Abstract
Reverse transcription quantitative polymerase chain reaction (qRT-PCR) has rapidly become the most sensitive and accurate method for the quantification of gene expression. To facilitate gene expression studies and obtain more accurate qRT-PCR data, normalization relative to stable housekeeping genes is required. These housekeeping genes need to show stable expression under the given experimental conditions for the qRT-PCR results to be accurate. Unfortunately, there are no studies on the stability of housekeeping genes used in Spodoptera litura. In this study, eight candidate reference genes, elongation factor 1 alpha (EF1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein L10 (RPL10), ribosomal protein S3 (RPS3), beta actin (ACTB), beta FTZ-F1 (FTZF1), ubiquinol-cytochrome c reductase (UCCR), and arginine kinase (AK), were evaluated for their suitability as normalization genes under different experimental conditions using the statistical software programs, BestKeeper, geNorm and Normfinder, and the comparative ΔCt method. We determined the expression levels of the candidate reference genes for three biotic factors (developmental stage, tissue and population), and four abiotic treatments (temperature, insecticide, food and starvation). The results indicated that the best sets of candidates as reference genes were as follows: GAPDH and UCCR for developmental stages; RPL10, AK and EF1 for different tissues; RPL10 and EF1 for different populations in China; GAPDH and EF1 for temperature-stressed larvae; AK and ACTB for larvae treated with different insecticides; RPL10, GAPDH and UCCR for larvae fed different diets; RPS3 and ACTB for starved larvae. We believe that these results make an important contribution to gene analysis studies in S. litura and form the basis of further research on stable reference genes in S. litura and other organisms.
Introduction
Reverse transcription quantitative polymerase chain reaction (qRT-PCR) has rapidly become the most sensitive, accurate and widely used method for gene expression analysis in order to understand biological processes and physiological functions, as well as for validation of the results of microarray analysis and other techniques [1], [2]. One of the critical challenges of qRT-PCR analysis for reliable mRNA quantification in any biological system is the availability of appropriate normalization genes, the expression level of which is considered stable, regardless of cell type and across various experimental conditions [3]. However, several studies have revealed that using different normalization genes which show variations in a biological system can result in appreciable errors owing to different treatments, sampling methods, total RNA extraction, reverse-transcription, etc., even up to 20-fold by some estimations [4]–[6]. Hence, the use of normalization genes should be experimentally validated for different developmental stages, tissues and specific experimental designs [3], [6], [7]. Furthermore, at least two or three reference genes should be used for accurate normalization based on the studies of Thellin et al. [8] and Vandesompele et al. [6]. In most studies, normalization has been described for certain systems but is frequently applied to other systems without an appropriate validation of their stability in that particular system. Therefore, it is necessary to select the most suitable genes for normalization from a panel of candidate genes in a given set of biological samples from a specific organism.
Spodoptera litura is an important polyphagous insect pest that causes widespread economic damage to vegetables and other crops, including ornamental plants in tropical and subtropical regions [9], [10]. As a polyphagous species, this pest has the potential to invade new areas and to adapt to new host plants. In recent years, molecular technology, particularly qRT-PCR for gene expression, has been widely used in genetic studies on S. litura [11], [12]. Changes in gene expression can often reflect biologically significant changes across insect developmental stages, tissues and other samples from different experimental conditions. Therefore, it is important to establish normalization genes so that specific changes in gene expression can be evaluated. However, no experimental data are available on the most appropriate normalization genes under different conditions and at different developmental stages for the sensitive detection of target gene transcripts in S. litura. In this study, we identified and examined eight normalization genes for S. litura in different developmental stages, tissues and under different treatments, in order to assess which of these genes were the most stable and therefore represented the best choice for qRT-PCR experiments on S. litura under different conditions. The eight selected genes were ribosomal protein S3 (RPS3), ribosomal protein L10 (RPL10), beta-actin (ACTB), ubiquinol-cytochrome c reductase (UCCR), transcription factor beta FTZ-F1 (FTZF1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), elongation factor (EF1) and arginine kinase (AK). These genes are commonly used as single normalization genes in gene expression studies of S. litura and other insects.
Materials and Methods
Insects
The laboratory strain of S. litura was established from field collections in June 2012 obtained from a lotus field at the Agricultural Experiment Station, Huazhong Agricultural University (Wuhan, Hubei, China). The larvae were reared on lotus leaves, and the adults were fed 10% honey solution in the laboratory. They were reared at a temperature of 30°C, under a photoperiod of 16∶8 h L: D, and relative humidity of 70%. Other populations used in this experiment were collected from lotus fields at Jiangsu, Zhejiang, Jiangxi, Anhui and Shandong provinces. The laboratory strain and other populations used in this experiment were from different fields. No specific permissions were required as these fields are experimental plots that belong to Huazhong Agricultural University, Wuhan, Hubei in China.
Biotic Factors
Developmental stage: Samples used comprised 300 first-day eggs, 50 first-instar larvae, 30 second-instar larvae, five third-instar larvae, five fourth-instar larvae, five fifth-instar larvae, five sixth-instar larvae, five pre-pupae, five first-day male and female pupae, five six-day male and female pupae, five 12-day-old male and female pupae, five first-day male and female adults, and five 7-day-old male and female adults for each replication. All the samples were collected in 1.5 mL microcentrifuge tubes, which were immediately frozen in liquid nitrogen and stored at −80°C.
Tissue: Tissue from the brain, midgut, fat body, epidermis and hemolymph were obtained from third-instar larvae using dissection needle in PBS solution on ice [13].
Population: One laboratory S. litura strain and five field collected populations from Jiangsu, Zhejiang, Jiangxi, Anhui and Shandong provinces were used. The laboratory strain was maintained without exposure to any insecticide within the laboratory setting.
Abiotic Stresses
Temperature-induced stress
Each group of five third-instar larvae were exposed to temperatures of 15°C (cold), 25°C (room temperature) or 35°C (hot) for 1 h in a glass tube placed in a water bath. Five insects in each temperature were then collected for RNA extraction.
Insecticide-induced stress
The insecticides used were chlorpyrifos, diafenthiuron, spinosad, indoxacarb and chlorantraniliprole, which are often used in Lepidopteran pest management programs. The leaf-dip bioassay method reported by Shelton et al. [14] and Liang et al. [15] was adopted for insecticide bioassay. Cabbage discs (6.5 cm diameter) were cut and dipped in various concentrations of insecticides prepared with distilled water containing 0.1% Triton X-100. Each disc was dipped for 10 s and allowed to air dry at room temperature. The discs were then placed individually inside plastic petri dishes (7.0 cm diameter). A total of 10–15 third-instar larvae were confined to each dish, and three replications were prepared. Controls were cabbage discs treated with distilled water containing 0.1% Triton X-100. The treated larvae were reared routinely and mortality was checked after 48 h. The 48-h LC15 (sublethal dose) values for the insecticides were estimated by probit analysis (Table 1). Third-instar larvae were then treated using the LC15 value of each insecticide. The surviving insects after 48 h were collected for RNA extraction.
Table 1. The toxicity of insecticides to the third-instar larvae of S. litura.
| Insecticides | Na | Slope ± SEb | LC15 c | LC50 c | ?2 d |
| Chlorpyrifos | 240 | 1.71±0.20 | 8.08 (4.91–11.46) | 32.69 (24.83–43.45) | 1.38 |
| Diafenthiuron | 210 | 1.90±0.25 | 7.69 (5.07–10.35) | 27.07 (20.74–37.56) | 1.06 |
| Spinosad | 210 | 1.85±0.24 | 10.64 (6.05–15.41) | 38.68 (29.18–50.61) | 2.26 |
| Indoxacarb | 210 | 2.21±0.27 | 10.28 (6.40–14.18) | 30.26 (23.46–38.27) | 2.35 |
| Chlorantraniliprole | 210 | 1.63±0.23 | 0.36 (0.18–0.56) | 1.58 (1.15–2.13) | 0.54 |
Number of tested larvae.
SE = standard error.
Expressed in mg/L; 95% fiducial limits (FL) of LC15, LC50 are given in parenthesis, respectively.
Chi-square testing linearity of dose-mortality responses.
Food
The newly hatched larvae were reared on an artificial diet [16], lotus leaves, taro leaves or water oats, until they reached third-instar stage. Then, the third-instar larvae were collected for RNA extraction.
Starvation-induced stress
Thirty third-instar larvae were starved for 6 h, and were collected for RNA extraction.
Reference Gene Selection and Primer Design
Eight commonly used reference genes were selected (Table 2). Based on the described insect reference genes in literature, the NCBI database (http://www.ncbi.nlm.nih.gov) was searched for available S. litura sequences: ACTB [17], UCCR, FTZF1, GAPDH [6], EF1 [2], [3] and AK [18]. We explored whether UCCR and FTZF1 could be used as reference genes. However, RPS3 [1], [19] and RPL10 (unpublished data from our laboratory) sequences were amplified based on the sequences from Spodoptera frugiperda and Spodoptera exigua (SfRPS3, accession no. AF429976; SeRPL10, accession no. EU258622). We have submitted RPS3 (accession No., KC866374) and RPL10 (accession No., KC866373) gene sequences from S. litura to GenBank. However, 28S rRNA was omitted in our study although it has been used in several qRT-PCR studies, because many literatures [3] suggested that 28S gene may not be an ideal gene for qRT-PCR due to its high expression level. All gene-specific primers were designed using Beacon Designer 8.0 software (Premier Biosoft International, Palo Alto, CA, USA; Table 2).
Table 2. Primer pairs used for quantitative real-time PCR.
| Gene name (Abbreviation) | Accession No. | Primer Namea | Sequence (5′-3′) | Product length (bp) | Tm (°C) | Primer efficiency (%) | R2 b |
| Elongation factor-1 | DQ192234 | SlN-F1 | CTCCTACATCAAGAAGATC | 295 | 55 | 96.7 | 0.997 |
| (EF1) | SlN-R1 | CTTGAGGATACCAGTTTC | 55 | ||||
| Ribosomal protein L10 | KC866373 | SlN-F2 | GACTTGGGTAAGAAGAAG | 189 | 55 | 109.7 | 0.998 |
| (RPL10) | SlN-R2 | GATGACATGGAATGGATG | 55 | ||||
| Actin | DQ494753 | SlN-F3 | GATCATGTTTGAGACCTT | 214 | 55 | 107.3 | 0.998 |
| (ACTB) | SlN-R3 | GATCTTCATGAGGTAGTC | 55 | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | HQ012003 | SlN-F4 | GGGTATTCTTGACTACAC | 184 | 55 | 109.6 | 0.996 |
| (GAPDH) | SlN-R4 | CTGGATGTACTTGATGAG | 55 | ||||
| Beta FTZ-F1 | HQ260326 | SlN-F5 | CTGATGAGACTACACTTC | 297 | 55 | 107.9 | 0.998 |
| (FTZF1) | SlN-R5 | CAGGAACTACCATTACTAG | 55 | ||||
| Ubiquinol-cytochrome c reductase | HQ599193 | SlN-F6 | GCCAAGATTGAGATCAAG | 204 | 55 | 109.7 | 0.998 |
| (UCCR) | SlN-R6 | GCATACTCCGATAACTAC | 55 | ||||
| Ribosomal protein S3 | KC866374 | SlN-F7 | CGGAGATCATCATTATGG | 191 | 55 | 105.6 | 0.997 |
| (RPS3) | SlN-R7 | GAGTTTGTATCTGAGAGAC | 55 | ||||
| Arginine kinase | HQ840714 | SlAK-F | CTGAAGAAGTACCTTACC | 80 | 55 | 105.2 | 0.989 |
| (AK) | SlAK-R | CAATCCAGCAGAGTTGAG | 55 |
F and R refer to forward and reverse primers, respectively;
R 2 refers to the coefficient of determination.
Total RNA Isolation and cDNA Synthesis
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the recommended procedures. The purity of all RNA samples was assessed at absorbance ratios of A260/A280 and A260/A230 with a UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan), and the integrity of the RNA was immediately checked using 1.0% agarose gel electrophoresis. Then, the RNA was treated with DNaseI (Fermentas, Glen Burnie, MD, USA) according to the manufacturer’s instructions, and the first-strand cDNA template was synthesized from 1.0 µg of total RNA using the First-Strand cDNA Synthesis Kit (Fermentas) with oligo (dT)18 as the primer, and stored at −20°C until use the next day. Our experimental processes were consistent for all the treatments.
Quantitative Real-time PCR
Reverse transcription quantitative PCR (qRT-PCR) was performed using SsoFast™ EvaGreen® Supermix (Bio-Rad, Hercules, CA, USA) on a Bio-Rad iQ2 Optical System (Bio-Rad) based on the method of Giulietti et al. [20]. The amplification conditions were as follows: 95°C for 30 s followed by 40 cycles of 95°C for 5 s and 55°C for 10 s. After the reaction, a melting curve analysis from 65°C to 95°C was applied to all reactions to ensure consistency and specificity of the amplified product. A 10-fold dilution series of cDNA from the whole body of five third-instar larvae was used to create the standard curve, and the qRT-PCR efficiency was determined for each gene and each treatment using the linear regression model [21]. The corresponding qRT-PCR efficiencies (E) were calculated according to the equation: E = (10[−1/slope]−1) × 100 [22].
Statistical Analysis
Expression levels were determined as the number of cycles needed for the amplification to reach a fixed threshold in the exponential phase of the PCR reaction [23]. The threshold was set at 500 for all genes to determine the Ct values. Gene stabilities of the eight candidate reference genes were evaluated using the software tools BestKeeper [21], geNorm version 3.5 (http://medgen.ugent.be/~jvdesomp/genorm/) [6] and NormFinder version 0.953 (http://www.mdl.dk/publications normfinder.htm) [24]. BestKeeper uses raw data and PCR amplification efficiency to determine the best-suited standards and combines them to create an index. Ct values were converted into relative quantities and imported into the geNorm and NormFinder software programs. The geNorm algorithm first calculates an expression stability value (M) for each gene and then compares the pair-wise variation (V) of this gene with the others. Using microarray data as a training set for the algorithm, a threshold of V <0.15 was suggested for valid normalization [6]. NormFinder also ranks the stability of the tested genes independently from each other. We also used a user-friendly web-based comprehensive tool, RefFinder (http://www.leonxie.com/referencegene.php?type=reference), including the comparative ΔCt method [21], to compare and rank the tested candidate reference genes. Based on the rankings from each program, RefFinder assigns an appropriate weight to an individual gene and calculates the geometric mean of their weights for the overall final ranking. The lower ranking indicated genes with more stable gene expression.
Results
Total RNA Quality and PCR Amplification Efficiencies
The concentration and purity of total RNA isolated from different samples were determined using the UV-1800 spectrophotometer. The A260/A280 ratios ranged from 1.80 to 2.20 for most RNA samples, indicating a high purity of total RNA for all samples. The integrity of all total RNA samples was confirmed using 1.0% agarose gel electrophoresis.
For each of the primer pairs, the single peak qPCR melting curves suggested that each of the primer pairs amplified a unique product. The products were sequenced and showed 100% identity with the fragment sequences on which the primer design was based (Fig. S1). The linear regression coefficients (R2) of each standard curve for PCR efficiency, which was determined for each gene using 10-fold serial dilutions of the cDNA generated from third-instar larvae of the laboratory strain, were 0.989 (AK), 0.996 (GAPDH), 0.997 (EF1 and RPS3) and 0.998 (RPL10, ACTB, FTZF1 and UCCR) (Table 2). The PCR efficiency of the eight candidate reference genes was excellent, ranging from the lowest for EF1 (96.7%) to the highest for RPL10 and UCCR (109.7%; Table 2).
Expression Profiles of Candidate Reference Genes
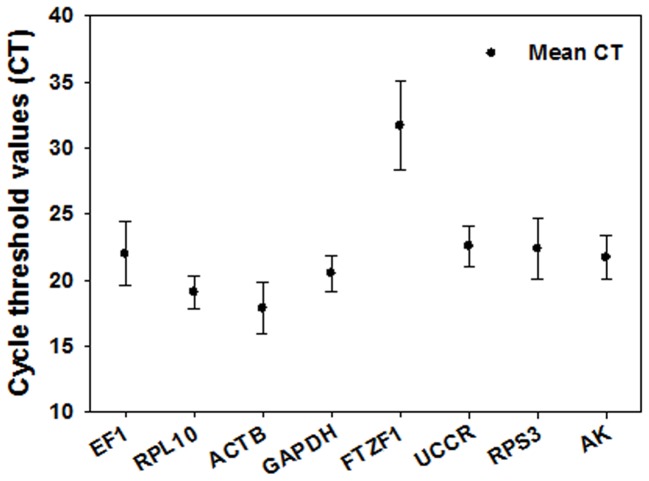
Expression levels were determined as the number of cycles needed for the amplification to reach a fixed threshold (500) in the exponential phase of the PCR reaction [23]. The gene expression analysis of seven candidate reference genes, with the exception of FTZF1, displayed a narrow range of mean Ct values across all experimental samples (Fig. 1). The raw Ct values ranged from 14.58 (ACTB) to 38.59 (FTZF1) in all samples, from 15.92 (ACTB) to 33.89 (FTZF1) in the developmental stages, from 14.58 (ACTB) to 33.59 (FTZF1) in the different tissues, from 16.08 (ACTB) to 33.14 (FTZF1) in the different populations, from 15.58 (ACTB) to 35.92 (FTZF1) in the different temperature treatments, from 14.92 (ACTB) to 38.59 (FTZF1) in the insecticide treatments, 14.95 (ACTB) to 32.43 (FTZF1) in the third-instar larvae reared on different diets, and from 17.48 (ACTB) to 35.92 (FTZF1) in the third-instar larvae starved for 6 h. The fluorescence peak after about 15 cycles showed that ACTB was the most abundantly transcribed, whereas FTZF1 was the least abundant transcript with a Ct value of 35 or higher. All candidate genes except FTZF1 exhibited relatively small variations in Ct values.
Figure 1. Expression levels of candidate reference genes in different samples of S. litura.
Expression levels are displayed as cycle threshold (Ct) values of the candidate S. litura reference genes used in this study. The black dot indicates the mean of duplicate samples (n = 270), and the bars indicate the standard deviation of the mean.
Analysis of Gene Expression Stability
Biotic Factors
Developmental stage
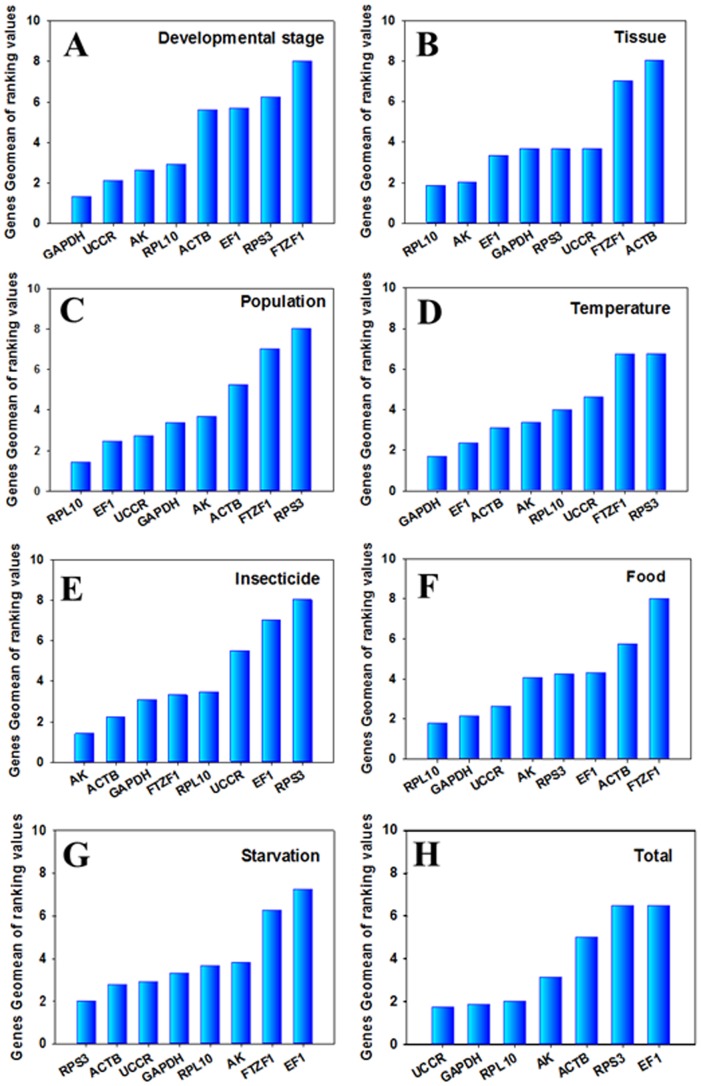
The stability rankings generated by the Delta Ct method were the same as those generated by NormFinder. Additionally, the stability rankings generated by geNorm were largely similar with the results obtained from ΔCt and Normfinder methods, even though the ranking order of the genes was different to some extent. However, the gene stability rankings by BestKeeper analysis were different to the results generated by the other three methods. All four programs, except for BestKeeper, identified GAPDH and UCCR as the most stable genes (Fig. S2). According to the results of RefFinder, the stability rankings across the developmental stages were in decreasing order of GAPDH, UCCR, AK, RPL10, ACTB, EF1, RPS3, and FTZF1 (Fig. 2A). For geNorm, the V value of 0.160 obtained by the RPS23-ACTB pair was close to the proposed 0.15 cut-off. Moreover, the inclusion of additional reference genes did not lower the V value below the proposed 0.15 cut-off until the eighth gene was added (Fig. 3).
Figure 2. Expression stability of the candidate reference genes as calculated by the Geomean method of RefFinder (http://www.leonxie.com/referencegene.php?type=reference).
A lower Geomean ranking indicates more stable expression. Expression stability of reference genes in the following samples: A) different developmental stages of S. litura; B) different S. litura tissues; C) different populations of S. litura; D) S. litura exposed to different temperatures; E) S. litura treated with different insecticides; F) S. litura fed with different diets; G) starved S. litura; and H) S. litura under all conditions.
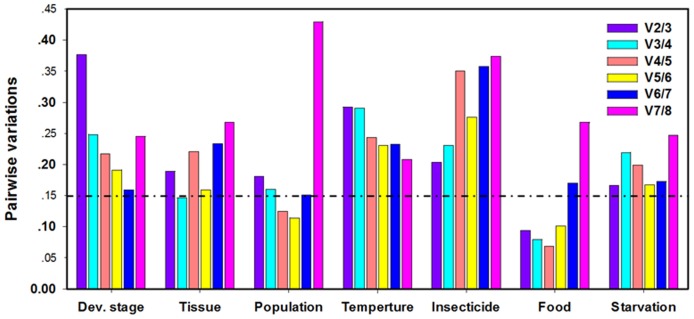
Figure 3. Determination of the optimal number of reference genes as calculated by geNorm for accurate normalization of gene expression.
Average pairwise variations (V) were calculated by geNorm between the normalization factors NFn and NFn+1 to indicate whether inclusion of an extra reference gene would add to the stability of the normalization factor. Values <0.15 indicate that additional genes are not required for the normalization of gene expression.
Tissue
The stability rankings generated by the Delta Ct method and NormFinder identified RPL10 and UCCR as the most stable pair of genes. However, gene stability, as ranked by BestKeeper and geNorm, differed from the results generated by the ΔCt and NormFinder methods (Fig. S3). According to the results of RefFinder, the stability rankings from the most stable to the least stable gene in different tissues were RPL10, AK, EF1, GAPDH, RPS3, UCCR, FTZF1 and ACTB (Fig. 2B). GeNorm analysis revealed that the pair-wise variation value V3/4 was below the proposed 0.15 cut-off (Fig. 3). An increase in variation in this value was related to a decrease in expression stability, because of the inclusion of a relatively unstable fourth gene (GAPDH). The inclusion of a fourth reference gene did not improve the statistical significance for each of the candidate reference gene pair groups.
Population
The stability rankings generated by BestKeeper and geNorm identified RPL10 and EF1 as the most stable pair of genes. However, gene stability as ranked by the Delta Ct method and NormFinder was different to the results generated by the BestKeeper and geNorm methods (Fig. S4). According to the RefFinder results, the stability rankings from the most stable to the least stable gene in the different populations were as follows: RPL10, EF1, UCCR, GAPDH, AK, ACTB, FTZF1, and RPS3 (Fig. 2C). GeNorm analysis revealed that the pair-wise variation value V4/5 was below the proposed 0.15 cut-off (Fig. 3). This result suggests that the inclusion of a fifth reference gene would not provide any additional improvement to the statistical significance for each of the candidate reference gene pair groups.
Abiotic Stresses
Temperature
All four programs, with the exception of geNorm, identified GAPDH as the most stable gene in the third-instar larvae treated at different temperatures. From the results of RefFinder, the stability rankings from the most stable to the least stable gene in the temperature-stressed samples were GAPDH, EF1, ACTB, AK, RPL10, UCCR, FTZF1 and RPS3 (Fig. 2D). However, GeNorm analysis revealed that all the pair-wise variation values were above the proposed 0.15 cut-off (Fig. 3) and identified EF1 as the most stable gene (Fig. S5). These results indicate that normalization with three stable reference genes was required (as suggested by the geNorm manual).
Insecticide
All four programs except for geNorm identified AK as the most stable gene in the third-instar larvae treated with different insecticides (Fig. S6). According to RefFinder, the stability rankings from the most stable to the least stable in the insecticide-stressed samples were AK, ACTB, GAPDH, FTZF1, RPL10, UCCR, EF1 and RPS3 (Fig. 2E). However, geNorm identified RPL10 as the most stable gene (Fig. S6). GeNorm analysis also revealed that all the pair-wise variation values were above the proposed 0.15 cut-off (Fig. 3). These results indicate that normalization with three stable reference genes was required (as suggested by the geNorm manual).
Food
The stability rankings generated by the Delta Ct method and geNorm identified RPL10 and GAPDH as the most stable pair of genes in the third-instar larvae reared on different diets (Fig. S7). Moreover, the results determined by NormFinder also identified RPL10 as the most stable gene. However, the stability ranking generated by BestKeeper and NormFinder identified UCCR as the most stable gene. Based on the RefFinder results, the stability rankings from the most stable to the least stable gene in the third-instar larvae reared on different diets were as follows: RPL10, GAPDH, UCCR, AK, RPS3, EF1, ACTB and FTZF1 (Fig. 2F). GeNorm analysis revealed that the pair-wise variation value V2/3 was below the proposed 0.15 cut-off (Fig. 3). This result suggests that the inclusion of a third reference gene would not improve the statistical significance of each of the candidate reference gene pair groups.
Starvation
The stability rankings generated by the Delta Ct method and NormFinder identified RPS3 and UCCR as the most stable pair of genes in the third-instar larvae starved for 6 h (Fig. S8). However, BestKeeper and geNorm identified RPL10 and ACTB as the most stable genes, respectively. According to RefFinder, the stability rankings from the most stable to the least stable in the third-instar larvae starved for 6 h were RPS3, ACTB, UCCR, GAPDH, RPL10, AK, FTZF1 and EF1 (Fig. 2G). GeNorm analysis revealed that all the pair-wise variation values were above the proposed 0.15 cut-off (Fig. 3). These results indicate that normalization with three stable reference genes was required (as suggested by the geNorm manual).
Total
We identified the ranking of S. litura reference genes across all of the investigated treatments. According to RefFinder, the stability rankings from the most stable to the least stable across the different developmental stages, tissues, populations, and stressors were as follows: UCCR, GAPDH, RPL10, AK, ACTB, RPS3 and EF1 (Fig. 2H).
Discussion
This study was conducted to identify the optimal reference genes for gene expression analyses of S. litura for studies of different developmental stages, tissues and abiotic stress conditions. The behaviors of eight candidate reference genes were analyzed using qRT-PCR studies. To our knowledge, this is the first study to evaluate the expression stability of different candidate reference genes for qRT-PCR in S. litura.
A major conclusion of this study is that few, if any, universally suitable reference genes in S. litura can be used for qRT-PCR analyses under various experimental conditions, as the candidate reference genes showed too much variation in expression among the different treatments (Table 3). RPL10 exhibited the most stable expression in different tissues, populations, and in the third-instar larvae reared on different diets. GAPDH displayed the most stable expression in different developmental stages and in samples treated at different temperatures. AK and RPS3 showed the most stable expression in samples treated with insecticides and starved for 6 h. These results indicate that the stability of reference gene expression in S. litura needs be investigated for each experimental treatment. These results were similar to the reference gene analysis of Drosophila [3].
Table 3. Preferable reference genes across different experimental conditions according to the software analysis.
| Experimental conditions | Preferable reference genes | |||
| Biotic factors | Developmental stage | GAPDH | UCCR | |
| Tissue | RPL10 | AK | EF1 | |
| Population | RPL10 | EF1 | ||
| Abiotic factors | Temperature | GAPDH | EF1 | |
| Insecticide | AK | ACTB | ||
| Food | RPL10 | GAPDH | UCCR | |
| Starvation | RPS3 | ACTB | ||
GAPDH plays a role in energy metabolism and is frequently used as a reference gene [25]. In this study, it was found to be the most stably expressed gene in the different developmental stages and temperature-stressed larvae, and the second most stable gene in the larvae fed on different foods. Scharlaken [26] also identified GAPDH as the most stable gene in the head of the honeybee after bacterial challenge. However, several studies have demonstrated that the stability of GAPDH expression was low in certain conditions, such as certain life stages of Tetranychus cinnabarinus, in the labial gland and fat body in Bombus terrestris and Bombus lucorum, and in the salivary glands after Trypanosoma cruzi injection [17], [18], [25]. These results further suggest that the expression stability of reference genes is affected by different experimental conditions.
The RPL10 gene showed the most stable expression in the tissues, different populations, and in the larvae fed on different diets, whereas RPS3 exhibited the most stable expression in the larvae starved for 6 h. The RPL10 gene encodes a ribosomal protein that is a component of the 60S subunit, whereas the RPS3 gene encodes a ribosomal protein that is a component of the 40S subunit where it forms part of the domain in which translation is initiated. The genes for various ribosomal proteins have been validated as normalization genes for qRT-PCR in many organisms, and these genes have also been reported to show the most stable expression in Tetranychus cinnabarinus (RPS18: [25]), Apis mellifera (RPS18: [26]), Rhodnius prolixus (RPS18: [27]), Cimex lectularius (RPL18: [28]), and Schistocerca gregaria (RP49: [29]). Although the genes for ribosomal proteins, including RPS3, RPL10, RPS6 and RPS18, were the most stable normalization genes for broad-scale gene expression analysis in most organisms, their stability ranking was dependent upon the instrument as well as the analysis program [1].
AK, which is the only phosphagen kinase in two major invertebrate groups, arthropods and mollusks, was the most stable gene in larvae treated with different insecticides, and the second most stable gene in tissue samples collected from third-instar larvae. Unfortunately, it has rarely been used as a reference gene in previous studies, with the exception of a single study that identified AK as the most stable gene in the labial gland and fat body of Bombus terrestris [18].
EF1, which plays an important role in translation by catalyzing the GTP-dependent binding of aminoacyl-tRNA to the acceptor site of the ribosome [3], exhibited the second most stable expression in the different populations and temperature-stressed larvae, and third most stable expression in the tissue samples. Our results were in accordance with reference gene analysis in Drosophila [3], Orthoptera [30] and Hymenoptera [18], in which EF1 was also identified as the most stable gene.
ACTB, which participates in many important cellular processes including muscle contraction, cell motility, cell division and cytokinesis, showed the second most stable expression in the temperature-stressed larvae and starved larvae, and a low stability in other treatments, including different developmental stages and tissues. It is not surprising that its transcript level varies among developmental stages and different cell types, as it has functions in various cellular processes.
In addition, UCCR, which plays a critical role in biochemical generation of adenosine-5′-triphosphate, has rarely been used as a reference gene in previous studies; in our study, it displayed the second and third most stable expression in the developmental stages and larvae fed on different diets.
FTZF1 was not a suitable reference gene in all experimental conditions owing to its higher Ct values and lower stability. These results emphasize that it is necessary to optimize normalization genes in all qRT-PCR experiments under different experimental conditions.
Our data reveal the optimal number of reference genes in different experimental conditions as calculated by geNorm, which determines the pairwise variations (V) in normalization factors (the geometric mean of multiple reference genes) using n or n +1 reference genes. When several reference genes are used simultaneously in a given experiment, the probability of biased normalization decreases. In our studies, V values for the developmental stages, temperatures, insecticides and starvation were higher than the threshold value (0.15); therefore, we propose that two or three of the best reference genes should be used to obtain accurate and reliable results under these conditions, because the manual of the geNorm software (2007) suggests that the 0.15 value must not be taken as a too strict cut-off.
In agreement with our results, increasing numbers of studies in recent years have clearly demonstrated that no single gene is expressed stably in all cell types and under all experimental conditions [3]. Therefore, the expression stability of a putative reference gene needs to be verified before starting each qRT-PCR experiment. In this study, we identified several optimal sets of genes that are suitable for qRT-PCR data normalization in S. litura under different biotic and abiotic stress conditions: GAPDH and UCCR for developmental stages; RPL10, AK and EF1 for the tissues dissected from third-instar larvae; RPL10 and EF1 for populations collected from different provinces in China; GAPDH and EF1 for temperature-stressed larvae; AK and ACTB for larvae treated with different insecticides; RPL10, GAPDH and UCCR for larvae fed different diets; RPS3 and ACTB for starved larvae. However, we did not find that the best-ranked reference genes were identical among treatments based on the analysis results of various software programs. To date, several strategies have recently been developed to evaluate the suitability of candidate reference genes as normalization genes in qRT-PCR gene expression quantification experiments, including BestKeeper, geNorm, NormFinder and RefFinder. A comparison of the rankings produced by the different approaches revealed important discrepancies, which appear to be caused by the differences between the algorithms used and their different sensitivities toward co-regulated reference gene candidates [3]. However, the analysis software programs can only suggest which genes are more suitable and the appropriate reference genes should be selected based on the experimental conditions. Therefore, each experimental investigation should establish which two or three reference genes are the most appropriate for the specific conditions (such as the developmental stages, tissues and populations) under investigation.
Supporting Information
Primer positions and amplicon sequences used for qRT-PCR. The DNA sequences are shown from the 5′ to 3′ end, and the primer positions are shaded. The products were first amplified by regular PCR and then sent to Invitrogen for sequencing.
(TIF)
Expression stability of the candidate reference genes across different developmental stages of S. litura . The expression stability of the reference genes in S. litura across developmental stages was measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in different tissues of S. litura . The expression stability of the reference genes in the different tissues of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in different populations of S. litura . The expression stability of the reference genes in the different populations of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in temperature-stressed samples of S. litura . The expression stability of the reference genes in the temperature-stressed samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in insecticide-stressed samples of S. litura . The expression stability of the reference genes in the insecticide-stressed samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in different food-reared samples of S. litura . The expression stability of the reference genes in the different food-reared samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in starvation-stressed samples of S. litura . The expression stability of the reference genes in the starvation-stressed samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Acknowledgments
The authors thank Prof. Kun Yan Zhu and Dr. Chuanwang Cao for their helpful comments on an earlier draft of this manuscript.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 31201544), the National Science and Technology Support Program (No. 2012BAD27B00), and Hubei Research and Development Project (No. 2011BBB043). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
References
- 1. Toutges MJ, Hartzer K, Lord J, Oppert B (2010) Evaluation of reference genes for quantitative polymerase chain reaction across life cycle stages and tissue types of Tribolium castaneum . J Agric Food Chem 58: 8948–8951. [DOI] [PubMed] [Google Scholar]
- 2. Shen GM, Jiang HB, Wang XN, Wang JJ (2010) Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol Biol 11: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponton F, Chapuis M-P, Pernice M, Sword GA, Simpson SJ (2011) Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster . J Insect Physiol 57: 840–850. [DOI] [PubMed] [Google Scholar]
- 4. Schmittgen TD, Zakrajsek BA (2000) Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46: 69–81. [DOI] [PubMed] [Google Scholar]
- 5. Lee PD, Sladek R, Greenwood CM, Hudson TJ (2002) Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res 12: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalization: strategies and considerations. Genes Immun 6: 279–284. [DOI] [PubMed] [Google Scholar]
- 8. Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, et al. (1999) Housekeeping genes as internal standards: use and limits. J Biotechnol 75: 291–295. [DOI] [PubMed] [Google Scholar]
- 9. Holloway JD (1989) The moths of Borneo: family Noctuidae, trifine subfamilies: Noctuinae, Heliothinae, Hadeninae, Acronictinae, Amphipyrinae, Agaristinae. Malayan Nat J 42: 57–226. [Google Scholar]
- 10. Qin H, Ye Z, Huang S, Ding J, Luo R (2004) The correlations of the different host plants with preference level, life duration and survival rate of Spodoptera litura (Fabricius). Chin J Eco-Agric 12: 40–42. [Google Scholar]
- 11. Sree K, Sowjanya S, Sachdev B, Padmaja V, Bhatnagar RK (2010) Electron spin resonance spectroscopic studies of free radical generation and tissue specific catalase gene expression in Spodoptera litura (Fab.) larvae treated with the mycotoxin, destruxin. Pestic Biochem Physiol 97: 168–176. [Google Scholar]
- 12. Chen H, Yin YP, Li Y, Mahmud MS, Wang ZK (2012) Identification and analysis of genes differentially expressed in the Spodoptera litura fat body in response to the biocontrol fungus, Nomuraea rileyi. Comp Biochem Physiol Part B 163: 302–210. [DOI] [PubMed] [Google Scholar]
- 13. Bear A, Simons A, Westerman E, Monteiro A (2010) The Genetic, morphological, and physiological characterization of a dark larval cuticlemutation in the butterfly, Bicyclus anynana . PLoS ONE 5(7): e11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shelton AM, Robertson JL, Tang JD, Perez C, Eigenbrode SD, et al. (1993) Resistance of diamondback moth (Lepidoptera: Yponomeutidae) to Bacillus thuringiensis subspecies in the field. J Econ Entomol 86: 697–705. [Google Scholar]
- 15. Liang P, Gao XW, Zheng BZ (2003) Genetic basis of resistance and studies on cross-resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag Sci 59: 1232–1236. [DOI] [PubMed] [Google Scholar]
- 16. Tu YG, Zeng JH (2010) A method for artificial rearing of common cutworm, Spodoptera litura . Acta Agricul Jiangxi 22: 87–88. [Google Scholar]
- 17. Paim RM, Pereira MH, Ponzio RD, Rodrigues JO, Guarneri AA, et al. (2012) Validation of reference genes for expression analysis in the salivary gland and the intestine of Rhodnius prolixus (Hemiptera, Reduviidae) under different experimental conditions by quantitative real-time PCR. BMC Research Notes 5: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horňáková D, Matoušková P, Kindl J, Valterová I, Pichová I (2010) Selection of reference genes for real-time PCR polymerase chain reaction analysis in tissues from Bombus terrestris and Bombus lucorum of different ages. Anal Biochem 397: 118–120. [DOI] [PubMed] [Google Scholar]
- 19. Lu Y, Pang Y-P, Park Y, Gao X, Yao J, et al. (2012) Genome organization, phylogenies, expression patterns, and three-dimensional protein models of two acetylcholinesterase genes from the red flour beetle. PLoS ONE 7(2): e32288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, et al. (2001) An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods 25: 386–401. [DOI] [PubMed] [Google Scholar]
- 21. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 22. Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, et al. (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313: 856–862. [DOI] [PubMed] [Google Scholar]
- 23. Walker NJ (2002) A technique whose time has come. Science 296: 557–559.24. [DOI] [PubMed] [Google Scholar]
- 24. Andersen CL, Ledet-Jensen J, Ørntoft T (2004) Normalization of real-time quantitative RT-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 25.Sun W, Jin Y, He L, Lu WC, Li M (2010) Suitable reference gene selection for the different strains and developmental stages of the carmine spider mite, Tetranychus cinnabarinus, using quantitative real-time PCR. J Insect Sci 10: 208, available online: insectscience.org/10.208. [DOI] [PMC free article] [PubMed]
- 26.Scharlaken B, de Graaf DC, Goossens K, Brunain M, Peelman LJ, et al. (2008) Reference gene selection for insect expression studies using quantitative real-time PCR: The honeybee, Apis mellifera, head after a bacterial challenge. J Insect Sci 8: 33, available online: insectscience.org/8.33.
- 27. Majerowicz D, Alves-Bezerra M, Logullo R, Fonseca-de-Souza AL, Meyer-Fernandes JR, et al. (2011) Looking for reference genes for real-time quantitative PCR experiments in Rhodnius prolixus (Hemiptera: Reduviidae) Insect Mol Biol. 20: 713–722. [DOI] [PubMed] [Google Scholar]
- 28. Mamidala P, Rajarapu SP, Jones SC, Mittapalli O (2011) Identification and validation of reference genes for quantitative real-time polymerse chain reaction in Cimex lectularius . J Med Entomol 48: 947–951. [DOI] [PubMed] [Google Scholar]
- 29. Van Hiel MB, Van Wielendaele P, Temmerman L, Van Soest S, Vuerinckx K, et al. (2009) Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Mol Biol 10: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chapuis MP, Tohidi-Estahani D, Dodgson T, Blondin L, Ponton F, et al. (2011) Assessment and validation of a suite of reverse transcription-quantitative PCR reference genes for analyses of density-dependent behavioral plasticity in the Australian plague locust. BMC Genomics 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer positions and amplicon sequences used for qRT-PCR. The DNA sequences are shown from the 5′ to 3′ end, and the primer positions are shaded. The products were first amplified by regular PCR and then sent to Invitrogen for sequencing.
(TIF)
Expression stability of the candidate reference genes across different developmental stages of S. litura . The expression stability of the reference genes in S. litura across developmental stages was measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in different tissues of S. litura . The expression stability of the reference genes in the different tissues of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in different populations of S. litura . The expression stability of the reference genes in the different populations of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in temperature-stressed samples of S. litura . The expression stability of the reference genes in the temperature-stressed samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in insecticide-stressed samples of S. litura . The expression stability of the reference genes in the insecticide-stressed samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in different food-reared samples of S. litura . The expression stability of the reference genes in the different food-reared samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)
Expression stability of the candidate reference genes in starvation-stressed samples of S. litura . The expression stability of the reference genes in the starvation-stressed samples of S. litura was also measured using the ΔCt method, BestKeeper, NormFinder and geNorm. A lower average stability value indicates more stable expression.
(TIF)