Abstract
Objectives
Male circumcision reduces penile high-risk human papillomavirus (HR-HPV) prevalence in randomised trials. The goal of this study was to examine the effect of circumcision on HPV viral load among HPV-infected men in a randomised trial of male circumcision.
Methods
In a randomised trial to assess the efficacy of circumcision on HIV acquisition in Rakai, Uganda, HIV-negative men were randomised to immediate (intervention) or delayed (control) circumcision and followed over 24 months. We performed quantitative-PCR HPV viral load assays on penile swabs which tested positive by Linear Array (LA) for six HR-HPV genotypes and estimated viral load in the remaining types by LA signal strength.
Results
At 24 months, circumcision intervention arm men infected with one of the six selected HR-HPV genotypes had a lower viral load and significantly reduced HR-HPV high LA band intensity (PRR=0.61, 95% CI 0.43 to 0.86) compared to infected men in the control arm of the trial. The decreased viral load associated with circumcision was seen among HPV infections acquired after enrolment but not among infections that persisted from trial enrolment to 24 months (p=0.80).
Conclusions
The decreased penile HR-HPV shedding observed among HPV-infected circumcised men may help to explain the protective association observed between circumcision and reduced acquisition of HR-HPV in female partners.
BACKGROUND
Genital high risk human papillomavirus (HR-HPV) is a common sexually transmitted infection and a causal agent of anogenital cancer, some oral cancers and cervical cancer,1 which is the leading cause of cancer mortality in women in Eastern Africa. There are currently 13 HPV genotypes that are considered high-risk for progression to cervical cancer; these are HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68, with HPV 16 and 18 accounting for the majority of the worldwide cervical cancer burden.2
Three randomised clinical trials and multiple observational studies demonstrate that male circumcision significantly decreases HIV acquisition in men.3–7 Additionally, two of these trials have shown that circumcision reduces the prevalence of HR-HPV infection or HPV-associated penile lesions in HIV-negative men.8,9 Male circumcision also reduces HR-HPV prevalence among HIV-positive men.10 Among HIV-negative men, male circumcision reduces the acquisition of new HR-HPV infection and increases the clearance of pre-existing HR-HPV infection for both the men and their female partners.8,9,11–13
While male circumcision was associated with a significant reduction in prevalent HR-HPV infections 24-months post surgery the absolute prevalence of HPV remained high.8,9,11,12 Detectable HPV viral load serves as evidence of a current HPV infection. Although the biological significance of HPV viral load is not well established; researchers have linked elevated HR-HPV viral loads in women before conisation of cervical intraepithelial neoplasia (CIN) lesions with risk of persistent HPV infection post conisation, as well as with risk of persistent or recurrent histological abnormalities in the cervix.14 Elevated HPV viral loads in male and female sex partners is associated with HPV genotype concordance, suggesting that viral load among HPV positive individuals may play a role in transmission of HPV.15 The Linear Array (LA) HPV genotyping assay results and band intensity have been shown previously to correspond to viral load in the cervix.16,17
In this study, we sought to determine whether HR-HPV viral load among men who had detectable HR-HPV infections was affected by circumcision.
METHODS
Study population
The penile swab samples tested in this study were collected from men enrolled in a randomised clinical trial of male circumcision for the prevention of HIV/sexually transmitted disease (STD) acquisition in Rakai, Uganda. The trial methods have been described previously7; briefly, 4996 uncircumcised, HIV-negative men aged 15–49 years were enrolled and randomised to receive either immediate circumcision (intervention) or circumcision delayed for 24 months (controls). At each study visit (enrolment and follow-up at 6, 12 and 24 months) men were interviewed and examined clinically, and provided venous blood, urine and penile swabs.
Moistened Dacron swabs were taken from the penile coronal sulcus/glans using a standard protocol, placed in Digene specimen transport medium, and stored at −80°C. A random sample of 972 men at the baseline visit were selected for HPV genotyping, and swabs from enrolment (N=972) and the 24-month follow-up visit (N=885; 91.4%) underwent testing with the LA assay (LA; Roche Molecular Diagnostics, Pleasanton, California, USA).16,18 At enrolment, 463 (47%) men were from the control arm and 509 (52%) men were from the intervention arm. Of the swabs tested at the 24-month postenrolment visit, 425 (47%) men were from the control arm and 460 (51.5%) were from the intervention arm. Samples that tested positive by LA for HPV16, HPV18, HPV31, HPV33, HPV35 or HPV52 at the baseline trial visit were included in this analysis of genotype-specific viral load assay by quantitative PCR.
All individuals tested were HIV-negative at the baseline study visit; eight individuals included in testing for HR-HPV viral load HIV-seroconverted by 24 months. In the secondary analysis of the LA band intensity of individuals who were sero-positive for a high-risk HPV genotype at either the baseline study visit or the 24-month study visit, 44 of the HPV-positive men at the baseline visit and 24 of the HPV-positive men at the 24-month visit HIV-seroconverted over the course of the trial.
The LA results and band intensity were evaluated by two independent observers using a labelled acetate overlay provided by Roche, which indicates the position of the genotype probes on the test strip. The two observers also subjectively scored each positive result band intensity from 1–4 in relation to the internal control low-intensity and high-intensity β-globin bands included on the strips. Band intensities similar to the low-intensity or high-intensity bands were scored as 2 and 3 respectively, while bands lighter than the low-intensity control band were scored as 1 and bands darker than the high intensity control band were scored as 4. Disagreement in strip intensity interpretation between observers was rare (1–4%), and usually was a matter of difference of 1° of band intensity. Disagreements were resolved by re-evaluation by the two observers.
The trial was approved by four Institutional Review Boards (IRBs): the Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, the Committee for Human Research at Johns Hopkins University, Bloomberg School of Public Health, and the Western Institutional Review Board. The trial was registered with ClinicalTrials.gov numbers NCT00425984.
High risk type HPV detection and viral load testing
The HPV genotypes that are considered primary carcinogenic types are HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. For each positive test, the band intensity was visually scored as 1–4 in relation to internal β-globin control high and low intensity bands and used as a semiquantitative measure of viral load.17 Samples which tested positive by LA for HR-HPV 16, 18, 31, 33, 35 or 52 underwent viral load testing by quantitative PCR for each specific HPV genotype. Samples from baseline and 24 month visits were tested in the same PCR runs on the same PCR plate if possible, and technicians were blinded to trial arm status to avoid bias.
Type-specific viral loads were quantified using TaqMan E6/E7 quantitative PCR assays on an Applied Biosystems (ABI) 7300 real-time PCR thermocycler. Reactions were carried out in 96 well, 0.2 ml PCR reaction plates with a 50 ml reaction volume comprised of forward and reverse primers, fluorescent probe, 1× Universal Mastermix with TaqGold (Applied Biosystems, Foster City, California, USA), diethyl pyrocarbonate-treated (DEPC) water and 5 μl of extracted DNA sample. Primer/probe sequences, concentrations and amplification conditions for each type-specific assay have been previously described.17,19–21
Viral load was quantified using postdetection software linked to the ABI 7300 thermocycler, which compares the detected fluorescence during amplification to the amplification of a standard curve of known concentrations of type-specific plasmid HPV DNA included on each reaction plate. Five serial 1 : 10 dilutions of purified HPV 16, 18, 31, 33, 35 or 52 plasmid with an initial concentration of 250 000 copies in a background of 50 ng/μl human placental DNA (Sigma) served as the standard curve. Human placental DNA at 50 ng/μl in low-salt Tris-EDTA buffer also served as a negative control on each plate. All standards and negative controls were run in duplicate.21 HR-HPV was expressed as copies per 5 μl. Due to low cellularity from penile swab specimens, we were unable to adjust for number of cells in our analysis of viral load.
Statistical analysis
We compared the viral load distributions among penile swab samples from men who tested positive for one of the six selected genotypes in the intervention and control arms at baseline and the 24-month visit in an intention to treat analysis. The distribution of HR-HPV viral loads was non-normal, so viral loads were transformed using the natural log of the viral copy number. Q-Q plots confirmed that log-transformation normalised the distribution. We compared log-transformed mean viral loads for each genotype individually between study arms at enrolment and at 24 months follow-up using unpaired t-tests. Analyses were performed for each of the six HPV genotypes tested, as well as for the pooled loads of all of the six tested types. We treated each HPV 16, 18, 31, 33, 35 or 52 positive sample as a single infection, and used standard t-tests to compare log-transformed mean viral loads in the intervention and control arms at the baseline visit and the 24 month visit. Fifty-five (20.6%) of the 266 HR-HPV infections positive by LA for one of the six tested genotypes at either the baseline or 24-month visit that were then tested by quantitative PCR occurred in men who were positive for multiple HPV genotypes. We estimated prevalence ratios of LA band intensity scores of 4 (strongest LA hybridisation intensity), relative to scores 1–3 (lower LA hybridisation band intensity), for all HR-HPV genotypes detected in the initial LA assays.
RESULTS
Male circumcision reduces HPV viral load
We directly measured HPV viral load by TaqMan PCR in 266 infections with HPV16, 18, 31, 33, 35 or 52 detected at either the baseline or 24 month visit in the 972 HIV-negative men selected for HPV testing by LA (27.4%). The demographic characteristics in these 266 infections are presented in online supplemental table S1. We compared the mean viral load (copies/5 μl) for the six HR-HPV genotypes by trial arm at the baseline and follow-up at 24 months (table 1).
Table 1.
HR-HPV viral load* in the control and intervention trial arms
| Trial arm | N | Mean viral load† | SE‡ | p>∣t∣§ | |
|---|---|---|---|---|---|
| Baseline visit | Control | 100 | 130.3 | 1.5 | 0.76 |
| Intervention | 127 | 109.9 | 1.4 | ||
| 24-month visit | Control | 74 | 164.0 | 1.6 | 0.001 |
| Intervention | 25 | 8.0 | 2.0 |
Any penile swab sample that tested positive for HPV 16, 18, 31, 33, 35, or 52 by Linear Array was then tested by quantitative PCR to quantify the number of HPV viral copies present in the sample.
Mean number of HPV viral copies detected per 5 μl of sample.
SE of the mean.
T-test probability for difference in means between trial arms at each visit.
The enrolment viral load of infections with these six genotypes did not differ significantly between study arms (p=0.76). However, at 24 months follow-up, the mean viral load in the intervention arm among men positive for one of the selected viral genotypes (8.0 copies/5 μl) was significantly lower than in the control arm (164.0 copies/5 μl, p=0.001). The mean viral load for HPV16 was equivalent at baseline between the two trial arms, but at 24 months the average viral load in the intervention arm was 43.5 copies/5 μl lower than the mean load in the control arm (95% CI −804.3 to −2.3). No other genotype demonstrated a statistically significant difference between the trial arms at 12-month or 24-month follow-up visits, likely due to the relatively lower prevalence of the non-HPV16 genotypes. The presence of multiple HPV infections did not appear to explain the variability in viral load. There were no statistically significant effects on viral load associated with co-infections with the most oncogenic genotypes HPV16 or HPV18, or with multiple demographic factors including age, marital status, number of sex partners in the last year, condom use, number of times the participant reported sexual intercourse in the last 7 days or reported genital washing after intercourse.
LA signal strength correlates with HR-HPV viral load
To determine whether the strength of the LA hybridisation signal could be used as a surrogate for viral load as has been found in cervical shedding studies,17 log-transformed viral loads were compared with their type-specific LA signal strength (figure 1). Among men who tested positive for HPV16, HPV18, HPV31, HPV33, HPV35 or HPV52, LA signal strength was highly correlated with log-transformed viral load (Spearman’s r=0.73), and a band signal strength of 4 was approximately equivalent to a viral load of >200 copies/5 μl. Using LA signal strength as a surrogate for relative viral load in this population, we estimated the proportion of LA results with band intensity 4, relative to lower intensity bands 1–3 (table 2).
Figure 1.
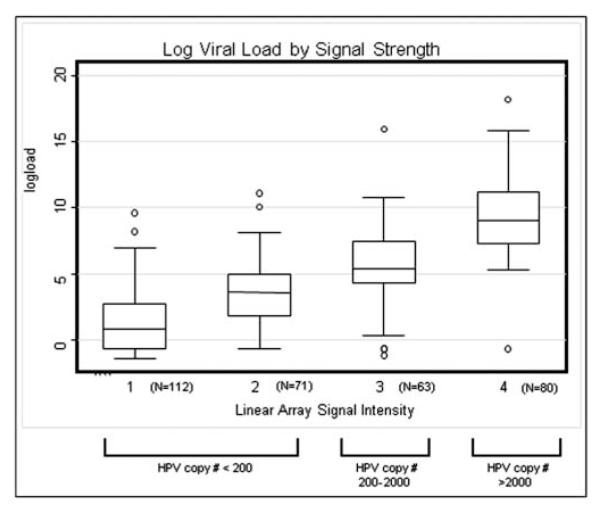
Box-plot of the distribution of log viral loads for HPV 16, 18, 31, 33, 35 and 52 by their corresponding Linear Array assay band intensities for all infections tested by quantitative PCR. The plot depicts the mean and IQR of the data, with whiskers representing the highest and lowest datum still within 1.5 IQR of the upper and lower quartile and points representing any observations outside this range. The average detected viral copy number (SD) in 5 μl of solution for samples with (A) signal strength=1 was 4.6 (12.4) copies. (B) signal strength=2 was 35.9 (15.3) copies. (C) signal strength=3 was 275.9 (21.8) copies. (D) signal strength=4 was 11 384.4 (19.9).
Table 2.
Linear array (LA) signal strength among HPV positive men in the Rakai circumcision trial
| Trial Arm | Frequency LA band intensity of 4/total* (%) |
Prevalence ratio of ‘4’ band intensity comparing intervention to control arm |
95% CI |
|---|---|---|---|
| (A) LA band intensities for all positive infections detected by LA for 37 HPV genotypes at baseline and 24 months | |||
| Baseline | |||
| Control | 126/628 (20.1%) | ||
| Intervention | 181/691 (26.2%) | 1.31 | (1.07 to 1.59) |
| 24 months | |||
| Control | 124/394 (31.4%) | ||
| Intervention | 32/166 (19.2%) | 0.61 | (0.43 to 0.86) |
| (B) LA intensities limited to HPV 16, 18, 31, 33, 35 and 52 | |||
| Baseline | |||
| Control | 22/111 (19.8%) | ||
| Intervention | 34/134 (24.3%) | 1.28 | (0.80 to 2.06) |
| 24 months | |||
| Control | 23/78 (29.4%) | ||
| Intervention | 4/25 (16.0%) | 0.54 | (0.21 to 1.42) |
| (C) LA positive results limited to infections that were detectable at 1 visit only (no persistent infections) | |||
| Baseline | |||
| Control | 99/547 (18.1%) | ||
| Intervention | 168/662 (25.3%) | 1.4 | (1.13 to 1.75) |
| 24 months | |||
| Control | 98/321 (30.5%) | ||
| Intervention | 26/137 (18.9%) | 0.62 | (0.42 to 0.91) |
Frequency of LA band intensities of ‘4’ on a scale of 1–4 among HPV positives. Data are presented as number of ‘4’ intensities/total number of HPV-positive infections, and percentage of ‘4’ intensities.
Panel A of table 2 presents the results of an analysis of LA band intensity for all 36 genotypes tested using all of the positive infections detected at the baseline visit and the 24-month visit. Frequencies of infections stratified by genotype and distribution of LA band intensities are presented in online supplemental tables S2 and S3. At enrolment the frequency of high intensity bands was slightly higher in the intervention arm (26.2%) than the control arm (20.1%, prevalence ratio PR=1.32, 95% CI 1.07 to 1.59), whereas at 24 months follow-up, the prevalence of band intensities ≥4 was lower in the intervention (19.4%) than the control arm (31.5%, PR=0.61, 95%CI 0.43 to 86). Similar, though attenuated, results were observed when using other cut-points for the band intensity (1 vs >1 and 1–2 vs 3–4). Our estimates were not affected when the analysis was limited to men who had HPV LA data at both visits.
Panel B of table 2 presents the same analysis limited to the genotypes with TaqMan measured viral load, and shows that the decreased viral load associated with circumcision at 24 months in the quantitative PCR analysis for these types is also observed when using LA band intensity as a semiquantitative surrogate of the viral load. Panel C presents the results when the HPV infections included are limited to those which were detected only at one of the visits and excluded HPV infections that likely persisted over the 24-month period. We observed similar decreased band intensity associated with circumcision at 24 months when the persistently detected infections were excluded.
To determine the effect of being in the circumcision arm on infections that were detected both at baseline and at 24 months, we examined whether assignment to the intervention arm affected change in band intensity (table 3). We found no significant effect of circumcision on the change in band intensity among persistently detected infections, nor did we observe a difference in the magnitude of the change across trial arms (see online supplemental table S4).
Table 3.
Changes in Linear Array (LA) band intensity among infections that did not clear and were detected both at baseline and 24 months (N=101)
| Among HPV infections detected at both visits | |||
|---|---|---|---|
| LA intensity* | Control arm | Intervention arm | χ2 Test |
| Decreased | 21 (28%) | 9 (32%) | |
| Stayed the same | 26 (36%) | 11 (39%) | |
| Increased | 26 (36% | 8 (28%) | |
| Total | 73 | 28 | Pr=0.798 |
*Direction of the change in magnitude of the LA intensity for the detected infection between the baseline visit and the 24-month visit.
DISCUSSION
In a randomised clinical trial male circumcision decreased penile HR-HPV viral load among men who tested positive for these genotypes at 24 months follow-up. This suggests that circumcision not only reduces the prevalence and incidence of HPV infection,9–12 but also reduces HPV DNA viral load among HR-HPV infected men. It is unlikely that sexual behaviours account for these differences because only men with detectable infections were included and thus were exposed to the virus, and adjustment for sexual behaviour risk factors did not affect the observed reduction in viral load associated with circumcision.
Using the LA hybridisation signal intensity as a surrogate for viral load, we also found that circumcision resulted in a lower proportion of high intensity infections (band intensity 4) at 24 months follow-up for all HR-HPV genotypes included in the LA assay, suggesting that circumcision decreases HPV viral load for a wide range of HR-HPV genotypes. Our analysis is limited by a lack of information on the exact duration of the infections we observed, but our results indicate that the differences in measured band intensity associated with circumcision were seen in infections acquired over the course of the trial but not in infections which persisted from the baseline visit to 24 months. A longitudinal study of circumcised and uncircumcised male university students in Washington did not observe a difference in rates of HPV infection associated with circumcision, but found that uncircumcised men were more likely to have HPV infections detected at multiple genital sites than circumcised men were, which could be a function of higher viral replication and viral shedding in those men,22 and may contribute to transmission rates between sex partners. Higher detectable viral load has been associated with higher frequency of HPV genotype concordance between male and female sex partners.15
The reduction in penile viral load among circumcised men observed in this study through quantitative and semiquantitative methods, combined with our previously observed decreased incidence and increased clearance of penile HPV associated with adult male circumcision9–11 together support the conclusion that the reduced 24-month HPV prevalence in female partners of the HIV-negative male trial participants13 represents a decrease in HR-HPV male-female transmission by circumcised men. These data provide further support for the potential public health benefit of adult male circumcision for prevention of HR-HPV infection in female partners.
Supplementary Material
Key messages.
▶ Reduced high-risk human papillomavirus (HR-HPV) viral load among infected men was observed in circumcised men in a randomised clinical trial of male circumcision.
▶ Reduced HPV Linear Array band intensity was observed among infected men in the circumcision trial arm.
▶ These results may help to explain the protective association between circumcision and reduced acquisition of HR-HPV in female partners.
Acknowledgements
We are most grateful to the study participants and the Rakai Community Advisory Board whose commitment and cooperation made this study possible.
Funding The trial was funded by the National Institutes of Health (#U1AI51171). The Fogarty International Center (#5D43TW001508 and #2D43TW00001019-AITRP) contributed to training. National Institute of Allergy and Infectious Diseases (NIAID), NIH grants U01-AI-068613 and 3U01-AI075115-03S1 and the NIAID Intramural Program provided laboratory support. AART was supported by the NIH 1K23AI093152-01A1, Doris Duke Charitable Foundation Clinician Scientist Development Award (#22006.02) and Johns Hopkins University Clinician Scientist Award. PG received research funding from Roche Molecular Diagnostics who manufacture the HPV genotyping test used in this study.
Footnotes
Contributors LEW designed and conducted the quantitative PCR assays and analysis, participated in all data analyses and writing the report. MJW, DS, and RHG oversaw the design and conduct of the trial, participated in all data analyses and writing the report. AART and PG oversaw the HPV assays, provided laboratory quality control and participated in all data analyses and writing the report. GK was responsible for study conduct in the field. SW supervised the trial surgeons and assisted in interpretation of data. FN participated in study implementation, data analysis and interpretation. All authors contributed to the preparation of the paper and approved the final version. The corresponding author had full access to all data in the study and final responsibility for the preparation and submission of the results for publication.
Competing interests None.
Ethics approval The Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, the Committee for Human Research at Johns Hopkins University, Bloomberg School of Public Health and the Western Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
▶ Additional supplementary tables are published online only. To view these files please visit the journal online (http://dx.doi.org/10.1136/sextrans-2012-050633)
REFERENCES
- 1.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 2.Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 3.Tobian A, Gray R, Quinn T. Male circumcision prevents acquisition and transmission of sexually transmitted infections: the case for neonatal circumcision. Arch Pediatr Adolesc Med. 2010;164:78–84. doi: 10.1001/archpediatrics.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobian A, Gray R. The medical benefits of male circumcision. JAMA. 2011;306:1479–80. doi: 10.1001/jama.2011.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey R, Moses S, Parker C, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 6.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray R, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 8.Tobian A, Serwadda D, Quinn T, et al. Male circumcision for the prevention of HSV-2, HPV infections, syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auvert B, Sobngwi-Tambekou J, Cutler E, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of the randomized clinical trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199:14–19. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serwadda D, Wawer M, Makumbi F, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1463–9. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray R, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–62. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobian A, Kong X, Gravitt P, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. Int J Cancer. 2011;129:2970–5. doi: 10.1002/ijc.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wawer M, Tobian A, Kigozi G, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomized trial in Rakai, Uganda. Lancet. 2011;377:209–18. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Lee K, Dong S, et al. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008;108:549–54. doi: 10.1016/j.ygyno.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Bleeker M, Hogewoning C, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis. 2005;41:612–20. doi: 10.1086/431978. [DOI] [PubMed] [Google Scholar]
- 16.Gravitt P, Peyton C, Apple R, et al. Genotyping of 27 human papillomavirus types by using L1 consensus PCR product by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentzensen N, Gravitt P, Long R, et al. Human papillomavirus load measured by Linear Array correlates with quantitative PCR in cervical cytology specimens. J Clin Microbiol. 2012;50:1564–70. doi: 10.1128/JCM.06240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravitt P, Schiffman M, Solomon D, et al. A comparison of Linear Array and hybrid capture 2 for detection of carcinogenic human papillomavirus and cervical precancer in ASCUS-LSIL Triage Study. Cancer Epidemiol Biomarkers Prev. 2008;17:1248–54. doi: 10.1158/1055-9965.EPI-07-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravitt P, Peyton C, Wheeler C, et al. Reproducibility of HPV 16 and HPV 18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003;112:23–33. doi: 10.1016/s0166-0934(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 20.Gillison M, D’Sousza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–40. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 21.Marks M, Gupta S, Liaw K, et al. Confirmation and quantitation of human papillomavirus type 52 by Roche Linear Array© using HPV52-specific TaqMan© E6/E7 quantitative real-time PCR. J Virol Methods. 2009;156:152–56. doi: 10.1016/j.jviromet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Vanbuskirk K, Winer R, Hughes J, et al. Circumcision and acquisition of human papillomavirus infection in young men. Sex Transm Dis. 2011;38:1074–81. doi: 10.1097/OLQ.0b013e31822e60cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


