Abstract
Background
Manual techniques of reproducing a preoperative plan for primary bone tumor resection using rudimentary devices and imprecise localization techniques can result in compromised margins or unnecessary removal of unaffected tissue. We examined whether a novel technique using computer-generated custom jigs more accurately reproduces a preoperative resection plan than a standard manual technique.
Description of Technique
Using CT images and advanced imaging, reverse engineering, and computer-assisted design software, custom jigs were designed to precisely conform to a specific location on the surface of partially skeletonized cadaveric femurs. The jigs were used to perform a hemimetaphyseal resection.
Methods
We performed CT scans on six matched pairs of cadaveric femurs. Based on a primary bone sarcoma model, a joint-sparing, hemimetaphyseal wide resection was precisely outlined on each femur. For each pair, the resection was performed using the standard manual technique on one specimen and the custom jig-assisted technique on the other. Superimposition of preoperative and postresection images enabled quantitative analysis of resection accuracy.
Results
The mean maximum deviation from the preoperative plan was 9.0 mm for the manual group and 2.0 mm for the custom-jig group. The percentages of times the maximum deviation was greater than 3 mm and greater than 4 mm was 100% and 72% for the manual group and 5.6% and 0.0% for the custom-jig group, respectively.
Conclusions
Our findings suggest that custom-jig technology substantially improves the accuracy of primary bone tumor resection, enabling a surgeon to reproduce a given preoperative plan reliably and consistently.
Introduction
With currently available advanced imaging modalities, orthopaedic surgeons can precisely identify boundaries of primary bone tumors before surgical resection [11, 26]. In theory, the surgeon can outline an accurate, ideal resection plan preoperatively to satisfy basic oncologic resection principles [29, 30] and maximize preservation of normal tissue. In reality, however, the standard tools and techniques used by orthopaedic surgeons can substantially limit the ability to reliably and consistently reproduce the ideal preoperative plan at the time of resection [6] with potentially serious consequences. For instance, the surgeon may inadvertently cut into the tumor during surgery. In the case of an osteogenic sarcoma [24], such imprecision can increase rates of local recurrence and mortality [3–5, 23]. Alternatively, the surgeon may opt to resect substantially more normal tissue to avoid tumor disruption. The latter approach satisfies the basic oncologic principles, but the resection of substantially more normal tissue can substantially affect functional outcome [2].
Custom jigs are currently used in knee arthroplasty to help the surgeon reproduce a well-defined preoperative plan at the time of surgery [9, 17]. The design of a custom jig typically is based on either preoperative CT or MRI scans. Constructed of plastic, the jig fits onto the bone surface in only one possible configuration, thereby orienting the surgeon to the proper bone location for correct jig placement. The jig can be designed to guide the path of surgical cutting tools, enabling the surgeon to exactly reproduce an ideal preoperative plan. To our knowledge, no group has reported on the use of custom-jig technology in orthopaedic oncology.
In this study on partially skeletonized cadaveric femurs, we developed and validated a novel technique for resection of primary bone sarcomas using computer-generated custom jigs. We evaluated whether a custom jig-assisted bone tumor resection technique yields substantial improvement in accurate and consistent reproduction of a well-defined preoperative plan compared with traditional manual resection techniques.
Surgical Technique
We first developed a detailed preoperative plan for six matched pairs of partially skeletonized cadaveric femurs. Each specimen was imaged using a CT scanner with 0.625-mm-thick axial slices (General Electric Healthcare, Tacoma, WA, USA). The images were reconstructed three-dimensionally using advanced imaging software (Mimics, Version 13.0; Materialise NV, Leuven, Belgium), and the three-dimensional model was imported into computer-aided design (CAD) software (Pro/Engineer Wildfire 5.0; Parametric Technology Corporation, Needham, MA, USA). The surgeon and engineer used the software to precisely outline the mentioned ideal joint-sparing hemimetaphyseal resection on all specimens.
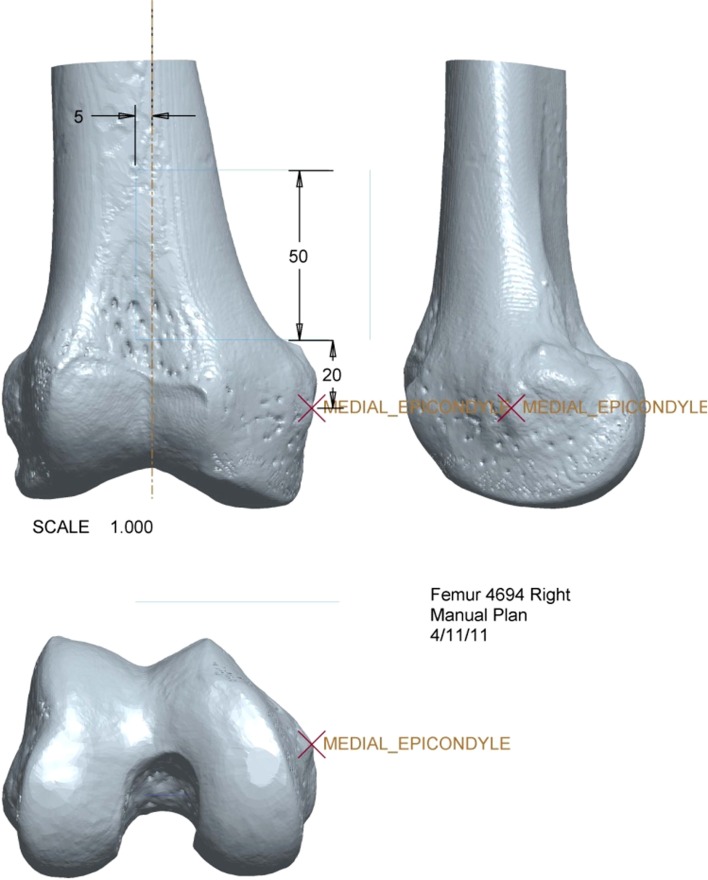
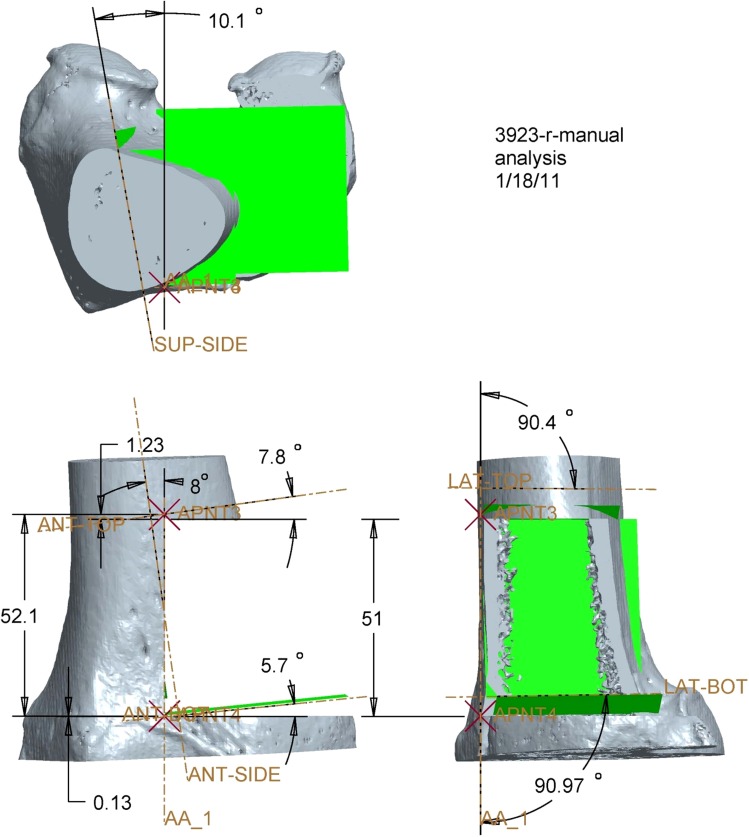
The medial epicondyle, distal medial articular surface, and anterior limit of the intercondylar notch were identified as anatomic landmarks on the three-dimensional reconstructed images of the specimen for use as reference points during surgery. The perpendicular distance from each anatomic landmark to the superior, inferior, and vertical target osteotomy planes was calculated (Fig. 1). The same resection dimensions and osteotomy plane locations with respect to anatomic landmarks were incorporated into manual and custom-jig preoperative plans. A printout of the preoperative plan served as a visual aid for the surgeons performing the manual procedure, similar to the procedure currently used by orthopaedic oncologists during traditional manual resections [31].
Fig. 1.

This is a three-dimensionally reconstructed CT image of a cadaveric femur with the distances from visible and/or palpable external landmarks depicted. The image was printed out for each manual resection specimen and used by the surgeon to perform the resection.
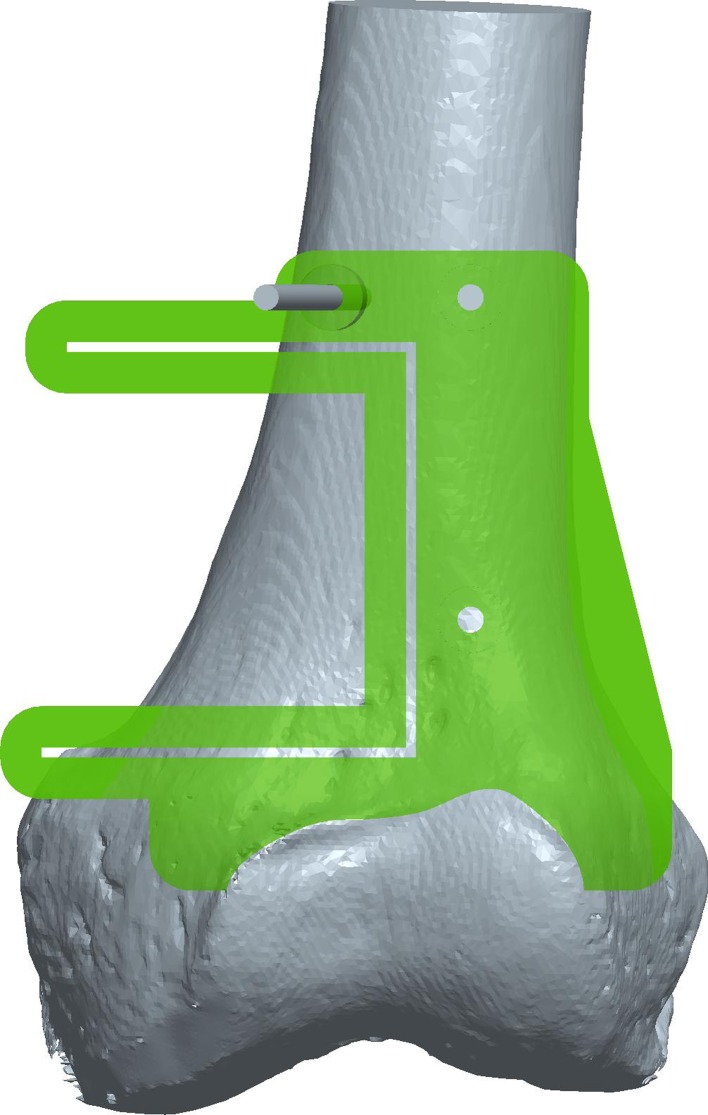
Using the preoperative plan and imaging data, we first created the custom jig virtually using CAD software (Fig. 2). The proximally based jig was collaboratively designed by an engineer with substantial custom-jig design experience and the surgeons, who helped to ensure the feasibility of the jig as a surgical tool. By subtracting the bony anatomy from the jig geometry with reverse engineering software (Geomagic Studio®; Geomagic, Inc, Research Triangle Park, NC, USA), the contacting surface of the jig was designed to conform to the bone surface in the region surrounding, but not superficial or into, the tumor. As an additional visual clue to assist in proper jig placement, the distal end of the jig was shaped to match to the superior ridge of the articular cartilage. Three perpendicular slots were created in the jig to accept a 0.89-mm-thick saw blade, creating a capture guide analogous to that used in traditional knee arthroplasty. Three holes, to accommodate 3.2-mm Steinmann pins placed outside the resection area, also were incorporated into the design for securing the jig in place while the surgeons performed the saw cuts.
Fig. 2.
This is a computer-aided design model of a computer-generated custom jig applied to the three-dimensionally reconstructed image of a cadaveric femur. The three holes depicted in the jig model allow for the introduction of Steinmann pins to secure the jig in place (for illustration, a pin is depicted in one of the three holes). The three slots built into the jig are designed to accept and capture a 0.89-mm-thick saw blade controlled by the surgeon. The placement and orientation of the slots are designed to precisely correspond to the desired resection plan as defined in the preoperative plan.
After finalization of the jig design and preoperative plans, the jig was fabricated using acrylonitrile butadiene styrene (ABS) plastic on a rapid prototyping machine (Dimension Elite; Stratasys, Inc, Eden Prairie, MN, USA). An ABS plastic reproduction of the specific cadaveric specimen on which each jig was based also was produced to facilitate familiarization with proper placement and seating of each jig on its corresponding bone before the procedure.
To perform the traditional manual resection, the surgeon identified appropriate anatomic landmarks on the cadaveric specimen and, guided by a printout of the preoperative plan, used a standard surgical (flexible metal) ruler and a marking pen to outline the three osteotomy planes (Fig. 3). A standard 0.89-mm-thick oscillating surgical saw then was used to create the three osteotomy planes of the resection.
Fig. 3.
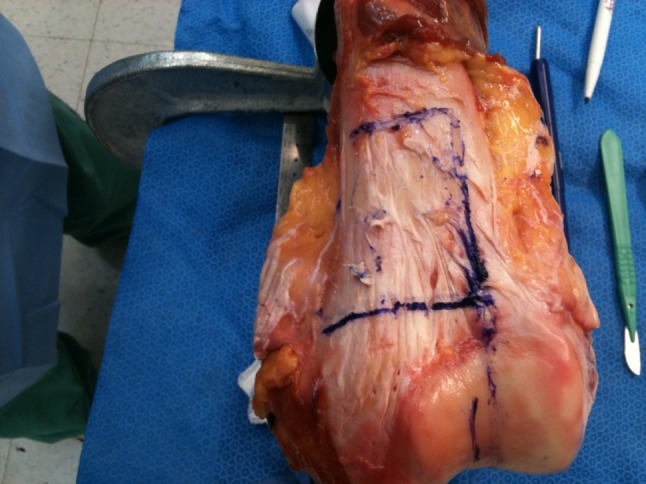
A joint-sparing hemimetaphyseal resection is outlined on a cadaveric femur using the traditional manual technique. Using a printout of the preoperative resection plan for this specific cadaveric femur as a reference, the surgeon used a standard flexible metal surgical ruler and a marking pen to outline each limb of the osteotomy with respect to key visible and/or palpable external landmarks. This replicates the manner in which traditional manual resections typically are performed.
For the custom jig-assisted resection, the soft tissue around the tumor (but not above or superficial to the tumor) was gently dissected back as a continuous sleeve from the bone such that it could be relaid over the bone in its original position after the resection; the periosteal sleeve was left on the bone. In this particular experiment, this dissection was relatively easy because the specimens were largely skeletonized. The jig then was applied to the bone. A three-dimensional plastic model of the cadaveric specimen was kept nearby for reference, as needed, to ensure that the jig was sitting correctly. Other visual clues such as the proximal ridge of articular cartilage and its position relative to the jig also were used to ensure adequate positioning of the jig (Fig. 4). The jig was applied with minimal uncertainty relative to its correct position on each of the six specimens. After appropriate seating, the jig was secured in a bicortical fashion with 3.2-mm Steinmann pins through holes designed in the jig for that purpose. Two orthopaedic oncology fellowship-trained surgeons then made the cuts through capture guide slots in the jig with a 0.89-mm-thick saw blade (Stryker, Kalamazoo, MI, USA). The resected bone, pins, and jig then were removed.
Fig. 4.
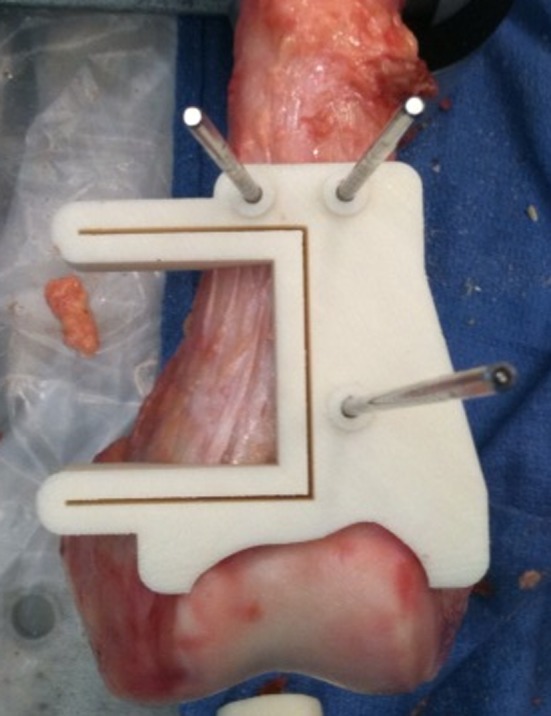
The photograph shows the computer-generated custom jig applied and secured with Steinmann pins to a cadaveric specimen. Because the jig was designed specifically to conform to one particular location on the distal femur, the surgeon could place the jig in the correct position by allowing it to key in to its appropriate location on the bone. To provide an additional visual clue to ensure its correct positioning, we designed the jig so that the shape of its distal surface corresponded to the proximal ridge of the articular cartilage.
Materials and Methods
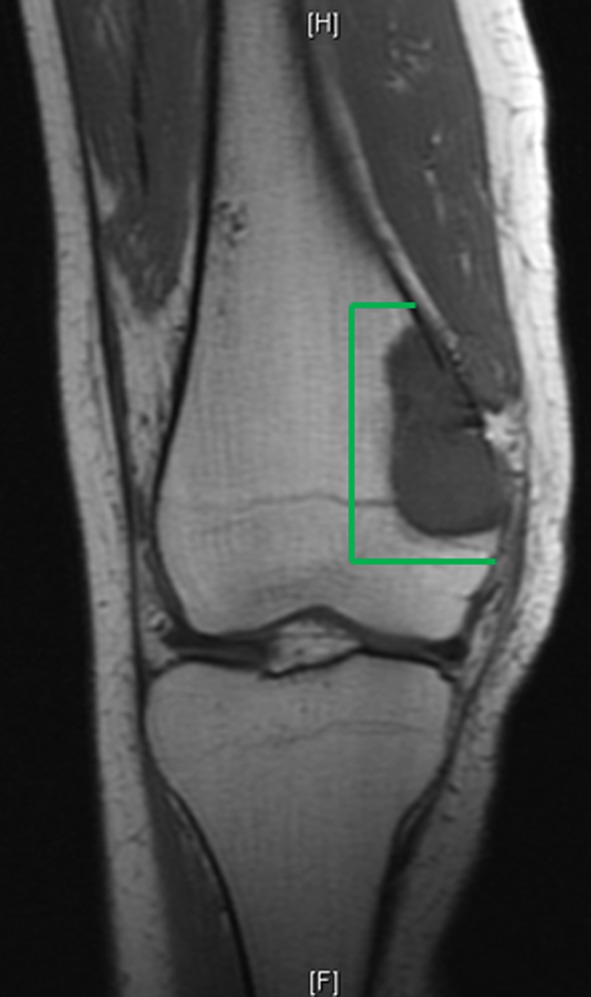
To compare manual and custom-jig resection accuracy, we used six matched pairs of cadaveric femurs. In each pair, one specimen was resected using the custom jig-assisted technique (custom-jig group); the other was resected using the traditional manual technique (manual group). We used a distal femur joint-sparing hemimetaphyseal resection as our model. After CT imaging of each cadaveric specimen, we detailed an exact preoperative resection plan for the joint-sparing resection and charged the surgeon with the task of identically reproducing this preoperative plan during surgery on the cadaveric specimen (Fig. 5). Development of the resection model was guided by MRI scans of an adolescent who presented to our clinic with a high-grade osteogenic sarcoma of the distal femur (Fig. 6); in this case, an ideal resection would remove the entire tumor en bloc with a surrounding cuff of normal tissue but spare the joint.
Fig. 5.
On this three-dimensionally reconstructed CT image of a cadaveric femur, a precisely defined preoperative plan of a joint-sparing hemimetaphyseal resection is outlined.
Fig. 6.
An MRI of a high-grade osteogenic sarcoma of the distal femur in a 16-year-old girl shows well-delineated tumor margins. An ideal resection, which removes the entire tumor en bloc along with a surrounding cuff of normal tissue and spares the articular surface, is outlined in green.
To analyze the accuracy of the two techniques, we obtained CT scans of each resected specimen with 0.625-mm-thick axial slices. A three-dimensional image of each specimen was created using the advanced imaging software (Mimics), and the reverse engineering software (Geomagic Studio®) was used to identify all points on each of the three cut surfaces. The software then was used to compile sets of discrete coordinate points for the cortical rims of the superior, inferior, and vertical cuts made to the specimens (Fig. 7) and to calculate best-fit planes corresponding to the three cortical rims in each bone. Cancellous surfaces were deliberately excluded during collection of the coordinate points for the resected specimens because incorporation of points from the cancellous voids would have confounded the best-fit plane calculations. For each specimen, a standard best-alignment function of the software was used to perfectly superimpose the preoperative and postoperative images, placing them in a common coordinate system for further analysis (Fig. 8).
Fig. 7.
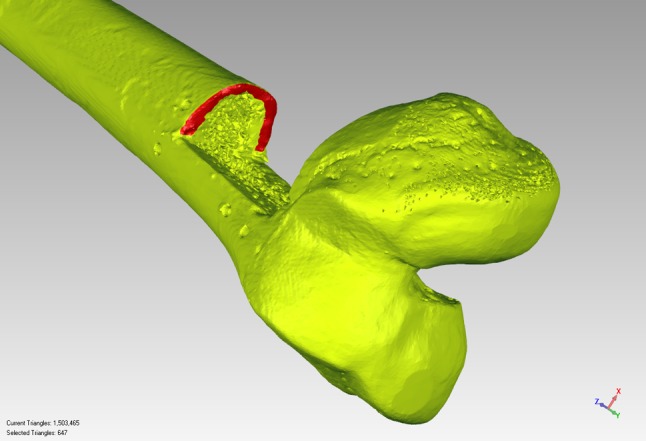
A three-dimensionally reconstructed image of a postresection specimen with the superior cortical rim isolated and selected for subsequent analysis is shown. As mentioned in the text, because the medullary cancellous bone contains multiple voids, attempts to select and include the cancellous surface in the analysis resulted in the software choosing points in the depths (rather than strictly on the surface) of the voids; points in the depth of the voids clearly do not represent the cut surface of the bone, and for this reason, the cancellous bone was deliberately excluded in the analysis.
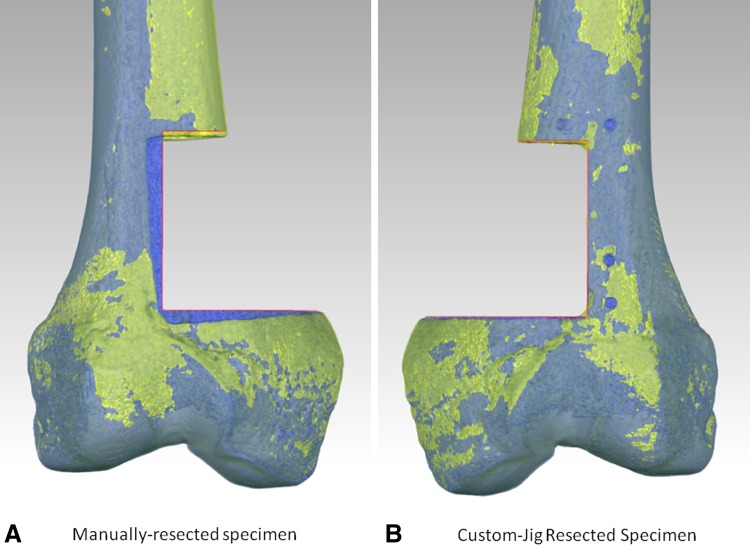
Fig. 8A–B.
These are the postresection images superimposed on the images of the preoperative plan for (A) a manually resected specimen and (B) a custom jig-resected specimen. On the manually resected specimen, there is substantial deviation from the preoperative plan (preoperative plan = red lines, which actually outline the femur depicting the preoperative plan—in pure ocean blue and directly visible in the vicinity of the resection). In contrast, on the custom-jig specimen, there is close adherence to the preoperative plan (again represented by the red lines). In the right image, the femur representing the preoperative plan coincides closely with the custom jig-resected specimen and thus is not directly visible.
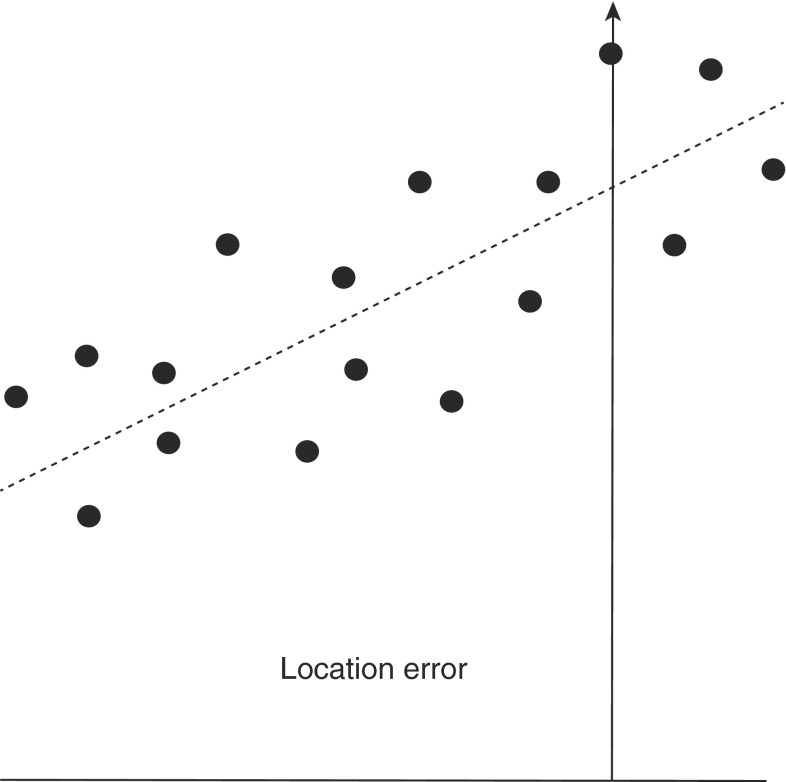
For each limb of the osteotomy, various geometric relationships between the resected cortical rim data point sets (and their best-fit planes) and the preoperative plan target planes were evaluated with the CAD software. For coordinate point data sets, the perpendicular distance between each point and the corresponding preoperative target plane was calculated. The data were reported in a form that was consistent with the standards of the International Organization of Standardization (ISO) [12]. According to ISO standards, the location error (the maximum deviation from the preoperative plan) is defined as the vertical distance from the target plane to the cut point that is farthest from the target plane (Fig. 9). Although not an ISO standard, we also calculated the mean deviation of the points from the target plane by calculating the mean of the absolute distance of each point from the target plane. Additionally, the angles of inclination between the target plane and best-fit planes were calculated. Similar to previous reports [6, 7] and to ISO parameters [12], the angles of inclination were measured by projecting the target and resection planes in a plane parallel to the approximate anatomic AP axis of the femur (the front angle) and in a plane approximately parallel to the anatomic sagittal axis of the femur (the depth angle) (Fig. 10). For simplicity, a single angular value also was calculated (the reduced angle) by measuring the absolute angular deviation between the target plane and the resection plane.
Fig. 9.
This graph shows the ISO location error, which in the text is called the maximum deviation from the preoperative plan. It is calculated by identifying the resection point that deviated most from the preoperative plan and measuring its vertical distance to the target plane.
Fig. 10.
Calculation of front and depth angles is shown. The error in the front angle is calculated by first intersecting the best-fit resection and target planes with an anatomic coronal plane slice taken at the anterior-most edge of the femur; the front angle is defined as the angle between the resulting projected target line and the corresponding resulting projected resection line. Similarly, the error in the depth angle is calculated by intersecting the resection and target planes with an anatomic sagittal plane slice that coincides with the vertical target plane.
The percentage of times that the manual and the custom jig-assisted resection techniques resulted in a maximum deviation exceeding a given threshold also was recorded. The percentage was calculated by dividing the number of planes that contained a point with a maximum deviation exceeding the specified threshold by the total number of planes in each group. The threshold values that were chosen were 2 mm, 3 mm, 4 mm, 5 mm, and 6 mm. Each of these values was chosen to represent an accepted error in surgical margins. For the custom-jig and manual resection groups, we compared the mean maximum deviation from the preoperative plan, the mean average deviation from the preoperative plan, angular deviations of the best-fit planes through the resection relative to the target planes, depth angle, reduced angle, single worst maximum deviation from the preoperative plan, and percentage of times the resection violated the chosen thresholds. Differences were compared using a two-sided Student’s t-test. The statistical analysis was performed with SPSS software (Version 15.0; IBM Corp, Armonk, NY, USA).
Results
The mean maximum deviation from the preoperative plan was greater (p = 0.002) for manually resected specimens than for custom jig-resected specimens: 9.0 mm versus 2.0 mm, respectively. The mean average deviation from the preoperative plan per specimen was greater (p < 0.001) for manually resected than for the custom jig-resected specimens: 3.1 mm versus 0.8 mm, respectively. For the angular deviations of the best-fit planes through the resection relative to the target planes, we found the manual group to have greater deviations compared with the custom-jig group with respect to the front angle (p = 0.001), depth angle (p = 0.02), and reduced angle (p < 0.001) (Table 1). Among all specimens and all planar cuts, the single worst maximum deviation from the preoperative plan was 14.2 mm for the manual group and 3.7 mm for the custom-jig group.
Table 1.
Absolute deviation of resections from the preoperative plan
| Outcome measure* | Manual | Custom jig | p value |
|---|---|---|---|
| Maximum deviation (mm) | 9.0 | 2.0 | 0.002 |
| Average deviation (mm) | 3.1 | 0.8 | < 0.001 |
| Error in front angle (degrees) | 4.7 | 0.9 | < 0.001 |
| Error in depth angle (degrees) | 3.5 | 1.4 | 0.02 |
| Error in reduced angle (degrees) | 6.6 | 1.8 | < 0.001 |
* The mean value per specimen is given.
We observed a difference in the percentage of times each of the two resection types violated the chosen thresholds (Table 2): at the 3-mm threshold, every manually resected plane exceeded this value whereas only 8.3% of the custom jig-resected planes did so. At thresholds of 4 mm and above, none of the custom jig-resected planes resulted in violations, whereas the manually resected planes resulted in violations 75% of the time at 4 mm, 58% of the time at 5 mm, and 41% of the time at 6 mm. The first (integral) threshold value at which the manually resected planes did not result in violations was 15 mm.
Table 2.
Percentage of times resection planes violated accepted error in surgical margins
| Resection type | Accepted error in surgical margins | ||||||
|---|---|---|---|---|---|---|---|
| 2 mm | 3 mm | 4 mm | 5 mm | 6 mm | 14 mm | 15 mm | |
| Custom jig | 50.0% | 8.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Manual | 100.0% | 100.0% | 75.0% | 58.3% | 41.7% | 8.3% | 0.0% |
Discussion
Imprecise reproduction of a given preoperative plan for bone tumor resection using standard manual techniques can have major clinical consequences. Inadvertently cutting into a malignant bone tumor contaminates the surgical margins and increases the likelihood of local recurrence [4], dramatically increasing mortality [19, 20, 23]. However, for bone tumors in certain locations (Fig. 6), resection of extra tissue, such as uninvolved joint surfaces, can have major long-term functional consequences [10, 13, 14, 21, 27]. Here, we evaluated whether a custom jig-assisted bone tumor resection technique reproduces a well-defined preoperative plan more accurately and consistently than traditional manual resection techniques.
The custom jig-assisted resection technique has several important limitations. First, our jig design was based on CT imaging, which is not always obtained in patients with a primary bone sarcoma and is associated with local radiation exposure [31]. Because MRI shows intramedullary tumor extent more accurately than CT [26], MRI findings should be incorporated into the jig design, perhaps via use of available MRI and CT fusion software [1, 33]. Second, our jig was proximally rather than distally based. The Appendix describes a preliminary experiment with a distally based jig (Appendix 1). A proximally based jig must make contact with the actual surface of the bone surrounding the tumor or with the periosteum for accurate positioning on the bone. Consequently, the surgeon must peel back soft tissue in the region next to the tumor to expose the underlying bone (or periosteum). In theory, soft tissue stripping could compromise the success of a biologically based reconstruction such as a structural allograft [10, 18, 22]. Furthermore, the additional soft tissue dissection could adversely affect healing and functional recovery. Third, holes left by the Steinmann pins used to secure the jig potentially could increase the fracture risk and may necessitate stricter postoperative restrictions. Nevertheless, the defect and pin holes would be spanned by a plate or other internal fixation device, and bone remodeling would be complete before bone healing occurred, so this theoretic point is unlikely to translate into a practical limitation. Fourth, jig construction requires substantial hardware, software, and engineering expertise, which may not be available at all institutions. Fifth, custom-jig use would have increased costs: the additional CT scan, three-dimensional printer, cost of jig manufacture and sterilization, and required engineering support must be considered.
Our experimental protocol also has a few important limitations. First, our cadaveric femurs were largely skeletonized and disarticulated. In a living patient, the presence of soft tissues and an articulated knee theoretically could alter the accuracy of the resections. Second, there was no actual tumor mass used in our cadaveric model. The presence of a mass might serve as yet another visible and palpable landmark that the surgeon performing a manual technique could use, therefore perhaps improving the accuracy of the manual technique. Third, there were limitations in the technique used to calculate the resection accuracy. For instance, the computer could not accurately identify the cut surface in its entirety as a result of voids in the cancellous bone, so only the cortical rim of each cut was used to define our measurement points on the resection surfaces. Theoretically, a greater deviation from the preoperative plan could have occurred within an osteotomy plane while the saw traversed the cancellous bone between the near and far cortical rims.
In this cadaveric study, we showed that a novel custom jig-assisted technique for the joint-sparing resection of a (hypothetical) distal femur metaphyseal tumor enables the surgeon to more accurately and reliably reproduce a given preoperative plan than a standard manual technique. Perhaps the most clinically important outcome variable is the percentage of times that each resection group violated the various accepted errors in surgical margins. For the manual group, violations were noted in the resection planes at every integral threshold value below 15 mm. In contrast, for the custom-jig group, no violations were noted in any resection plane for any specimen when the accepted error was 4 mm or greater. Based on our findings, the surgeon should aim to produce cuts that are at least 15 mm away from the ideal resection lines when using the manual resection technique. However, when using the custom jig-assisted resection technique, the surgeon can aim to produce cuts that are approximately only 4 mm away from the ideal resection lines. In practice, given the relatively close proximity of metaphyseal tumors to the joint, cutting an extra 15 mm beyond the ideal resection line may leave little or no possibility of sparing the epiphysis, whereas cutting only 4 mm beyond the ideal resection line would likely still allow for a satisfactory joint-sparing resection and reconstruction [2].
An important future study should compare the accuracy of recently introduced computer-navigated resections [8, 28, 32] with that of custom jig-assisted resections. For certain bone tumor resections, computer navigation has enabled the surgeon to achieve negative margins while also preserving key anatomic structures such as joint surfaces [8, 28, 32]. We surmise that the custom-jig technique may produce more accurate results than the computer-navigated technique. The jig and computer navigation orient the surgeon to the starting point of the cut, but only use of the custom jig can ensure adherence to a given trajectory for the entire cut. Another important relative advantage of the custom jig is that although it requires substantial preoperative planning, its intraoperative use is quick and simple compared with computer navigation, which requires substantial instrumentation to be placed in appropriate positions at the time of surgery and a relatively complex and occasionally unsuccessful registration process [25, 28]. Computer navigation and custom-jig technology do not have to be mutually exclusive; in fact, one may conceive of a setup in which computer navigation can be used to confirm correct positioning of the custom jig.
The accuracy achieved with the custom jigs allows for new surgical advances in reconstructive orthopaedic surgery. For example, our group is investigating the use of custom prefabricated implants to correct the defect that remains after a custom jig-assisted bone tumor resection. In such an application, the mean deviation from the preoperative plan must be small, because a prefabricated implant could be rendered useless at the time of surgery if it does not fit the defect. In this study, the small average deviation (0.8 mm) and the small deviations of the other parameters (eg, flatness, Euler angles) suggest that custom-jig technology may facilitate the practical use of custom implant fabrication. We also are investigating the possibility of using custom jigs for structural bone allograft reconstruction, where a second custom jig is designed to aid the surgeon in shaping a segment of cadaver bone to precisely fit the defect left in the host bone after sarcoma resection.
We showed that a novel custom jig-assisted technique for a joint-sparing resection of a distal femur metaphyseal primary bone sarcoma facilitates reliable, consistent reproduction of a given preoperative resection plan substantially more accurately than a traditional manual technique. Further cadaveric studies are warranted to evaluate the use and accuracy of this technique in increasingly realistic clinical scenarios before its acceptance as a surgical tool.
Acknowledgments
We thank the Musculoskeletal Transplant Foundation (Edison, NJ, USA) for providing the cadaver femurs for this investigation, and we thank Clara Hilario for assistance in the Hospital for Special Surgery’s Computer Assisted Surgery Center Laboratory.
Appendix 1. Proximally based versus distally based jig design
One limitation of our study is that the jig design was proximally based rather than distally based. Because the jig is constructed to mate with the bone surface, a proximally based jig must make contact either with the actual surface of the bone surrounding the tumor or with the periosteum and requires the surgeon peel back soft tissue in the region next to, but not on top of, the tumor to expose the underlying bone (or periosteum). Such soft tissue stripping may decrease the success of a biologically based reconstruction [23, 27, 28].
A distally based jig that contacts the articular cartilage (which already is exposed and does not require stripping) would forego these theoretical concerns about stripping. Interestingly, our first jig design was distally based but was abandoned because our CT imaging alone did not adequately account for the articular cartilage surface. Thus, this jig did not seat well grossly on the articular cartilage. However, after shaving down enough cartilage to expose subchondral bone in certain locations, the jig did seat well, and the resulting resection was quite accurate (data not shown). Obviously, extensive cartilage shaving is not a realistic option when attempting a joint-sparing resection. Cartilage shaving to expose subchondral bone sometimes is used when custom jigs are used for knee arthroplasty (during which the joint surface is to be resected anyway) [9]. For a joint-sparing hemimetaphyseal resection, there are two potential ways to avoid this problem, which we are currently investigating. The first is to design distally based jigs that would avoid contact with the cartilage in areas where there is a significant difference between the shape of the subchondral bone and the articular cartilage surface (and building in a small offset to account for the approximate estimated thickness of articular cartilage). The second is to image the cartilage using MRI, although imaging cartilage with enough accuracy to determine the shape of its surface in an intact knee, where subtle deforming forces also can enter the picture, can be challenging [16]. MRI-based jigs have been used clinically for knee arthroplasty [17], but data raise questions about the accuracy of this approach [15].
Footnotes
The institution of one or more of the authors (JHH) received funding from the Major Family Fund/Major Fellowship in Musculoskeletal Oncology.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This research was performed at Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
References
- 1.Alyafei S, Inoue T, Zhang H, Ahmed K, Oriuchi N, Sato N, Suzuki H, Endo K. Image fusion system using PACS for MRI, CT, and PET Images. Clin Positron Imaging. 1999;2:137–143. doi: 10.1016/S1095-0397(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 2.Avedian RS, Haydon RC, Peabody TD. Multiplanar osteotomy with limited wide margins: a tissue preserving surgical technique for high-grade bone sarcomas. Clin Orthop Relat Res. 2010;468:2754–2764. doi: 10.1007/s11999-010-1362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacci G, Briccoli A, Longhi A, Ferrari S, Mercuri M, Faggioli F, Versari M, Picci P. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. 2005;44:748–755. doi: 10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Ferrari S, Mercuri M, Bertoni F, Picci P, Manfrini M, Gasbarrini A, Forni C, Cesari M, Campanacci M. Predictive factors for local recurrence in osteosarcoma: 540 patients with extremity tumors followed for minimum 2.5 years after neoadjuvant chemotherapy. Acta Orthop Scand. 1998;69:230–236. doi: 10.3109/17453679809000921. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 6.Cartiaux O, Docquier PL, Paul L, Francq BG, Cornu OH, Delloye C, Raucent B, Dehez B, Banse X. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: an experimental study. Acta Orthop. 2008;79:695–702. doi: 10.1080/17453670810016731. [DOI] [PubMed] [Google Scholar]
- 7.Cartiaux O, Paul L, Docquier PL, Francq BG, Raucent B, Dombre E, Banse X. Accuracy in planar cutting of bones: an ISO-based evaluation. Int J Med Robot. 2009;5:77–84. doi: 10.1002/rcs.237. [DOI] [PubMed] [Google Scholar]
- 8.Cho HS, Oh JH, Han I, Kim HS. Joint-preserving limb salvage surgery under navigation guidance. J Surg Oncol. 2009;100:227–232. doi: 10.1002/jso.21267. [DOI] [PubMed] [Google Scholar]
- 9.Fitz W. Unicompartmental knee arthroplasty with use of novel patient-specific resurfacing implants and personalized jigs. J Bone Joint Surg Am. 2009;91(suppl 1):69–76. doi: 10.2106/JBJS.H.01448. [DOI] [PubMed] [Google Scholar]
- 10.Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–196. [PubMed] [Google Scholar]
- 11.Hoffer FA, Nikanorov AY, Reddick WE, Bodner SM, Xiong X, Jones-Wallace D, Gronemeyer SA, Rao BN, Kauffman WM, Laor T. Accuracy of MR imaging for detecting epiphyseal extension of osteosarcoma. Pediatr Radiol. 2000;30:289–298. doi: 10.1007/s002470050743. [DOI] [PubMed] [Google Scholar]
- 12.International Organization for Standardization (ISO). Geometrical Product Specifications (GPS)—Geometrical Tolerancing—Tolerances of Form, Orientation, Location and Run-out [ISO Standard 1101:2004]. Geneva, Switzerland: ISO; 2004.
- 13.Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon DG. A rotating-hinge knee replacement for malignant tumors of the femur and tibia. J Arthroplasty. 1999;14:187–196. doi: 10.1016/S0883-5403(99)90124-9. [DOI] [PubMed] [Google Scholar]
- 14.Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70:109–115. doi: 10.1002/(SICI)1096-9098(199902)70:2<109::AID-JSO9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Klatt BA, Goyal N, Austin MS, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–29. doi: 10.1016/j.arth.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Koff MF, Chong le R, Virtue P, Chen D, Wang X, Wright T, Potter HG. Validation of cartilage thickness calculations using indentation analysis. J Biomech Eng. 2010;132:041007. [DOI] [PubMed]
- 17.Lombardi AV, Jr, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty. Orthopedics. 2008;31:927–930. doi: 10.3928/01477447-20080901-21. [DOI] [PubMed] [Google Scholar]
- 18.Mankin HJ. The changes in major limb reconstruction as a result of the development of allografts. Chir Organi Mov. 2003;88:101–113. [PubMed] [Google Scholar]
- 19.Meyers PA, Healey JH, Chou AJ, Wexler LH, Merola PR, Morris CD, Laquaglia MP, Kellick MG, Abramson SJ, Gorlick R. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 2011;117:1736–1744. doi: 10.1002/cncr.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE, Children’s Oncology Group Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 21.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Abalo E, Farfalli G. Unicondylar osteoarticular allografts of the knee. J Bone Joint Surg Am. 2007;89:2137–2142. doi: 10.2106/JBJS.F.01277. [DOI] [PubMed] [Google Scholar]
- 22.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop Relat Res. 2004;426:97–102. doi: 10.1097/01.blo.0000141652.93178.10. [DOI] [PubMed] [Google Scholar]
- 23.Nathan SS, Gorlick R, Bukata S, Chou A, Morris CD, Boland PJ, Huvos AG, Meyers PA, Healey JH. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107:1607–1616. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 24.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 25.Pearle AD, Kendoff D, Musahl V. Perspectives on computer-assisted orthopaedic surgery: movement toward quantitative orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 1):7–12. doi: 10.2106/JBJS.H.01510. [DOI] [PubMed] [Google Scholar]
- 26.Saifuddin A. The accuracy of imaging in the local staging of appendicular osteosarcoma. Skeletal Radiol. 2002;31:191–201. doi: 10.1007/s00256-001-0471-y. [DOI] [PubMed] [Google Scholar]
- 27.Shin DS, Choong PF, Chao EY, Sim FH. Large tumor endoprostheses and extracortical bone-bridging: 28 patients followed 10–20 years. Acta Orthop Scand. 2000;71:305–311. doi: 10.1080/000164700317411933. [DOI] [PubMed] [Google Scholar]
- 28.So TY, Lam YL, Mak KL. Computer-assisted navigation in bone tumor surgery: seamless workflow model and evolution of technique. Clin Orthop Relat Res. 2010;468:2985–2991. doi: 10.1007/s11999-010-1465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Springfield DS, Enneking WF, Neff JR, Makley JT. Principles of tumor management. Instr Course Lect. 1984;33:1–25. [PubMed] [Google Scholar]
- 30.Springfield DS, Schmidt R, Graham-Pole J, Marcus RB, Jr, Spanier SS, Enneking WF. Surgical treatment for osteosarcoma. J Bone Joint Surg Am. 1988;70:1124–1130. [PubMed] [Google Scholar]
- 31.Sugarbaker PH, Malawer MM. Musculoskeletal Surgery for Cancer: Principles and Techniques. Stuttgart, Germany: Thieme Medical Publishers; 1992. [Google Scholar]
- 32.Wu K, Webber NP, Ward RA, Jones KB, Randall RL. Intraoperative navigation for minimally invasive resection of periarticular and pelvic tumors. Orthopedics. 2011;34:372. doi: 10.3928/01477447-20110317-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanaka Y, Kamogawa J, Katagi R, Kodama K, Misaki H, Kamada K, Okuda S, Morino T, Ogata T, Yamamoto H. 3-D MRI/CT fusion imaging of the lumbar spine. Skeletal Radiol. 2010;39:285–288. doi: 10.1007/s00256-009-0788-5. [DOI] [PubMed] [Google Scholar]