Abstract
BACKGROUND
Neurointervention is an ever-evolving specialty with tools including microcatheters, microwires, and coils that allow treatment of pathological conditions in increasingly smaller intracranial arteries, requiring increasing accuracy. As endovascular tools evolve, so too should the imaging.
OBJECTIVE
To detail the use of microangiography performed with a novel fluoroscope during coiling of intracranial aneurysms in 2 separate patients and discuss the benefits and potential limitations of the technology.
METHODS
The microangiographic fluoroscope (MAF) is an ultra high-resolution x-ray detector with superior resolution over a small field of view. The MAF can be incorporated into a standard angiographic C-arm system for use during endovascular procedures.
RESULTS
The MAF was useful for improved visualization during endovascular coiling of 2 unruptured intracranial aneurysms, without adding significant time to the procedure. No significant residual aneurysm filling was identified post-coiling, and no complications occurred.
CONCLUSION
The MAF is a high-resolution detector developed for use in neurointerventional cases in which superior image quality over a small field of view is required. It has been used with success for coiling of 2 unruptured aneurysms at our institution. It shows promise as an important tool in improving the accuracy with which neurointerventionists can perform certain intracranial procedures.
Keywords: Intracranial aneurysms, Microangiographic fluoroscope
Diagnostic and interventional neuroendovascular procedures involve imaging of the brain vasculature and catheter manipulation. These procedures entail fluoroscopic exposure and digital subtraction angiography (DSA), which is the gold standard in vascular diagnosis. DSA has the advantages of a decreased contrast agent requirement for diagnostic studies and the capabilities for image post-processing and to perform measurements.
As x-ray image receptors improve, endovascular treatment options have been evolving to enable greater accuracy of diagnosis. As the tools for neurointervention continue to evolve and become smaller to allow treatment of pathological conditions in 1- to 2-mm intracranial arteries, there is increasingly a need to provide finer detail with magnification and to increase image resolution in a small field, enabling road-mapping navigation in the vessels and accurate performance of procedures.
The microangiographic fluoroscope (MAF)1,2 is an ultra high-resolution x-ray detector developed at the University at Buffalo–Toshiba Stroke Research Center for cases in which superior resolution is required within a small field of view (FOV), ie, during aneurysm coiling. To date, this novel system has been used at our hospital (Millard Fillmore Gates–Kaleida Health) for coiling and stent-assisted coiling in 2 cases of unruptured intracranial aneurysms, with excellent results. The system and procedures are detailed in this article.
MICROANGIOGRAPHIC FLUOROSCOPE
The MAF can be incorporated into a standard angiographic C-arm system. The detector consists of a 300-µm cesium iodide input phosphor coupled to a dual-stage second-generation microchannel plate light image intensifier, followed by minifying fiberoptic taper coupled to a charged-coupled device chip. The detector has a round FOV with 3.5-cm diameter and 35-µm pixels. The light image intensifier has a large variable gain, which allows use of the detector at the very low exposures characteristic of fluoroscopic ranges while maintaining superior image quality (approximately 2–3× the resolution of standard x-ray detectors). The detector is attached using a specially designed changer onto the anteroposterior (AP) C-arm of an x-ray image intensifier biplane angiographic unit (Toshiba America Medical Systems, Tustin, California) and is controlled by specially designed Lab-VIEW-based software (National Instruments Corp., Austin, TX, USA) designated CAPIDS (Control, Acquisition, Processing, and Image Display).2
Clinical testing of the system has begun in neuroendovascular cases where superior image quality over a small FOV is required. In general, most of the procedure is done using the standard biplane imagers. Once the microcatheters are in place and x-ray guidance is required only in the small FOV, the MAF is brought in by swinging it into place over the AP fluoroscope tube. The system automatically collimates the x-ray beam to the detector active area; however, at present, the x-ray parameters must be adjusted manually. Because the prototype MAF system described here was not integrated into the commercial system, some of the automatic dose rate and x-ray parameter selection controls had to be deactivated, which would not be necessary in an integrated commercial product. The MAF system currently uses only the x-ray triggering signal from the Toshiba C-arm system and provides no feedback to the C-arm automatic exposure control software.
To reach optimal image quality, the detector is used preferably with a small x-ray focal spot and minimum geometrical magnification. In some cases, the exposure parameters are increased if necessary to twice those selected by the x-ray system; however, because the small irradiation FOV of the MAF, the effective dose to the patient is actually reduced by more than 10× when compared with a standard commercial x-ray neuroimaging system.3 Because of the superior resolution of the detector, there is no reason to increase the physical magnification. Quite the opposite is possible by binning to increase the effective pixel size and enable higher frame rates. In that case, electronic magnification or zooming the display is done. The MAF is stable, and imaging can be done for extensive periods of time for as long as required by the interventionist. Additional features, such as road-mapping, digital angiography, and digital subtraction angiography, are available for clinical use. Other features, such as region-of-interest dual-detector cone-beam computed tomography (CT),1 were tested in animal subjects only and will be available for clinical use in the near future.2
CLINICAL APPLICATION
Case 1
A 65-year-old woman presented to the emergency department with a right frontal headache, diplopia, and right eye ptosis that had become progressively worse over the past week. She denied smoking and any family history significant for vascular disease. She was on aspirin at home.
Examination revealed a right-sided ptosis and minimal anisocoria with a slightly larger, but reactive, right pupil. The patient’s extraocular muscle function was intact, although subjectively she reported diplopia. No other deficits were noted.
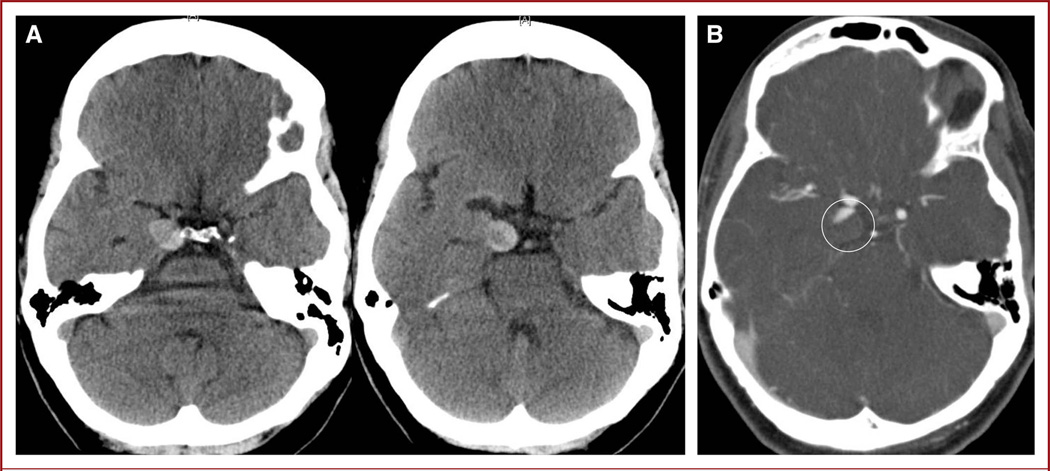
A noncontrast cranial CT scan was suggestive of a partially thrombosed 15 × 5.7-mm aneurysm in the region of the right posterior communicating artery with no evidence of subarachnoid hemorrhage (Figure 1A). Cranial CT angiography (CTA) revealed a 9 × 5.7-mm, partially thrombosed right posterior communicating artery aneurysm (Figure 1B). Cervical CTA revealed bilateral distal cervical internal carotid artery (ICA) fusiform aneurysmal dilations with bilateral intimal flaps, all of which were non–flow limiting. These lesions were thought likely related to a motor vehicle accident 3 years earlier. Lumbar puncture revealed no xanthochromia of the cerebrospinal fluid. Cerebral DSA confirmed the CTA findings (Figure 2).
FIGURE 1.
A, noncontrast computed tomography (CT) images reveal what appears to be a partially thrombosed aneurysm in the region of the right posterior communicating artery. B, CT angiography showing partially filling of the right posterior communicating artery aneurysm (circle).
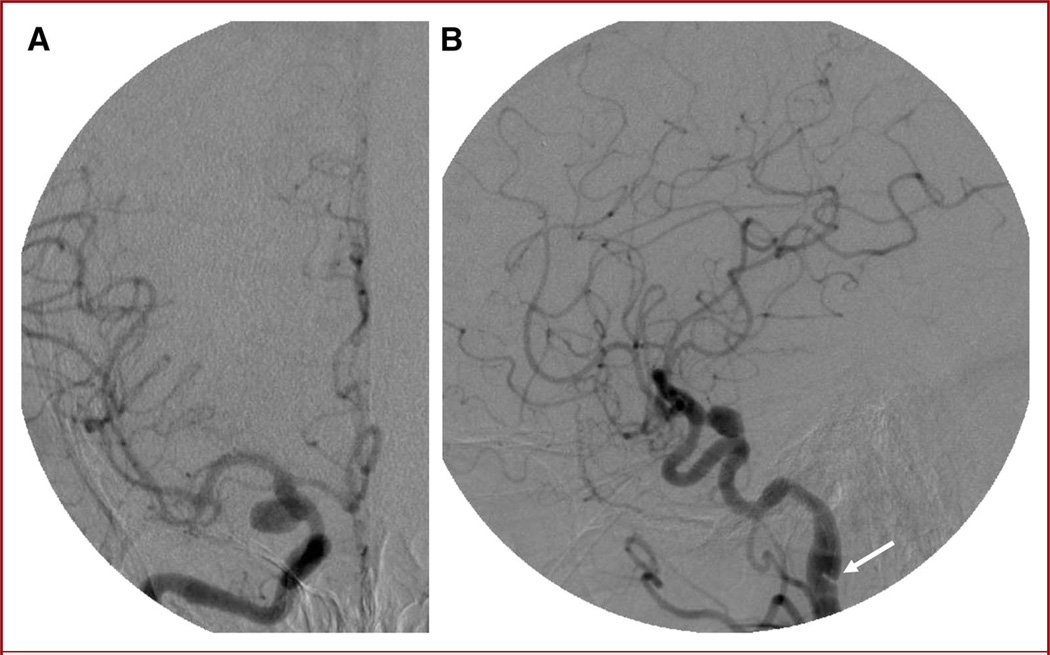
FIGURE 2.
Anteroposterior (A) and lateral (B) digital subtraction angiography images showing a 10 × 6-mm posterior communicating artery aneurysm. The pseudoaneurysm and dissection flap can be seen on the lateral view (arrow).
After counseling the patient and her family about the options of open microsurgical clipping vs an endovascular approach, the decision was made to proceed with endovascular coiling of the aneurysm. Two days before the procedure, she received a loading dose of aspirin (650 mg) and clopidogrel (600 mg) and then was maintained on a daily regimen of aspirin (325 mg) clopidogrel (75 mg) and found to have adequate platelet responses before the procedure.
The procedure was performed with the patient under conscious sedation and via right femoral artery access. To safely access the intracranial ICA, the prepetrous cervical ICA dissection was stented along the full length of the dissection and pseudoaneurysm with a Wallstent (Boston Scientific, Natick, Massachusetts). This allowed for improved guide catheter purchase with less risk of propagating the dissection. Although the aneurysm was a narrow-necked saccular aneurysm, a decision was made to place a stent across the neck of the aneurysm to minimize the risk of distal embolization of intraluminal thrombus from the aneurysm. Using a Prowler Select Plus microcatheter (Codman & Shurtleff, Inc., Raynham, Massachusetts), an Enterprise stent (Codman & Shurtleff, Inc.) was placed across the neck of the aneurysm. A Prowler Select LPES 45-degree microcatheter (Codman & Shurtleff, Inc.) was then used to catheterize the aneurysm, which was coiled using microangiography for the AP projection (Figure 3). Microangiography was useful in identifying small pockets within the aneurysm to reposition the microcatheter for more complete coiling (Figure 3; see Video 1, Supplemental Digital Content 1, http://links.lww.com/NEU/A399). Complete aneurysm occlusion was achieved (Figure 4). The procedure was uneventful, and the patient was discharged to home the next morning on a daily regimen of aspirin (325 mg) and clopidogrel (75 mg).
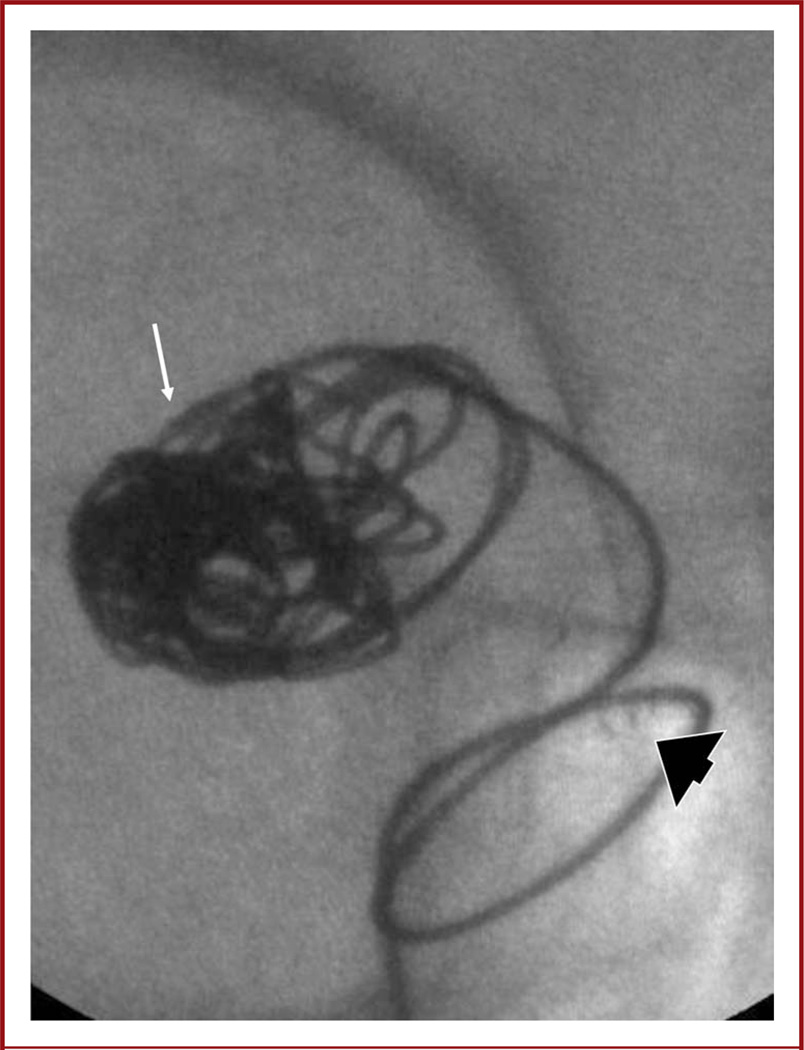
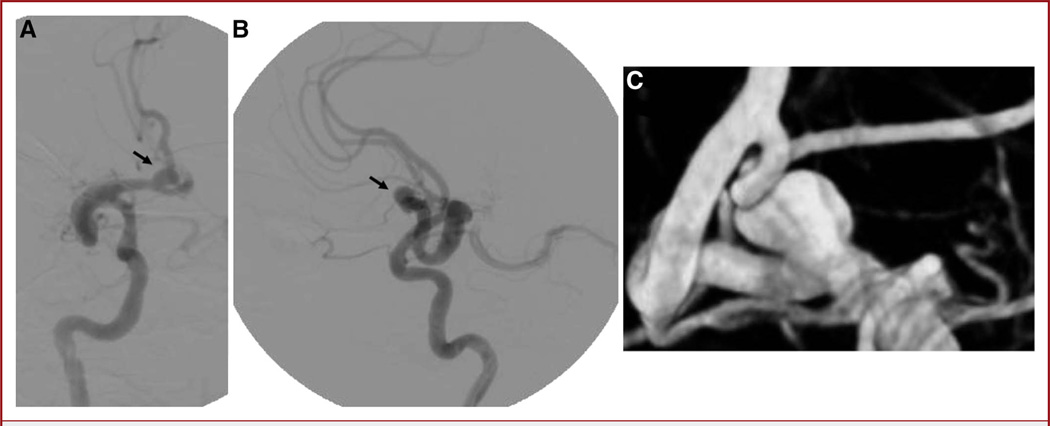
FIGURE 3.
Image from a microangiographic fluoroscope (MAF) (anteroposterior projection). The tip of the microcatheter (arrow) can be seen easily. Before coiling, the MAF was used for placement of an Enterprise stent. The stent markers (arrowhead) are easily visualized.
FIGURE 4.
Anteroposterior (A) and lateral (B) digital subtraction angiography images show no residual aneurysm filling and a widely patent stented internal carotid artery.
Case 2
A 53-year-old woman presented as an outpatient with headaches and a history of a fall down multiple steps 7 months earlier. Her workup included a cranial CT scan (not available), and CTA that demonstrated a possible dissection in the right ICA and middle cerebral artery in addition to an anterior communicating artery aneurysm. She was neurologically intact.
The patient initially underwent a CTA that demonstrated the lesions, followed by a subsequent diagnostic cerebral angiogram (DSA) that confirmed a 5.3 × 6.5-mm anterior communicating artery aneurysm that was supplied by the right carotid artery. On the basis of 2- and 3-dimensional DSA (Figure 5), the aneurysm was found to have a reasonably narrow neck, and the patient was deemed a good candidate for endovascular coil embolization. The options of surgical clipping as well as endovascular embolization were discussed with the patient and her family, and it was decided to proceed with coiling. The patient was started on a daily regimen of clopidogrel (75 mg) and aspirin (325 mg) 1 week before the procedure (in case stenting was necessary) and was found to have adequate platelet responses before performance of the procedure.
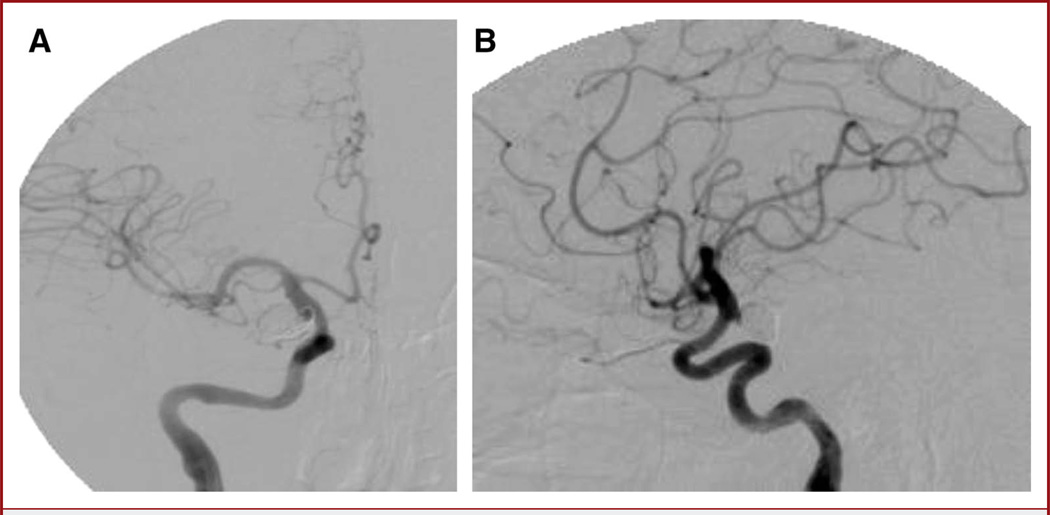
FIGURE 5.
Anteroposterior (A) and lateral (B) pretreatment digital subtraction angiography images before coiling reveal a serpiginous and likely chronic dissection of the right middle cerebral artery (MCA). The right MCA ends in a blind pouch and curves around to give rise to the right anterior cerebral artery. The A1–A2 junction aneurysm can be seen on both projections (arrows). C, 3-dimensional angiography reveals a reasonably narrow neck for attempted primary coiling.
The endovascular procedure was performed with the patient under conscious sedation and after femoral artery access had been established. Using a 6-French Envoy guide catheter (Codman & Shurtleff, Inc.), a Prowler Select Plus microcatheter was first advanced over a Synchro-2 standard microwire (Boston Scientific) past the aneurysm and left in the parent artery in preparation for stenting if necessary. The Prowler Select LPES 45-degree microcatheter and Synchro-2 microwire were then used to catheterize the aneurysm. The MAF was integral in this case to visualize small kickbacks of the microcatheter during coiling (Figure 6) to readjust the catheter before losing access to aid in accurate placement and deployment of the coils within the dome of the aneurysm (see Video 2, Supplemental Digital Content 2, http://links.lww.com/NEU/A400). On the basis of the posttreatment DSA images (Figure 7), we concluded that the treatment was complete. DSA acquired with MAF allowed for excellent visualization of the parent artery, giving added confidence that stenting would not be necessary, and there was no need for additional radiation dose or contrast injections that an extra cone-beam CT or contrast-based CT scan would have entailed. The ability to perform primary aneurysm coiling without stenting enabled us to stop the patient’s clopidogrel, which she would have been maintained on for 3 months had a stent been placed; moreover, it prevented the added risk and expense of an intracranial stent. There was no significant residual aneurysm filling at the end of the case and the patient was discharged home on postoperative day 1 on aspirin monotherapy.
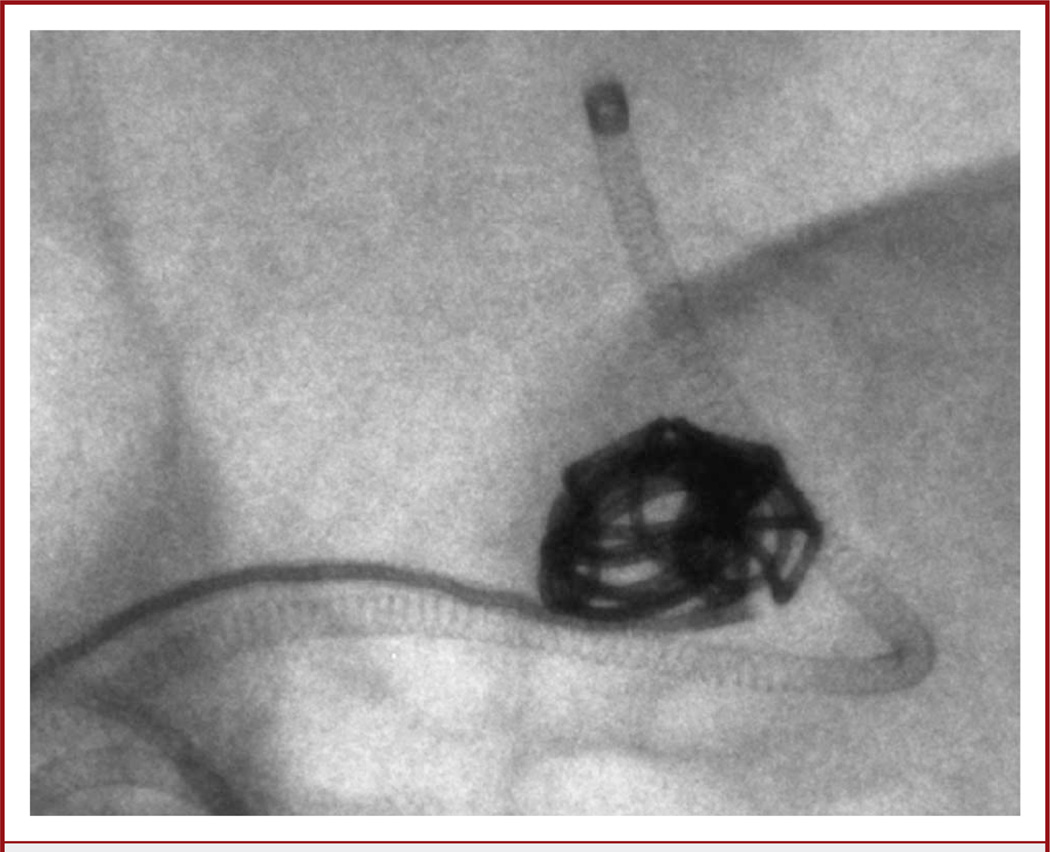
FIGURE 6.
Fluoroscopic snapshot using a microangiographic fluoroscope shows individual coils and accurate catheter positioning with respect to the coil mass.
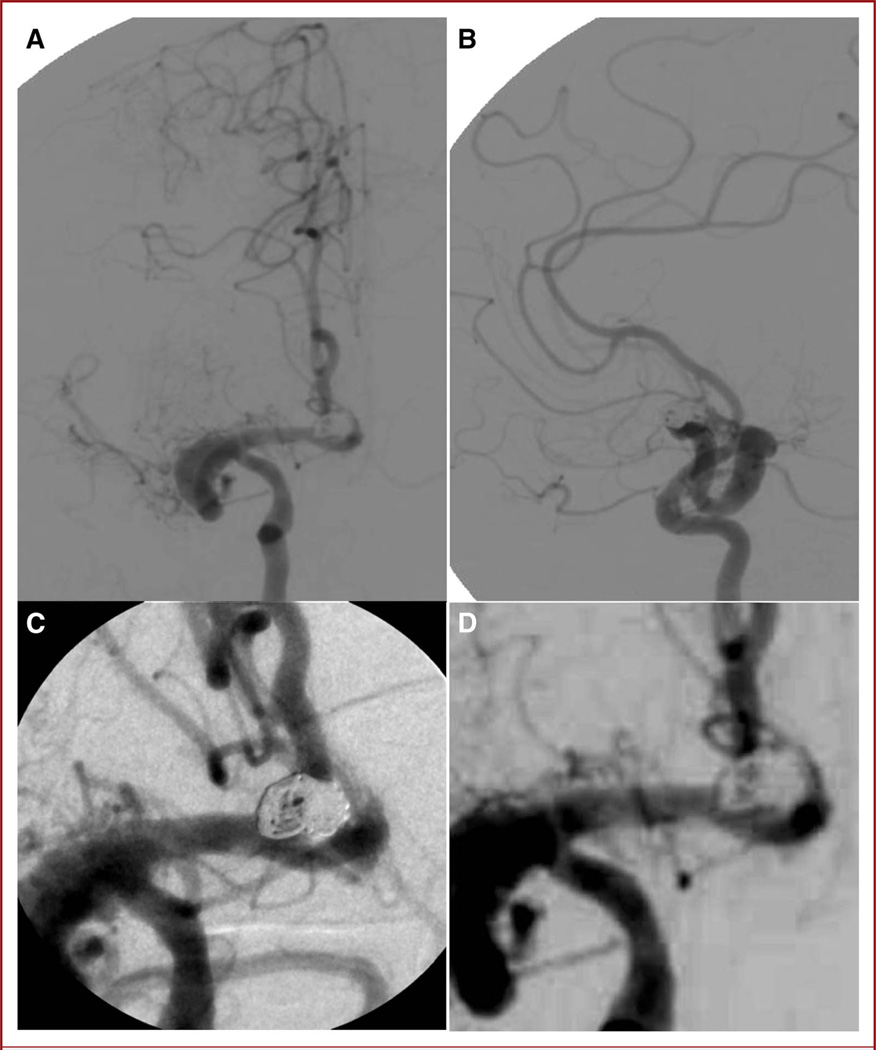
FIGURE 7.
Anteroposterior (A) and lateral (B) posttreatment digital subtraction angiography (DSA) images acquired with the standard x-ray image intensifier demonstrate no residual filling of the aneurysm. Microangiographic fluoroscope (C) and image intensifier (D) images show comparison of the DSA runs between the 2 detectors.
DISCUSSION
Major advances in imaging have been made since Roentgen’s discovery of x-rays. DSA was first discovered and developed by Mistretta et al4 in 1973 and was introduced into clinical use in 1980.5 Digital road-mapping and rotational cone-beam CT DSA have further improved image guidance for neurointerventions.6 These advances in computer imaging technology as well as the evolution of catheter-based technology and techniques have allowed neurointerventionists to treat a wide variety of intracranial pathologies with greater accuracy and precision. As microcatheter and microcoil technology advances, thereby allowing treatment of lesions in small intracranial arteries, continuing advances in imaging technology are necessary for treatment of lesions in increasingly smaller intracranial arteries.
Microangiography is one of the newest tools in our armamentarium to aid in visualization of intracranial vessels. To date, it has been used for coiling of one unruptured aneurysm and stent-assisted coiling of a second unruptured aneurysm. Currently, endovascular treatment of intracranial aneurysms seems its most useful application. The smallest movements of the microcatheters and coils can be seen, allowing the operator to visualize kickback of the catheter before it actually kicks out of the aneurysm. The microcatheter can be repositioned with great precision, which is especially helpful when repositioning the microcatheter within small intra-aneurysmal pockets. Small movements of the catheter tip can be better observed with the MAF than with the images offered by conventional systems. If the coil mass is very large, visualization of the catheter tip is indeed difficult; using the MAF, we are able to see the position of the tip through the small spaces between the coils; however, this is not in the case with the standard x-ray image intensifier system because of its much lower resolution (Video 2).
Microangiography can be used without a significant delay in the procedure, as the MAF is simply a unit that swings into place on the standard AP fluoroscope. We have also used microangiography for surveillance angiography of a previously stent-coiled aneurysm (M.J. Binning, personal communication, September 2010). The MAF was useful in evaluating for residual filling, although recoiling would not have been performed if filling could have been seen on microangiography alone because the small residual in this case would likely be too small to be of concern clinically. Other possible useful applications are evaluation and treatment of intracranial atherosclerosis and intracranial in-stent stenosis.
Preclinical investigations of the MAF system compared with standard x-ray imagers showed a vast improvement in the capability of the MAF over conventional commercial x-ray imaging systems.7–12 The current systems are limited by large pixel size and inherent detector noise. In the best case scenario (small focal spot, no scattering material, and no overlapping anatomic features), a standard commercial x-ray system (either with an x-ray image intensifier or a flat panel) may visualize 2 to 3 line pairs per millimeter, considering that for a pixel size of 150 to 200 µm the Nyquist frequency (the ultimate maximum spatial frequency) is 3.3 and 2.5 cycles per mm, respectively. Under similar conditions, the MAF will allow visualization of 8 to 10 line pairs per millimeter, considering that its Nyquist frequency for the 35-µm pixel is 14 cycles per mm. The expectation that the MAF system could offer improved image guidance was shown in the 2 cases reported here.
Although moderate geometrical magnification may work somewhat for conventional detectors by overcoming to some extent their inherent spatial resolution limitation, too much magnification results in geometric unsharpness caused by the finite focal spot size that degrades or blurs the image more than the potential benefit of image magnification overcoming the inherent detector resolution limits. The current flat panel system uses different processing techniques during dynamic imaging to enhance displayed images, such as temporal filtering and adaptive spatial filtering to reduce noise as well as edge-enhancement filters to make devices more visible. For the MAF, we have thus far implemented temporal filtering during fluoroscopy; other noise-filtering and edge-enhancement techniques are under development and will further improve the comparative quality of the MAF images.
When using the MAF, the effective radiation dose to the patient is decreased, whereas in the small FOV, the skin exposure could increase.3 The MAF pixel size is 35 µm, roughly 4 to 5 times less than the current x-ray imaging detectors. Theoretically, the number of photons per pixel should be increased somewhat to maintain adequate signal compared with noise response. However, there are a few important factors that influence the dose considerations:
It is envisioned that the MAF will be used only when the high-resolution detector is needed over a small area, such as during aneurysm coiling or placement of stents and other devices. In this case, generally a much smaller area is irradiated than is done using a conventional detector.
Because of the improved resolution and image quality, the accuracy of an interventional procedure may improve and may take a shorter amount of time, hence, reducing the total patient skin integrated dose.
Because of the small FOV, the skin dose can be spread if necessary by angulating the gantry slightly while keeping the region of intervention within the FOV. This assumes that the object of interest is not at the surface, which is consistent with most neurovascular pathological conditions. In actuality, our experience has been that it is often difficult to increase the exposure parameters because during fluoroscopy they were set to near their maximum, especially while maintaining the small focal spot in use. Thus, in practice, a substantially increased dose to the patient above the fluoroscopy parameters selected by the automatic exposure control of the commercial system cannot occur.
Other applications, such as visualization of the stent struts, are highly dependent on the anatomic background and the amount of bone in the path of the x-ray beam. There were instances when we could visualize briefly the stent structure with the given x-ray parameters. To visualize the stent struts more consistently, we may need to increase the x-ray parameters while keeping a small focal spot; however, as previously mentioned, the x-ray parameters were already near maximum for the commercial system.
The MAF is currently in its infancy, and further iterations of the system will likely be necessary to streamline its use during live interventions. For example, because the MAF changer was originally designed for single-plane use, the MAF must be physically swung down onto the AP fluoroscope by a technologist, which often requires moving the lateral fluoroscope temporarily to make room for the technologist and raising the image intensifier to make room for the MAF. This can affect the working views, which may need to be re-obtained once the MAF is in place. Overall, however, this process takes less than 2 minutes of actual on-table time. In addition, the applications for the MAF appear initially to be very specific; for example, it may be less useful for procedures to treat lesions that require a larger FOV, such as arteriovenous malformation embolization or cervical carotid artery stenting.
CONCLUSION
The MAF is a high-resolution detector developed for use in neurointerventional cases in which superior image quality over a small FOV is required. It has been used with success for coiling of 2 unruptured aneurysms at our institution. Although refinements are necessary, it shows promise as an important tool in improving the accuracy with which neurointerventionists can perform certain intracranial procedures.
Acknowledgments
The authors thank Paul H. Dressel, BFA, for videography and preparation of the illustrations and Debra J. Zimmer, AAS CMA-A, for editorial assistance.
Disclosures
Funding received for this study from NIH grants R01NS43924 and R01EB002873. IRB Project approval no. NSG0280702A. Dr Hopkins receives research support from NIH grants R01NS43924 and R01EB002873 (co-investigator), research study grants from Abbott (ACT 1 Choice), Boston Scientific (CABANA), Cordis (SAPPHIRE WW), and ev3/Covidien Vascular Therapies (CREATE) and a research grant from Toshiba (for the Toshiba Stroke Research Center); has an ownership/financial interest in AccessClosure, Boston Scientific, Cordis, Micrus, and Valor Medical; serves on the Abbott Vascular Speakers’ Bureau; receives honoraria from Bard, Boston Scientific, Cordis, and from the following for speaking at conferences: Complete Conference Management, Cleveland Clinic, and SCAI; receives royalties from Cordis (for the AngioGuard device); serves as a consultant to or on the advisory board for Abbott, AccessClosure, Bard, Boston Scientific, Cordis, Gore, Lumen Biomedical,Micrus, and Toshiba; and serves as the conference director for Nurcon Conferences/Strategic Medical Seminars LLC. Dr Ionita receives research support from NIH grants R01NS43924 and R01EB002873. Dr Jain receives research support from NIH grants R01NS43924 and R01EB002873. Dr Levy receives research support from NIH grants R01NS43924 and R01EB002873 (co-investigator), research grant support (principal investigator: Stent-Assisted Recanalization in acute Ischemic Stroke, SARIS), other research support (devices), and honoraria from Boston Scientific and research support from Codman & Shurtleff, Inc. and ev3/Covidien Vascular Therapies; has ownership interests in Intratech Medical Ltd. and Mynx/Access Closure; serves as a consultant on the board of Scientific Advisors to Codman & Shurtleff, Inc.; serves as a consultant per project and/or per hour for Codman & Shurtleff, Inc., ev3/Covidien Vascular Therapies, and TheraSyn Sensors, Inc.; and receives fees for carotid stent training from Abbott Vascular and ev3/Covidien Vascular Therapies. Dr Levy receives no consulting salary arrangements. All consulting is per project and/or per hour. Dr Rudin receives research support from NIH grants R01NS43924 and R01EB002873 (principal investigator) and equipment from Toshiba Corporation. Dr Siddiqui receives research support from NIH grants R01NS43924 and R01EB002873 (coinvestigator), research grants from the National Institutes of Health (co--principal investigator: NINDS 1R01NS064592-01A1, Hemodynamic induction of pathologic remodeling leading to intracranial aneurysms) and the University at Buffalo (Research Development Award); holds financial interests in Hotspur, Intratech Medical, StimSox, and Valor Medical; serves as a consultant to Codman & Shurtleff, Inc., Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, and Penumbra; belongs to the speakers’ bureaus of Codman & Shurtleff, Inc. and Genentech; serves on an advisory board for Codman & Shurtleff; and has received honoraria from American Association of Neurological Surgeons’ courses, an Emergency Medicine Conference, Genentech, Neocure Group LLC, and from Abbott Vascular and Codman & Shurtleff, Inc. for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. Dr Siddiqui receives no consulting salary arrangements. All consulting is per project and/or per hour. Drs Binning, Orion, Yashar, and Webb have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
ABBREVIATIONS
- AP
anteroposterior
- CT
computed tomography
- CTA
CT angiography
- DSA
digital subtraction angiography
- FOV
field of view
- ICA
internal carotid artery
- MAF
microangiographic fluoroscope
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the print text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.neurosurgery-online.com).
REFERENCES
- 1.Patel V, Hoffmann KR, Ionita CN, Keleshis C, Bednarek DR, Rudin S. Rotational micro-CT using a clinical C-arm angiography gantry. Med Phys. 2008;35(10):4757–4764. doi: 10.1118/1.2989989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Ionita CN, Keleshis C, et al. Progress in the development of a new angiography suite including the high resolution micro-angiographic fluoroscope (MAF), a control, acquisition, processing, and image display system (CAPIDS), and a new detector changer integrated into a commercial C-arm angiography unit to enable clinical use. Proc SPIE. 2010;7622(76225I):pii762251. doi: 10.1117/12.844909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill K, Bednarek DR, Rudin S. Evaluation of effective dose in region-of-interest neuroimaging (abstract) Med Phys. 2010;37(6):3118. [Google Scholar]
- 4.Mistretta CA, Ort MG, Kelcz F, Cameron JR, Siedband MP, Crummy AB. Absorption edge fluoroscopy using quasi-monoenergetic x-ray beams. Invest Radiol. 1973;8(6):402–412. [PubMed] [Google Scholar]
- 5.Mistretta CA, Crummy AB, Strother CM. Digital angiography: a perspective. Radiology. 1981;139(2):273–276. doi: 10.1148/radiology.139.2.7012918. [DOI] [PubMed] [Google Scholar]
- 6.Korner M, Weber CH, Wirth S, Pfeifer KJ, Reiser MF, Treitl M. Advances in digital radiography: physical principles and system overview. Radiographics. 2007;27(3):675–686. doi: 10.1148/rg.273065075. [DOI] [PubMed] [Google Scholar]
- 7.Ionita CN, Dohatcu A, Jain A, et al. Modification of the NEMA XR21-2000 cardiac phantom for testing of imaging systems used in endovascular image guided interventions. Proc Soc Photo Opt Instrum Eng. 2009;7258:72584R. doi: 10.1117/12.813533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain A, Kuhls-Gilcrist AT, Gupta SK, Bednarek DR, Rudin S. Generalized two-dimensional (2D) linear system analysis metrics (GMTF, GDQE) for digital radiography systems including the effect of focal spot, magnification, scatter, and detector characteristics. Proc Soc Photo Opt Instrum Eng. 2010;7622(76220K):pii76220K. doi: 10.1117/12.845293. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyprianou IS, Rudin S, Bednarek DR, Hoffmann KR. Study of the generalized MTF and DQE for a new microangiographic system. Proc Soc Photo Opt Instrum Eng. 2004;(5368):349–360. doi: 10.1117/12.533512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyprianou IS, Rudin S, Bednarek DR, Hoffmann KR. Generalizing the MTF and DQE to include x-ray scatter and focal spot unsharpness: application to a new microangiographic system. Med Phys. 2005;32(2):613–626. doi: 10.1118/1.1844151. [DOI] [PubMed] [Google Scholar]
- 11.Yadava GK, Kyprianou IS, Rudin S, Bednarek D, Hoffmann KR. Generalized performance evaluation of x-ray image intensifier compared with a micro-angiographic system. Proc Soc Photo Opt Instrum Eng. 2005;5745(1):419–429. doi: 10.1117/12.594593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadava GK, Rudin S, Kuhls-Gilcrist AT, Bednarek DR. Generalized objective performance assessment of a new high-sensitivity microangiographic fluoroscopic (HSMAF) imaging system. Proc Soc Photo Opt Instrum Eng. 2008;6913(69130u) doi: 10.1117/12.769808. nihpa68288. [DOI] [PMC free article] [PubMed] [Google Scholar]