Abstract
The Ikaros gene encodes a Krüppel-like zinc-finger transcription factor involved in hematopoiesis regulation. Ikaros has been established as one of the most clinically relevant tumor suppressors in several hematological malignancies. In fact, expression of dominant negative Ikaros isoforms is associated with adult B-cell acute lymphoblastic leukemia, myelodysplastic syndrome, acute myeloid leukemia and adult and juvenile chronic myeloid leukemia. Here, we report the isolation of a novel, non-canonical Ikaros splice variant, called Ikaros 11 (Ik11). Ik11 is structurally related to known dominant negative Ikaros isoforms, due to the lack of a functional DNA-binding domain. Interestingly, Ik11 is the first Ikaros splice variant missing the transcriptional activation domain. Indeed, we demonstrated that Ik11 works as a dominant negative protein, being able to dimerize with Ikaros DNA-binding isoforms and inhibit their functions, at least in part by retaining them in the cytoplasm. Notably, we demonstrated that Ik11 is the first dominant negative Ikaros isoform to be aberrantly expressed in B-cell lymphoproliferative disorders, such as chronic lymphocytic leukemia. Aberrant expression of Ik11 interferes with both proliferation and apoptotic pathways, providing a mechanism for Ik11 involvement in tumor pathogenesis. Thus, Ik11 could represent a novel marker for B-cell lymphoproliferative disorders.
Introduction
Ikaros is a member of the Krüppel-like zinc finger transcription factor family. It is encoded by the IKZF1 gene, which consists of eight exons alternatively spliced to produce different isoforms able to homo- and heterodimerize [1]–[3]. All isoforms share two C-terminal zinc-finger domains that allow for homo- and heterodimerization between Ikaros family members, while they differ in the number of N-terminal zinc-finger motifs, which form the DNA-binding domain. Ikaros proteins with fewer than three N-terminal zinc-fingers act as dominant negative (DN) factors, being able to impair the activity of the DNA-binding isoforms [1], [4].
Ikaros has been shown to act both as a canonical transcriptional activator and repressor. In addition, a large body of literature has demonstrated that it may modulate gene expression by taking part in chromatin remodeling [1]–[3], [5].
Ikaros is a master regulator of hematopoiesis, especially of lymphoid development. Initial studies in Ikaros null mice showed an early and complete block of B-cell development, the absence of natural killer and dendritic cells, as well as a decreased number of T cells and perturbed myelopoiesis [6], [7]. In addition, mice lacking Ikaros or expressing DN isoforms developed T-cell leukemia, suggesting that Ikaros acts as a tumor suppressor gene in the lymphoid lineage [8].
Ikaros’ role in tumor suppression is due to its ability to negatively regulate the G1/S transition through the modulation of both positive and negative effectors of the cell cycle [9]–[11]. Ikaros is also involved in apoptosis. Ikaros null mice showed decreased apoptosis in response to oxidative stress in bone marrow erythroid cells [12]. In addition, the overexpression of full-length Ikaros increased apoptosis in leukemic cell lines [13].
Genetic inactivation of Ikaros and the aberrant expression of DN isoforms have been demonstrated in different types of human leukemia, such as B and T acute lymphoblastic leukemia (ALL) [14]–[27], chronic myeloid leukemia (CML) [15], [28], [29] and acute myeloid leukemia (AML) [4]. Mechanisms underlying the generation of DN isoforms in tumors are still debated, but intragenic deletions were shown to be involved [15].
We identified a novel, non-canonical splice variant of the Ikaros gene, which we called Ik11. Here, we showed that Ik11 is a novel DN isoform capable of inactivating functional Ikaros isoforms by, at least in part, cytoplasmic sequestration. Ik11 promoted cell proliferation and counteracted apoptosis, in vitro. Finally, in order to investigate a possible involvement of Ik11 in tumor pathogenesis, we have also analyzed Ik11 expression in human CML, ALL, myelodysplastic syndromes, lymphomas and chronic lymphocytic leukemia (CLL). Interestingly, we found that Ik11 was aberrantly expressed particularly in B-cell lymphoproliferative disorders. To our knowledge, this is the first evidence for DN Ikaros expression in CLL.
Materials and Methods
Ethics Statement
This study was reviewed and approved by the Institutional Review Board of Molecular and Clinical Pathology and Oncology Section, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila (L’Aquila, Italy) in accordance with Declaration of Helsinki. Blood samples were collected with the written informed consent of the donors.
IK11 Cloning
Ikaros11 was isolated from Ficoll-separated normal human peripheral blood lymphocytes (hPBLs) using the SMART RACE kit (BD Biosciences, San Jose, CA, USA). 1 µg of RNA was subjected to reverse transcription PCR (RT-PCR) and subsequently amplified according to the manufacturer’s specifications. PCR products were cloned into the pcRII vector (TOPO-TA cloning, Life Technology, Carlsbad, CA, USA) and sequenced.
Plasmids and Reagents
pcDNA3.1-Ik2, pcDNA3.1-Ik6, pcDNA3.1-Ik11, pcDNA3.1/Myc-HysB-Ik2, pcDNA3.1/Myc-HysB-Ik6 and pcDNA3.1/Myc-HysB-Ik11 constructs are described in Supporting Text S1. The pGL3 luciferase reporter vector containing an Ikaros responsive gene was previously described [30]. Staurosporine was purchased from Sigma Aldrich (St. Louis, Missouri USA). zVAD-FMK was purchased from Promega (Madison, WI, USA).
Tumor Samples
cDNAs from patients with poorly differentiated malignant lymphoma (n = 1), Hodgkin’s lymphoma (n = 1) and non-Hodgkin’s lymphoma (n = 1) were purchased from BD Biosciences. Samples from patients with CML (n = 21), ALL (n = 11), myelodysplastic syndromes (n = 7), Hodgkin’s and non-Hodgkin’s lymphoma (n = 6) and CLL (n = 22) were provided from the Internal Medicine and Hematology Unit of San Salvatore Hospital-L’Aquila. Diagnosis was based on morphology, cytogenetics, immunophenotype and molecular biology analyses.
Cell Lines and Transfection
293T HEK, Cos7 and RAW 264 (ATCC, Manassas, VA, USA) cell lines were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine and 1% Penicillin Streptomycin. K562 (ATCC) and BJAB [31] cell lines were cultured in RPMI 1640 with 10% FBS, 1% L-glutamine and 1% Penicillin Streptomycin. PBLs were obtained by Ficoll density-gradient centrifugation of heparinized blood from healthy donors. Leukocyte subset isolation was performed by magnetic cell sorting (MACS, Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instructions. CD14 or CD19 mAb-coated microbeads and CD4 or CD8 cell isolation kits (Miltenyi Biotec) were used to purify monocytes, B and T cells, respectively.
The transfections were performed using Lipofectamine 2000 Reagent (Life Technology), Fugene HD (Promega) and Attractene Transfection Reagent (Qiagen, Valencia, CA, USA) according to the manufacturer’s specifications. K562 and BJAB were electroporated by using Amaxa-Nucleofector II™ according to the manufacturer’s specifications (Lonza, Walkersville, MD, USA).
RNA Extraction, PCR and Real-time PCR
RNA was extracted with Trizol reagent (Life Technology) according to the manufacturer’s specifications and reverse transcribed using the GeneAmp® Gold RNA PCR Reagent Kit (Life Technology). cDNAs of spleen, thymus, lymph nodes and bone marrow were commercially available (BD Biosciences). Semiquantitative PCR was performed using Go-Taq Green Master Mix (Promega). Real-time PCR was performed by using Mastercycler® ep realplex and RealMasterMix Sybr Rox (Eppendorf, Barkhausen, Hamburg, Germany). The gapdh gene was used as a normalizing control. Primer sequences and Real-time PCR conditions are available upon request.
Sequence Analysis
PCR products were sequenced using the ABI PRISM Big Dye terminator v3.1 cycle sequencing kit and the automated sequencer ABI PRISM 310 Genetic Analyzer (Life Technology) in accordance with the manufacturer’s instructions.
In vitro Binding Assay, Immunoprecipitation and Western Blotting
In vitro translation of the Ikaros 2-myc protein was performed using the TNT Quick Coupled Transcription/Translation System (Promega), according to manufacturer’s instructions. pcDNA3.1/Myc-HysB-Ik2 construct was used as template. In vitro translation of the Ikaros 6 and Ikaros 11 proteins was performed using the Transcend Non-Radioactive Translation Detection System (Promega), according to manufacturer’s instructions. pcDNA3.1-Ik6 and pcDNA3.1-Ik11 were used as template. Translated proteins were incubate at 37° for 1 hour. IP buffer (20 mM Tris-HCl pH 7.4, 140 mM NaCl, 10% glycerol, 1 mM CaCl2, 0.1% Triton X, 1 tablet of complete mini EDTA-free protease inhibitors (Roche Molecular Biochemicals, Mannheim, Germany) and anti-Myc antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) were subsequently added to the protein mix. After 1 hour incubation at RT on a rotating wheel, Protein A/G Plus-Agarose (Santa Cruz Biotechnology) was added to each protein mix and incubated at 4°C overnight.
Cell extracts were prepared in Giordano buffer (50 mM Tris-HCl pH 7.4, 250 mM NaCl, 5 mM EDTA, 25 mM NaF, 0.1% Triton X-100, 0.1 mM PMSF, 1 µg/ml Leupeptine, 10 µg/ml trypsin inhibitor from soybean, 10 µg/ml TPCK, 5 µg/ml TLCK, 1 µg/ml Aprotinine, 0.1 mM Na3VO4) or RIPA buffer (1x phosphate buffered saline, 1% NP40, 0.5% sodium deoxycholate, 1% SDS, 0.1 mM PMSF, 1 µg/ml aprotinine, 0.1 M Na3VO4) containing complete mini EDTA-free protease inhibitors (Roche Molecular Biochemicals). Immunoprecipitation was performed using anti-Myc antibody and Protein A/G Plus-Agarose (Santa Cruz Biotechnology), according to the manufacturer’s specifications. Primary antibodies were: Ikaros, Cyclin E, BAX, Actin (Santa Cruz Biotechnology), p27 (BD Biosciences), p21 and PARP (Cell Signaling Technologies, Inc Beverly, MA, USA).
Luciferase Assay
The Ikaros-regulated promoter of the KCTD11(REN) gene was cloned into the pGL3 luciferase reporter vector as previously described [30]. Promoter activity was analyzed using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. Promoter activity was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity.
Immunofluorescence Assay
Transfected cells were cultured on poly-D-Lysine, 8 wells culture slides (BD) and fixed in 4% paraformaldehyde. Cells were incubated with anti-Myc and/or anti-Ikaros antibodies (Santa Cruz Biotechnology) followed by Fluorescein (FITC)-conjugated AffiniPure Goat anti-Rabbit IgG (H+L) and Texas Red dye conjugated AffiniPure Goat anti-mouse IgG (H+L) secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA). Nuclei were stained with Hoechst 33258 (Life Technology). The subcellular localizations were analyzed using a Leica TCS SP5 Confocal Microscopy. 40x objective was used.
MTS Assay
CellTiter 96® Aqueous One Solution Reagent (Promega) was added to transfected cells according to the manufacturer’s instructions. Cell viability was determined by measuring the absorbance at 490 nm using a µ-Quant plate-reader (Bio-Tek Instruments, Winooski, VT, USA) and calculated as the percent of control (pcDNA3.1). Statistical analysis was performed using unpaired 2-tailed Student’s t test. P values less than 0.05 were considered significant.
Apoptosis Evaluation
Caspase-3 activity was measured by using the CaspACE™ Assay System, Colorimetric (Promega), according to the manufacturer’s instructions and calculated as the relative absorbance (mean induced apoptosis sample A405 -mean negative control sample A405). Measurement of mono- and oligonucleosome enrichment was carried out by ELISA (Cell Death Detection ELISA, Roche Molecular Biochemicals), according to the manufacturer’s instructions. The mono and oligonucleosome enrichment was calculated as the ratio of mean induced apoptosis sample A405 and mean negative control sample A405. Statistical analysis was performed using the unpaired 2-tailed Student’s t test. P values less than 0.05 were considered significant.
Results
Ik-11: A Novel, Non-canonical Splice Variant of Ikaros
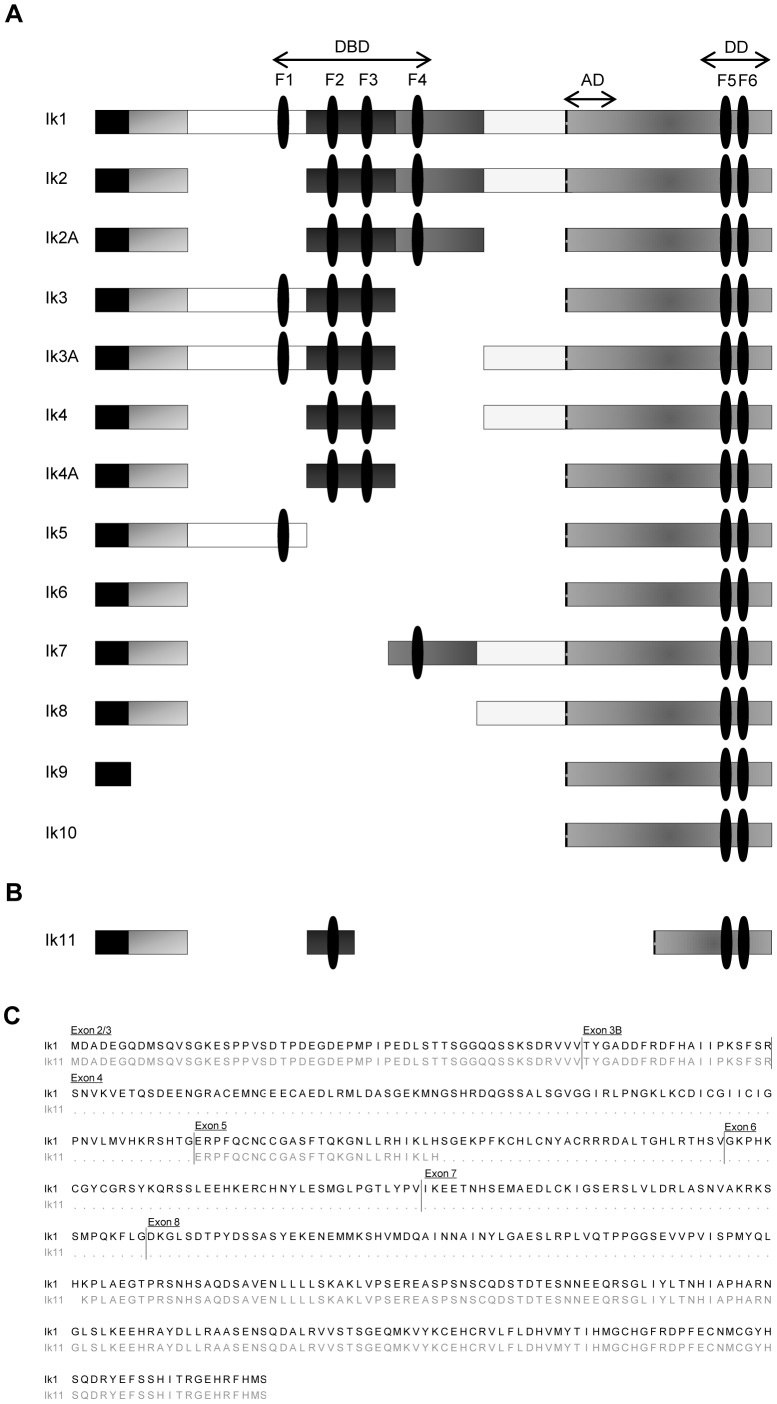
Several isoforms of the Ikaros gene have been previously identified (Figure 1A) [1]–[3], [15], [16], [23]–[25], [32]–[35], as either full-length proteins with transcriptional activity or splice variants with dominant-negative functions. Due to the relevance of these latter forms in the development of hematological malignancies, we decided to perform a PCR-based screening to identify novel Ikaros splice variant mRNAs. To this end, mRNA from hPBLs was reverse transcribed and cDNA was amplified by using the SMART RACE kit. We isolated several isoforms of Ikaros, which have been sub-cloned and sequenced. Among these, one was unknown and it was called Ik11 (GeneBank Acc. N. JX459579). Ik11 is a non-canonical splice variant, as revealed by DNA sequencing. The Ik11 sequence contains exon 2, exon 3, the first half of exon 5 (from the 1st amino acid [E] to the 26th amino acid [H]) and the second half of exon 8 (from the 68th amino acid [K] to the last amino acid [S]) (Figure 1B–C). Thus, the Ik11 protein presents two C-terminal and only one N-terminal zinc-finger domains, whereas it completely lacks the transcriptional activation domain (AD) (Figure 1A–B and Figure S1), suggesting that Ik11 might dimerize with other Ikaros isoforms but not induce gene transcription. In addition, Ik11 had the 60-bp insertion at the end of exon 3 that was already described in other Ikaros isoforms [16], [25] and referred to as exon 3B [36].
Figure 1. Ikaros 11 is a novel, non-canonical splice variant of the Ikaros gene.
(A) Diagrammatic representation of Ikaros isoforms 1–10 with their functional domains. (B) Schematic representation of the novel splice variant Ik11. (DBD, DNA Binding Domain; AD, Activation Domain; DD, Dimerization Domain, F1–F6, Zinc Finger modules). (C) Amino acid sequence alignment of the full-length Ik1 and Ik11. Exon positions are indicated. Exon 3B has been previously described [16], [25], [36] but is currently not identified as an exon in Gene Bank.
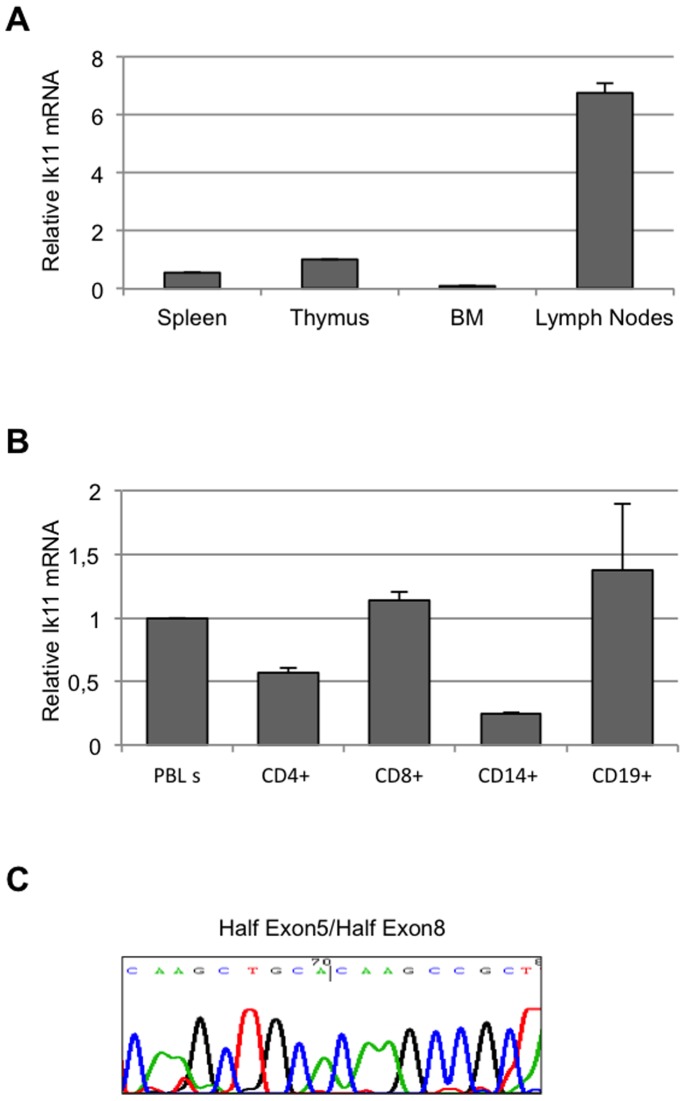
Next, the existence of this novel Ikaros splice variant was confirmed. A significant expression of Ik11 was found in lymph nodes, and less was found in the spleen and thymus, whereas no expression was observed in bone marrow (Figure 2A). These data suggest that this short isoform of Ikaros could have physiological functions in peripheral lymphocytes.
Figure 2. Ik11 expression is restricted to lymph nodes and peripheral lymphocytes.
(A) Real-time PCR analysis of Ikaros mRNA in human cDNAs from normal thymus, spleen, lymph nodes and bone marrow. The Ik11 levels are expressed as fold change relative to expression in thymus and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates. (B) Real-time PCR analysis of Ik11 transcripts in magnetic bead-purified human leukocyte subsets (CD4+ and CD8+ T cells, CD19+ B cells and CD14+ monocytes). The Ik11 levels are expressed as fold change relative to expression in PBLs obtained from the healthy donor #1 (see Figure S3) and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates. (C) Sequencing of Ik11 real-time PCR products from lymph nodes. The electropherogram shows the sequence corresponding to the junction fragments half exon 5/half exon 8.
To further characterize Ik11 expression in PBL sub-populations, CD4+, CD8+, CD14+ and CD19+ cells were isolated. Ik11 was mainly expressed in lymphocyte fractions (Figure 2B). Ik11 amplification products have been confirmed by sequencing (Figure 2C).
Ik-11 Functions as a Dominant Negative Isoform of Ikaros
Based on its sequence, Ik11 resembled the DN isoforms of Ikaros proteins (Figure 1A–B). There is evidence in the literature that Ik6, the most studied Ikaros dominant negative isoform, exerts its inhibitory effect by binding the functional splice variants and inhibiting their ability to bind DNA [3], [8], [36]. Therefore, to shed light on Ik11 function, we first analyzed its capability to form heterodimers with the transcriptionally active form Ik2, in vitro. Ik2-myc, biotinylated-Ik11 and biotinylated-Ik6 proteins were generated by in vitro transcription/translation. Then, co-immunoprecipitations of Ik2-myc with either biotinylated-Ik11 or biotinylated-Ik6 were performed by using an anti-Myc antibody. Immune-complexes were separated by SDS-PAGE and analyzed by streptavidin-HRP Western blot (Figure 3A, first and second lanes). Ik11 was found in Ik2-immunoprecipitated complexes, demonstrating that Ik11 is effectively able to bind Ik2 (Figure 3A, second lane). Ik-2/Ik-6 complexes were shown as a control (Figure 3A, first lane) [1]–[3]. Translated Ikaros proteins were loaded to control the efficiency of the in vitro transcription/translation system (Figure 3A, third, fourth and fifth lanes). Anti-Myc Western blot was performed as control of the immunoprecipitation (Figure 3A, lower panel).
Figure 3. Ik11 acts as a dominant negative isoform.
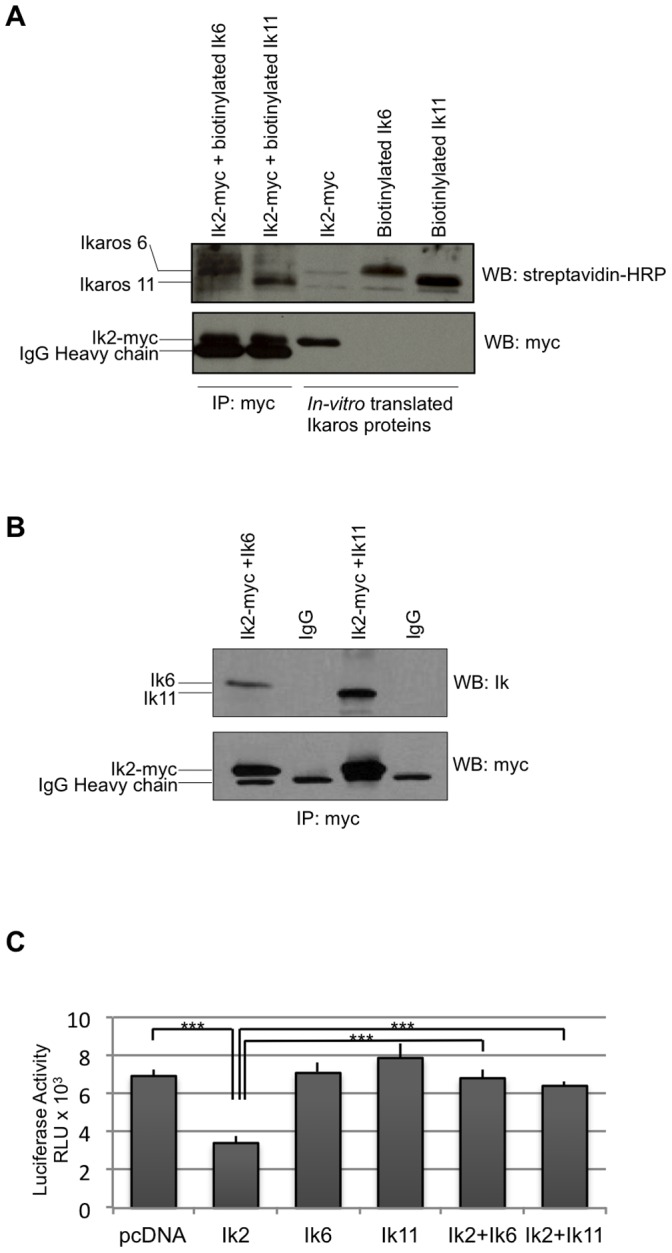
(A) In vitro co-immunoprecipitation of Ik2 with the short isoforms Ik11 or Ik6. Ik2-myc, biotinylated-Ik11 and biotinylated-Ik6 were generated by in vitro transcription/translation. After 1 h incubation of Ik2-Myc with Ik11 or Ik6, the Ik2 complexes were immunoprecipitated with an anti-Myc antibody and subjected to Western blot analysis as indicated (lanes 1 and 2). Lanes 3, 4 and 5 contained the three in vitro translated proteins without immunoprecipitation. (B) In vivo heterodimerization of Ik2 with the splice variants Ik11 or Ik6. The 293T HEK cell line was co-transfected with pcDNA3.1/Myc-HysB-Ik2 along with pcDNA3.1-Ik6 or pcDNA3.1-Ik11. The Ik2 immunoprecipitation was performed with an anti-Myc antibody and the immune-complexes were analyzed by Western blotting as indicated. (C) Luciferase assay showing functional dominant-negative activity of Ik11. The 293T HEK cell line was co-transfected with pcDNA3.1-Ik2, pcDNA3.1-Ik6 or pcDNA3.1-Ik11 or their combinations along with a reporter-LUC construct driven by a promoter containing Ikaros-binding sites. Mean ± SD of triplicate wells is shown (***p<0.001). Data shown are representative of three different experiments.
We confirmed Ik2/Ik11 interaction by immunoprecipitating protein extracts from 293T cells co-transfected with pcDNA3.1-myc-Ik2 and pcDNA3.1-Ik11, by using an anti-Myc antibody (Figure 3B). Ik11 resulted to be co-immunoprecipitated by the anti-Myc antibody but not by a control IgG. Ik-2/Ik-6 complexes were shown as a control [1]–[3]. This analysis indicates that the interaction between Ik2 and Ik11 also takes place in vivo.
Next, the effect of Ik11 on the transcriptional activation of an Ik2-regulated promoter has been evaluated. As expected by the lack of the AD, overexpression of Ik11 alone has no effect on a promoter containing Ikaros consensus sites (Figure 3C). Importantly, Ik11 is able to revert the Ik2-dependent inhibition of the promoter (Figure 3C), demonstrating that Ik11 works as a DN isoform. The choice of a negatively Ik-regulated promoter was due to the fact that most of the known Ik-responsive elements are repressed by this transcription factor, whereas only a few of them are activated.
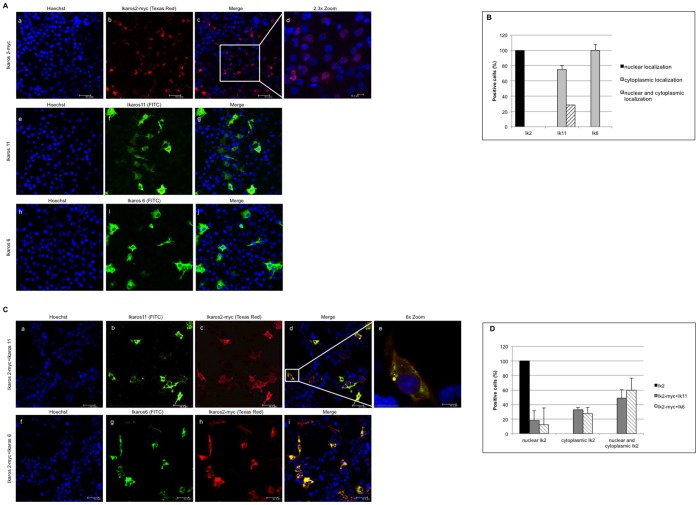
To investigate the mechanism by which Ik11 is able to block Ik2’s transcriptional activity, we looked at the sub-cellular localization of these proteins in the Ikaros-null COS-7 cell line [4], either when overexpressed alone or in combination. The transfection efficiency was evaluated by FACS (Figure S2A). As previously reported in the literature, our immunofluorescence images confirmed that the Ik-2 active isoform localized wholly in the nucleus [1]–[3] (Figure 4A, panels a-d and Figure 4B). Interestingly, the novel DN splice variant Ik-11 showed a predominant cytoplasmic localization (Figure 4A, panels e-g and Figure 4B). A ∼20% of Ik11-transfected cells exhibited both cytoplasmic and nuclear staining, but none of them showed solely nuclear localization of the protein (Figure 4A–B). As known from the literature, Ik-6 localized predominantly in the cytoplasm (Figure 4A, panels h-j and Figure 4B) [4]. Notably, Ik2 changed its localization from the nucleus to the cytoplasm in the presence of Ik11 (Figure 4C, panels a-e and Figure S2B), showing almost a complete merge with this isoform (Figure 4C, panels d, e). Indeed, this translocation was not always complete. In fact, ∼50% of positive cells showed both cytoplasmic and nuclear staining for Ik2-myc in presence of Ik11 (Figure 4C–D and Figure S2B). Cytoplasmic Ik2 was not detectable in cells negative for Ik11. Ik2+Ik11 immunofluorescence data were comparable with those obtained from the co-transfection of Ik6 with Ik2, suggesting that Ik11 resemble Ik6 in its ability of sequestering and inactivating Ik2.
Figure 4. Ik2 subcellular localization changes in presence of Ik11.
(A) Subcellular localization of Ik2 (panels a–d), Ik11 (panels e-g) and Ik6 (panels h–j). The Cos7 cell line was transfected with pcDNA3.1/Myc-HysB-Ik2, pcDNA3.1-Ik6 or pcDNA3.1-Ik11 constructs and the cellular localization of each isoform was analyzed by confocal microscopy. Immunofluorescence localization of Ik2 was assessed by anti-Myc antibody and Texas Red dye conjugated AffiniPure Goat anti-mouse IgG (H+L) (red fluorescence); Ik6 and Ik11 were detected with anti-Ikaros antibody and Fluorescein (FITC)-conjugated AffiniPure Goat anti-Rabbit IgG (H+L) (green fluorescence). Nuclei were stained with Hoechst 33258 (panels a, e, h, blue fluorescence). Merged images of double fluorescence (Hoechst localization of nuclei plus Ikaros staining) are shown for all of the three isoforms (Ik2: panels c and d, scale bar equals to 50 and 10 microns respectively; Ik11: panel g; Ik6: panel j). x40 magnification (panels a–c, e–j). Panel d was an x2.3 zoom of the white box field indicated in panel c. (B) Graphic representation of Ik2, Ik11 and Ik6 subcellular localization. The analysis was conducted counting nuclear or cytoplasmic staining, or both (nuclear+cytoplasmic), of the three isoforms as percent point. 5 fields were counted for each transfection. (C) Confocal triple immunofluorescence images of Hoechst 33258 plus Ik2-myc and Ik11 or Ik6. Cos7 cells were co-transfected with either pcDNA3.1/Myc-HysB-Ik2 and pcDNA3.1-Ik11 (a–e) or pcDNA3.1/Myc-HysB-Ik2 and pcDNA3.1-Ik6 (f–i) expression vectors. Staining for Ik11 (green fluorescence), Ik6 (green fluorescence), Ik2 (red fluorescence) and Hoechst 33258 (blue fluorescence) were performed as described in (A). Merged images of triple fluorescence (Hoechst localization of nuclei plus co-localization of Ik2/Ik11 or Ik2/Ik6) were illustrated in panels d-e and panel j, respectively. Scale bar were equals to 50 microns (panels a-d and panels f–j, x40 objective) and 10 microns (panel e, x6 zoom of the white box field indicated in panel d). (D) Graphic representation of Ik2 subcellular localization when it is transfected alone or in combination with Ik11 and Ik6. The analysis was conducted counting Ik2 nuclear or cytoplasmic staining, or both (nuclear+cytoplasmic), as percent point. 5 fields were counted for each transfection.
Taken all together, these data demonstrated that Ik11 is a novel Ikaros splice variant, which acts as a dominant negative isoform. Indeed, the ability of Ik11 to block Ik2’s transcriptional functions could be due, at least in part, to Ik11-mediated cytoplasmic sequestration of Ik2.
Ik-11 Enhances Cell Proliferation and Impairs Apoptosis
Several lines of evidence have suggested that Ikaros acts as a tumor suppressor gene [8]. In fact, Ikaros-deficient mice revealed that the absence of Ikaros expression, or the presence of DN Ikaros mutants, leads to the development of T-cell leukemia in mice [8]. Moreover, Ikaros provides thresholds that regulate proliferation at key stages of T-cell development. Furthermore, T cells from Ikaros-deficient mice showed facilitated cell-cycle entry in response to minimal TCR engagement and accelerated G1-S transition in response to IL2R signaling [37]. In addition, Ikaros suppresses pre-B cell proliferation, thus allowing for a correct differentiation program [11]. Loss of Ikaros activity is indeed observed in more than 80% of Ph+ ALL [38]. Ikaros proteins also play an important role in monocyte/macrophage development [4].
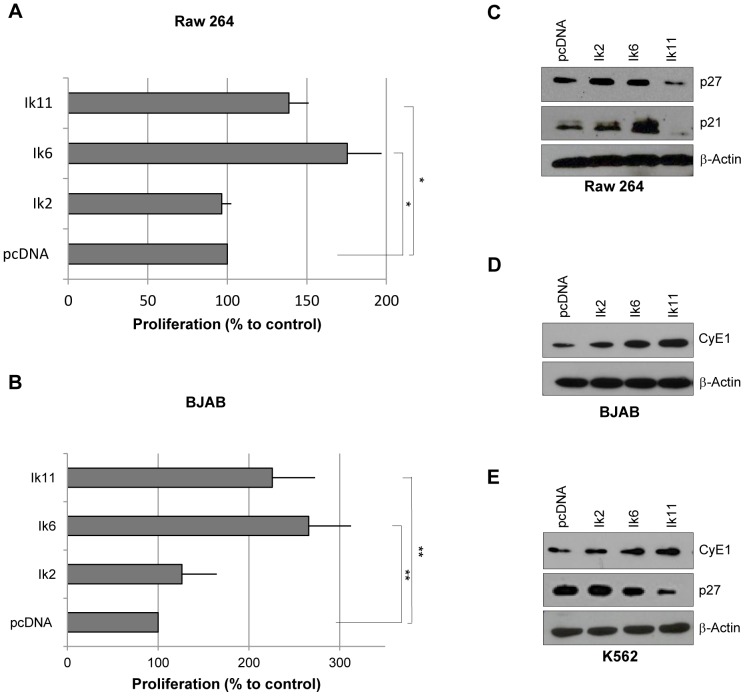
The overexpression of DN Ikaros isoforms impaired the growth-inhibitory function of Ikaros in several systems [39]–[41]. Therefore, we tested whether the novel DN Ik11 isoform was capable of promoting cell proliferation. Transient overexpression of Ik2 did not impact Raw 264 cell growth, whereas transfection of Ik11 induced a significant increase of cell proliferation (Figure 5A). The same results have been obtained by using the BJAB cell line (Figure 5B). Increased proliferation by Ik11 overexpression was associated with a down-regulation of cyclin-dependent kinase inhibitors p21 and p27 in Raw264 cells (Figure 5C), as well as with an up-regulation of cyclin E in BJAB cells (Figure 5D). A decrease of p27 protein levels, as well as the up-regulation of cyclin E was also detected in K562 cells overexpressing Ik11 (Figure 5E). The proliferation rate induced by Ik11 overexpression was similar to that obtained upon Ik6 transfection in both cell lines (Figure 5A–B). High cyclin E levels were also observed in the Ik6-transfected BJAB cell line, while no down-regulation of p21 and p27 was found in Ik6-expressing Raw264 and K562 cell lines, suggesting that Ik11 and Ik6 might promote proliferation through different mechanisms.
Figure 5. Ik11 overexpression promotes cell proliferation.
(A–B) Analysis of cell proliferation in Raw 264 (A) and BJAB (B) cell lines. Cells were transfected with pcDNA3.1-Ik2, pcDNA3.1-Ik6, pcDNA3.1-Ik11 or empty vector. Proliferation was determined by the MTS assay at 24 h (BJAB) or 48 h (RAW 264) and calculated as the percent of control (pcDNA3.1). Mean ± SD is shown (*p<0.05; **p<0.001). Data shown are representative of three different experiments. (C–E) Western blot analysis of p27, p21 and cyclin E in RAW 264 (C), BJAB (D) and K562 (E) cell lines.
Ikaros is also known to play a role in the control of apoptosis. Bone marrow erythroid cells from Ikaros-null mice were less susceptible to oxidative stress-induced apoptosis than control cells [12]. Moreover, apoptosis was increased upon overexpression of full-length Ikaros in leukemic cell lines [13]. Furthermore, the overexpression of Ik6 can delay apoptotic cell death upon growth factor withdrawal in myeloid and lymphoid cytokine-dependent cell lines and confer resistance to dexamethasone and anti-IgM-induced apoptosis in B cells [42], [43].
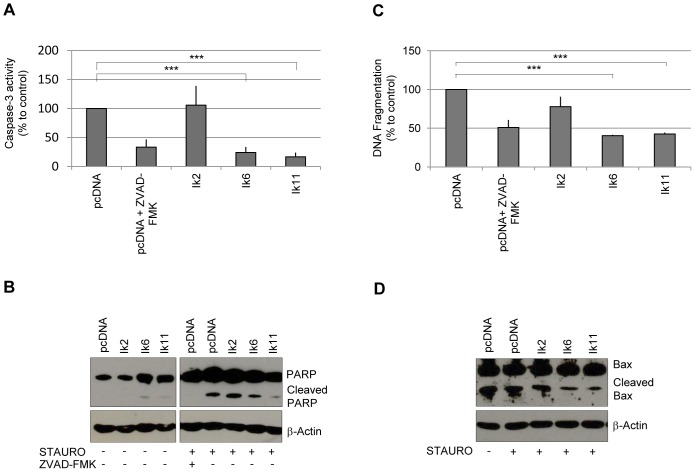
Therefore, we assessed the role of Ik11 in staurosporine-induced apoptosis. As shown in Figure 6, overexpression of Ik11 strongly protected Raw264 cells against staurosporine-induced apoptosis (Figure 6A–C). In this cell context, Ik11 protected against staurosporine-induced apoptosis by inhibiting Bax cleavage, with the consequent decrease of the potent proapoptotic molecule p18-Bax (Figure 6D).
Figure 6. Ik11 overexpression protects against apoptosis.
Evaluation of apoptosis by measurement of caspase-3 activity (A), examination of PARP cleavage products (B) and determination of mono- and oligo-nucleosome enrichment (C). Raw 264 cells were transfected with pcDNA3.1-Ik2, pcDNA3.1-Ik6, pcDNA3.1-Ik11 or empty vector and were incubated with 100 nM staurosporine or vehicle for 14 hours. Empty vector-transfected cells were also treated with 50 µM Z-VAD-FMK, a general caspase inhibitor. Values were calculated as percent of control (pcDNA3.1). Mean ± SD is shown (***p<0.0001) (A,C). Data shown are representative of three different experiments. (D) Western blot analysis of Bax protein. Raw 264 cells were transfected with Ikaros isoforms and treated with staurosporine as previously described. Protein levels of Bax and Bax/p18 cleavage products are shown.
Therefore, Ik11 expression impacts both cell proliferation and cell death, suggesting that this new DN Ikaros isoform might play a role in the development of hematological cancers.
Ik11 is Aberrantly Expressed in B-cell Lymphoproliferative Disorders
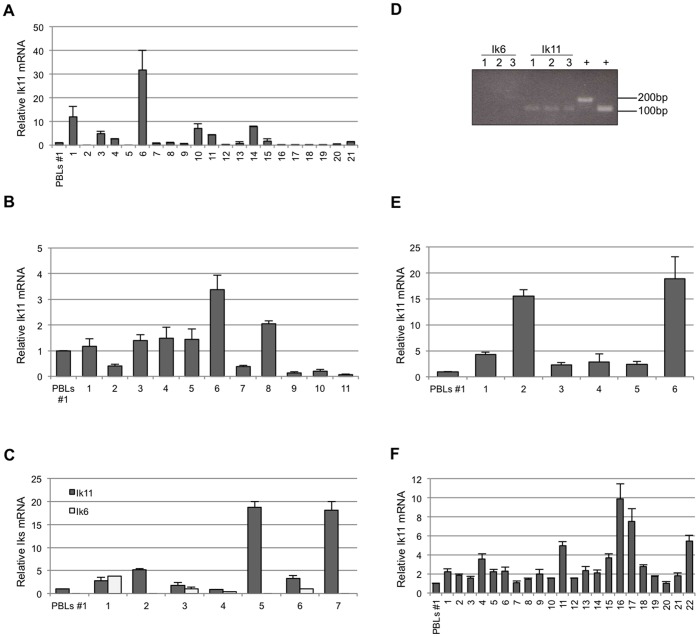
Aberrant expression of DN Ikaros isoforms, particularly Ik6, has been found in adult B cell ALL [22], [27], as well as in myelodysplastic syndrome [44], AML [4] and adult and juvenile CML [29]. Therefore, we investigated the involvement of the novel DN Ik11 in hematological tumors. Expression of Ik11 in PBLs was used as reference value. To this end, we evaluated Ik11 mRNA levels in 10 different PBLs samples obtained from healthy donors, proving a similar expression values in all samples (Figure S3). Therefore, we assessed the expression of Ik11 in several lymphoid and myeloid cell lines, detecting no significant increase with respect to PBLs (Figure S4). Next, we analyzed Ik11 mRNA in those hematological disorders in which Ik6 overexpression was previously demonstrated [4], [22], [26], [27], [29], [44]. In particular, our analysis focused on 21 samples of CML (Figure 7A and Table S1), 11 samples of ALL (Figure 7B and Table S2) and 7 samples of myelodysplastic syndromes (Figure 7C and Table S3). Ik11 mRNA was increased more than 2-fold in 7 of 21 (31.8%) samples of CML (Figure 7A) and in 2 of 11 (18.2%) samples of ALL (Figure 7B). Notably, Ik6 expression was not detectable in all CML and ALL samples analyzed (see discussion). Ik11 expression was also increased in 5 of 7 (71.4%) samples of myelodysplastic syndromes (Figure 7C). Nevertheless, the relevance of this latter data needs to be confirmed by using a wider set of myelodysplastic samples.
Figure 7. Increased Ik11 expression in several hematological malignancies.
(A, B, C) Real-time PCR analysis of Ik11 and Ik6 mRNAs in samples of chronic myeloid leukemia (A, n = 21), acute lymphoblastic leukemia (B, n = 11) and myelodysplastic syndromes (C, n = 7). The Ik11 levels are expressed as fold change relative to expression in PBLs obtained from the healthy donor #1 (see Figure S3) and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates. (D) Expression of Ik11 and Ik6 in commercial samples of lymphoproliferative disorders (n = 3). 1 = Poorly differentiated malignant lymphoma; 2 = Hodgkin’s lymphoma; 3 = non-Hodgkin’s lymphoma, diffuse; + = PCR positive controls. (E, F) Real-time PCR analysis of Ik11 and Ik6 mRNAs in samples of lymphoma (E, n = 7) and chronic lymphoblastic leukemia (F, n = 22). The Ik11 levels are expressed as fold change relative to expression in PBLs obtained from the healthy donor #1 (see Figure S3) and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates.
Finally, we analyzed Ik11 expression in the B-cell lymphoproliferative disorders in which DN Ikaros isoforms have not been reported to play a role. The mRNA of three commercially available samples of lymphoma (Figure 7D), six samples of Hodgkin’s and non-Hodgkin’s lymphoma (Figure 7E and Table 1) and 22 samples of CLL (Figure 7F and Table 2) have been analyzed. Conventional semi-quantitative PCR demonstrated that Ik11 was expressed in the three commercially available samples of lymphoma (Figure 7D). Ik11 PCR-amplified products were then confirmed by sequencing (Figure S5). Indeed, increased expression of Ik11 was found in all lymphoma samples (n = 6) (Figure 7E), with two samples showing strikingly high levels of Ik11 (Figure 7E). Moreover, Ik11 was also increased in 12 of 22 CLL samples (54.5%) (Figure 7F). Given that the unmutated IgVH gene status or some cytogenetic abnormalities are associated with a worse clinical outcome, we analyzed the level of Ik11 expression in these CLL subgroups. No correlations of these clinical markers with Ik11 were observed. Notably, the highest level of Ik11 expression was observed in those patients (cases 16, 17 and 22) with rapidly progressive disease (cases 16 and 22 for leukocytosis and case 17 for anemia and thrombocytopenia). Remarkably, no signal for Ik6 was revealed in both lymphoma and CLL samples.
Table 1. Clinical data in patients with Lymphoma.
| N° | Sex | Age | WHO | Stage | IPI/FLIPI or IPS | Karyotype | Disease status at sampling | Outcome |
| 1 | F | 69 | Diffuse Large B-cell Non-Hodgkin lymphoma | III A | 2 | NA | Relapse | Alive |
| 2 | F | 85 | Diffuse Large B-cell Non-Hodgkin lymphoma | II E | 1 | NA | Relapse | Dead |
| 3 | M | 36 | Hodgkin lymphoma | III B | 2 | NA | Diagnosis | Alive |
| 4 | F | 85 | Diffuse Large B-cell Non-Hodgkin lymphoma | II A | 3 | NA | Diagnosis | Dead |
| 5 | M | 63 | Diffuse Large B-cell Non-Hodgkin lymphoma | III B | 5 | Normal | Diagnosis | Dead |
| 6 | F | 89 | Marginal Zone Non-Hodgkin lymphoma | II | 1 | NA | Diagnosis | Dead |
NA indicates not available.
Table 2. Clinical data in patients with Chronic Lymphocytic Leukemia.
| N° | Sex | Age | Disease stage at diagnosis | VH gene status | Treatment | Karyotype | WBC/mmc | % CD19+/CD5+ |
| 1 | F | 63 | II | Unmut | No | Del 13 | 82600 | 85% |
| 2 | M | 78 | IV | Mut | Yes | Del 13q14; Del 17p | 117000 | 76% |
| 3 | M | 66 | II | Mut | Yes | NA | 32000 | 65% |
| 4 | F | 60 | I | Mut | No | normal | 80000 | NA |
| 5 | F | 75 | III | Mut | No | trisomy 12 | 74080 | 80% |
| 6 | M | 73 | NA | Mut | Yes | normal | 122400 | 77% |
| 7 | M | 78 | I | Unmut | Yes | Del 11q | 64000 | 15% |
| 8 | F | 85 | I | Mut | Yes | NA | 46180 | 76.70% |
| 9 | M | 56 | II | Mut | No | normal | 13450 | 40% |
| 10 | M | 82 | II | Unmut | Yes | normal | 21630 | 74.20% |
| 11 | F | 76 | 0 | Mut | No | trisomy 12 | 12000 | 20% |
| 12 | F | 71 | 0 | Mut | No | NA | 14670 | 23% |
| 13 | F | 81 | 0 | Mut | No | NA | 19570 | 60% |
| 14 | F | 52 | 0 | Mut | No | Del 13q14 | 44100 | 84% |
| 15 | F | 59 | I | Mut | No | normal | 19580 | 62% |
| 16 | M | 55 | I | Mut | Yes | normal | 81220 | 88% |
| 17 | M | 81 | I | Mut | Yes | Del 13 | 18460 | 80% |
| 18 | F | 80 | 0 | Mut | no | normal | 17860 | 49% |
| 19 | M | 66 | 0 | Mut | no | NA | 23160 | 59% |
| 20 | M | 66 | II | Mut | no | NA | 11890 | 58% |
| 21 | M | 60 | II | Mut | Yes | normal | 16250 | NA |
| 22 | M | 74 | 0 | NA | Yes | Del 13 | 104000 | 96% |
NA indicates not available.
Taken together, all these data indicated that Ik11 aberrant expression is strongly associated with B-cell lymphoproliferative disorders and, to a lesser degree, with B-ALL and CML.
Discussion
During the past years, Ikaros has been established as one of the most clinically relevant tumor suppressors in several hematological malignancies. Expression of DN isoforms is associated with adult B-cell ALL, as well as with myelodysplastic syndrome, AML, and adult and juvenile CML. In addition, multiple microarray-based analyses of genetic changes and alterations in gene expression have revealed that Ikaros plays a key role in tumor suppression in pediatric B-cell ALL. Indeed, a modest decrease in Ikaros activity is sufficient to contribute to leukemogenesis [3], [4], [17], [19]–[27], [29], [44].
In this study we reported the isolation of a novel, non-canonical, Ikaros DN isoform, named Ik11. Ik11 protein has two C-terminal and only one N-terminal zinc-finger domains, thus lacking the functional DNA binding domain, but able to form homo- and heterodimers. All known DN Ikaros isoforms share these structural characteristics. However, in contrast to all other DN Ikaros proteins, Ik11 completely skips the AD due to alternative splicing. To our knowledge, this is the first evidence of a non-canonical splice variant of Ikaros. How the absence of the AD can impact on Ik11 protein structure–ie., tertiary folding, stability, phosphorylation acceptor sites etc.–needs to be established with further experiments. Nevertheless, we demonstrated that Ik11 functionally acts as a DN protein. In fact, Ik11 is able to block the activity of transcriptionally active isoforms at least in part by binding them and inducing their cytoplasmic sequestration, as previously demonstrated also for other DN Ikaros isoforms [4].
Notably, Ik11 was aberrantly expressed in B-cell lymphoproliferative disorders, such as CLL. This disease is characterized by the monoclonal expansion of B lymphocytes in the peripheral blood, bone marrow and lymphoid organs with an indolent course that can become aggressive or even fatal [45]. The pathogenic events of CLL are not well known. Here, we showed that Ik11 is overexpressed in 12 of 22 (54.5%) cases of CLL, with the highest expressions of Ik11 observed in those patients in a rapidly progressed disease state. To our knowledge, this is the first evidence of aberrant expression of Ikaros DN isoforms in B-cell lymphoproliferative disorders. An increased expression of Aiolos, another member of Ikaros family, has been recently demonstrated in CLL. The authors showed that Aiolos overexpression confers a survival advantage to the CLL population [46]. In CLL cells, whether Ik11 and Aiolos play distinct pathogenic roles or they work in concert forming a heterodimer remains to be determined.
The most studied dominant-negative isoform of Ikaros is Ik6, whose aberrant expression has been found in adult B-cell ALL [22], [27], as well as in myelodysplastic syndrome [44], AML [4] and adult and juvenile CML [29]. Therefore, we also analyzed the expression of a DN Ik6 isoform in our hematological cancer samples. Surprisingly, we did not detect Ik6 mRNA in any case, except for four samples of myelodysplastic syndromes. Conflicting data have been reported in the literature on the frequency of Ik6 expression in hematological cancers. Our data are in accordance with previous data on U.S. patients reported by Sun and colleagues [23]–[25], but in contrast with other studies on Japanese and Chinese patients [20]–[22], [26], [27], [29]. The differences between the Japanese and Chinese reports on the one hand and our and Sun’s studies on the other hand could be explained by ethnic diversity, as already suggested by Takanashi et al. [21].
Given the large amount of research on Ikaros DN isoforms in cancer, it might seem surprising that Ik11 was not previously identified. However, Ikaros expression has most frequently been analyzed by nested-PCR, using a single primer pair common to all isoforms. In many of these works, the antisense primer annealed to the first part of exon 8, corresponding to the AD, which is missing in the Ik11 transcript [19]–[29], [47]. In a previous work by Iacobucci and colleagues [16], the detection of Ikaros isoforms was performed by PCR followed by capillary electrophoresis, a highly sensitive, accurate and standardized method. Also in this case, the reverse primer was complementary to the exon 8 region, which is absent in Ik11. However, Ik11 has not been detected by PCR even when a primer pair complementary to the start and the stop codons of full-length Ikaros was used [22]. A possible explanation is that a single PCR reaction may not have sufficient sensitivity to detect all different isoforms, because some transcripts may amplify more efficiently than others. Therefore, the existence of this novel non-canonical splice variant of Ikaros indicates that screening methods to detect and quantify Ikaros short splice variants missing the AD needs to be performed.
The expression of Ik11 is not restricted to malignant cells, as also demonstrated for other DN isoforms [16], suggesting that alternative splicing of the Ikaros gene might be responsible for the generation of a complex regulatory network that controls normal hematopoiesis. Our expression pattern analysis showed that Ik11 mRNA is mainly present in lymph nodes and, to a lesser extent, in spleen and thymus. Yet, Ik11 is mainly expressed in B- and T-cell fractions of PBLs, indicating that it could have physiological roles in peripheral lymphocytes. Further experiments are necessary to better define this issue.
Ikaros DN isoforms have been shown to affect cell proliferation. Ectopic expression of Ik6 can immortalize murine hematopoietic progenitor cells in myeloid conditions resulting in growth factor-independent proliferation of a myeloid cell line [4], [39]. Our results showed that overexpression of Ik11 enhances cell proliferation of both myeloid and lymphoid cell lines by modulating the protein levels of three of the major actors involved in the G1 checkpoint and G1-S transition, such as p21 and p27 cyclin-dependent kinase inhibitors and cyclin E. Interestingly, p27 and cyclin E deregulation has been already found in lymphomas [48].
Apoptosis escape has been indicated as another relatively common mechanism by which Ikaros DN proteins can promote oncogenesis [4]. Overexpression of the Ik6 DN isoform in myeloid and lymphoid cytokine-dependent cell lines can delay apoptosis upon growth factor withdrawal [41] and results in the modulation of Bcl-2 family members in different systems [4], [41]. We found that Ik11 strongly protects Raw264 from staurosporine-induced apoptosis by inhibiting Bax cleavage and the consequent generation of the potent pro-apoptotic molecule p18-Bax.
Unlike Ik11 and Ik6, the overexpression of Ik2 seems to have no significant effects on both cell proliferation and apoptosis. Therefore, the modulation of cell cycle- and apoptosis-related proteins by these short isoforms could not be entirely related to their ability to inhibit the active Ikaros isoforms, leading to the hypothesis that other mechanisms in terms of gene regulation are involved. Recently, it has been demonstrated that Ik6 can prompt survival of pituitary tumor cells by acetylating Bcl-XL promoter and that this effect was not mediated entirely by disruption of Ik1 action [41]. Thus, it seems that not only the full-length isoforms, but also the short ones can influence gene expression by means of epigenetic mechanisms; it remains to be clarified whether also Ik11 can directly induce epigenetic modifications.
In conclusion, our data identify Ik11 as a novel Ikaros DN isoform generated by non-canonical splicing. Aberrant expression of Ik11 was mainly found in CLL and lymphomas. Ik11 overexpression interferes with both proliferation and apoptotic pathways, providing a mechanism for DN Ik11 isoform involvement in human hematological malignancies, primarily in B-cell lymphoproliferative disorders. Taken together, these findings suggest that Ik11 could represent a novel marker for CLL.
Supporting Information
Nucleotide sequence alignment of the full-length Ik1 and Ik11 . Nucleotide sequence alignment of the full-length Ik1 and the novel isoform Ik11. The start of each exon are represented by grey letters.
(TIF)
Ik2 subcellular localization changes in presence of Ik11. (A) Cos7 cells were transfected with peGFP vector and transfection efficiency was evaluated by FACS analysis. (B) Confocal triple immunofluorescence images of Hoechst 33258 plus Ik2-myc and Ik11. Cos7 cells were co-transfected with pcDNA/Myc-HysB-Ik2 and pcDNA3.1-Ik11. Staining for Ik11 (green fluorescence), Ik2 (red fluorescence) and Hoechst 33258 (blue fluorescence) were performed as described in Figure 4A. Scale bars were equals to 50 microns (panels a–d, x40 objective) and 10 microns (panels e–h, x6.5 zoom of the white box field indicated in panels a–d).
(TIF)
IK11 expression in hPBLs from healthy donors. Real-time PCR analysis of Ik11 mRNAs in 10 different samples of hPBLs obtained from healthy donors. The Ik11 levels are expressed as fold change relative to expression in PBLs #1 and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates.
(TIF)
IK11 expression in myeloid and lymphoid cell lines. Real-time PCR analysis of Ik11 and Ik6 mRNAs in myeloid and lymphoid cell lines (see Supporting Text S1). The Ik11 levels are expressed as fold change relative to expression in PBLs obtained from the healthy donor #1 (see Figure S3) and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates.
(TIF)
Ik11 PCR-amplified products were confirmed by sequencing. Sequencing of Ik11 semi-quantitative PCR products shown in Figure 6D. The electropherograms show the sequences corresponding to the junction fragments exon 3/exon 5 and half exon 5/half exon 8.
(TIF)
Clinical data in patients with Chronic Myeloid Leukemia.
(TIF)
Clinical data in patients with Acute Lymphoblastic Leukemia.
(TIF)
Clinical data in patients with Myelodysplastic Syndromes.
(TIF)
Supplementary Material and Methods.
(DOCX)
Acknowledgments
The authors thank Dr. Mariagrazia Perilli for her help with sequencing. The authors are sincerely grateful to the colleagues of University “G. D’Annunzio” of Chieti-Pescara for their kind hospitality after the 2009 L’Aquila earthquake, thus giving us the possibility to conclude this project.
Funding Statement
This work was supported by The Italian Ministry of Education, University and Research Fund for Investments in Basic Research MIUR-FIRB grant No. RBAP10A9H9 to E.A. and to A.G. (http://firb.miur.it/) and by MIUR-PRIN grant No. 2009EWAW4M_003 to F.Z. (http://prin.miur.it/). D.V. was supported by the Ph.D. program in Experimental Medicine, and M.F. was supported by the Ph.D program in Biotechnology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Li Z, Perez-Casellas LA, Savic A, Song C, Dovat S (2011) Ikaros isoforms: The saga continues. World J Biol Chem 2: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. John LB, Ward AC (2011) The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol 48: 1272–1278. [DOI] [PubMed] [Google Scholar]
- 3. Payne KJ, Dovat S (2011) Ikaros and tumor suppression in acute lymphoblastic leukemia. Crit Rev Oncog 16: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yagi T, Hibi S, Takanashi M, Kano G, Tabata Y, et al. (2002) High frequency of Ikaros isoform 6 expression in acute myelomonocytic and monocytic leukemias: implications for up-regulation of the antiapoptotic protein Bcl-XL in leukemiogenesis. Blood 99: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 5. Oestreich KJ, Weinmann AS (2011) Ikaros changes the face of NuRD remodeling. Nat Immunol 13: 16–18. [DOI] [PubMed] [Google Scholar]
- 6. Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, et al. (1994) The Ikaros gene is required for the development of all lymphoid lineages. Cell 79: 143–156. [DOI] [PubMed] [Google Scholar]
- 7. Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, et al. (1996) Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 5: 537–549. [DOI] [PubMed] [Google Scholar]
- 8. Winandy S, Wu P, Georgopoulos K (1995) A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 83: 289–299. [DOI] [PubMed] [Google Scholar]
- 9. Gomez-del Arco P, Maki K, Georgopoulos K (2004) Phosphorylation controls Ikaros’s ability to negatively regulate the G1-S transition. Mol Cell Biol 24: 2797–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kathrein KL, Lorenz R, Minniti IA, Griffiths E, Winandy S (2005) Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol Cell Biol 25: 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma S, Pathak S, Mandal M, Trinh L, Clark MR, et al. (2010) Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Mol Cell Biol 30: 4149–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pulte D, Lopez RA, Baker ST, Ward M, Ritchie E, et al. (2006) Ikaros increases normal apoptosis in adult erythroid cells. Am J Hematol 81: 12–18. [DOI] [PubMed] [Google Scholar]
- 13. He LC, Xu HZ, Gu ZM, Liu CX, Chen GQ, et al. (2011) Ikaros is degraded by proteasome-dependent mechanism in the early phase of apoptosis induction. Biochem Biophys Res Commun 406: 430–434. [DOI] [PubMed] [Google Scholar]
- 14. Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, et al. (2009) Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 360: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, et al. (2008) BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453: 110–114. [DOI] [PubMed] [Google Scholar]
- 16. Iacobucci I, Lonetti A, Cilloni D, Messa F, Ferrari A, et al. (2008) Identification of different Ikaros cDNA transcripts in Philadelphia-positive adult acute lymphoblastic leukemia by a high-throughput capillary electrophoresis sizing method. Haematologica 93: 1814–1821. [DOI] [PubMed] [Google Scholar]
- 17. Iacobucci I, Lonetti A, Messa F, Cilloni D, Arruga F, et al. (2008) Expression of spliced oncogenic Ikaros isoforms in Philadelphia-positive acute lymphoblastic leukemia patients treated with tyrosine kinase inhibitors: implications for a new mechanism of resistance. Blood 112: 3847–3855. [DOI] [PubMed] [Google Scholar]
- 18. Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, et al. (2007) Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 446: 758–764. [DOI] [PubMed] [Google Scholar]
- 19. Tonnelle C, Imbert MC, Sainty D, Granjeaud S, N’Guyen C, et al. (2003) Overexpression of dominant-negative Ikaros 6 protein is restricted to a subset of B common adult acute lymphoblastic leukemias that express high levels of the CD34 antigen. Hematol J 4: 104–109. [DOI] [PubMed] [Google Scholar]
- 20. Nishii K, Katayama N, Miwa H, Shikami M, Usui E, et al. (2002) Non-DNA-binding Ikaros isoform gene expressed in adult B-precursor acute lymphoblastic leukemia. Leukemia 16: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 21. Takanashi M, Yagi T, Imamura T, Tabata Y, Morimoto A, et al. (2002) Expression of the Ikaros gene family in childhood acute lymphoblastic leukemia. Br J Haematol 117: 525–530. [DOI] [PubMed] [Google Scholar]
- 22. Nakase K, Ishimaru F, Avitahl N, Dansako H, Matsuo K, et al. (2000) Dominant negative isoform of the Ikaros gene in patients with adult B-cell acute lymphoblastic leukemia. Cancer Res 60: 4062–4065. [PubMed] [Google Scholar]
- 23. Sun L, Crotty ML, Sensel M, Sather H, Navara C, et al. (1999) Expression of dominant negative Ikaros isoforms in T-cell acute lymphoblastic leukemia. Clin Cancer Res 5: 2112–2120. [PubMed] [Google Scholar]
- 24. Sun L, Goodman PA, Wood CM, Crotty ML, Sensel M, et al. (1999) Expression of aberrantly spliced oncogenic Ikaros isoforms in childhood acute lymphoblastic leukemia. J Clin Oncol 17: 3753–3766. [DOI] [PubMed] [Google Scholar]
- 25. Sun L, Heerema N, Crotty L, Wu X, Navara C, et al. (1999) Expression of dominant negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukaemia. Proc Natl Acad Sci USA 96: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou F, Mei H, Jin R, Li X, Chen X (2011) Expression of Ikaros isoform 6 in Chinese children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 33: 429–432. [DOI] [PubMed] [Google Scholar]
- 27. Liu P, Lin Z, Qian S, Qiao C, Qiu H, et al. (2012) Expression of dominant-negative Ikaros isoforms and associated genetic alterations in Chinese adult patients with leukemia. Ann Hematol 91: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 28. Olivero S, Maroc C, Beillard E, Gabert J, Nietfeld W, et al. (2000) Detection of different Ikaros isoforms in human leukaemias using real-time quantitative polymerase chain reaction. Br J Haematol 110: 826–830. [DOI] [PubMed] [Google Scholar]
- 29. Nakayama H, Ishimura F, Avithal N (1999) Decreases in Ikaros activity correlate with blast crisis in patients with chronic myelogenous leukaemia. Cancer Res 59: 3931–3934. [PubMed] [Google Scholar]
- 30. Mancarelli MM, Zazzeroni F, Ciccocioppo L, Capece D, Po A, et al. (2010) The tumor suppressor gene KCTD11REN is regulated by Sp1 and methylation and its expression is reduced in tumors. Mol Cancer 9: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zazzeroni F, Papa S, Algeciras-Schimnich A, Alvarez K, Melis T, et al. (2003) Gadd45 beta mediates the protective effects of CD40 costimulation against Fas-induced apoptosis. Blood 102: 3270–3279. [DOI] [PubMed] [Google Scholar]
- 32. Klug CA, Morrison SJ, Masek M, Hahm K, Samle SR, et al. (1999) Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc Natl Acad Sci USA 95: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Payne KJ, Nicolas JH, Zhu JY, Barsky LW, Crooks GM (2001) Cutting edge: predominant expression of a novel Ikaros isoform in normal human hemopoiesis. J Immunol 167: 1867–1870. [DOI] [PubMed] [Google Scholar]
- 34. Payne KJ, Huang G, Sahakian E, Zhu JY, Barteneva NS, et al. (2003) Ikaros isoform x is selective expressed I myeloid differentiation. J Immunol 170: 3091–3098. [DOI] [PubMed] [Google Scholar]
- 35. Beverly LJ, Capobianco AJ (2003) Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in Notch(IC)-induced T cell leukemogenesis. Cancer Cell 3: 551–564. [DOI] [PubMed] [Google Scholar]
- 36. Dovat S (2011) Regulator of Myeloid differentiation and function: The secret life of Ikaros. World J Biol Chem 2: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, et al. (1999) Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity 10: 333–343. [DOI] [PubMed] [Google Scholar]
- 38. Trageser D, Iacobucci I, Nahar R, Duy C, von Levetzow G, et al. (2006) Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J Exp Med 206: 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruiz A, Williams O, Brady HJ (2008) The Ikaros splice isoform, Ikaros 6, immortalizes murine haematopoietic progenitor cells. Int J Cancer 123: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 40. Tonnelle C, Bardin F, Maroc C, Imbert AM, Campa F, et al. (2001) Forced expression of the Ikaros 6 isoform in human placental blood CD34(+) cells impairs their ability to differentiate toward the B-lymphoid lineage. Blood 98: 2673–2680. [DOI] [PubMed] [Google Scholar]
- 41. Ezzat S, Zhu X, Loeper S, Fischer S, Asa SL (2006) Tumor-derived Ikaros 6 acetylates the Bcl-XL promoter to up-regulate a survival signal in pituitary cells. Mol Endocrinol 20: 2976–2986. [DOI] [PubMed] [Google Scholar]
- 42. Kano G, Morimoto A, Takanashi M, Hibi S, Sugimoto T, et al. (2008) Ikaros dominant negative isoform (Ik6) induces IL-3-independent survival of murine pro-B lymphocytes by activating JAK-STAT and up-regulating Bcl-xl levels. Leuk Lymphoma 49: 5965–5973. [DOI] [PubMed] [Google Scholar]
- 43. Sezaki N, Ishimaru F, Takata M, Tabayashi T, Nakase K, et al. (2003) Over-expression of the dominant-negative isoform of Ikaros confers resistance to dexamethasone-induced and anti-IgM-induced apoptosis. Br J Haematol 121: 165–169. [DOI] [PubMed] [Google Scholar]
- 44. Crescenzi B, La Starza R, Romoli S, Beacci D, Matteucci C, et al. (2004) Submicroscopic deletions in 5q-associated malignancies. Haematologica 89: 281–285. [PubMed] [Google Scholar]
- 45. Chiorazzi N, Rai KR, Ferrarini M (2005) Chronic lymphocytic leukemia. N Engl J Med 352: 804–815. [DOI] [PubMed] [Google Scholar]
- 46. Billot K, Soeur J, Chereau F, Arrouss I, Merle-Béral H, et al. (2011) Deregulation of Aiolos expression in chronic lymphocytic leukemia is associated with epigenetic modifications. Blood 117: 1917–1927. [DOI] [PubMed] [Google Scholar]
- 47. Ruiz A, Jiang J, Kempski H, Brady HJ (2004) Overexpression of the Ikaros 6 isoform is restricted to t(4;11) acute lymphoblastic leukaemia in children and infants and has a role in B-cell survival. Br J Haematol 125: 31–37. [DOI] [PubMed] [Google Scholar]
- 48. Erlanson M, Landberg G (2001) Prognostic implications of p27 and cyclin E protein contents in malignant lymphomas. Leuk Lymphoma 40: 461–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nucleotide sequence alignment of the full-length Ik1 and Ik11 . Nucleotide sequence alignment of the full-length Ik1 and the novel isoform Ik11. The start of each exon are represented by grey letters.
(TIF)
Ik2 subcellular localization changes in presence of Ik11. (A) Cos7 cells were transfected with peGFP vector and transfection efficiency was evaluated by FACS analysis. (B) Confocal triple immunofluorescence images of Hoechst 33258 plus Ik2-myc and Ik11. Cos7 cells were co-transfected with pcDNA/Myc-HysB-Ik2 and pcDNA3.1-Ik11. Staining for Ik11 (green fluorescence), Ik2 (red fluorescence) and Hoechst 33258 (blue fluorescence) were performed as described in Figure 4A. Scale bars were equals to 50 microns (panels a–d, x40 objective) and 10 microns (panels e–h, x6.5 zoom of the white box field indicated in panels a–d).
(TIF)
IK11 expression in hPBLs from healthy donors. Real-time PCR analysis of Ik11 mRNAs in 10 different samples of hPBLs obtained from healthy donors. The Ik11 levels are expressed as fold change relative to expression in PBLs #1 and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates.
(TIF)
IK11 expression in myeloid and lymphoid cell lines. Real-time PCR analysis of Ik11 and Ik6 mRNAs in myeloid and lymphoid cell lines (see Supporting Text S1). The Ik11 levels are expressed as fold change relative to expression in PBLs obtained from the healthy donor #1 (see Figure S3) and normalized to the expression of GAPDH. Each bar represents the average ± SD of three replicates.
(TIF)
Ik11 PCR-amplified products were confirmed by sequencing. Sequencing of Ik11 semi-quantitative PCR products shown in Figure 6D. The electropherograms show the sequences corresponding to the junction fragments exon 3/exon 5 and half exon 5/half exon 8.
(TIF)
Clinical data in patients with Chronic Myeloid Leukemia.
(TIF)
Clinical data in patients with Acute Lymphoblastic Leukemia.
(TIF)
Clinical data in patients with Myelodysplastic Syndromes.
(TIF)
Supplementary Material and Methods.
(DOCX)