Abstract
Background
Metal-on-metal (MOM) THA bearing technology has focused on improving the arc of motion and stability and minimizing wear compared with traditional metal-on-polyethylene (MOP) bearing couples. It is unclear whether this more costly technology adds value in terms of improved implant survival.
Questions/purposes
This study evaluated Kaplan-Meier survival, revisions for dislocation, and cost of MOM THA compared with metal-on-cross-linked polyethylene (MOXP) THA in a community joint registry, with subset analysis of the recalled Depuy ASR™ implant.
Methods
All MOM THAs (resurfacings excluded) performed between January 2002 and December 2009 were included (n = 1118) and compared with a control group of MOXP THAs (n = 1286) done during the same time. Analysis was performed to compare age, gender, cost of implant, length of stay, year of index procedure, diagnosis, head size (< 32 mm versus ≥ 32 mm), revision and revision reason for both groups. Analysis at a mean of 3.6 years was done using Wilcoxon rank sum tests, Pearson’s chi-square tests, Kaplan Meier methods, and Cox regression.
Results
The cumulative revision rate (CRR) was higher in MOM implants than in MOXP implants (MOM CRR = 13%; MOXP CRR = 3%). MOM implants were three times as likely to be revised as MOXP implants after adjustment for age, head size, and year of procedure. The recalled DePuy ASR™ implant was six times as likely to be revised as other MOM THAs. After removing the ASR™ implants from analysis, survivorship of MOM implants was not better than that of the MOXP hips.
Conclusions
During the study time, MOM THAs showed inferior survival to MOXP THAs after adjusting for age, head size, and year of procedure. Longer followup is necessary to see whether MOM THAs add value in younger patient groups.
Level of Evidence
Level III, retrospective case-control study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA has emerged as one of the 20th century’s most common and reliable surgical procedures [6, 15]. The aim of the surgery is to provide a pain-free, stable, mobile, and durable joint. Advances in THA bearing technology have focused on improving the arc of motion and stability and minimizing wear compared with traditional metal-on-polyethylene (MOP) bearing couples. However, implementation of this new technology comes at an additional cost to our patients and the healthcare system. Implant expense traditionally has been one of the costlier parts of the hospital bill for a THA [15, 18, 20] and efforts continue to cut costs in an era of dwindling Medicare reimbursements and the push for national healthcare reform [6, 16]. Surgeons may reasonably be expected to weigh implant cost considerations against their desire to use new technology that may afford better function, fewer complications, and/or improved longevity of THA for their patients. In recent years, the most commonly used THA bearing surfaces have been metal-on-highly cross-linked polyethylene (MOXP) and MOM interfaces. Although similar in function, the tribology and biologic interaction between these two options are substantially different [7, 9, 19, 21, 23, 25, 26, 34]. Recently, concerns over periprosthetic adverse reactions to metallic debris including pseudotumors and local tissue necrosis (aseptic lymphocyte-dominated vasculitis-associated lesion [ALVAL]; adverse reaction to metal debris [ARMD]) have surfaced in reports of MOM THA. The United Kingdom’s Medical products and Healthcare Devices Regulatory Agency (MHRA), the United Kingdom’s equivalent of the U.S. Food and Drug Administration, issued a report based on registry experience recommending close followup of MOM devices and measurements of cobalt and chromium ions in the blood for patients with painful prostheses [29, 31]. A second report detailed concerns regarding a higher-than-expected revision rate associated with a specific MOM implant, the DePuy ASR™ (DePuy, Inc, Warsaw, IN, USA), which eventually led to its worldwide recall [30].
MOM bearing surfaces have recently come under increased scrutiny [14]. Bernthal et al. [2] reported their DePuy ASR™ results and noted a high rate of implant dysfunction and failure. Of their 70 patients, 28% experienced implant dysfunction and 17% required revision surgery within 3 years. They did not find cup positioning to be a factor contributing to failure, and they concluded that caution should be used when MOM large-diameter heads are paired with a monoblock acetabular cup design. Bolland et al. [3] similarly reviewed the midterm results of 199 hips and found 14 with evidence of ARMD and a revision rate of 15% at 5 years. According to their data, there was evidence of high wear at the trunnion-head interface and passive corrosion of stem surfaces.
Whether the results of MOM technology in THA justify the increased cost over MOXP implants provides an important part of the rationale behind joint registries, as evidenced by the DePuy ASR™ recall. In this study, we sought to determine the following from our joint registry database: (1) whether higher-cost MOM THA implants were superior to MOXP THA implants in terms of implant survival; (2) whether survival differed between the aforementioned DePuy ASR™ MOM implant and other MOM THA implants; and (3) whether revision for dislocation was more common in MOXP or MOM THAs.
Materials and Methods
The HealthEast Joint Registry is a total joint registry that prospectively tracks hip and knee arthroplasties performed by 40 surgeons at six community hospitals in the St Paul, MN, USA, metropolitan area since its inception in 1991. Details of the data collection methods and application of statistical analyses in the HealthEast Joint Registry have been reported [11].
We considered all MOM and MOXP THAs from January 1, 2002, to March 31, 2011, for inclusion in this study. Earlier THAs performed from 1999 to 2001 were excluded because MOM designs were not used consistently in HealthEast Joint Registry until 2002. Other hips excluded included those in which conventional (rather than MOXP) polyethylene liners were implanted. We did include THAs involved in the ASR™ recall in the study.
Of the patients who had the 2404 THAs (1118 MOM, 1286 MOXP), 54% were female, the average age was 66 years (range, 21–94 years), and the minimum followup was 0 years (average, 3.6 years; range, 0–8.5 years). We used revision of the index THA before March 31, 2011, as our primary end point and defined revision to be the removal, exchange, or addition of any prosthetic component. Revision surgery for dislocation was used as a secondary end point. We compared MOM and MOXP THAs using the following variables: age, sex, year of index procedure, followup, cost of the index implant, length of stay in the hospital for the index procedure, primary diagnosis, head size, and reason for revision (Table 1). A subgroup analysis was performed on the recalled ASR™ MOM implants to determine if their inclusion had a major effect on MOM THA survival. Of the 1118 MOM THAs, 423 used ASR™ implants, 692 used Depuy Pinnacle™ implants, and there were three other MOM devices.
Table 1.
Univariate analysis
| Variables | MOM implant (n = 1118) | MOXP implant (n = 1286) | p value* |
|---|---|---|---|
| Followup (years) – mean (SD) | 3.2 (1.5) | 4.3 (2.9) | < 0.001 |
| Age (years) – mean (SD) | 62 (11.5) | 68 (10.6) | < 0.001 |
| Cost of implant – mean (SD) | $6150 ($7654) | $5480 ($733) | < 0.001 |
| Length of stay (days) – mean (SD) | 3.3 (1.3) | 3.5 (1.2) | < 0.001 |
| Sex | |||
| Male | 535 (48%) | 571 (44%) | 0.09 |
| Female | 583 (52%) | 715 (56%) | |
| Age categories | |||
| < 60 years | 477 (43%) | 266 (21%) | < 0.001 |
| 60–69 years | 337 (30%) | 356 (28%) | |
| ≥ 70 years | 304 (27%) | 664 (52%) | |
| Year of index procedure | |||
| 2002–2005 | 269 (24%) | 716 (56%) | < 0.001 |
| 2006–2009 | 849 (76%) | 570 (40%) | |
| Primary diagnosis | |||
| Osteoarthritis | 1056 (94%) | 1198 (93%) | 0.28 |
| Aseptic necrosis | 35 (3%) | 43 (3%) | |
| Other | 27 (2%) | 45 (4%) | |
| Head size categories | |||
| < 36 | 22 (2%) | 630 (52%) | < 0.001 |
| ≥ 36 | 1036 (98%) | 580 (48%) | |
| Revision | |||
| Yes | 73 (7%) | 29 (2 %) | < 0.0001 |
| No | 1045 (93%) | 1257 (98%) | |
| Revision reason | |||
| Dislocation | 6 (8%) | 17 (59%) | < 0.0001 |
| Periprosthetic fracture | 8 (11%) | 4 (14%) | |
| Failure of bone ingrowth | 15 (20%) | 4 (14%) | |
| Aseptic loosening | 9 (12%) | 0 (0%) | |
| Infection | 5 (7%) | 2 (8%) | |
| Pain | 16(22%) | 0 (0%) | |
| Other/unknown | 3 (4%) | 4 (14%) | |
| Metal hypersensitivity | 2 (3%) | ||
| Pseudotumor | 1 (1%) | ||
| Metallosis | 8 (11%) | ||
* P values were calculated using the Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test for categorical variables.
We compared MOM and MOXP THAs using Wilcoxon rank sum text for continuous variables (age, followup, cost of the index implant, and length of stay) and Pearson’s chi-square test for categorical variables (sex, year of the index procedure, primary diagnosis, head size, and reason for revision). Cost comparisons were performed using the contract price of each implant averaged over the years of the study. Cumulative revision rates (CRRs) were calculated using the Kaplan-Meier survival method and compared using the log-rank test. We calculated relative risk of revision using Cox proportional hazards regression and all variables mentioned previously (except reason for revision) were considered for potential confounding. A confounder was defined as a variable of interest that changed the main effect estimate by greater than 10%. We included variables meeting this definition in the final Cox regression model.
Compared with MOXP THAs, MOM THAs on average, were performed in younger patients who had a shorter length of stay and a shorter followup. The MOM THAs on average, had a higher implant cost, larger head size, and more often the prosthesis was implanted from 2006 to 2011 (Table 1).
Results
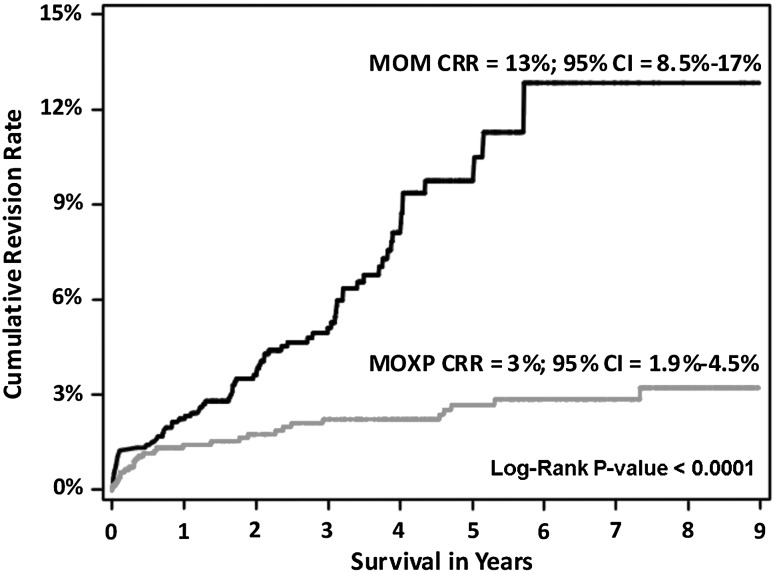
Patients undergoing MOM THAs had a significantly higher (p = 0.0001) overall CRRs compared with MOXP THAs (13% versus 3%) (Fig. 1). MOM THAs were three times as likely to be revised compared with MOXP THAs after adjusting for age, head size, and year of the index procedure (Table 2).
Fig. 1.
There was a significant difference in the cumulative revision rates between the MOXP THA and MOM THA. MOM THAs have a higher CRR than MOXP CRRs.
Table 2.
Cox proportional hazards regression model for MOXP versus MOM implants
| Implant | Crude hazards regression (95% CI) | p value | Adjusted hazards regression (95% CI)* | p value | Adjusted hazards regression (95% CI)** | p value |
|---|---|---|---|---|---|---|
| MOXP implant | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| MOM implant | 3.5 (2.2–5.4) | < 0.001 | 3.0 (1.6–5.6) | < 0.001 | 1.5 (0.7–3.2) | 0.29 |
* Adjusted for age, head size, and year of index procedure; **adjusted for all of the above and whether hey are part of the ASR™ recall.
Subgroup analysis revealed that after removing the recalled ASR™ implants from the MOM group, there was no significant difference (p = 0.3) in the overall CRR between the MOM and MOXP THAs (7% versus 3%). After adjusting for age, head size, year of the index procedure, and ASR™ recall, there was no difference (p = 0.3) in the risk of revision between the MOM and MOXP THAs. ASR™ implants were six times as likely to be revised (95% CI, 3.4–11; p = 0.0001) as other MOM implants after adjustment for year of the index procedure (Table 3).
Table 3.
Cox proportional hazards regression model for other MOM versus ASR™ implants
| Implant | Crude hazards regression (95% CI) | p value | Adjusted hazards regression (95% CI)* | p value |
|---|---|---|---|---|
| Other MOM | 1.0 (reference) | 1.0 (reference) | ||
| ASR | 6.1 (3.5–11) | < 0.001 | 6.1 (3.4–11) | < 0.001 |
* Adjusted for year of index procedure.
More detailed analysis of the MOM implant failures revealed that the majority were revised for pain (22%), with the most common intraoperative finding being failure of bony ingrowth of the acetabular component (20%). Notably, in this registry, there was only a 1% incidence of pseudotumor related to metallic debris as documented by the operative surgeons, although preoperative concern for metallosis was cited in 11% of the MOM revisions. No MOM THAs were revised solely on the basis of pending litigation surrounding the implant recall.
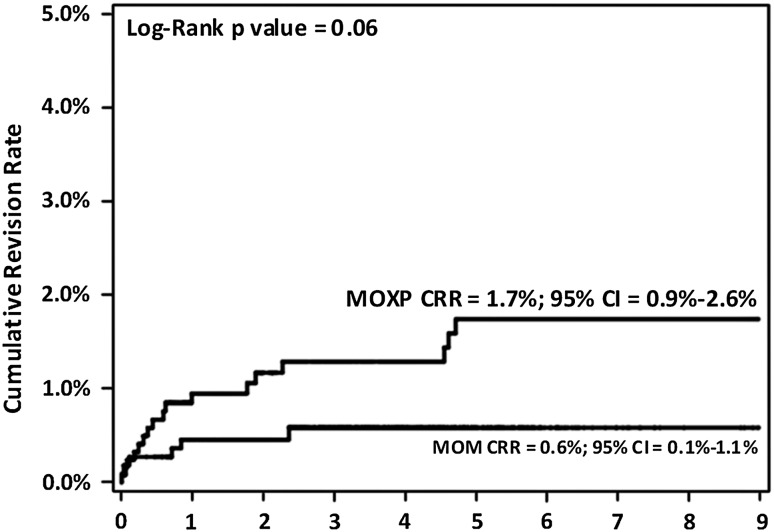
Although a higher percentage of patients in the MOXP group underwent revision surgery for dislocation (Table 1), there was no difference (p = 0.06) in the dislocation CRRs between the MOM and MOXP THAs (0.6% versus 1.7) (Fig. 2). Finally, there was no difference in the risk of revision for dislocation between the MOM and MOXP THAs after adjustment for age, head size (< 36 mm, ≥ 36 mm) and year of the index procedure (hazard ratio, 1.36; CI, 0.4–4.6; p = 0.6) (Table 4).
Fig. 2.
There was no difference in the CRR for dislocation between the MOM and MOXP implants.
Table 4.
Cox proportional hazards regression model for revision
| Implant | Crude hazards regression (95% CI) | p value | Adjusted hazards regression (95% CI)* | p value |
|---|---|---|---|---|
| MOXP | 1.0 (reference) | 1.0 (reference) | ||
| MOM | 2.4 (0.9–6.2) | 0.07 | 1.4 (0.4–4.6) | 0.6 |
* Adjusted for age, head size (< 36, ≥ 36), and year of index procedure.
Discussion
Primary and revision total joint arthroplasties account for a higher percentage of Medicare spending than any other inpatient procedures and the numbers of these procedures are expected to increase [4, 15, 18]. Implant expense is a major portion of the total cost of the procedure [14, 16–18, 20, 27] and the HealthEast Joint Registry noted an increased proportion of more costly MOM THAs from 16% of the procedures done in 2002 to 2005 to 70% from 2005 to 2009. Given increasing attention to MOM THA failures including the widely publicized United Kingdom MHRA report [29], we sought to determine whether MOM THA could be shown to have better survival than MOXP THA in early- to midterm followup in our community registry.
Inherent to our study are the obvious limitations of any implant and explant joint registry. First, revision is a crude end point, and we are unable to identify patients who may have had poor clinical or functional results or those who were too medically infirm to undergo revision surgery. Similarly, we cannot identify individuals with superior function or activity level; therefore, registry studies cannot be applied on an individual level. Second, there may be patients who had revision surgery performed elsewhere who were not included in our study; we assumed these events occurred in an equal proportion between the MOM and MOXP groups. However, a prior validation analysis of our registry suggests a 94% capture rate [12], similar to that of the Scandinavian registries. Third, our study only captured early to midterm events in the anticipated life expectancy of total joint devices, particularly in the more recently introduced MOM designs. Despite these limitations, registries have the unique advantage of offering a broad representation of contemporary total joint practice. Unique to our registry is that none of the 40+ surgeons in the HealthEast Joint Registry have major industry relationships, and we presumed implant choices were not biased toward specific companies.
Current studies comparing MOM with MOP or MOXP bearing surfaces have yielded mixed results [1, 22–24]. Comparison with other registry reports is difficult, because of the varying level of detail provided in annual report summaries. The Australian Orthopaedic Association National Joint Replacement Registry has the largest data set of MOM THAs. Data gathered from the 2009 Australian Orthopaedic Association annual report suggest a CRR of 4.4% in MOM THAs and 3.3% in MOP THAs at 7 years [1]. They noted 1.2 versus 0.7 revisions per 100 observed implant years in MOM versus MOP THAs, respectively. For the 8-year period followed by the registry, the hazard ratio for MOM versus MOP THA CRR was 1.4 (p < 0.001) [1]. The New Zealand Joint Registry also noted 0.86 MOM versus 0.60 MOP THA revisions per 100 observed implant years, consistent with the Australian registry, and the CRR for MOM THAs of 2.9% compared with a CRR of 2.6% for MOP THAs [24]. The 2011 England and Wales registry showed MOM THAs to have a 14% CRR compared with MOP THAs 3.4% CRR at 7 years [22]. A matched case-control study using the hip database of the Maurice E. Müller Institute for Evaluative Research comparing MOM THAs with MOXP THAs did show a slightly lower risk of aseptic component loosening for the MOM group, which did not reach statistical significance [23]. Additional studies looking at the risk of cancer, wear rate, and survivability have not shown considerable benefit of MOM over MOP THA systems in the times noted [10, 13, 28, 33, 35].
One important confounding factor in our study is the inclusion of DePuy ASR™ acetabular cups in our MOM analysis. A global recall for this device was issued by the company on August 24, 2010, after reports of higher than anticipated rates of revision by the National Joint Registry for England and Wales. Between April 2001 and March 2010, the registry data predicted 80 revisions and noted 130 revisions (n = 2769). Additional analysis for cups paired with extra large femoral heads reported a predicted 80 revisions and found 126 (n = 3155) [32]. The MHRA subsequently issued reports recommending close followup of patients with MOM devices [29, 30]. The Australian Orthopaedic Association National Joint Replacement Registry reported a hazard ratio of 3.9 for THAs performed with the ASR™ prosthesis compared with all conventional THAs [1]. The New Zealand Registry also found the ASR™ to have a higher revision rate than other MOM implants, but ASR implants alone did not account for the higher rate of revision of MOM when compared with MOXP [24]. DePuy ASR™ THA implants in our registry were six times as likely to be revised as other MOM implant THAs (p < 0.0001).
We confirmed a substantially decreased (p = 0.0001) rate of dislocation as a revision reason for MOM THA (8%) versus MOXP THA (59%). For MOM and MOXP THAs, head sizes less than 36 mm were most commonly revised for dislocation (60%), whereas 36 mm or larger heads had dislocation as the revision reason only 14% of the time (p < 0.0001). However, only 2% (22 of 1118) of the MOM THAs had head sizes less than 36 mm, and head sizes of 40 mm or greater were not uncommon in this group. We hypothesize that our surgeons used MOM THAs, particularly in an older age group, precisely because of the increased arc of motion and stability associated with larger head sizes [8]. In our registry, this perceived advantage of MOM THA over MOXP THA disappeared at head sizes of 36 mm or greater. This tendency toward MOM THA may become less pronounced as higher cumulative revision rates from this and other studies are reported and also as larger heads for MOXP THAs become readily available on the market. Larger head sizes in MOXP shells clearly affect polyethylene liner thickness and the long-term impact on wear rates with minimum 5 to 6 mm polyethylene thickness remains unknown. Although cost-effectiveness analysis was not performed, MOM implants were on average $700 more expensive than MOXP implants. The shorter hospital length of stay for patients with MOM THAs undoubtedly reflects younger population and an increasing trend toward use of these devices from 2005 to 2009 when rehabilitation advances encouraged rapid discharge from the acute care setting.
In a time of intensifying debate over healthcare reform, in which cost-effectiveness of health care has become paramount, orthopaedic surgeons are in the best position to select the appropriate implant for their patients. A review of the Nationwide Inpatient Sample Database revealed that 35% of a sample of 112,095 THAs performed in the United States between October 2005 and December 2006 had a MOM bearing surface [5]. Currently in the United States, there is little aftermarket data directly comparing various implants, and delayed product recalls such as that seen with the ASR™ are only the most obvious result. Smaller registry studies, randomized clinical trials, and formal cost-effectiveness analysis will continue to allow surgeons an evidence-based approach to implant selection for their patients, as the national American Joint Replacement Registry gains momentum and critical mass. In the HealthEast Registry, MOM implants did not show superior performance when compared with MOXP implants during the time of this study. As orthopaedic surgeons, we must remain cognizant that all innovation may not offer improvement, and higher cost may not necessarily equate to higher quality.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at HealthEast Hospitals, St Paul, MN, USA.
References
- 1.Annual Report. Adelaide, Australia: AOA; 2009. [Google Scholar]
- 2.Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27:539–544. doi: 10.1016/j.arth.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Bolland BJ, Culliford DJ, Langton DJ, Millington JP, Arden NK, Latham JM. High failure rates with a large-diameter hybrid metal-on-metal total hip replacement. J Bone Joint Surg Br. 2011;93:608–615. doi: 10.1302/0301-620X.93B5.26309. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Durbhakula S, Berry DJ, Naessens JM, Rappaport K, Cisternas M, Saleh KJ, Rubash HE. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(suppl 3):17–25. doi: 10.1016/j.arth.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Saleh KJ, Rosenberg AG, Rubash HE. Economic evaluation in total hip arthroplasty: analysis and review of the literature. J Arthroplasty. 2004;19:180–189. doi: 10.1016/S0883-5403(03)00456-X. [DOI] [PubMed] [Google Scholar]
- 7.Callaghan JJ, Cuckler JM, Huddleston JL, Galante JO. How have alternative bearings (such as metal-on-metal, highly cross linked polyethylene and ceramic-on-ceramic) affected the prevention and treatment of osteolysis? J Am Acad Orthop Surg. 2008;16:S33–S38. doi: 10.5435/00124635-200800001-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cuckler JM, Moore KD, Lombardi AV, Jr, McPherson E, Emerson R. Large versus small femoral heads in metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19(suppl 3):41–44. doi: 10.1016/j.arth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Doorn PF, Campbell PA, Amstutz HC. Metal versus polyethylene wear particles in total hip replacement. Clin Orthop Relat Res. 1996;329(suppl):S206–S216. doi: 10.1097/00003086-199608001-00018. [DOI] [PubMed] [Google Scholar]
- 10.Garellick G, Malchau H, Herberts P. Survival of hip replacements. A comparison of a randomized trial and a registry. Clin Orthop Relat Res. 2000;375:157–167. doi: 10.1097/00003086-200006000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Gioe TJ, Killeen KK, Mehle S, Grimm K. Implementation and application of a community total joint registry: a twelve-year history. J Bone Joint Surg Am. 2006;88:1399–1404. doi: 10.2106/JBJS.E.01198. [DOI] [PubMed] [Google Scholar]
- 12.Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all-polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88–92. doi: 10.1097/BLO.0b013e31812f7879. [DOI] [PubMed] [Google Scholar]
- 13.Grubl A, Marker M, Brodner W, Giurea A, Heinze G, Meisinger V, Zehetgruber H, Kotz R. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25:841–848. doi: 10.1002/jor.20381. [DOI] [PubMed] [Google Scholar]
- 14.Haddad FS, Thakrar RR, Hart AJ, Skinner JA, Nargol AV, Nolan JF, Gill HS, Murray DW, Blom AW, Case CP. Metal-on-metal bearings-the evidence so far. J Bone Joint Surg Br. 2011;93:572–579. doi: 10.1302/0301-620X.93B4.26429. [DOI] [PubMed] [Google Scholar]
- 15.Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57–63. doi: 10.1097/BLO.0b013e31803372e0. [DOI] [PubMed] [Google Scholar]
- 16.Healy WL, Iorio R, Lemos MJ, Patch DA, Pfeifer BA, Smiley PM, Wilk RM. Single price/case price purchasing in orthopaedic surgery: experience at the Lahey Clinic. J Bone Joint Surg Am. 2000;82:607–612. doi: 10.2106/00004623-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Healy WL, Iorio R, Richards JA. Opportunities for control of hospital cost for total knee arthroplasty. Clin Orthop Relat Res. 1997;345:140–147. doi: 10.1097/00003086-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Healy WL, Iorio R, Richards JA, Lucchesi C. Opportunities for control of hospital costs for total joint arthroplasty after initial cost containment. J Arthroplasty. 1998;13:504–507. doi: 10.1016/S0883-5403(98)90048-1. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. Metal-on-metal bearing surfaces. J Am Acad Orthop Surg. 2009;17:69–76. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Levine DB, Cole BJ, Rodeo SA. Cost awareness and cost containment at the Hospital for Special Surgery: strategies and total hip replacement cost centers. Clin Orthop Relat Res. 1995;311:117–124. [PubMed] [Google Scholar]
- 21.Malviya A, Ramaskandhan JR, Bowman R, Hashmi M, Holland JP, Kometa S, Lingard E. Metal-on-metal total hip arthroplasty: current concepts review. J Bone Joint Surg Am. 2010;92:1675–1683. doi: 10.2106/JBJS.I.01426. [DOI] [PubMed] [Google Scholar]
- 22.8th Annual Report. London, UK: Pad Creative Ltd; 2011. [Google Scholar]
- 23.Naudie D, Roeder CP, Parvizi J, Berry DJ, Eggli S, Busato A. Metal-on-metal versus metal-on-polyethylene bearings in total hip arthroplasty: a matched case-control study. J Arthroplasty. 2004;19(suppl 2):35–41. doi: 10.1016/j.arth.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 24.New Zealand Orthopaedic Association. The New Zealand Joint Registry—Ten Year Report. 1999–2008. Christchurch, New Zealand: Cantebury District Health Board Ltd.
- 25.Park MS, Chung WC, Yoon SJ, Cho HM, Kwon SH. Eleven-year follow-up of second-generation metal-on-metal total hip arthroplasty. J Orthop Surg (Hong Kong). 2010;18:15–21. doi: 10.1177/230949901001800104. [DOI] [PubMed] [Google Scholar]
- 26.Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005;87:1515–1521. doi: 10.2106/JBJS.D.02641. [DOI] [PubMed] [Google Scholar]
- 27.Sharkey PF, Sethuraman V, Hozack WJ, Rothman RH, Stiehl JB. Factors influencing choice of implants in total hip arthroplasty and total knee arthroplasty: perspectives of surgeons and patients. J Arthroplasty. 1999;14:281–287. doi: 10.1016/S0883-5403(99)90052-9. [DOI] [PubMed] [Google Scholar]
- 28.Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Soballe K, Menne T. The association between metal allergy, total hip arthroplasty and revision. Acta Orthop. 2009;80:646–652. doi: 10.3109/17453670903487008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MHRA. Medical Device Alert: All metal-on-metal (MOM) hip replacements. (MDA/2010/033). April 22, 2010. Available at: www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON079157 Accessed December 14, 2012.
- 30.MHRA. Medical Device Alert: ASR™ Hip Replacement Implants Manufactured by DePuy International Ltd (MDA/2010/069). September 7, 2010. Available at: www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON093789 Accessed December 14, 2012.
- 31.MHRA. Medical Device Alert: MDA/2007/054. Total Hip Replacement: DePuy Ultima TPS Femoral Stem Used in Combination With Ultima Metal-on-metal Articulation. June 14, 2007. Available at: www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON2031467 Accessed December 14, 2012.
- 32.MHRA. UKMHRA Medical Device Alert: DePuy ASR™ acetabular cups used in hip resurfacing arthroplasty and total hip replacement. MDA/2010/044. Available at: www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con082125.pdf. Accessed December 14, 2012.
- 33.Visuri T, Pukkala E, Paavolainen P, Pulkkinen P, Riska EB. Cancer risk after metal on metal and polyethylene on metal total hip arthroplasty. Clin Orthop Relat Res. 1996;329(suppl):S280–289. doi: 10.1097/00003086-199608001-00025. [DOI] [PubMed] [Google Scholar]
- 34.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 35.Zijlstra WP, Cheung J, Sietsma MS, van Raay JJ, Deutman R. No superiority of cemented metal-on-metal vs metal-on-polyethylene THA at 5-year follow-up. Orthopedics. 2009;32:479–485. doi: 10.3928/01477447-20090527-06. [DOI] [PubMed] [Google Scholar]