Abstract
Background
Limb salvage surgery (LSS) with endoprosthetic replacement is the most common method of reconstruction following bone tumor resection in the adult population. The risk of a postoperative infection developing is high when compared with conventional arthroplasty and there are no appropriate guidelines for antibiotic prophylaxis.
Questions/purposes
We sought to answer the following questions: (1) What is the overall risk of deep infection and the causative organism in lower-extremity long-bone tumor surgery with endoprosthetic reconstruction? (2) What antibiotic regimens are used with endoprosthetic reconstruction? (3) Is there a correlation between infection and either duration of postoperative antibiotics or sample size?
Methods
We conducted a systematic review of the literature for clinical studies that reported infection rates in adults with primary bony malignancies of the lower extremity treated with surgery and endoprosthetic reconstruction. The search included articles published in English between 1980 and July 2011.
Results
The systematic literature review yielded 48 studies reporting on a total of 4838 patients. The overall pooled weighted infection rate for lower-extremity LSS with endoprosthetic reconstruction was approximately 10% (95% CI, 8%–11%), with the most common causative organism reported to be Gram-positive bacteria in the majority of cases. The pooled weighted infection rate was 13% after short-term postoperative antibiotics and 8% after long-term postoperative antibiotics. There was no correlation between sample size and infection rate.
Conclusions
Infection rates of 10% are high when compared with rates for conventional arthroplasty. Our results suggest that long-term antibiotic prophylaxis decreases the risk of deep infection. However, the data should be interpreted with caution owing to the retrospective nature of the studies.
Introduction
Limb salvage surgery (LSS) by means of tumor resection and reconstruction has become the standard of care in the treatment of long-bone sarcomas, resulting in limb salvage rates greater than 90% [32]. Endoprostheses provide several advantages over other reconstructive methods. They are readily available, can be appropriately sized to the patient, and are durable and immediately reliable, allowing patients to rapidly regain full weightbearing function. However, owing to the length and complexity of the surgical procedure, and the immunocompromised nature of patients receiving chemotherapy, the risk of endoprosthetic infection remains high in patients having LSS, regardless of the method of reconstruction [33, 46].
Deep infection after endoprosthetic limb reconstruction is a devastating complication and its treatment is costly to the patient and the healthcare system [7]. Patients who have a deep infection often require staged revision operations and long-term intravenous antibiotics. Infection can further delay adjuvant therapy. In approximately 20% of cases, deep infection ultimately results in limb salvage failure or amputation [32]. The risk of infection also can vary dramatically with tumor site. The tibia and femur (lower extremities), for example, are at a greater risk of having an infection develop than the humerus (upper extremities) [32]. Given the dire consequences associated with endoprosthetic infections, strategies to reduce infection risk and optimize quality of life are needed.
Prophylactic antibiotics are given routinely preoperatively and postoperatively to minimize the incidence of surgical site infections (SSIs). The standard guidelines for antibiotic prophylaxis in total joint arthroplasty, as outlined by the National Surgical Infection Prevention Program [7] and the American Academy of Orthopaedic Surgeons [4], state a single preoperative dose of antibiotics should be administered 60 minutes before the procedure and discontinued within 24 hours of the procedure completion. The preoperative dose, administered as close to the incision time as possible, was shown to be the most effective dose in preventing postoperative infections [7, 8]. However, there are currently no such guidelines or recommendations in place for endoprosthetic reconstruction in sarcoma surgery.
We performed a comprehensive and systematic review of the scientific literature to determine (1) the overall risk of deep infection and the bacterial causative organism in long-bone tumor resection operations with endoprosthetic reconstruction; (2) what antibiotic regimens are used following long-bone tumor surgery with endoprosthetic reconstruction; and (3) if is there a correlation between infection rate outcomes and antibiotic duration (short-term and long-term) or sample size.
Search Strategy and Criteria
We conducted a systematic literature search through MEDLINE®, EMBASE®, and all Evidence Based Medicine (EBM) Reviews databases, including Cochrane Database of Systematic Reviews, ACP Journal Club®, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment, and National Health Service (NHS) Economic Evaluation Database. The proceedings for past American Society for Clinical Oncology (ASCO) Annual Meetings also were searched for any abstracts containing the words sarcoma and endoprosthetic in the abstract body. The MEDLINE® and EMBASE® database searches were performed by combining exploded Medical Search Headings (MeSH® terms) and free text words using Boolean operators “OR” and “AND” as follows: (“prosthesis infection” OR “prosthesis related infections”) AND (“bone neoplasms” OR “bone tumor”) AND (“anti-infective agents” OR “antiinfective agents” OR “bacterial infections and mycoses” OR “infection”) AND (“prosthesis and implants” OR “prosthesis implantation” OR “prosthesis failure” OR “prosthesis and orthoses”) OR (“infection*.ti,ab” OR “antibiotic*.ti,ab” OR “anti-infectiv*.ti,ab” OR “antiinfectiv*.ti,ab”) OR (“septic.ti,ab” OR “sepsis.ti,ab”) AND (“prosthes*.ti,ab” OR “prosthetic*.ti,ab” OR “endoprosthe*.ti,ab” OR “endoprosthetic*.ti,ab”) AND ((“bone*.ti,ab” OR “osseous.ti,ab” OR “cartilage*.ti,ab”) AND (“cancer*.ti,ab” OR “neoplasm*.ti,ab” OR “tumor*.ti,ab” OR “tumour*.ti,ab”)). The EBM Reviews database search was performed in the same manner. The search was limited to articles published in the English language. No restrictions were placed on study types during the initial screening phase. All databases were searched from 1980 up to July 2011, and duplicates were eliminated. Studies with any sample sizes were included to maximize overall sample size. The studies were not assessed for quality as there are no reliable evaluation tools for retrospective case series.
Two independent reviewers (AR, TP) assessed the eligibility of each study. Any discrepancies in evaluation were discussed and reconciled accordingly. Studies that reported infection rates for long-bone resection and endoprosthetic reconstructions of malignant or benign aggressive neoplasms were included. Only studies concerning lower-extremity lesions in skeletally mature patients were considered for further review. Case reports were excluded. Studies where pooled infection rates included lesions from the upper extremities and/or pelvis were excluded. Similarly, studies where pooled infection rates included patients with allograft or allograft-prosthesis composite reconstructions were excluded. Studies that involved solely soft tissue sarcomas also were excluded, as were studies that reported infection rates exclusively in metastatic lesions, recurrent lesions, or lesions that had received prior surgical treatment.
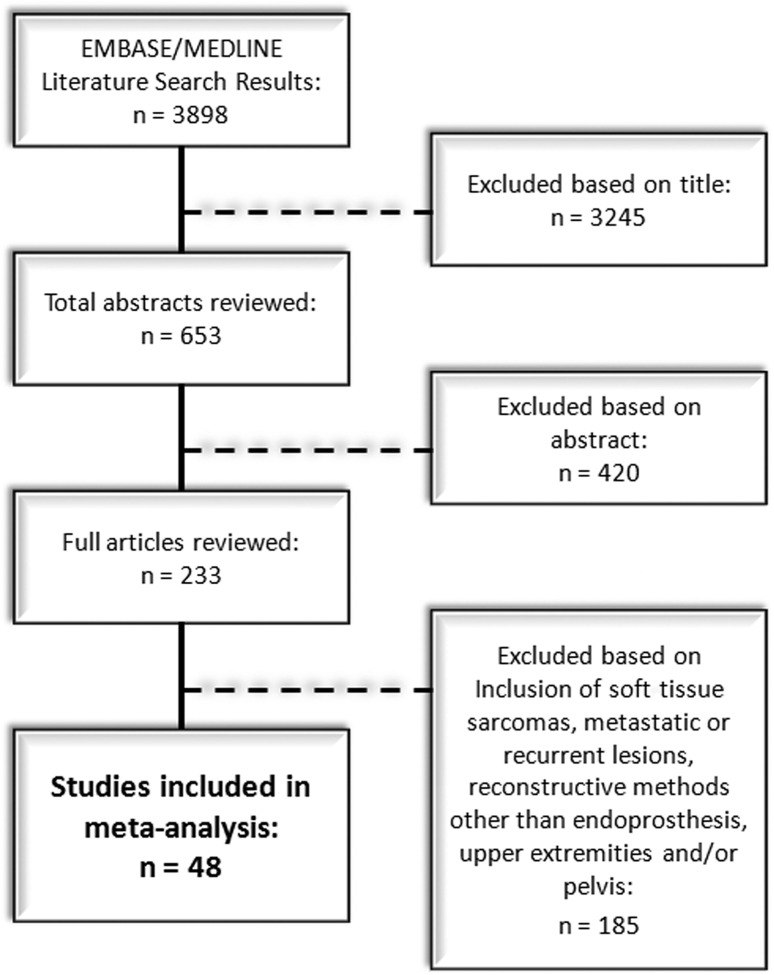
Based on these criteria, a search of the EBM Reviews database yielded no results. The ASCO database search generated 141 results; however, none of these results met our predefined inclusion criteria. The MEDLINE® and EMBASE® database searches initially generated 3898 titles (Fig. 1). Overall, 3245 studies were excluded on the basis of their titles, leaving 653 abstracts for review. These abstracts then were screened for applicability to our clinical question, after which 420 additional studies were excluded. Full-text articles then were retrieved and reviewed, in full, for the remaining 233 studies. Of these, 185 papers were further excluded for one, or a combination, of the following: inability to extract relevant data owing to the inclusion of a large number of ineligible soft tissue sarcomas, metastatic lesions, or recurrent lesions in the overall reported infection rates; inclusion of reconstructive methods other than an endoprosthesis pooled in the calculation of infection rates; and failure to report infection outcome based on tumor site (ie, upper extremities versus lower extremities versus pelvis) or pooled calculation of infection rates based on all reported sites. After these exclusions, a total of 48 studies were left for review [1–3, 5, 6, 10–13, 16, 17, 19–21, 23, 24, 28–31, 33–40, 42, 44–52, 54–63]. Publication dates of the included studies ranged from 1990 to 2011 and sample sizes varied from five to 1036.
Fig. 1.
A flow diagram illustrates the EMBASE® and MEDLINE® literature review.
We tested the heterogeneity among the studies using the Cochran Q test with a p value set at 0.1 for significance. The I2 statistic is reported, representing the percentage of total variation across studies owing to heterogeneity. We used a random-effects model (DerSimonian-Laird) that accounted for between-study heterogeneity owing to the inherent heterogeneity of case series to calculate the pooled weighted proportion. The pooled weighted proportion of infection, with 95% CI for single-group studies, is reported. We compared weighted proportions using normal approximation to binomial distribution when comparing short- versus long-duration antibiotic regimens, where reported. The Pearson correlation coefficient was used to determine the linear correlation between infection proportions and sample size of the included studies. We used StatsDirect 2.7 (StatsDirect Ltd, Cheshire, UK) for data analysis.
Results
All 48 studies included in our analysis were retrospective chart reviews that reported on infection after the excision of lower-extremity tumors (femur, tibia, and fibula) with endoprosthetic reconstruction (Table 1). A total of 4838 patients were included across all 48 studies. Three studies were retrospective comparative studies, while the remaining 45 were either retrospective noncomparative observational studies or retrospective case series. Based on these data, all 48 studies are considered Level IV evidence. The most common tumor diagnoses among these studies were osteosarcoma, Ewing’s sarcoma, chondrosarcoma, malignant fibrous histiocytoma, fibrosarcoma, and giant cell tumor of bone.
Table 1.
Systematic review of deep infection rates after lower-extremity tumor resection and endoprosthetic reconstruction
| Study | Year | Number of patients | Tumor site | Deep infection rate (%) |
|---|---|---|---|---|
| Lee and Baek [39] | 1990 | 17 | PF, DF, PT, PFib | 0.0 |
| Horowitz et al. [28] | 1991 | 12 | PT | 25.0 |
| Eckardt et al. [11] | 1991 | 68 | PF, DF, total F, PT | 1.5 |
| Roberts et al. [52] | 1991 | 133 | DF | 7.5 |
| Shih et al. [59] | 1993 | 61 | DF | 6.6 |
| Morris et al. [45] | 1995 | 31 | PF | 3.2 |
| Malawer and Chou [42] | 1995 | 51 | PF, DF, PT | 19.6 |
| Abudu et al. [2] | 1996 | 16 | F, T | 0.0 |
| Zehr et al. [63] | 1996 | 17 | PF | 5.9 |
| Abudu et al. [2] | 1999 | 5 | DT | 20.0 |
| Lee et al. [38] | 1999 | 5 | DT, DFib | 20.0 |
| Kawai et al. [36] | 1999 | 32 | DF, PT | 6.3 |
| Kabukcuoglu et al. [35] | 1999 | 54 | PF | 1.9 |
| Grimer et al. [20] | 1999 | 151 | PT | 18.5 |
| Natarajan et al. [48] | 2000 | 6 | DT | 16.7 |
| Ilyas et al. [31] | 2000 | 15 | PT | 13.3 |
| Donati et al. [10] | 2001 | 25 | PF | 4.2 |
| Ilyas et al. [29] | 2001 | 48 | DF | 8.3 |
| Wunder et al. [62] | 2001 | 64 | DF, PT | 6.3 |
| Anract et al. [5] | 2002 | 9 | DF, PT | 22.2 |
| Ilyas et al. [30] | 2002 | 15 | PF | 6.7 |
| Sokolov [61] | 2002 | 30 | DF, PT | 13.3 |
| Bickels et al. [6] | 2002 | 110 | DF | 5.5 |
| Griffin et al. [19] | 2005 | 99 | DF, PT | 10.1 |
| Natarajan et al. [50] | 2005 | 246 | DF | 6.9 |
| Jeys et al. [33] | 2005 | 1036 | DF, PF, total F, F diaphysis, T | 11.9 |
| Farid et al. [12] | 2006 | 52 | PF | 3.8 |
| Sharma et al. [56] | 2006 | 77 | DF | 7.8 |
| Orlic et al. [51] | 2006 | 82 | PF, DF, PT | 4.9 |
| Gosheger et al. [17] | 2006 | 199 | PF, DF, total F, PT | 13.6 |
| Akahane et al. [3] | 2007 | 11 | Around knee | 9.1 |
| Sim et al. [60] | 2007 | 50 | DF, PT | 12.0 |
| Finstein et al. [13] | 2007 | 62 | PF | 4.8 |
| Sharma et al. [57] | 2007 | 112 | PF, DF, PT | 9.8 |
| Myers et al. [47] | 2007 | 194 | PT | 19.6 |
| Myers et al. [46] | 2007 | 335 | DF | 9.6 |
| Gitelis et al. [16] | 2008 | 80 | DF, PT, PFib | 2.5 |
| Guo et al. [21] | 2008 | 104 | DF, PT | 6.7 |
| Jeys et al. [34] | 2008 | 530 | DF, PF, total F, F diaphysis, T | 12.8 |
| Shekkeris et al. [58] | 2009 | 6 | DT, ankle | 16.7 |
| Natarajan et al. [49] | 2009 | 17 | Total F | 11.8 |
| Sewell et al. [55] | 2009 | 22 | Total F | 0.0 |
| Lee et al. [37] | 2009 | 256 | DF, PT | 9.8 |
| Hanna et al. [23] | 2010 | 22 | F diaphysis | 4.5 |
| Morii et al. [44] | 2010 | 82 | PT, DT | 17.1 |
| Hardes et al. [24] | 2010 | 125 | PF, PT | 12.8 |
| Sewell et al. [54] | 2011 | 14 | T diaphysis | 7.1 |
| Li et al. [40] | 2011 | 50 | PF, DF, PT | 8.0 |
| 4838 (Total) | 10 (95% CI, 8%–11%) (Weighted average and CI) | |||
P = proximal; F = femur; D = distal; T = tibia; Fib = fibula.
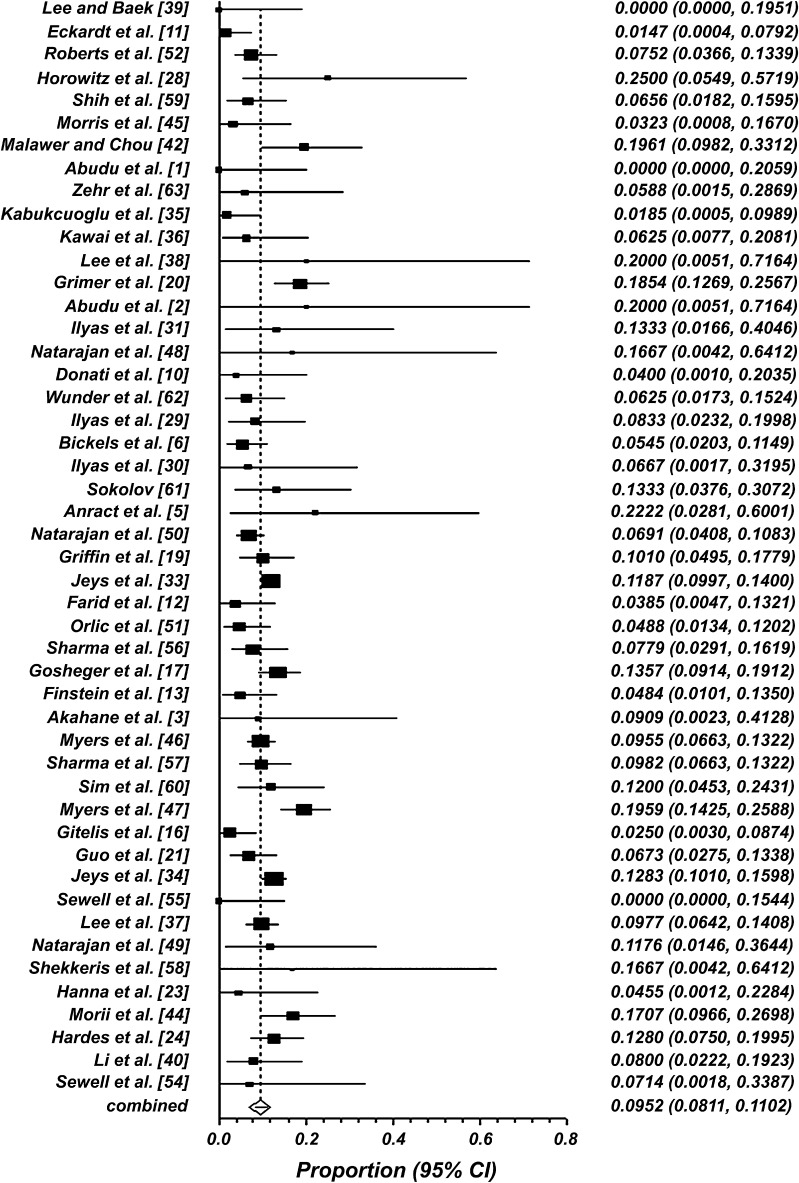
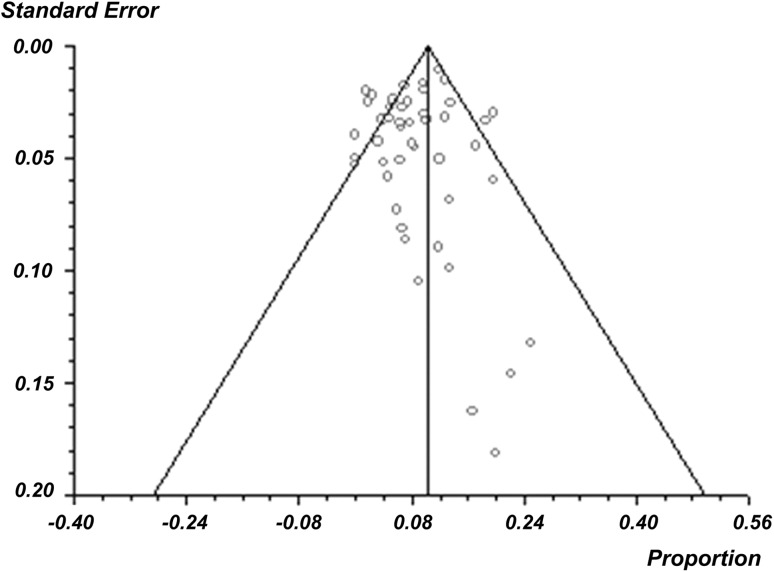
Infection rate outcomes across all studies ranged from 0% [1, 39, 55] to 25% [28] (Table 1). Overall, the pooled weighted infection rate was 10% (95% CI, 8%–11%) (Fig. 2). When the infection rates reported among the 48 studies were further assessed for publication bias using a funnel plot, no publication bias was uncovered, as the associated funnel plot appeared symmetric (Fig. 3). The Cochran Q and I2 statistics suggest significant heterogeneity in infection rates across all studies (p < 0.001). The I2 statistic, representing the percentage of total variation across studies owing to heterogeneity, was found to be moderate at 54.2% (95% CI, 33.3%–66.4%). Thirteen studies further specified the organism associated with infection [20, 21, 23, 24, 30, 31, 33, 37, 40, 44, 52, 55, 57]. The most common causative organisms were Staphylococcus aureus and coagulase-negative staphylococci, such as S. epidermis, all of which are Gram-positive.
Fig. 2.
A forest plot of deep infection rates using a random-effects model is shown. Overall, the pooled weighted infection rate is 10% (95% CI, 8%–11%).
Fig. 3.
A funnel plot of publication bias for included studies appears symmetric, suggesting there is no publication bias.
Of the 48 studies, 21 reported information on the antibiotic regimens administered during the course of the study [1, 2, 6, 11, 13, 17, 20, 23, 24, 30, 33, 40, 44–47, 51, 52, 55, 56, 58] (Table 2). There is considerable variation in the antibiotic regimens reported by these studies. Only seven studies specified the dose (ie, 1 g) and/or the type of prophylactic antibiotics administered (ie, first-, second-, or third-generation Gram-positive cephalosporin) [1, 2, 13, 17, 24, 33, 40]. Two studies specified giving additional coverage against Gram-negative bacteria as well [17, 24]. Twenty-seven of the 48 studies provided no details regarding the antibiotic prophylaxis used.
Table 2.
Systematic review of antibiotic regimens in lower-extremity tumor resection with endoprosthetic reconstruction
| Study | Year | Antibiotic regimens reported |
|---|---|---|
| Eckardt et al. [11] | 1991 | Intravenous antibiotics administered immediately before surgery; continued for 3–4 days postoperatively, until drains removed |
| Roberts et al. [52] | 1991 | Prophylactic antibiotics administered parenterally during surgery |
| Morris et al. [45] | 1995 | Intravenous antibiotics administered preoperatively; continued for 3 days postoperatively when drains removed |
| Abudu et al. [1] | 1996 | Continuous intravenous cefuroxime administered throughout procedure; no postoperative antibiotics given |
| Abudu et al. [2] | 1999 | Continuous intravenous antibiotics (usually cefuroxime) administered throughout surgery |
| Grimer et al. [20] | 1999 | Antibiotics administered at the induction of anesthesia but not repeated |
| Ilyas et al. [30] | 2002 | Antibiotics administered postoperatively for 72 hours |
| Bickels et al. [6] | 2002 | Intravenous prophylactic antibiotics continued until drainage tubes removed |
| Jeys et al. [33] | 2005 | Intravenous broad-spectrum cephalosporin administered preoperatively |
| Sharma et al. [56] | 2006 | Prophylactic antibiotics administered perioperatively for 48 hours |
| Gosheger et al. [17] | 2006 | Prophylactic antibiotics administered locally with a gentamicin-containing collagenous drug carrier; intravenous cephalosporin administered postoperatively for 3 days, followed by oral therapy until wound healed |
| Orlic et al. [51] | 2006 | Prophylactic antibiotics administered perioperatively according to hospital guidelines |
| Myers et al. [47] | 2007 | Prophylactic antibiotics administered at time of surgery; continued for up to 24 hours postoperatively |
| Finstein et al. [13] | 2007 | Intravenous antibiotics (1st-generation cephalosporin) administered preoperatively and for 3 days postoperatively, followed by 5 days of oral antibiotics; antibiotics given while drains in place |
| Myers et al. [46] | 2007 | Prophylactic antibiotics administered at time of surgery; continued for up to 24 hours postoperatively |
| Sewell et al. [55] | 2009 | Intravenous antibiotics continued for 3 days postoperatively |
| Shekkeris et al. [58] | 2009 | Intravenous antibiotics continued for 3 days postoperatively |
| Morii et al. [44] | 2010 | Antibiotics administered preoperatively within 2 hours of surgery; continued for > 72 hours postoperatively |
| Hanna et al. [23] | 2010 | Intravenous antibiotics continued for 3 days postoperatively |
| Hardes et al. [24] | 2010 | Antibiotics administered locally with a gentamicin-containing collagenous drug carrier; intravenous 3rd-generation cephalosporin administered for 3–7 days postoperatively, followed by a 2nd-generation cephalosporin administered orally until wound healed |
| Li et al. [40] | 2011 | Intravenous cephalosporin (1 g) administered intraoperatively; continued for 3 days postoperatively, then administered orally for an additional 5 days |
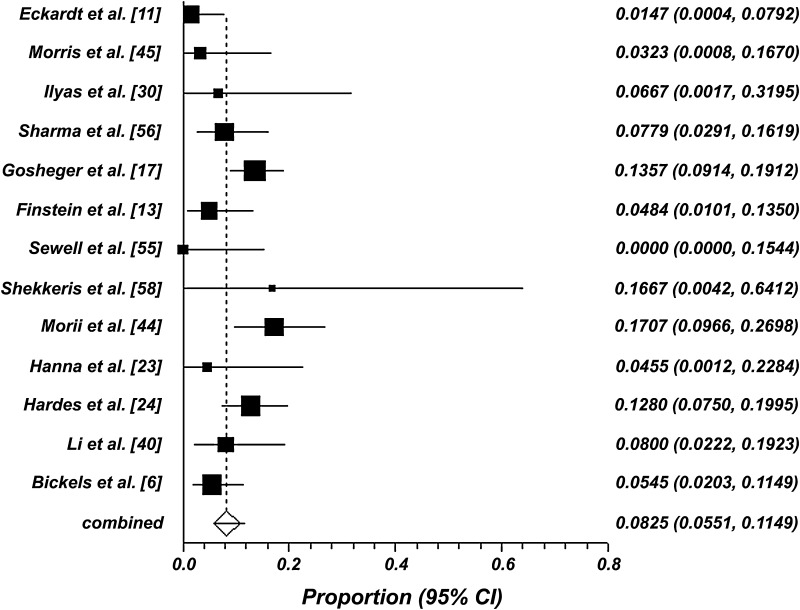
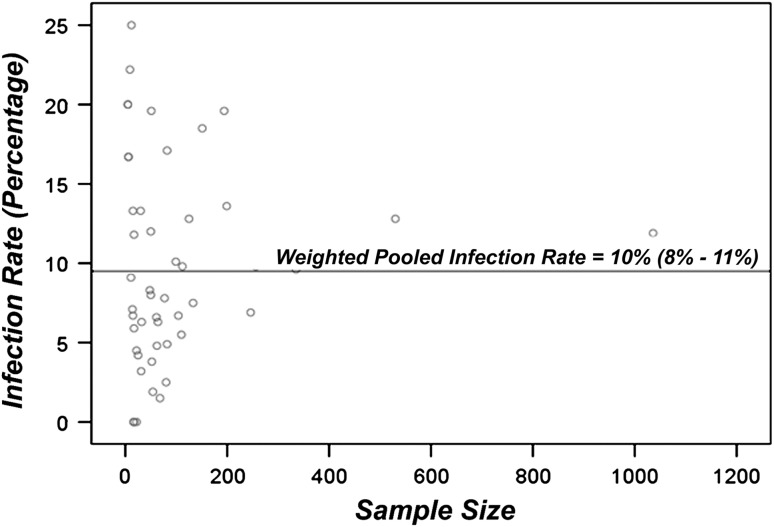
Twenty studies reported postoperative antibiotic regimens. These studies were further subdivided into short-term regimens (0 to 24 hours of postoperative antibiotics) and long-term regimens (greater than 24 hours of postoperative antibiotics) and compared. The pooled infection rate following short-term postoperative antibiotic prophylaxis was 13% (95% CI, 9% to 17%; p < 0.001) (Fig. 4), which is slightly higher than the overall pooled infection rate. The pooled infection rate for the long-term postoperative antibiotic prophylaxis was 8% (95% CI, 6% to 12%; p < 0.05) (Fig. 5), which is slightly lower than the overall pooled infection rate. This difference in the pooled infection rates following short-term and long-term postoperative antibiotics was statistically significant (p < 0.05). No further correlation (correlation coefficient, −0.05; p > 0.05) was found between sample size and infection rate among the 48 included studies (Fig. 6).
Fig. 4.
A forest plot of deep infection rates using a random-effects model is shown. The pooled weighted infection rate is 13% (95% CI, 9%–17%) following 0 to 24 hours of postoperative antibiotics.
Fig. 5.
A forest plot of deep infection rates using a random-effects model is shown. The pooled weighted infection rate is 8% (95% CI, 6%–12%) following greater than 24 hours of postoperative antibiotics.
Fig. 6.
A scatter plot of deep infection rates versus sample size is shown. No correlation is evident (correlation coefficient: −0.05).
Discussion
LSS with endoprosthetic replacement is the most common method of reconstruction in adults with sarcoma. However, the risk of having postoperative infections develop is relatively high in this immunocompromised patient population when compared to the risk with conventional arthroplasty. Currently, no guidelines exist for antibiotic prophylaxis in tumor surgery. We assessed the overall risk of deep infection after primary lower-extremity long-bone tumor surgery with endoprosthetic reconstruction, reviewed prophylactic antibiotic regimens used, and assessed if short-term or long-term antibiotics are better at reducing overall infection risk.
Within the studies included in our systematic review, there may be several factors that can influence infection outcomes. The most striking factor is the inconsistency in antibiotic prophylaxis used in each study. These observations are in line with the results of a previous survey which revealed similar inconsistencies in practice among orthopaedic oncologists [25]. Another important fact to consider when examining infection rates is that the majority of studies included in this systematic review are retrospective studies, with some included cases dating to the late 1960s and early 1970s [12, 35, 46, 52, 59, 63]. Moreover, it is likely that there exists a certain degree of patient overlap among studies produced by the same group of authors; however it is not possible to determine the exact amount of overlap based on the information provided. It also is unknown whether the definition of infection remained constant during the course of these studies. For example, definitions may have varied from erythema at the incision site to sepsis from an infected endoprosthetic device. In addition, not all studies included in this systematic review defined infection in the same way. In 1992, the CDC defined deep SSI as infection occurring within 1 year of the operative procedure, if an implant is in place [27]. According to the CDC, infection must be related to the surgical procedure and must include the skin incision, fascia, or muscle layers opened or manipulated during surgery [27]. The patient also must have purulent drainage, an organism-positive fluid or tissue culture, an abscess, or any other evidence of infection on direct examination or by histopathologic or radiographic measures [27]. Only two studies defined infection according to the CDC; however, neither study limited infection to 1 year after surgery [24, 44]. For the remainder of the studies, efforts were made to separate deep infection from superficial infection, where distinctions were made. Finally, none of the included studies reported on white-blood cell count, absolute neutrophil count, nutritional parameters, or the use of growth factors. Therefore the importance of these factors could not be determined. However, there is a multicenter prospective randomized study underway that may answer these questions as such important data points will be prospectively included [15].
In our systematic review, infection rates varied from 0% to 25%, with an overall infection risk of approximately 10% in this patient population. This rate is high compared with those of conventional knee and hip arthroplasties, which are reported to be between 0.5% and 2% [32, 40]. The high infection rate among orthopaedic oncology patients is not surprising, however, and can be attributed to numerous factors. For instance, orthopaedic oncology patients generally are immunocompromised as a result of preoperative chemotherapy. In addition, these patients undergo long and complex surgical procedures, are left with a large dead space after removal of bone and surrounding tissue, and have large wounds that often lack adequate soft tissue coverage. All of these factors contribute to an increased risk of having endoprosthetic infections develop after surgery. Therefore, not only do our findings support that orthopaedic oncology patients and patients having conventional arthroplasty are diverse populations with likely diverse prophylaxis needs, but they also support the increasing need to limit infections and establish guidelines for antibiotic prophylaxis in tumor surgery. Significant advances aimed at improving SSI rates have been made during the last few decades. These improvements include different prostheses (eg, silver-coated versus uncoated titanium) [18, 24]; enhanced soft tissue reconstruction techniques (eg, use of a gastrocnemius flap, a free tissue transfer, and/or antibiotic cement) [17, 20, 33, 47, 57]; use of laminar air flow and aspiration suits during surgery to minimize the number of airborne bacteria [14]; and, possibly, shorter surgical times at each site [26]. Without the ability to control for these variables, it is difficult to draw precise conclusions about current infection rates. As a result, our quoted SSI rate of 10% reflects the rate over two decades and, therefore, may not precisely reflect the current rate of infection, which is unknown. A multicenter prospective randomized study is underway to answer this question [15].
We found that antibiotic regimens reported in the studies varied substantially from center to center. Although most surgeons provide Gram-positive coverage, others also provide Gram-negative coverage. In addition, the length of administration of postoperative antibiotics varied from none to several days. These results are consistent with those of a recent survey of orthopaedic oncologists, which revealed there is no consensus on antibiotic prophylaxis in tumor surgery among experts in the field [25]. Moreover, the survey revealed that the current state of practice varies widely, particularly with respect to the duration of antibiotic administration [25]. Specifically, that survey found 46% of surgeons prescribe multiple days of antibiotics after tumor resection with endoprosthetic reconstruction, whereas 36% prescribe only 24 hours of postoperative antibiotics [25]. The survey also revealed, while antibiotics with Gram-positive coverage are routinely prescribed, 11% of surgeons surveyed prescribe additional coverage against Gram-negative bacteria [25]. Antibiotic overuse is associated with risks [22, 41, 53]; thus, it is imperative to develop evidence-based practice guidelines for antibiotic prophylaxis in tumor surgery.
Of the studies that do report antibiotic regimes, all provided preoperative treatment, while postoperative treatment ranged from none to 24 hours to 7 days or until surgical drains were removed. Studies comparing single-dose prophylaxis and multiple-dose prophylaxis, in a general surgery setting, have not shown any benefit to having the added doses [43]. Interestingly, our results support the use of long-term (greater than 24 hours) postoperative antibiotics in patients undergoing primary tumor surgery with endoprosthetic reconstruction. We also found that no correlation exists between infection rate outcomes and sample size. In conventional arthroplasty, infection outcomes generally are lower in higher volume centers [9], whereas our results show that in tumor surgery, volume has little effect on infection outcomes. However, these data must be interpreted with caution given the retrospective nature of these studies. Accordingly, a multicenter randomized controlled trial is currently underway to determine whether long- versus short-term antibiotic prophylaxis is more effective in preventing deep infection in this patient population with increased susceptibility to bacteremia [15].
The results of this systematic review indicate current infection rates are high in lower-extremity tumor surgery and common practice varies with respect to antibiotic prophylaxis. Moreover, the results of this review suggest that long-term antibiotic prophylaxis is more effective at minimizing infection risk in patients with lower extremity long-bone tumors that require surgery and endoprosthetic reconstruction. However, these comparative data should be interpreted with caution given the retrospective nature of the included studies. Randomized controlled trials, such as the PARITY Trial [15], will provide higher-level evidence to answer the important clinical questions asked in this study.
Acknowledgments
We thank Mike Fraumeni for technical assistance with the systematic database search.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Abudu A, Carter SR, Grimer RJ. The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br. 1996;78:652–657. [PubMed] [Google Scholar]
- 2.Abudu A, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement of the distal tibia and ankle joint for aggressive bone tumours. Int Orthop. 1999;23:291–294. doi: 10.1007/s002640050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akahane T, Shimizu T, Isobe K, Yoshimura Y, Fujioka F, Kato H. Evaluation of postoperative general quality of life for patients with osteosarcoma around the knee joint. J Pediatr Orthop B. 2007;16:269–272. doi: 10.1097/BPB.0b013e3280925670. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Orthopaedic Surgeons. Antibiotic Prophylaxis for Bacteremia in Patients with Joint Replacements. Information Statement 1033. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010. [PubMed]
- 5.Anract P, Missenard G, Jeanrot C, Dubois V, Tomeno B. Knee reconstruction with prosthesis and muscle flap after total arthrectomy. Clin Orthop Relat Res. 2001;384:208–216. doi: 10.1097/00003086-200103000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer MM. Distal femur resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235. doi: 10.1097/00003086-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Bratzler DW, Houck PM; Surgical Infection Prevention Guidelines Writers Workgroup; American Academy of Orthopaedic Surgeons; American Association of Critical Care Nurses; American Association of Nurse Anesthetists; American College of Surgeons; American College of Osteopathic Surgeons; American Geriatrics Society; American Society of Anesthesiologists; American Society of Colon and Rectal Surgeons; American Society of Health-System Pharmacists; American Society of PeriAnesthesia Nurses; Ascension Health; Association of periOperative Registered Nurses; Association for Professionals in Infection Control and Epidemiology; Infectious Diseases Society of America; Medical Letter; Premier; Society for Healthcare Epidemiology of America; Society of Thoracic Surgeons; Surgical Infection Society. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706–1715. [DOI] [PubMed]
- 8.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 9.Critchley RJ, Baker PN, Deehan DJ. Does surgical volume affect outcome after primary and revision knee arthroplasty? A systematic review of the literature. Knee. 2012;19:513–518. doi: 10.1016/j.knee.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Donati D, Zavatta M, Gozzi E, Giacomini S, Campanacci L, Mercuri M. Modular prosthetic replacement of the proximal femur after resection of a bone tumour: a long-term follow-up. J Bone Joint Surg Br. 2001;83:1156–1160. doi: 10.1302/0301-620X.83B8.12165. [DOI] [PubMed] [Google Scholar]
- 11.Eckardt JJ, Eilber FR, Rosen G, Mirra JM, Dorey FJ, Ward WG, Kabo JM. Endoprosthetic replacement for stage IIB osteosarcoma. Clin Orthop Relat Res. 1991;270:202–213. [PubMed] [Google Scholar]
- 12.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 13.Finstein JL, King JJ, Fox EJ, Ogilvie CM, Lackman RD. Bipolar proximal femoral replacement prostheses for musculoskeletal neoplasms. Clin Orthop Relat Res. 2007;459:66–75. doi: 10.1097/BLO.0b013e31804f5474. [DOI] [PubMed] [Google Scholar]
- 14.Franco JA, Baer H, Enneking WF. Airborne contamination in orthopedic surgery: evaluation of laminar air flow system and aspiration suit. Clin Orthop Relat Res. 1977;122:231–243. [PubMed] [Google Scholar]
- 15.Ghert M, Deheshi B, Holt G, Randall RL, Ferguson P, Wunder J, Turcotte R, Werier J, Clarkson P, Damron T, Benevenia J, Anderson M, Gebhardt M, Isler M, Mottard S, Healey J, Evaniew N, Racano A, Sprague S, Swinton M, Bryant D, Thabane L, Guyatt G, Bhandari M. PARITY Investigators Prophylactic antibiotic regimens in tumour surgery (PARITY) protocol for a multicentre randomised controlled study. BMJ Open. 2012;2:e002197. doi: 10.1136/bmjopen-2012-002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gitelis S, Yergler JD, Sawlani N, Schiff A, Shott S. Short and long term failure of the modular oncology knee prosthesis. Orthopedics. 2008;31:362. doi: 10.3928/01477447-20080401-10. [DOI] [PubMed] [Google Scholar]
- 17.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 18.Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, Gunsel A, Kemper FH, Winkelmann W, Von Eiff C. Silver-coated megaendoprostheses in a rabbit model: an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25:5547–5556. doi: 10.1016/j.biomaterials.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–79. doi: 10.1097/01.blo.0000180050.27961.8a. [DOI] [PubMed] [Google Scholar]
- 20.Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494. doi: 10.1302/0301-620X.81B3.9234. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Ji T, Yang R, Tang X, Yang Y. Endoprosthetic replacement for primary tumours around the knee: experience from Peking University. J Bone Joint Surg Br. 2008;90:1084–1089. doi: 10.1302/0301-620X.90B8.20240. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Biyani M, Khaira A. Vancomycin nephrotoxicity: myths and facts. Neth J Med. 2011;69:379–383. [PubMed] [Google Scholar]
- 23.Hanna SA, Sewell MD, Aston WJ, Pollock RC, Skinner JA, Cannon SR, Briggs TW. Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumours. J Bone Joint Surg Br. 2010;92:867–874. doi: 10.1302/0301-620X.92B6.23449. [DOI] [PubMed] [Google Scholar]
- 24.Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, Hauschild G, Ahrens H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 25.Hasan K, Racano A, Deheshi B, Farrokhyar F, Wunder J, Ferguson P, Holt G, Schwartz H, Petrisor B, Bhandari M, Ghert M. Prophylactic antibiotic regimens in tumor surgery (PARITY) survey. BMC Musculoskelet Disord. 2012;13:91. doi: 10.1186/1471-2474-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J. 2007;83:777–779. doi: 10.1136/pgmj.2007.057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. doi: 10.1086/646436. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz SM, Lane JM, Otis JC, Healey JH. Prosthetic arthroplasty of the knee after resection of a sarcoma in the proximal end of the tibia: a report of sixteen cases. J Bone Joint Surg Am. 1991;73:286–293. [PubMed] [Google Scholar]
- 29.Ilyas I, Kurar A, Moreau PG, Younge DA. Modular megaprosthesis for distal femoral tumors. Int Orthop. 2001;25:375–377. doi: 10.1007/s002640100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilyas I, Pant R, Kurar A, Moreau PG, Younge DA. Modular megaprosthesis for proximal femoral tumors. Int Orthop. 2002;26:170–173. doi: 10.1007/s00264-002-0335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilyas I, Younge D, Pant R, Moreau P. Limb salvage for proximal tibial tumours using a modular prosthesis. Int Orthop. 2000;24:208–211. doi: 10.1007/s002640000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeys L, Grimer R. The long-term risks of infection and amputation with limb salvage surgery using endoprostheses. Recent Results Cancer Res. 2009;179:75–84. doi: 10.1007/978-3-540-77960-5_7. [DOI] [PubMed] [Google Scholar]
- 33.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87:842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 34.Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271. doi: 10.2106/JBJS.F.01324. [DOI] [PubMed] [Google Scholar]
- 35.Kabukcuoglu Y, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement for primary malignant tumors of the proximal femur. Clin Orthop Relat Res. 1999;358:8–14. doi: 10.1097/00003086-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon DG. A rotating-hinge knee replacement for malignant tumors of the femur and tibia. J Arthroplasty. 1999;14:187–196. doi: 10.1016/S0883-5403(99)90124-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee JA, Kim MS, Kim DH, Lim JS, Park KD, Cho WH, Song WS, Lee SY, Jeon DG. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol. 2009;16:147–151. doi: 10.1245/s10434-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Kim HS, Park YB, Rhie TY, Lee HK. Prosthetic reconstruction for tumours of the distal tibia and fibula. J Bone Joint Surg Br. 1999;81:803–807. doi: 10.1302/0301-620X.81B5.9588. [DOI] [PubMed] [Google Scholar]
- 39.Lee SY, Baek GH. Limb-salvage operations in primary malignant tumors of the bone: interim report. J Korean Med Sci. 1990;5:205–212. doi: 10.3346/jkms.1990.5.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Moretti VM, Ashana AO, Lackman RD. Perioperative infection rate in patients with osteosarcomas treated with resection and prosthetic reconstruction. Clin Orthop Relat Res. 2011;469:2889–2894. doi: 10.1007/s11999-011-1877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin CM, Chen YM, Po HL, Hseuh IH. Acute neurological deficits caused by cefipime: a case report and review of literature. Acta Neurol Taiwan. 2006;15:269–272. [PubMed] [Google Scholar]
- 42.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 43.McDonald M, Grabsch E, Marshall C, Forbes A. Single- versus multiple-dose antimicrobial prophylaxis for major surgery: a systematic review. Aust N Z J Surg. 1998;68:388–396. doi: 10.1111/j.1445-2197.1998.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 44.Morii T, Yabe H, Morioka H, Beppu Y, Chuman H, Kawai A, Takeda K, Kikuta K, Hosaka S, Yazawa Y, Takeuchi K, Anazawa U, Mochizuki K, Satomi K. Postoperative deep infection in tumor endoprosthesis reconstruction around the knee. J Orthop Sci. 2010;15:331–339. doi: 10.1007/s00776-010-1467-z. [DOI] [PubMed] [Google Scholar]
- 45.Morris HG, Capanna R, Del Ben M, Campanacci D. Prosthetic reconstruction of the proximal femur after resection for bone tumors. J Arthroplasty. 1995;10:293–299. doi: 10.1016/S0883-5403(05)80177-9. [DOI] [PubMed] [Google Scholar]
- 46.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 47.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89:1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 48.Natarajan MV, Annamalai K, Williams S, Selvaraj R, Rajagopal TS. Limb salvage in distal tibial osteosarcoma using a custom mega prosthesis. Int Orthop. 2000;24:282–284. doi: 10.1007/s002640000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Natarajan MV, Balasubramanian N, Jayasankar V, Sameer M. Endoprosthetic reconstruction using total femoral custom mega prosthesis in malignant bone tumours. Int Orthop. 2009;33:1359–1363. doi: 10.1007/s00264-009-0737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natarajan MV, Sivaseelam A, Ayyappan S, Bose JC, Sampath Kumar M. Distal femoral tumours treated by resection and custom mega-prosthetic replacement. Int Orthop. 2005;29:309–313. doi: 10.1007/s00264-005-0677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30:458–464. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts P, Chan D, Grimer RJ, Sneath RS, Scales JT. Prosthetic replacement of the distal femur for primary bone tumours. J Bone Joint Surg Br. 1991;73:762–769. doi: 10.1302/0301-620X.73B5.1894662. [DOI] [PubMed] [Google Scholar]
- 53.Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 54.Sewell MD, Hanna SA, McGrath A, Aston WJ, Blunn GW, Pollock RC, Skinner JA, Cannon SR, Briggs TW. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg Br. 2011;93:1111–1117. doi: 10.1302/0301-620X.93B8.25750. [DOI] [PubMed] [Google Scholar]
- 55.Sewell MD, Spiegelberg BG, Hanna SA, Aston WJ, Bartlett W, Blunn GW, David LA, Cannon SR, Briggs TW. Total femoral endoprosthetic replacement following excision of bone tumours. J Bone Joint Surg Br. 2009;91:1513–1520. doi: 10.1302/0301-620X.91B11.21996. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28–32. doi: 10.1097/01.blo.0000229316.66501.fc. [DOI] [PubMed] [Google Scholar]
- 57.Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res. 2007;459:54–59. doi: 10.1097/BLO.0b013e3180514c8e. [DOI] [PubMed] [Google Scholar]
- 58.Shekkeris AS, Hanna SA, Sewell MD, Spiegelberg BG, Aston WJ, Blunn GW, Cannon SR, Briggs TW. Endoprosthetic reconstruction of the distal tibia and ankle joint after resection of primary bone tumours. J Bone Joint Surg Br. 2009;91:1378–1382. doi: 10.1302/0301-620X.91B10.22643. [DOI] [PubMed] [Google Scholar]
- 59.Shih LY, Sim FH, Pritchard DJ, Rock MG, Chao EY. Segmental total knee arthroplasty after distal femoral resection for tumor. Clin Orthop Relat Res. 1993;292:269–281. [PubMed] [Google Scholar]
- 60.Sim IW, Tse LF, Ek ET, Powell GJ, Choong PF. Salvaging the limb salvage: Management of complications following endoprosthetic reconstruction for tumours around the knee. Eur J Surg Oncol. 2007;33:796–802. doi: 10.1016/j.ejso.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Sokolov T. Prosthetic knee replacement after resections for tumors. Ortopediya i Travmatologiya. 2002;38:151–160. [Google Scholar]
- 62.Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99; discussion 100. [DOI] [PubMed]
- 63.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. doi: 10.1097/00003086-199601000-00026. [DOI] [PubMed] [Google Scholar]