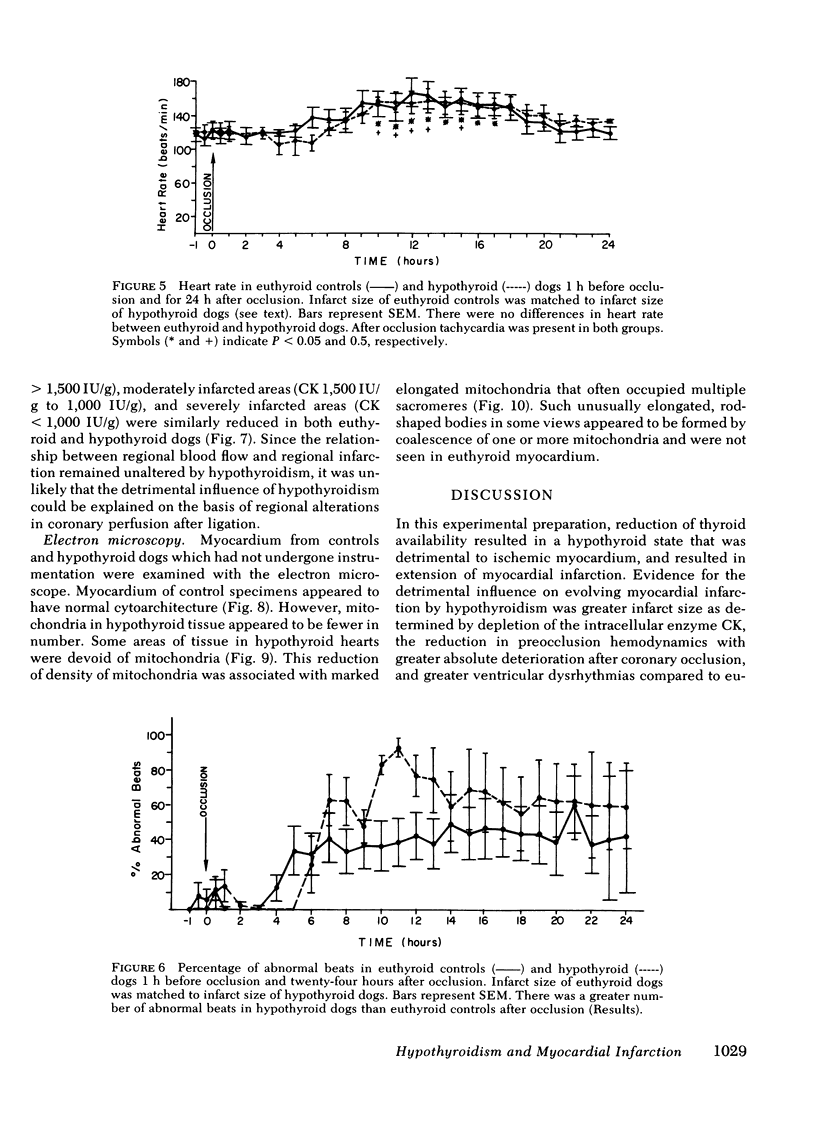
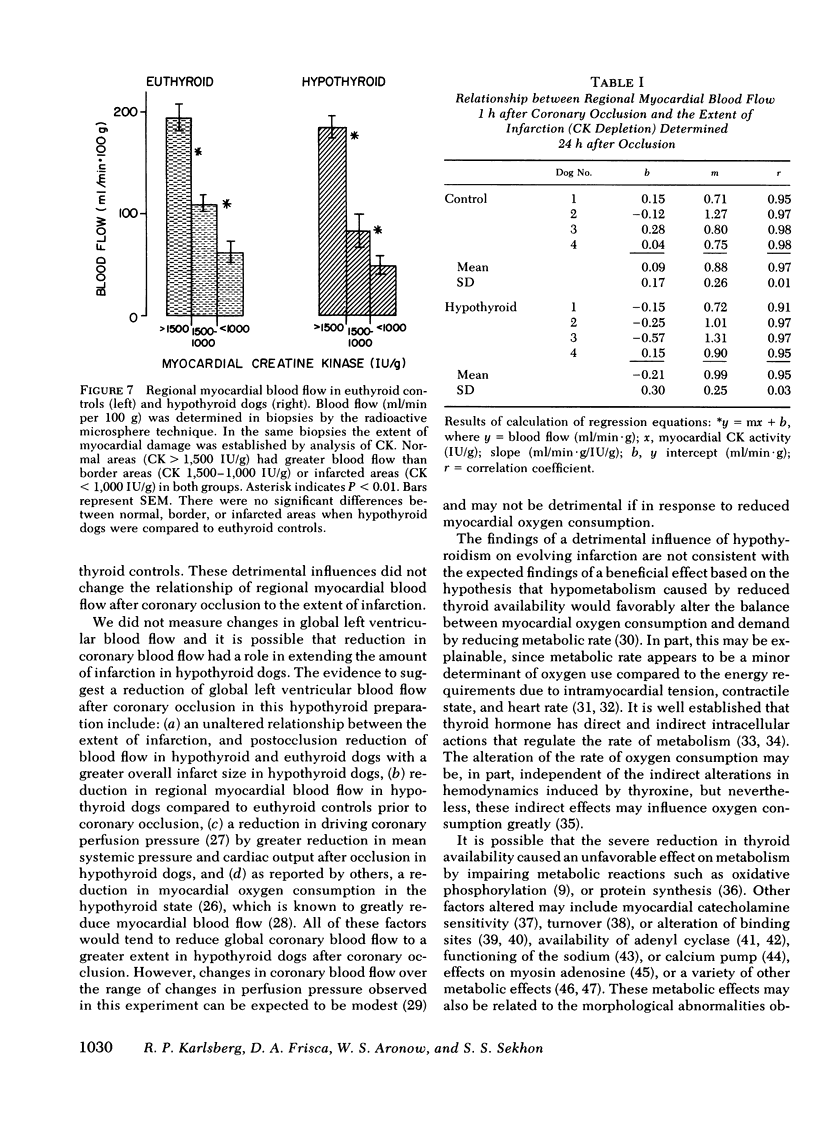
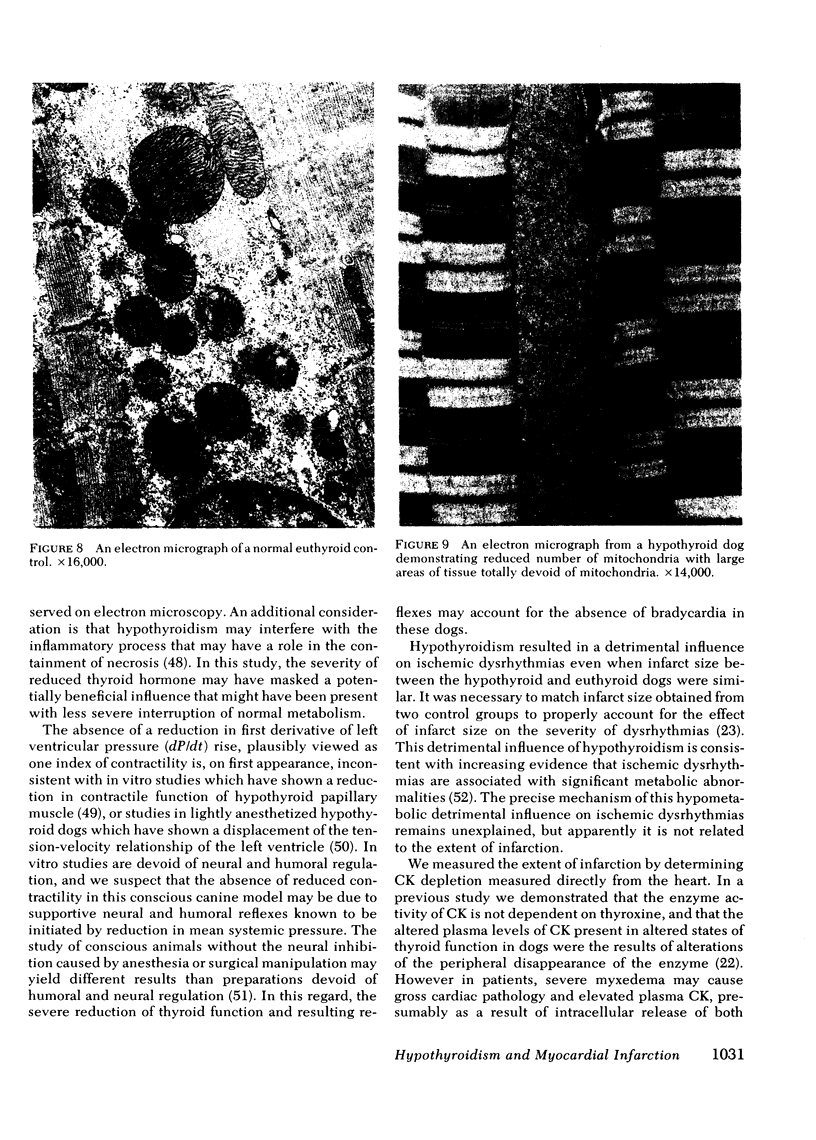
Abstract
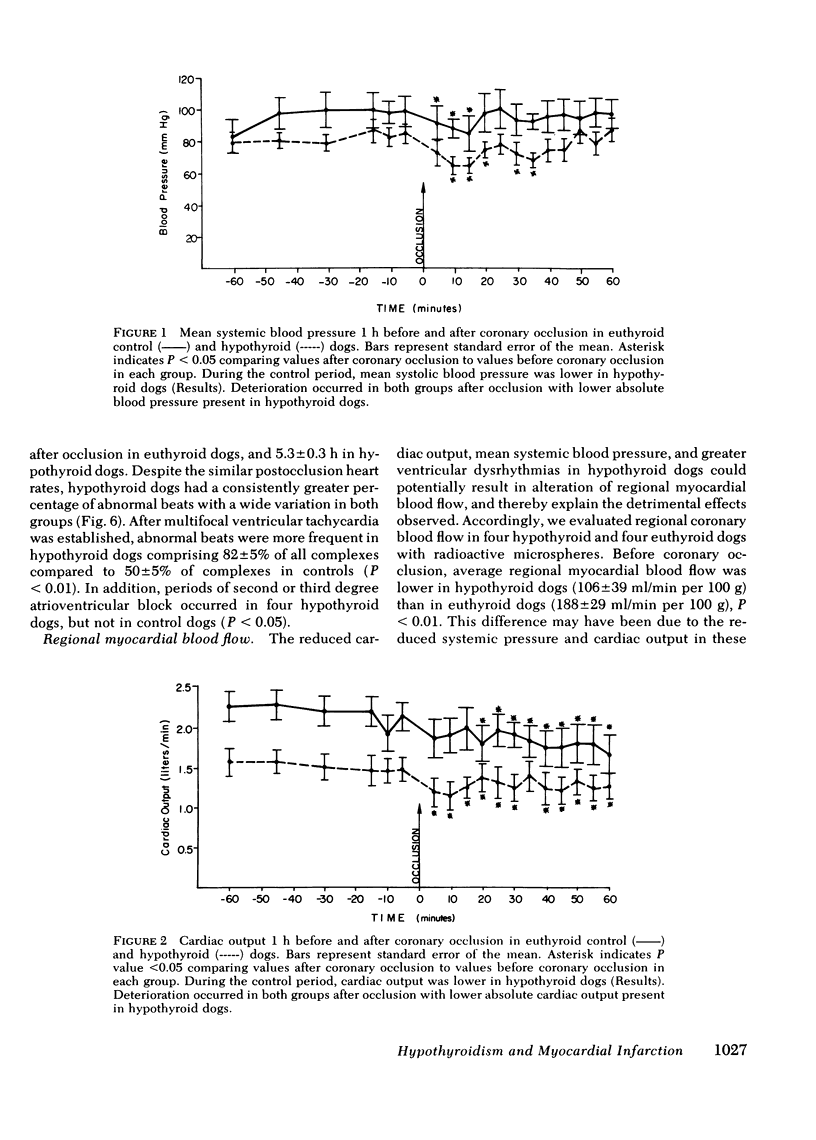
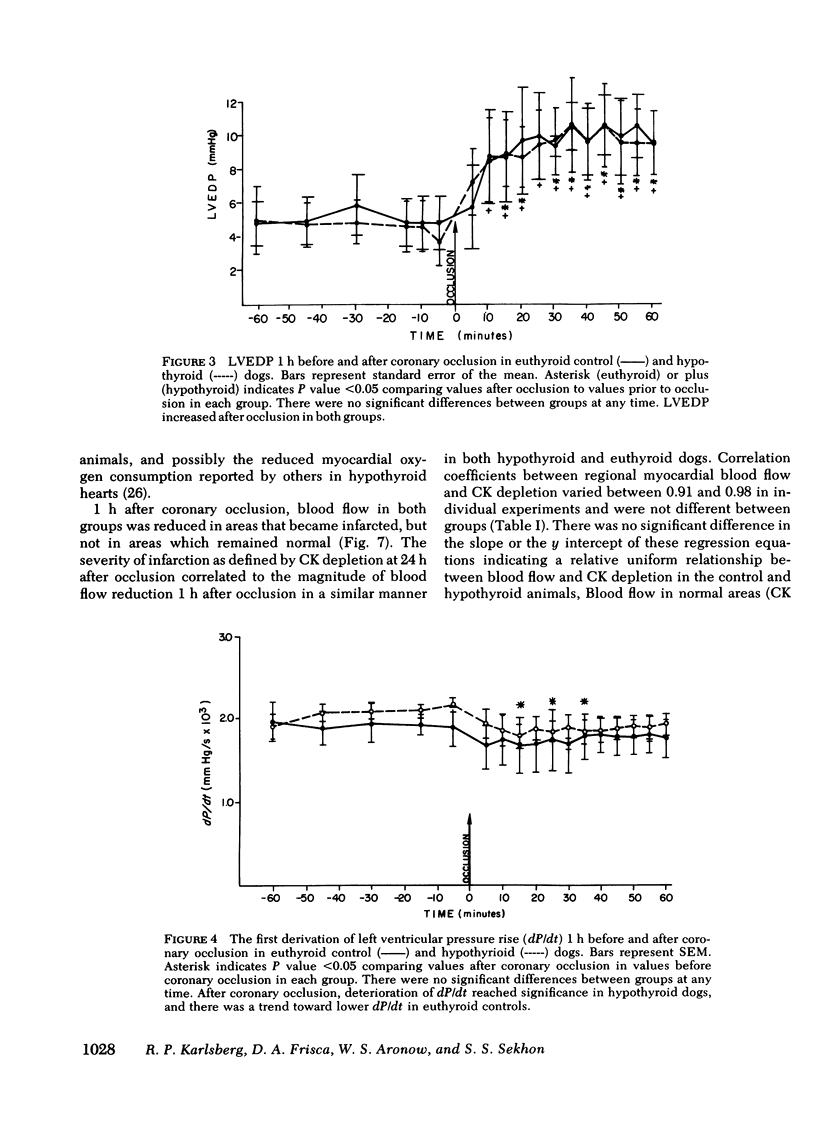
To study the influence of hypometabolism on evolving myocardial infarction in a model with intact autoregulation, we investigated 53 awake dogs after coronary artery occlusion. Severe hypothyroidism was induced by the intravenous administration of 131I. Animals were instrumented to obtain hemodynamic measurements, and regional myocardial blood flow was measured with radioactive microspheres. Infarct size was determined by the creatine kinase depletion method, and dysrhythmia analysis was performed from 24-h Holter monitor tapes in animals matched for infarct size. The microarchitecture of hypothyroid myocardium was determined by the electron microscope. Before coronary occlusion, mean systemic pressure in hypothyroid dogs was reduced by 14% and cardiac output reduced by 32%, with no change in left ventricular end-diastolic pressure, first derivative of left ventricular pressure rise, (dP/dt), or heart rate. After coronary occlusion, there was deterioration in hemodynamic measurements in both groups, with lower absolute levels of mean systemic blood pressure and cardiac output obtained in hypothyroid dogs. Hypothyroidism was detrimental to evolving infarction with a 36% increase in infarct size present in hypothyroid dogs (30 +/- 2%) compared to euthyroid controls (22 +/- 3%), P less than 0.05. Dysrhythmias were more severe in hypothyroid dogs. There were no changes in the relationship between regional myocardial blood flow and the extent of infarction after coronary occlusion. Abnormalities in microarchitecture were present in hypothyroid dog myocardium. Severe hypometabolism in this model was associated with alterations in hemodynamics, more severe dysrhythmias and changes in microarchitecture. The combined effect of these alterations resulted in an overall detrimental influence of hypothyroidism on evolving myocardial necrosis in this model.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braunwald E. Control of myocardial oxygen consumption: physiologic and clinical considerations. Am J Cardiol. 1971 Apr;27(4):416–432. doi: 10.1016/0002-9149(71)90439-5. [DOI] [PubMed] [Google Scholar]
- Broekhuysen J., Ghislain M. Increased heart adenylcyclase activity in the hypothyroid rat. Biochem Pharmacol. 1972 May 15;21(10):1493–1500. doi: 10.1016/0006-2952(72)90374-7. [DOI] [PubMed] [Google Scholar]
- Buccino R. A., Spann J. F., Jr, Pool P. E., Sonnenblick E. H., Braunwald E. Influence of the thyroid state on the intrinsic contractile properties and energy stores of the myocardium. J Clin Invest. 1967 Oct;46(10):1669–1682. doi: 10.1172/JCI105658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD J. B. Fine structure of normal and diseased heart. Prog Cardiovasc Dis. 1963 May;5:610–630. doi: 10.1016/s0033-0620(63)80019-5. [DOI] [PubMed] [Google Scholar]
- CORDAY E., JAFFE H. L., IRVING D. W. Hypometabolic treatment of heart disease. Am J Cardiol. 1960 Nov;6:952–960. doi: 10.1016/0002-9149(60)90298-8. [DOI] [PubMed] [Google Scholar]
- CROSS C. E., RIEBEN P. A., SALISBURY P. F. Coronary driving pressure and vasomotor tonus as determinants of coronary blood flow. Circ Res. 1961 May;9:589–600. doi: 10.1161/01.res.9.3.589. [DOI] [PubMed] [Google Scholar]
- Domenech R. J., Hoffman J. I., Noble M. I., Saunders K. B., Henson J. R., Subijanto S. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res. 1969 Nov;25(5):581–596. doi: 10.1161/01.res.25.5.581. [DOI] [PubMed] [Google Scholar]
- Doran G. R., Wilkinson J. H. The origin of the elevated activities of creatine kinase and other enzymes in the sera of patients with myxoedema. Clin Chim Acta. 1975 Jul 23;62(2):203–211. doi: 10.1016/0009-8981(75)90229-6. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J., Massumi R. A., Shugoll G. I., Ali N., Roberts W. C. Ultrastructural studies of myocardial biopsies in 45 patients with obstructive or congestive cardiomyopathy. Recent Adv Stud Cardiac Struct Metab. 1973;2:231–272. [PubMed] [Google Scholar]
- GOH K., DALLAM R. D. Oxygen consumption of the auricles, right and left ventricles of the normal, hypothyroid rat heart. Am J Physiol. 1957 Mar;188(3):514–518. doi: 10.1152/ajplegacy.1957.188.3.514. [DOI] [PubMed] [Google Scholar]
- Graham T. P., Jr, Covell J. W., Sonnenblick E. H., Ross J., Jr, Braunwald E. Control of myocardial oxygen consumption: relative influence of contractile state and tension development. J Clin Invest. 1968 Feb;47(2):375–385. doi: 10.1172/JCI105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallfelz F. A., Erali R. P. Thyroid function tests in domesticated animals: free thyroxine index. Am J Vet Res. 1973 Nov;34(11):1449–1451. [PubMed] [Google Scholar]
- Karlsberg R. P., Aronow W. S. Reduction of myocardial infarct size: approach for the 1980s. Arch Intern Med. 1980 May;140(5):616–619. [PubMed] [Google Scholar]
- Karlsberg R. P., Henry P. D., Ahmed S. A., Sobel B. E., Roberts R. Lack of protection of ischemic myocardium by verapamil in conscious dogs. Eur J Pharmacol. 1977 Apr 21;42(4):339–346. doi: 10.1016/0014-2999(77)90167-4. [DOI] [PubMed] [Google Scholar]
- Karlsberg R. P., Penkoske P. A., Cryer P. E., Corr P. B., Roberts R. Rapid activation of the sympathetic nervous system following coronary artery occlusion: relationship to infarct size, site, and haemodynamic impact. Cardiovasc Res. 1979 Sep;13(9):523–531. doi: 10.1093/cvr/13.9.523. [DOI] [PubMed] [Google Scholar]
- Karlsberg R. P., Roberts R. Effect of altered thyroid function on plasma creatine kinase clearance in the dog. Am J Physiol. 1978 Dec;235(6):E614–E618. doi: 10.1152/ajpendo.1978.235.6.E614. [DOI] [PubMed] [Google Scholar]
- Kjekshus J. K., Sobel B. E. Depressed myocardial creatine phosphokinase activity following experimental myocardial infarction in rabbit. Circ Res. 1970 Sep;27(3):403–414. doi: 10.1161/01.res.27.3.403. [DOI] [PubMed] [Google Scholar]
- Kranz D., Hecht A., Fuhrmann I. The influence of hyperthyroidism and hypothyroidism on the wound healing of experimental myocardial infarction in the rat. Exp Pathol (Jena) 1976;12(3-4):129–136. doi: 10.1016/s0014-4908(76)80035-x. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Skelton C. L., Epstein S. E. Decreased myocardial adenyl cyclase activity in hypothyroidism. J Clin Invest. 1969 Dec;48(12):2244–2250. doi: 10.1172/JCI106190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroko P. R., Kjekshus J. K., Sobel B. E., Watanabe T., Covell J. W., Ross J., Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971 Jan;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Ginks W. R., Bloor C. M., Shell W. E., Sobel B. E., Ross J., Jr Coronary artery reperfusion. I. Early effects on local myocardial function and the extent of myocardial necrosis. J Clin Invest. 1972 Oct;51(10):2710–2716. doi: 10.1172/JCI107090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkin E. Stimulation of cardiac myosin adenosine triphosphatase in thyrotoxicosis. Circ Res. 1979 Jan;44(1):1–7. doi: 10.1161/01.res.44.1.1. [DOI] [PubMed] [Google Scholar]
- Nagamine M., Furukawa I. Increased activities of serum enzymes predominating in heart muscle in patients with hypothyroidism. Report of two cases. Jpn Circ J. 1972 Jan;36(1):47–53. doi: 10.1253/jcj.36.47. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Bruyneel K., Owen P. Effects of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation. 1975 Jul;52(1):49–57. doi: 10.1161/01.cir.52.1.49. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Metabolism of free fatty acids, glucose and catecholamines in acute myocardial infarction. Relation to myocardial ischemia and infarct size. Am J Cardiol. 1975 Dec;36(7):938–953. doi: 10.1016/0002-9149(75)90086-7. [DOI] [PubMed] [Google Scholar]
- Page E., McCallister L. P. Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol. 1973 Feb;31(2):172–181. doi: 10.1016/0002-9149(73)91030-8. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Edelman I. S. Thyroid hormone control of Na+-K+-ATPase and K+-dependent phosphatase in rat heart. Am J Physiol. 1977 May;232(5):C196–C201. doi: 10.1152/ajpcell.1977.232.5.C196. [DOI] [PubMed] [Google Scholar]
- Rasmussen M. M., Reimer K. A., Kloner R. A., Jennings R. B. Infarct size reduction by propranolol before and after coronary ligation in dogs. Circulation. 1977 Nov;56(5):794–798. doi: 10.1161/01.cir.56.5.794. [DOI] [PubMed] [Google Scholar]
- Roberts R., Husain A., Ambos H. D., Oliver G. C., Cox J. R., Jr, Sobel B. E. Relation between infarct size and ventricular arrhythmia. Br Heart J. 1975 Nov;37(11):1169–1175. doi: 10.1136/hrt.37.11.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph A. M., Heymann M. A. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967 Aug;21(2):163–184. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- SCOTT J. B., HARDIN R. A., HADDY F. J. Pressure-flow relationships in the coronary vascular bed of the dog. Am J Physiol. 1960 Nov;199:765–769. doi: 10.1152/ajplegacy.1960.199.5.765. [DOI] [PubMed] [Google Scholar]
- Sanford C. F., Griffin E. E., Wildenthal K. Synthesis and degradation of myocardial protein during the development and regression of thyroxine-induced cardiac hypertrophy in rats. Circ Res. 1978 Nov;43(5):688–694. doi: 10.1161/01.res.43.5.688. [DOI] [PubMed] [Google Scholar]
- Shell W. E., Sobel B. E. Protection of jeopardized ischemic myocardium by reduction of ventricular afterload. N Engl J Med. 1974 Sep 5;291(10):481–486. doi: 10.1056/NEJM197409052911001. [DOI] [PubMed] [Google Scholar]
- Skelton C. L., Karch F. E., Wildenthal K. Lack of acute effects of thyroid hormones on myocardial contractility. Am J Physiol. 1973 Apr;224(4):957–962. doi: 10.1152/ajplegacy.1973.224.4.957. [DOI] [PubMed] [Google Scholar]
- Sobel B. E. Salient biochemical features in ischemic myocardium. Circ Res. 1974 Sep;35 (Suppl 3):173–181. [PubMed] [Google Scholar]
- Sodi-Pallares D., Ponce de León J., Bisteni A., Medrano G. A. Potassium, glucose, and insulin in myocardial infarction. Lancet. 1969 Jun 28;1(7609):1315–1316. doi: 10.1016/s0140-6736(69)92251-x. [DOI] [PubMed] [Google Scholar]
- Sonnenblick E. H., Ross J., Jr, Braunwald E. Oxygen consumption of the heart. Newer concepts of its multifactoral determination. Am J Cardiol. 1968 Sep;22(3):328–336. doi: 10.1016/0002-9149(68)90117-3. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O., Brenner M. A., Lazarus J. H. Thyroid hormone action: the mitochondrial pathway. Science. 1977 Sep 2;197(4307):996–999. doi: 10.1126/science.196334. [DOI] [PubMed] [Google Scholar]
- Sterling K. Thyroid hormone action at the cell level (first of two parts). N Engl J Med. 1979 Jan 18;300(3):117–123. doi: 10.1056/NEJM197901183000304. [DOI] [PubMed] [Google Scholar]
- Suko J. The calcium pump of cardiac sarcoplasmic reticulum. Functional alterations at different levels of thyroid state in rabbits. J Physiol. 1973 Feb;228(3):563–582. doi: 10.1113/jphysiol.1973.sp010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. R., Covell J. W., Ross J., Jr Influence of the thyroid state on left ventricular tension-velocity relations in the intact, sedated dog. J Clin Invest. 1969 Apr;48(4):775–784. doi: 10.1172/JCI106035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco J. L., Flattery K. V., Sellers E. A. Effects of thyroid hormones and cold exposure on turnover of norepinephrine in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1977 Jun;55(3):515–522. doi: 10.1139/y77-073. [DOI] [PubMed] [Google Scholar]
- Vatner S. F., Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med. 1975 Nov 6;293(19):970–976. doi: 10.1056/NEJM197511062931906. [DOI] [PubMed] [Google Scholar]
- WALLACE A. G., SKINNER N. S., Jr, MITCHELL J. H. Hemodynamic determinants of the maximal rate of rise of left ventricular pressure. Am J Physiol. 1963 Jul;205:30–36. doi: 10.1152/ajplegacy.1963.205.1.30. [DOI] [PubMed] [Google Scholar]
- WHITE P. D. The choice of therapy in the management of refractory angina pectoris. Prog Cardiovasc Dis. 1960 Sep;3:97–107. doi: 10.1016/s0033-0620(60)80073-4. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., KRAUSE E. G., MACHO L. THYROID STATE AND THE ACTIVITY OF GLYCOGEN PHOSPHORYLASE IN ISCHAEMIC MYOCARDIUM. Nature. 1964 Feb 22;201:789–791. doi: 10.1038/201789a0. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., SCHULZE W. Mitochondrial alterations in the myocardium of dogs with aortic stenosis. J Biophys Biochem Cytol. 1961 Jun;10:285–288. doi: 10.1083/jcb.10.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. S., Ahmed S. A., Welch M. J., Williamson J. R., Ter-Pogossian M. M., Sobel B. E. Quantification of infarction in cross sections of canine myocardium in vivo with positron emission transaxial tomography and 11C-palmitate. Circulation. 1977 Jan;55(1):66–73. doi: 10.1161/01.cir.55.1.66. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Lefkowitz R. J. Thyroid hormone regulation of alpha-adrenergic receptors: studies in rat myocardium. J Cardiovasc Pharmacol. 1979 Mar-Apr;1(2):181–189. doi: 10.1097/00005344-197903000-00002. [DOI] [PubMed] [Google Scholar]
- Williams T. H., Jew J. Y. An improved method for perfusion fixation of neural tissues for electron microscopy. Tissue Cell. 1975;7(3):407–418. doi: 10.1016/0040-8166(75)90015-4. [DOI] [PubMed] [Google Scholar]
- Wollenberger A., Will-Shahab L. Influence of thyroid state on the specific binding of noradrenaline to a cardiac particulate fraction and on catecholamine-sensitive cardiac adenylate cyclase activity. Recent Adv Stud Cardiac Struct Metab. 1976;9:193–203. [PubMed] [Google Scholar]
- Zaimis E., Papadaki L., Ash A. S., Larbi E., Kakari S., Matthew M., Paradelis A. Cardiovascular effects of thyroxine. Cardiovasc Res. 1969 Apr;3(2):118–133. doi: 10.1093/cvr/3.2.118. [DOI] [PubMed] [Google Scholar]