NFκB by IKKα siRNA assay, TNF-α–/IFN-γ–induced upregulation of SMAD7 and downregulation of p-SMAD1, RUNX2 and ALP were abolished, leading to the rescue of TNF-α–/IFN-γ–induced reduction of mineralized nodule formation in BMMSCs. (I, J) Systemic administration of NFκB inhibitor Bay 11-7082 in OVX mice was able to rescue mineralized nodule formation capacity of BMMSCs, by downregulating SMAD7 and upregulating p-SMAD1, RUNX2, and ALP in BMMSCs, as shown by Western blot analysis. (K, L) Western blot showed that OVX failed to induce upregulation of NFκBp65 and p-IκB in IFN-γ or TNF-α null BMMSCs, respectively. (M) Either IFN-γ or TNF-α neutralizing antibody in vivo treatment was able to downregulate NFκBp65 and p-IκB in BMMSCs. Error bars represent mean ± SEM, and all experiments were performed in triplicate. n = 5 animals per group. *, P < 0.05.
To further confirm the role of the NFκB pathway in TNF-α–/IFN-γ–induced MSC deficiency, we showed that NFκB inhibitor Bay 11-7082 treatment was able to rescue osteogenic differentiation of OVX-derived BMMSCs by downregulating SMAD7 and upregulating p-SMAD1, RUNX2, and ALP (Fig. 4I, 4J). To confirm the synergistic effects of IFN-γ and TNF-α that resulted in MSC deficiency in vivo, we demonstrated that OVX failed to induce MSC NFκB activation in IFN-γ and TNF-α knockout mice, respectively (Fig. 4K, 4L). Moreover, either IFN-γ or TNF-α neutralizing antibody treatment in vivo was sufficient to downregulate NFκB pathway in BMMSCs to the level observed in the sham-operated group (Fig. 4M). These in vivo results confirm that IFN-γ and TNF-α work synergistically to induce osteogenic deficiency in MSCs via NFκB pathway.
IFN-γ and TNF-α increase the tendency toward MSC malignant transformation via NFκB-mediated upregulation of oncogenes c-Fos and c-Myc
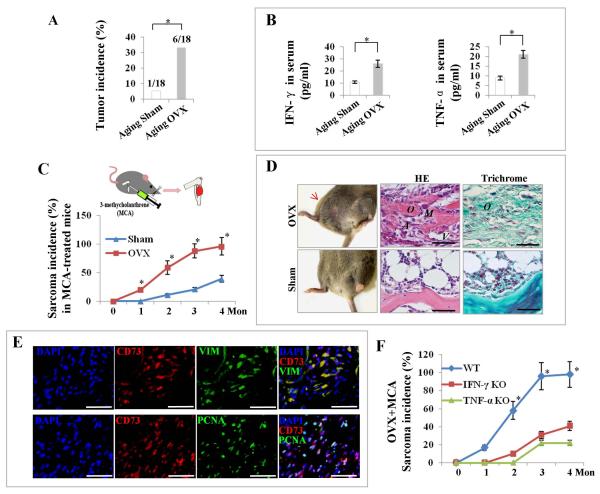
Given the facts that degenerative diseases may be a risk factor for cancer occurrence [14] and that NFκB activation may upregulate oncogenes c-Fos and c-Myc expression [39], we next asked whether IFN-γ–/TNF-α–induced NFκB activation could increase the tendency toward MSC malignant transformation. When we extended the observation time to one-year post-OVX, the aging OVX mice showed a markedly increased tumor incidence when compared with aging sham-operated mice (Fig. 5A). Interestingly, ELISA showed that both IFN-γ and TNF-α concentration in serum remained markedly higher in OVX mice when compared with sham-operated mice (Fig. 5B), suggesting that long-term overexposure of MSCs to IFN-γ and TNF-α may be associated with higher susceptibility to tumorigenesis in OVX mice. To confirm that such higher susceptibility of tumorigenesis involves MSCs, we locally applied 3-methycholanthrene (MCA) as a carcinogen to induce osteosarcoma [36, 40], and found that the MCA-treated OVX mice showed a higher incidence of sarcoma with earlier sarcoma occurrence in femurs when compared with sham-operated mice (Fig. 5C). In tumors that occurred in MCA-treated OVX mice, but not sham-operated mice, histological analysis showed the production of osteoid by atypical tumor cells with high mitotic activity and abundant neovascularization, as well as osteogenic differentiation of the osteosarcoma-like tumor cells, as indicated by Trichrome staining (Fig. 5D). Immunohistochemical co-staining showed that CD73 was highly overlapped with VIM or PCNA (Fig. 5E), confirming that the highly proliferating tumor cells mainly originated from mesenchymal cells, while excluding the possibility of epithelial cell origin, as indicated by negative staining of pan-CK (Supporting Information Fig. S9 A). Furthermore, MCA-treated OVX mice showed a markedly lower incidence of sarcoma with delayed sarcoma occurrence in IFN-γ knockout and TNF-α knockout mice, respectively, when compared with the sham-operated mice (Fig. 5F), suggesting again that IFN-γ and TNF-α work synergistically to, in this case, induce sarcomagenesis.
Figure 5. Long-term exposure of MSCs to IFN-γ and TNF-α synergistically contributes to increased susceptibility to tumor occurrence in OVX mice.
(A) OVX mice at age 1 year showed a markedly increased tumor incidence when compared with age-matched sham mice. (B) ELISA showed that both IFN-γ and TNF-α concentration in serum remained markedly higher in OVX mice when compared with sham mice. (C) When MCA was injected to tibias, OVX mice showed a markedly higher sarcoma incidence with earlier sarcoma occurrence in tibias when compared with sham mice. (D) H&E staining showed the production of osteoid (O) by atypical tumor cells (A) with high mitotic activity (M) and abundant neovascularization (V) in the tumors of MCA-treated OVX mice. Trichrome staining showed osteogenic differentiation (O) of osteosarcoma-like tumor cells in MCA-treated OVX mice. (E) Immunostaining showed that CD73 was highly overlapped with VIM or PCNA in the tumors of MCA-treated OVX mice. (F) MCA-treated OVX mice showed a markedly lower sarcoma incidence with delayed sarcoma occurrence in IFN-γ or TNF-α knockout mice, respectively, when compared with the sham-operated mice. Error bars represent mean ± SEM, n = 10–18 animals per group. *, P < 0.05. Scale bars, 50 μm.
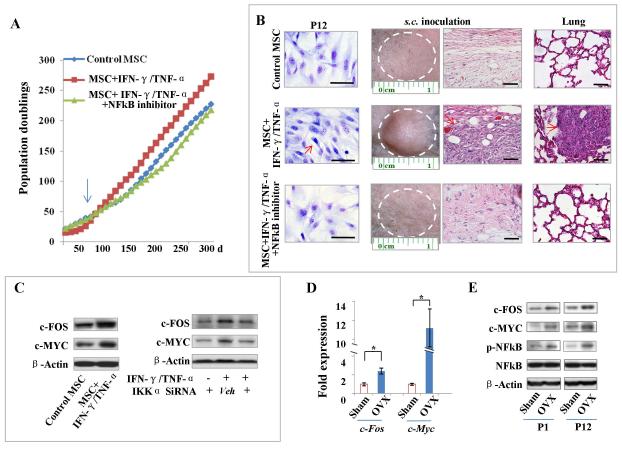
These findings provided a strong rationale for further exploring the mechanisms by which long-term IFN-γ/TNF-α overexposure could induce MSC malignant transformation. To achieve this, we used an established spontaneous malignant transformation assay [18] by continuously passaging mouse BMMSCs to gradually acquire unlimited population doublings. When treated with long-term IFN-γ/TNF-α in vitro, the BMMSCs showed a markedly quicker increase in population doublings after passage 10, whereas NFκB inhibitor was able to delay such increase in population doublings (Fig. 6A). At passage 12, IFN-γ–/TNF-α–treated BMMSCs showed markedly smaller morphology with frequent abnormal mitosis and the generation of sarcoma-like tumors upon s.c. inoculation, which, when infused to nude mice via tail vein settled in the lungs with in situ tumorigenesis (Fig. 6B). In contrast, neither NFκB inhibitor-treated mice nor control mice showed any change in cell morphology or generated any tumor upon s.c. inoculation or infusion into nude mice (Fig. 6B). Immunohistochemical staining of the tumors derived from s.c. inoculation of IFN-γ–/TNF-α–treated BMMSCs revealed that the highly proliferating tumor cells originated from mesenchymal cells, as indicated by high expressions of VIM and PCNA and negative staining of pan-CK (Supporting Information Fig. S9 B).
Figure 6. Long-term IFN-γ/TNF-α exposure increases susceptibility to MSC malignant transformation via NFκB-induced oncogenes.
(A) In an in vitro spontaneous malignant transformation assay, long-term treatment of IFN-γ/TNF-α showed a markedly quicker increase in population doublings after passage 10 (arrow), whereas NFκB inhibitor was able to delay such increase. (B) Giemsa staining showed that IFN-γ/TNF-α-treated BMMSCs presented markedly smaller morphology with frequent abnormal mitosis. Upon s.c. inoculation and tail vein infusion, sarcoma-like tumors grow in situ and settled in lungs with tumorigenesis in immunocompromised mice (red arrows), as assessed by H&E staining. In contract, NFκB inhibitor-treated and control BMMSCs maintained large cell morphology, with no tumor generation upon s.c. inoculation or infusion into immunocompromised mice. Scale bars, 50 μm. (C) Western blot showed that expression of c-FOS and c-MYC was markedly elevated in long-term IFN-γ/TNF-α-treated BMMSCs when compared with control BMMSCs. Knockdown of NFκB by IKKα siRNA treatment was able to abolish elevated expression of c-FOS and c-MYC. (D) Real-time PCR showed that c-Fos and c-Myc were markedly elevated in BMMSCs derived from aging OVX mice when compared with sham mice. (E) Western blot showed that c-FOS and c-MYC expression was enhanced, along with NFκB activation, in BMMSCs derived from aging OVX mice. Error bars represent mean ± SEM, n = 4 animals per group, and all experiments were performed in triplicate. *, P < 0.05.
To further dissect the molecular mechanism of such IFN-γ–/TNF-α–induced MSC tumorigenesis, we demonstrated that the expression of c-MYC and c-FOS, was markedly elevated in long-term IFN-γ–/TNF-α–treated BMMSCs when compared with control BMMSCs (Fig. 6C), but that knockdown of NFκB by IKKα siRNA assay was able to abolish the elevation in c-MYC and c-FOS (Fig. 6C). To confirm this result in vivo, we showed that c-Myc and c-Fos were markedly elevated in BMMSCs derived from aging OVX mice when compared with sham-operated ones by using real-time PCR (Fig. 6D), which was confirmed by Western blot and immunohistochemistry showing that c-MYC and c-FOS expression was enhanced along with NFκB activation in aging OVX mice when compared with sham-operated mice (Fig. 6E, Supporting Information Fig. S9 C).
Aspirin blocks OVX-induced MSC deficiency and tumorigenesis by inhibiting IFN-γ and TNF-α
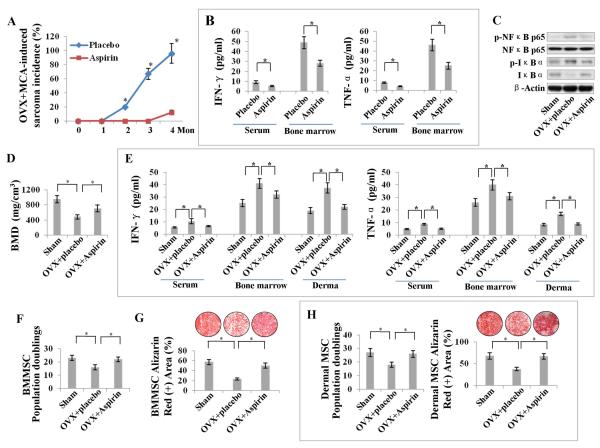
Next, we investigated whether blocking the levels of IFN-γ and TNF-α by pharmacological approach could rescue MSC deficiency and tumorigenesis in vivo. Since aspirin has been reported to inhibit the function of IFN-γ and TNF-α [28, 41], we examined the effect of systemic aspirin treatment in OVX mice which had undergone MCA injections in their tibias. Interestingly, aspirin treatment dramatically reduced the concentrations of IFN-γ and TNF-α in both serum and bone marrow, resulting in a decreased and delayed osteosarcoma occurrence following MCA treatment (Fig. 7A, 7B), with marked inhibition of NFκB activity in BMMSCs, as shown by Western blot analysis (Fig. 7C). The data suggest that aspirin treatment may decrease the tendency toward MSC malignant transformation by inhibiting IFN-γ–/TNF-α–induced NFkB activation.
Figure 7. Aspirin blocks OVX-induced tumorigenesis and MSC deficiency by inhibiting IFN-γ and TNF-α.
(A) Systemic aspirin treatment resulted in a markedly decreased and delayed osteosarcoma occurrence in MCA-treated OVX mice. (B) ELISA showed that aspirin treatment dramatically reduced the levels of IFN-γ and TNF-α in both serum and bone marrow. (C) Western blot showed markedly downregulated p-NFκBp65 and p-IκB in BMMSCs derived from aspirin-treated OVX mice when compared with placebo-treated OVX mice. (D) Aspirin-treated OVX mice showed increased BMD when compared to un-treated OVX mice. (E) ELISA showed that aspirin treatment reduced the concentrations of IFN-γ and TNF-α in serum, bone marrow and derma. (F–H) Aspirin administrated in vivo was able to markedly improve population doublings and osteogenic differentiation of both BMMSCs and DMSCs in OVX mice. Error bars represent mean ± SEM, n = 10–18 animals per group, and all experiments were performed in triplicate. *, P < 0.05. Scale bars, 50 μm.
In order to determine the efficacy of aspirin administration to rescue IFN-γ–/TNF-α–induced MSC deficiency in organ degenerative diseases, we systemically applied aspirin to OVX mice and found that aspirin could markedly improve BMD and rescue the deteriorated trabecular bone structures and derma atrophy in OVX mice (Fig. 7D; Supporting Information Fig. S10 A, S10 B), at least partly by reducing the concentrations of IFN-γ and TNF-α in serum, bone marrow and derma (Fig. 7E). Importantly, in vivo aspirin administration was able to markedly improve population doublings and differentiation capacities of both BMMSCs and DMSCs in OVX mice (Fig. 7F–7H), suggesting that aspirin treatment may provide an ideal MSC-based therapy for OVX mice by preventing MSC deficiency which is induced by medium-term IFN-γ and TNF-α overexposure.
In summary, we have uncovered a previously unrecognized mechanism whereby elevated levels of IFN-γ and TNF-α synergistically, but not independently, cause MSC deficiency via NFκB–mediated activation of SMAD7 in OVX mice, whereas long-term elevated levels of IFN-γ and TNF-α synergistically result in increased tendency toward MSC malignant transformation through NFκB–mediated upregulation of the oncogenes c-Fos and c-Myc (Supporting Information Fig. S11). Thus, as a therapeutic regimen, systemic administration of aspirin provides a promising approach in rescuing MSC deficiency in organ degenerative diseases, as well as preventing MSC tumorigenesis through reducing the levels of IFN-γ and TNF-α.
DISCUSSION
It has been well documented that estrogen deficiency increases levels of IFN-γ and TNF-α thereby enhancing osteoclast activity in OVX mice [3–5]. However, it has been unclear whether elevated levels of IFN-γ and TNF-α contribute to the impairment of MSCs/osteoblasts in OVX mice or osteoporotic humans [2, 10–13]. In this study, we found that elevated levels of IFN-γ and TNF-α synergistically induce osteogenic deficiency of MSCs through NFκB/SMAD7, whereas long-term overexposure of IFN-γ and TNF-α can induce malignant transformation of MSCs.
TNF-α is widely accepted as a proinflammatory cytokine, serving as a prerequisite in several models of inflammatory and autoimmune diseases. However, IFN-γ is believed to possess both proinflammatory and anti-inflammatory properties, such as inhibiting IL-1 production, and, as such, it also plays a complex role in inflammation and autoimmune diseases [42]. The respective effects of IFN-γ and TNF-α in osteogenic differentiation of MSCs have remained controversial [28, 43–47]. The discrepancies in the effects of IFN-γ and TNF-α among these studies may be attributed to the different doses of IFN-γ and TNF-α, treatment, variations between the microenvironments and MSC growth status. Nonetheless, IFN-γ and TNF-α do not present independently in vivo; instead, they are simultaneously secreted by activated Th1 and other immune cells and cooperatively create proinflammatory microenvironments. Indeed, our data demonstrated that high levels of IFN-γ and TNF-α synergistically play a crucial role in causing MSC deficiency in OVX mice, thereby causing the phenotypes of osteoporotic bone or derma atrophy in OVX mice. Blockage of either IFN-γ or TNF-α with their neutralizing antibodies in vivo is sufficient to effectively rescue MSC deficiency. Furthermore, OVX is not able to induce MSC deficiency in IFN-γ and TNF-α KO mice respectively, suggesting that IFN-γ and TNF-α act together, not independently, to induce MSC deficiency in the OVX model. Since OVX can induce elevation of multiple inflammatory cytokines, it is possible that other cytokines, with the exception of IFN-γ and TNF-α, may contribute to OVX-induced MSC impairment in OVX mice. For example, interleukin-1 (IL-1) has been proved to enhance osteoclastogenesis in OVX mice [48]. Thus, further study is required to clarify the role of other inflammatory cytokines in OVX-induced osteoporosis. In this study, we demonstrate that IFN-γ and TNF-α play a crucial role in synergistically inducing MSC deficiency in OVX mice. Given the fact that MSCs derived from derma and subcutaneous fat have different changes in population doublings and osteogenic differentiation after OVX, the effects of IFN-γ and TNF-α in inducing MSC deficiency may be associated with tissue levels of IFN-γ and TNF-α.
It has been demonstrated that IFN-γ and TNF-α may activate STAT-1 and NFκB pathways, thereby interacting with their common basal transcription complexes to affect coactivator recruitment in fibroblasts and asthmatic airway smooth muscle cells, respectively [49, 50]. IFN-γ and TNF-α have also been reported to synergistically activate STAT-1/IFN regulatory factor-1 and c-Jun NH2-terminal kinase (JNK) pathways to induce apoptosis of pancreatic beta-cells in autoimmune diabetes [51, 52]. However, it is unclear how overexposure of stem cells to the synergistically activity of IFN-γ and TNF-α affects their behavior during the course of degenerative diseases. In this study, we demonstrated that IFN-γ binds its receptor IFNGR1 to strongly enhance TNF-α/TNFR1-upregulated NFκB, which, in turn, mediates inhibitory SMAD7 to downregulate the osteogenic master gene Runx2 [53] and, hence, induce osteogenic deficiency in MSCs, both in vivo and in vitro. In our previous studies, we proved that TNF-α converts the signaling of the IFN-γ–activated, nonapoptotic form of FAS in BMMSCs to a caspases-associated proapoptotic cascade, resulting in the apoptosis of implanted BMMSCs [28]. Such difference in the final fates of MSCs may be caused by high levels of IFN-γ and TNF-α produced by more intensive local infiltration of Th1 cells in tissue-engineered transplants when compared with those in a degenerative disease model, such as OVX mice. The synergistic dose-dependent effects of IFN-γ and TNF-α indicate that pro-inflammatory microenvironments in degenerative diseases and tissue engineering are hierarchical and complex for the alteration of MSCs.
It has been suggested that TNF-α may drive malignant tumor progression and that NF-κB signaling may be involved in tumor development [16, 54, 55]. However, it has been unknown whether long-term overexposure of synergistically active IFN-γ and TNF-α could cause malignant transformation of MSCs. In this study, we extended the period of IFN-γ and TNF-α overexposure and found that the ultimate effect was upregulation of the oncogenes c-Fos and c-Myc via NF-κB signaling, thereby causing increased tendency toward MSC tumorigenesis, both in vitro and in vivo. Furthermore, carcinogen-treated OVX IFN-γ and TNF-α knockout mice showed a markedly lower incidence of sarcoma with delayed sarcoma occurrence, indicating that IFN-γ and TNF-α must work together, not independently, to upregulate NF-κB-mediated oncogenes c-Fos and c-Myc. Thus, it is plausible that both organ degenerative diseases and malignant tumor might be “stem cell diseases”, in which IFN-γ and TNF-α overexposure plays a dynamic role in inducing MSC deficiency and MSC tumorigenesis through activation of NFκB-mediated SMAD7 and the oncogenes c-Fos/c-Myc, respectively.
As a widely used anti-inflammatory drug, aspirin is able to inhibit osteoclastogenesis and improve osteogenesis in OVX mice by inhibiting cyclooxygenases and upregulating telomerase in BMMSCs [10, 56]. Since aspirin can block Th1 cell development [57] and reduce TNF-α– and IFN-γ–producing cells in implanted tissue-engineered bones [28], we hypothesized that aspirin should be able to block TNF-α and IFN-γ in OVX mice and therefore rescue MSC deficiency and MSC tumorigenesis. Indeed, we demonstrated that systemic administration of aspirin was able to decrease concentrations of TNF-α and IFN-γ, both in serum and bone marrow, and prevent MSC deficiency and carcinogen-induced MSC tumorigenesis in OVX mice. Therefore, aspirin may be a promising drug to treat estrogen deficiency-induced MSC deficiency and reduce the risk of tumor occurrence. However, cautious evaluation is needed before these findings in mice would accurately translate to humans. On one hand, although aspirin was shown to be associated with higher BMD at multiple skeletal sites in a cohort study of men and women [58], Bauer et al. [59] only observed a modest beneficial effect on BMD in another cohort study of postmenopausal women. On the other hand, although daily aspirin has been proved to decrease the risk of adenocarcinoma metastasis in a randomized controlled trial [17], a recent study indicated that regular administration of aspirin and other nonsteroidal anti-inflammatory drugs was associated with a slightly higher incidence of follicular lymphoma incidence [60]. Thus, the daily use of aspirin or nonsteroidal anti-inflammatory drugs and the risk of cancer in human should be investigated carefully using large cohorts of patients. Also, large cohort studies are required to determine the effects of aspirin in treating post-menopausal osteoporosis and other organ degenerative diseases. Moreover, it is known that MSCs possess such immunomodulatory properties as immunosuppression [61]. Therefore, it will be interesting to examine whether host MSCs show any immunosuppressive effects, such as inhibition of activated T cells and NK cells, thereby serving as a self-protecting mechanism against potential autoimmune reactions by the host immune system.
CONCLUSION
In summary, this study demonstrated that TNF-α and IFN-γ, as two important representative inflammatory factors, play a crucial role in synergistically inducing MSC deficiency and eventual MSC tumorigenesis via the NFκB pathway in OVX–induced osteoporotic mice. Anti-inflammatory treatment, such as aspirin administration, prevents MSC deficiency and malignant transformation in OVX mice.
Supplementary Material
Figure S1. Elevated levels of IFN-γ and TNF-α in OVX mice. Immunostaining showed increased IFN-γ and TNF-α positive cells (white arrows) in bone marrow and derma, but not in subcutaneous fat, in OVX mice when compared with sham-operated mice. Scale bars, 50 μm.
Figure S2. The number of dermal MSCs is reduced in OVX mice. Immunohistochemical staining of MSC markers CD105, CD44 and CD73 showed markedly reduced number of dermal MSCs in OVX mice when compared with sham-operated mice. Scale bars, 50 μm.
Figure S3. OVX results in trabecular bone deterioration and derma atrophy in mice. (A, B) H&E staining showed markedly thinner trabecular bone and reduced dermal thickness, respectively, in OVX mice when compared with sham-operated mice. n = 8 animals per group. *, P < 0.05. Scale bars, 50 μm.
Figure S4. IFN-γ and TNF-α synergistically cause MSC deficiency in nude mice. (A, B) H&E staining showed that only IFN-γ/TNF-α treatment group showed marked loss of trabecular bone and derma atrophy. n = 8 animals per group. Scale bars, 100 μm.
Figure S5. Local administration of IFN-γ/TNF-α causes MSC deficiency in nude mice. When IFN-γ and TNF-α, either alone or in combination, were subcutaneously transplanted with hydrogel as a controlled release system to nude mice, only IFN-γ–/TNF-α–infused group showed significantly reduced population doublings (A) and capacity to form mineralized nodules (B) in DMSCs. (C) H&E staining demonstrated that only IFN-γ/TNF-α locally transplanted with hydrogel showed significant derma atrophy. n = 5 animals per group. *, P < 0.05. Scale bars, 100 μm.
Figure S6. IFN-γ and TNF-α impair MSC differentiation via NFκB/SMAD7 pathway. (A) Western blot showed upregulation of p-NFκBp65, p-IκB, SMAD7 and downregulation of p-SMAD1, RUNX2 and ALP in OVX-derived DMSCs. (B) TNF-α treatment showed a dose-dependent NFκB activation. (C, D) Alizarin red staining showed that IFN-γ and TNF-α in combination but not alone caused a markedly reduction in mineralized nodule formation in DMSCs, with upregulated p-IκB, SMAD7 and down-regulated p-SMAD1, RUNX2 and ALP. (E, F) With knockdown of NFκB by IKKα siRNA, IFN-γ-/TNF-α-induced upregulation of SMAD7 and downregulation of p-SMAD1, RUNX2 and ALP were abolished, leading to the rescue of IFN-γ-/TNF-α–induced reduction of mineralized nodule formation in DMSCs. All experiments were performed in triplicate. *, P < 0.05.
Figure S7. IFNGRα and TNFR1 are required for IFN-γ/TNF-α to snergistically induce activation of NFκB. (A) Combined knockdown of IFNGRα and TNFR1 resulted in markedly inhibition of IFN-γ–/TNF-α–induced activation of NFκBp65 and p-IκB when compared with TNFR1 knockdown group. (B) Knockdown of STAT-1 abolished TNF-α-/IFN-γ–induced activation of NFκB pathway. (C, D) Western blot analysis confirmed efficacy of siRNA in inhibiting IKKα, IFNGRα, TNFR1, STAT-1 and SMAD7.
Figure S8. SMAD7 knockdown rescues impaired differentiation of BMMSCs and DMSCs. (A) Western blot showed that knockdown of SMAD7 downregulated p-SMAD1, RUNX2 and ALP in BMMSCs without downregulating p-IκB. (B) Alizarin red staining showed that knockdown of SMAD7 resulted in rescuing of IFN-γ-/TNF-α–induced reduction of mineralized nodule formation in BMMSCs. (C) Western blot showed that knockdown of SMAD7 downregulated RUNX2 and ALP in DMSCs without downregulating p-IκB. (D) Alizarin red staining showed that knockdown of SMAD7 resulted in rescuing of IFN-γ–/TNF-α–induced reduction of mineralized nodule formation in DMSCs. All experiments were performed in triplicate. *, P < 0.05.
Figure S9. Long-term IFN-γ/TNF-α exposure increases susceptibility to MSC malignant transformation via NFκB-induced oncogenes. (A) Immunostaining of pan-CK showed negative cells in MCA–induced tumor in OVX mice. (B) Immunostaining of the tumors derived from s.c. inoculation of IFN-γ–/TNF-α–treated BMMSCs showed elevated expressions of VIM and PCNA, but negative expression of pan-CK. (C) Immunostaining of p-NFκB, c-FOS and c-MYC in bone marrow showed more positive cells in aging OVX mice. Scale bars, 50 μm.
Figure S10. Aspirin rescues osteoporosis and derma atrophy in OVX mice. H&E staining showed that systemically applied aspirin rescued the deteriorated trabecular bone structures (A) and derma atrophy (B) in OVX mice. Scale bars, 100 μm. *, P < 0.05.
Figure S11. Schematic diagram illustrating the mechanism in IFN-γ– and TNF-α–induced MSC deficiency and MSC tumorigenesis. Elevated levels of IFN-γ and TNF-α together, but not independently, synergistically cause MSC deficiency via NFκB–mediated activation of SMAD7, whereas long-term elevated levels of IFN-γ and TNF-α synergistically result in increased tendency toward MSC malignant transformation through NFκB–mediated upregulation of the oncogenes c-Fos and c-Myc.
ACKNOWLEDGEMENTS
This work was supported by grants from National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449, R01DE019932, and R01DE019413 to S.S.), grant from California Institute for Regenerative Medicine (RN1-00572 for S.S.), grant from the National Natural Science Foundation of China (No. 81020108019 to Y.J. and S.S.), and grant from the National Basic Research Program (973 Program) of China (No. 2011CB964700 to Y.J.).
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Author Contributions: S.S., Y.J. and L.W.: conception and design, writing manuscript, and final approval of manuscript; L.W., Y.Z., Y.L., K.A., C.C. and C.Q.: collection and assembly of data; S.S., L.W., Y.Z. and Y.L.: data analysis and interpretation.
DISCLOSURE OF POTENTIAL AND CONFLICTS OF INTEREST The authors declare that they have no competing interests.
REFERENCES
- 1.Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. 2010;6:698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y, Grassi F, Ryan MR, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roggia C, Gao Y, Cenci S, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuiri T, Kim I, Yamaza T, et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010;25:1668–1679. doi: 10.1002/jbmr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 10.Yamaza T, Miura Y, Bi Y, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 2008;3:e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaza T, Ren G, Akiyama K, et al. Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res. 2011;90:317–324. doi: 10.1177/0022034510387796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benisch P, Schilling T, Klein-Hitpass L, et al. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS One. 2012;7:e45142. doi: 10.1371/journal.pone.0045142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cebioglu M, Schild HH, Golubnitschaja O. Diabetes mellitus as a risk factor for cancer: stress or viral etiology? Infect Disord Drug Targets. 2008;8:76–87. doi: 10.2174/187152608784746501. [DOI] [PubMed] [Google Scholar]
- 15.Suba Z. Gender-related hormonal risk factors for oral cancer. Pathol Oncol Res. 2007;13:195–202. doi: 10.1007/BF02893499. [DOI] [PubMed] [Google Scholar]
- 16.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 18.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 19.Mohseny AB, Szuhai K, Romeo S, et al. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- 20.Riggi N, Cironi L, Provero P, et al. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 21.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045–1056. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Zhao Y, Shi S. Interplay between Mesenchymal Stem Cells and Lymphocytes: Implications for Immunotherapy and Tissue Regeneration. J Dent Res. 2012;91:1003–1010. doi: 10.1177/0022034512460404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 26.Li JY, Tawfeek H, Bedi B, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Wang S, Shi S. The role of recipient T cells in mesenchymal stem cell-based tissue regeneration. Int J Biochem Cell Biol. 2012;44:2044–2050. doi: 10.1016/j.biocel.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Wang L, Kikuiri T, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cenci S, Weitzmann M, Roggia C, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cenci S, Toraldo G, Weitzmann M, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lallemand F, Mazars A, Prunier C, et al. Smad7 inhibits the survival nuclear factor kappaB and potentiates apoptosis in epithelial cells. Oncogene. 2001;20:879–884. doi: 10.1038/sj.onc.1204167. [DOI] [PubMed] [Google Scholar]
- 32.Schiffer M, Bitzer M, Roberts I, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bitzer M, von Gersdorff G, Liang D, et al. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- 34.Baugé C, Attia J, Leclercq S, et al. Interleukin-1beta up-regulation of Smad7 via NF-kappaB activation in human chondrocytes. Arthritis Rheum. 2008;58:221–226. doi: 10.1002/art.23154. [DOI] [PubMed] [Google Scholar]
- 35.Miura M, Chen XD, Allen MR, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanuki T, Ogawa M, Yamada M. Methylcholanthrene-induced sarcoma in singly fractured bone of mice. Tohoku J Exp Med. 1967;93:125–129. doi: 10.1620/tjem.93.125. [DOI] [PubMed] [Google Scholar]
- 37.Devens BH, Lundak RL, Byus CV. Induction of murine fibrosarcomas by low dose treatment with 3-methylcholanthrene followed by promotion with 12-O-tetradecanoylphorbol-13-acetate. Cancer Lett. 1984;21:317–324. doi: 10.1016/0304-3835(84)90011-9. [DOI] [PubMed] [Google Scholar]
- 38.Tsai C, Su P, Huang Y, et al. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–82. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Majumder S, Roy S, Kaffenberger T, et al. Loss of metallothionein predisposes mice to diethylnitrosamine-induced hepatocarcinogenesis by activating NF-kappaB target genes. Cancer Res. 2010;70:10265–10276. doi: 10.1158/0008-5472.CAN-10-2839. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Liu C, Chen Z, Zhang T, et al. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–724. doi: 10.1593/neo.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon MS, Shim EJ, Seo YJ, et al. Effect of aspirin and acetaminophen on proinflammatory cytokine-induced pain behavior in mice. Pharmacology. 2005;74:152–156. doi: 10.1159/000084548. [DOI] [PubMed] [Google Scholar]
- 42.Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3:1247–1255. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 43.Lu X, Beck GR, Jr., Gilbert LC, et al. Identification of the homeobox protein Prx1 (MHox, Prrx-1) as a regulator of osterix expression and mediator of tumor necrosis factor alpha action in osteoblast differentiation. J Bone Miner Res. 2011;26:209–219. doi: 10.1002/jbmr.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duque G, Huang DC, Macoritto M, et al. Autocrine regulation of interferon gamma in mesenchymal stem cells plays a role in early osteoblastogenesis. Stem Cells. 2009;27:550–558. doi: 10.1634/stemcells.2008-0886. [DOI] [PubMed] [Google Scholar]
- 45.Dighe AS, Yang S, Madhu V, et al. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. J Orthop Res. 2013;31:227–234. doi: 10.1002/jor.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glass GE, Chan JK, Freidin A, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Huang J, Zhang H, et al. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells. 2011;29:1601–1610. doi: 10.1002/stem.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimble R, Srivastava S, Ross F, et al. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271:28890–28897. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- 49.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272:14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 50.Clarke DL, Clifford RL, Jindarat S, et al. TNFalpha and IFNgamma synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-kappaB, and the transcriptional coactivator CREB-binding protein. J Biol Chem. 2010;285:29101–29110. doi: 10.1074/jbc.M109.0999952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim WH, Lee JW, Gao B, et al. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal. 2005;17:1516–1532. doi: 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Suk K, Kim S, Kim YH, et al. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 53.Itoh F, Asao H, Sugamura K, et al. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J. 2001;20:4132–4142. doi: 10.1093/emboj/20.15.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 57.Mazzeo D, Panina-Bordignon P, Recalde H, et al. Decreased IL-12 production and Th1 cell development by acetyl salicylic acid-mediated inhibition of NF-kappaB. Eur J Immunol. 1998;28:3205–3213. doi: 10.1002/(SICI)1521-4141(199810)28:10<3205::AID-IMMU3205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 58.Carbone L, Tylavsky F, Cauley J, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18:1795–1802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- 59.Bauer D, Orwoll E, Fox K, et al. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:29–35. doi: 10.1002/jbmr.5650110106. [DOI] [PubMed] [Google Scholar]
- 60.Teras L, Gapstur S, Patel A, et al. Aspirin and Other Non-steroidal Anti-Inflammatory Drugs and Risk of Non-Hodgkin Lymphoma. Cancer Epidemiol Biomarkers Prev. 2013 Jan 17; doi: 10.1158/1055-9965.EPI-12-1158. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Spaggiari G, Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol. 2013;91:27–31. doi: 10.1038/icb.2012.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Elevated levels of IFN-γ and TNF-α in OVX mice. Immunostaining showed increased IFN-γ and TNF-α positive cells (white arrows) in bone marrow and derma, but not in subcutaneous fat, in OVX mice when compared with sham-operated mice. Scale bars, 50 μm.
Figure S2. The number of dermal MSCs is reduced in OVX mice. Immunohistochemical staining of MSC markers CD105, CD44 and CD73 showed markedly reduced number of dermal MSCs in OVX mice when compared with sham-operated mice. Scale bars, 50 μm.
Figure S3. OVX results in trabecular bone deterioration and derma atrophy in mice. (A, B) H&E staining showed markedly thinner trabecular bone and reduced dermal thickness, respectively, in OVX mice when compared with sham-operated mice. n = 8 animals per group. *, P < 0.05. Scale bars, 50 μm.
Figure S4. IFN-γ and TNF-α synergistically cause MSC deficiency in nude mice. (A, B) H&E staining showed that only IFN-γ/TNF-α treatment group showed marked loss of trabecular bone and derma atrophy. n = 8 animals per group. Scale bars, 100 μm.
Figure S5. Local administration of IFN-γ/TNF-α causes MSC deficiency in nude mice. When IFN-γ and TNF-α, either alone or in combination, were subcutaneously transplanted with hydrogel as a controlled release system to nude mice, only IFN-γ–/TNF-α–infused group showed significantly reduced population doublings (A) and capacity to form mineralized nodules (B) in DMSCs. (C) H&E staining demonstrated that only IFN-γ/TNF-α locally transplanted with hydrogel showed significant derma atrophy. n = 5 animals per group. *, P < 0.05. Scale bars, 100 μm.
Figure S6. IFN-γ and TNF-α impair MSC differentiation via NFκB/SMAD7 pathway. (A) Western blot showed upregulation of p-NFκBp65, p-IκB, SMAD7 and downregulation of p-SMAD1, RUNX2 and ALP in OVX-derived DMSCs. (B) TNF-α treatment showed a dose-dependent NFκB activation. (C, D) Alizarin red staining showed that IFN-γ and TNF-α in combination but not alone caused a markedly reduction in mineralized nodule formation in DMSCs, with upregulated p-IκB, SMAD7 and down-regulated p-SMAD1, RUNX2 and ALP. (E, F) With knockdown of NFκB by IKKα siRNA, IFN-γ-/TNF-α-induced upregulation of SMAD7 and downregulation of p-SMAD1, RUNX2 and ALP were abolished, leading to the rescue of IFN-γ-/TNF-α–induced reduction of mineralized nodule formation in DMSCs. All experiments were performed in triplicate. *, P < 0.05.
Figure S7. IFNGRα and TNFR1 are required for IFN-γ/TNF-α to snergistically induce activation of NFκB. (A) Combined knockdown of IFNGRα and TNFR1 resulted in markedly inhibition of IFN-γ–/TNF-α–induced activation of NFκBp65 and p-IκB when compared with TNFR1 knockdown group. (B) Knockdown of STAT-1 abolished TNF-α-/IFN-γ–induced activation of NFκB pathway. (C, D) Western blot analysis confirmed efficacy of siRNA in inhibiting IKKα, IFNGRα, TNFR1, STAT-1 and SMAD7.
Figure S8. SMAD7 knockdown rescues impaired differentiation of BMMSCs and DMSCs. (A) Western blot showed that knockdown of SMAD7 downregulated p-SMAD1, RUNX2 and ALP in BMMSCs without downregulating p-IκB. (B) Alizarin red staining showed that knockdown of SMAD7 resulted in rescuing of IFN-γ-/TNF-α–induced reduction of mineralized nodule formation in BMMSCs. (C) Western blot showed that knockdown of SMAD7 downregulated RUNX2 and ALP in DMSCs without downregulating p-IκB. (D) Alizarin red staining showed that knockdown of SMAD7 resulted in rescuing of IFN-γ–/TNF-α–induced reduction of mineralized nodule formation in DMSCs. All experiments were performed in triplicate. *, P < 0.05.
Figure S9. Long-term IFN-γ/TNF-α exposure increases susceptibility to MSC malignant transformation via NFκB-induced oncogenes. (A) Immunostaining of pan-CK showed negative cells in MCA–induced tumor in OVX mice. (B) Immunostaining of the tumors derived from s.c. inoculation of IFN-γ–/TNF-α–treated BMMSCs showed elevated expressions of VIM and PCNA, but negative expression of pan-CK. (C) Immunostaining of p-NFκB, c-FOS and c-MYC in bone marrow showed more positive cells in aging OVX mice. Scale bars, 50 μm.
Figure S10. Aspirin rescues osteoporosis and derma atrophy in OVX mice. H&E staining showed that systemically applied aspirin rescued the deteriorated trabecular bone structures (A) and derma atrophy (B) in OVX mice. Scale bars, 100 μm. *, P < 0.05.
Figure S11. Schematic diagram illustrating the mechanism in IFN-γ– and TNF-α–induced MSC deficiency and MSC tumorigenesis. Elevated levels of IFN-γ and TNF-α together, but not independently, synergistically cause MSC deficiency via NFκB–mediated activation of SMAD7, whereas long-term elevated levels of IFN-γ and TNF-α synergistically result in increased tendency toward MSC malignant transformation through NFκB–mediated upregulation of the oncogenes c-Fos and c-Myc.