Abstract
Our laboratory at Rice University has forged numerous collaborations with clinicians and basic scientists over the years to advance the development of novel biomaterials and modification of existing materials to meet clinical needs. This review highlights collaborative advances in biomaterials research from our laboratory in the areas of scaffold development, drug delivery and gene therapy, especially as related to applications in bone and cartilage tissue engineering.
Keywords: biomaterials, scaffolds, drug delivery, cartilage tissue engineering, bone tissue engineering
1. Introduction
Although most tissues in the body can undergo self-repair to varying extents, injuries beyond the reparative threshold may benefit from therapeutic intervention to facilitate healing. The gold standard for many of these treatment strategies involves the use of autografts, such as bone grafts harvested from the iliac crest to fill bone defects.[1]–[4] However, harvesting healthy tissue from other sites within the body is typically constrained by limited supply and donor site morbidity. To address this demand for donor tissue, tissue engineering has been recognized as a potential therapeutic solution.
Tissue engineering generally involves the use of biomaterials, cells and bioactive factors in various combinations to facilitate the regeneration of lost or injured tissue.[5] For example, primary progenitor cells can be harvested from a patient, expanded ex vivo to sufficient numbers, and implanted together with a biomaterial scaffold at the site of injury to effect tissue repair. Alternatively, scaffolds can be leveraged for the controlled release of bioactive factors at the defect site to recruit host cells and promote tissue regeneration.[6] Regardless of the specific tissue engineering strategy employed, biomaterial scaffolds generally serve as the foundation to guide and support tissue formation, while delivering cells and bioactive factors to promote regeneration.
Developing clinically relevant biomaterials for use in tissue engineering scaffolds presents distinct challenges, as it requires a strong understanding of materials science in combination with extensive knowledge of the clinical challenge, cell biology, native tissue properties, and controlled therapeutic delivery, among other considerations. Consequently, interdisciplinary collaboration between material scientists, engineers, clinicians, cell biologists, and others may be leveraged to harness collectively the respective expertise of each field toward the development of biomaterials for tissue engineering applications. Situated within the Texas Medical Center, our laboratory at Rice University is in a prime location to facilitate this critical crosstalk between clinicians and materials scientists and engineers. Indeed, over the past two decades, we have forged numerous collaborations with clinicians and basic scientists to drive the development of novel biomaterials and modification of existing materials for tissue engineering, drug delivery and gene therapy.
Developing successful biomaterials is a non-trivial task and requires years of extensive in vitro characterization and comprehensive investigation in pre-clinical in vivo models along the pathway toward regulatory approval or clearance for clinical application. In this review, we will highlight ongoing biomaterials research in our laboratory, with a special focus on bone and cartilage tissue engineering.
2. Bone Tissue Engineering
2.1. Clinical Need
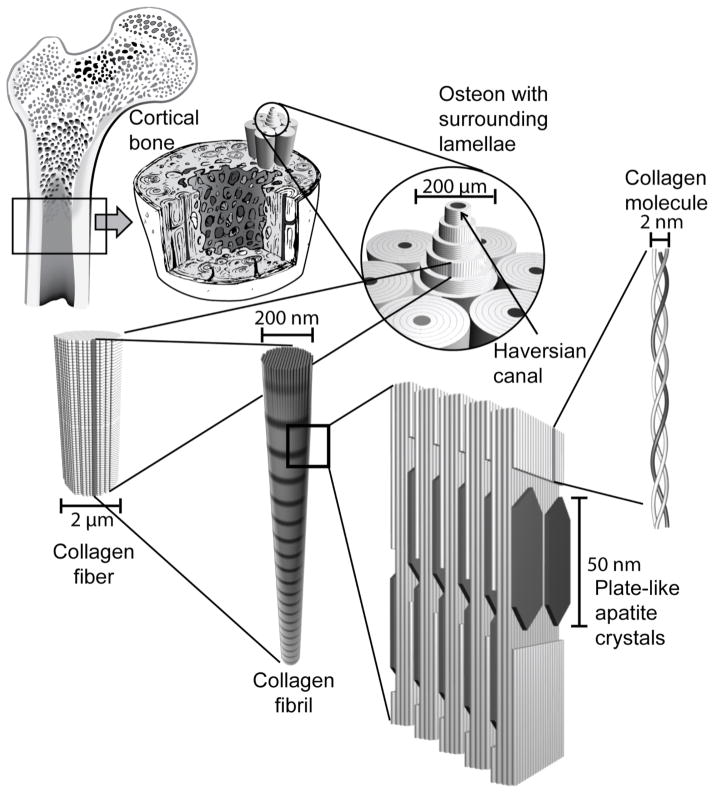
One of the primary functions of the skeletal system is to provide mechanical support to the body. The association of bone’s inorganic components, composed primarily of hydroxyapatite, with its organic components, mostly type I collagen, with smaller quantities of various proteoglycans and glycoproteins, is responsible for the strength of bone tissue.[7] Mechanical support is largely provided by cortical bone, which is a dense solid tissue composed mainly of hydroxyapatite arranged in a compact pattern that forms the outer wall of bones (Figure 1). Cortical bone is supported by blood vessels located within the Haversian canals. Cancellous bone is a lighter, less dense form of bone consisting of trabecular plates and bars that are found in the highly vascular inner parts of bone where hematopoesis and ion exchange occur. These two types of bone have differing mechanical properties reflecting their different functions, with cortical bone having tensile strength (3.1–180 GPa) and modulus (3.9–71 GPa) two to three orders of magnitude greater than that of cancellous bone.[8],[9] Though the compressive properties of cancellous bone vary greatly depending on location in the body, the compressive strength (130–180 MPa) and modulus (4.9–34 GPa) of cortical bone are also greater than those of cancellous bone (0.2–310 MPa and 1.4–9800 MPa, respectively).[8]–[10] It is important, therefore, that materials used for bone repair have the ability to provide adequate mechanical support in bone defects.
Figure 1.
The hierarchical structure of bone. Cortical bone is composed of densely packed osteons made up of lamellae of collagen fibers surrounding a central Haversian canal. Collagen fibers are composed of bundles of collagen molecules called collagen fibrils. Plate-like hydroxyapatite crystals are deposited in the gaps of the collagen molecule structures within collagen fibrils.
According to Wolff’s law, bone tissue has the ability to continuously remodel itself to meet changing mechanical needs such as those associated with growth, development, and exercise.[11] For example, in space flight, astronaut exposure to zero gravity initiates pathological bone resorption.[12] This maintenance is performed by osteoblasts, cells that deposit bone where it is needed, and osteoclasts, cells that resorb bone. Additionally, bone has the ability to regenerate following most injuries.[13] However, in cases of non-union fractures or severe traumatic bone injuries, the intrinsic capacity of bone to self-repair is not sufficient for complete healing to occur. In these cases, the current gold standard of care is the use of a bone autograft, which can typically bridge the defect and facilitate healing.[3],[4] In the United States alone, over 500,000 bone-grafting procedures are carried out annually.[4] However, besides being limited in supply, autografts must be harvested from a secondary site on the patient, which results in donor site morbidity.[1] An alternative solution is the use of allografts harvested from cadavers, which partially resolves the issue of limited supply, but carries the risk of disease transmission as well as other complications that may result in graft failure. Taking into consideration the limitations of these current strategies and the high demand for bone grafts, other materials and methods are being investigated for use in bone regeneration.[14]
2.2. Scaffold Criteria for Bone Tissue Engineering
For the past 20 years, our laboratory has focused on the development of new biomaterials for use as scaffolds in bone tissue engineering strategies to address the clinical need for bone repair. An ideal material for this purpose needs to fulfill several design criteria. The first of these is osteoconductivity, the ability of a material to facilitate cell attachment, proliferation, and migration through the scaffold, as well as nutrient-waste exchange and new vessel penetration.[14] Additionally, osteoinductivity, the ability to induce the differentiation of osteoprogenitor cells such as mesenchymal stem cells (MSCs) into osteoblasts,[14] is necessary for scaffolds to enable bone formation to occur where it would otherwise not occur, a requirement for the healing of critical-size defects.[13] A bone tissue engineering scaffold should also promote vascularization for the delivery of blood to the newly formed bone tissue, occurring mainly via capillary in-growth into the scaffold pores from the surrounding tissue. Vessel in-growth can be induced by the incorporation of angiogenic factors within the scaffold.[13]
There are a variety of techniques that have been developed for the fabrication of scaffolds aimed at facilitating bone regeneration. Regardless of the method, it would be advantageous if the scaffolds are manipulable and easily shaped to conform to irregularly-shaped bone defects.[13] It is also critically important for scaffolds used in load-bearing applications to possess mechanical properties that are similar to bone tissue surrounding the defect. Since bone is constantly remodeling to adapt to new stresses, scaffolds with mechanical strength greater than that of bone can result in bone resorption, while those with mechanical strength less than that of bone may not provide adequate support to the tissue in the region of the defect.[14],[15] Ideally, the scaffold should be designed with a degradation rate such that the strength of the scaffold is maintained until the regenerating tissue can provide the mechanical support necessary to withstand in vivo stresses. Furthermore, degradation products of the scaffold should be biocompatible and removed from the body via physiological pathways to avoid long-term biocompatibility problems.[13]
An important determinant of a scaffold’s ability to support tissue regeneration is the pore size and porosity of the structure. It has been reported that the pore size of scaffolds needs to be greater than 30 microns to allow for bone in-growth.[9] The porosity of a scaffold must be high enough to allow for cellular migration as well as nutrient and waste exchange. Pore interconnectivity is also a necessary feature to enable cells to migrate within the scaffold and allow for the formation of a contiguous tissue that can more uniformly distribute load within the scaffold.[14] Undifferentiated cells such as MSCs and other osteogenic precursor cells are popular cell sources in many bone tissue engineering strategies as they possess a higher proliferative capacity and are often more readily available than mature osteoblastic cells. As such, it is important for bone regeneration constructs to provide osteogenic cues to facilitate the differentiation of cells seeded in them so that they can generate bone-like extracellular matrix (ECM) to fill the defect or support the growth of bone when exogenous growth factors are delivered.[16] Finally, perhaps the most critical scaffold requirement is biocompatibility. Scaffolds should not only be non-toxic to the cells around them, but also not trigger a sustained response from the host’s immune system.[13]
To fulfill different scaffold design criteria, a variety of scaffolding materials have been developed and investigated for use in bone tissue engineering, ranging from natural polymers, such as collagen and hyaluronan, to synthetic polymers, such as poly(lactic acid), to bioactive inorganic materials, such as calcium phosphate.[14] In the next section, we will highlight materials that have been developed or investigated in our laboratory.
2.3. Bone Tissue Engineering Materials
2.3.1. Poly(Propylene Fumarate) Cross-Linked Networks
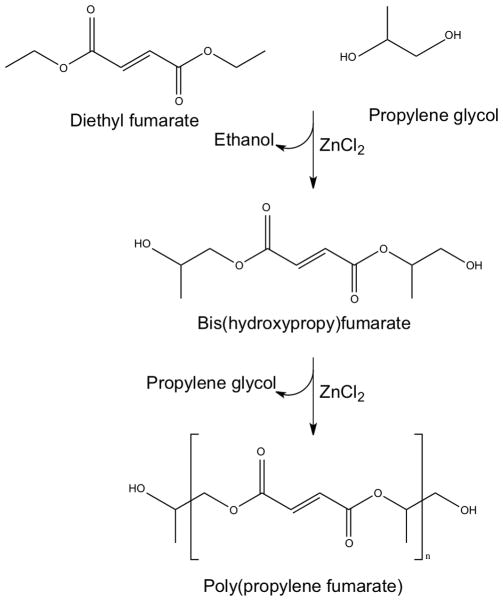
Poly(propylene fumarate) (PPF) is a biodegradable, cross-linkable macromer that was developed in our laboratory for use in bone tissue engineering applications in collaboration with Dr. Michael Yaszemski of the Mayo Clinic. It is most commonly prepared via a two-step synthesis from diethyl fumarate and propylene glycol (Figure 2).[17] PPF is a viscous liquid, which contains unsaturated carbon-carbon double bonds that allow for cross-linking.[18]
Figure 2.
Two-step synthesis of poly(propylene fumarate) from diethyl fumarate and propylene glycol catalyzed by ZnCl2. Reproduced with permission [17].
In collaboration with Dr. Paul Engel of Rice University, Peter et al. demonstrated that synthesis techniques can be modified to produce different molecular weights of PPF, with higher molecular weights resulting in a more viscous liquid.[19] Increased molecular weight of the PPF macromer has been shown to result in improved mechanical properties following the formation of a polymer network.[20] Furthermore, the liquid nature of PPF prior to the formation of cross-linked networks allows it to be molded into a variety of shapes during the cross-linking process and endows it with the potential to be injected and cross-linked in situ.[21] Timmer et al. have shown that once cross-linked, PPF composites containing beta-tricalcium phosphate (β-TCP) exhibit good mechanical properties with modulus and yield strength (approximately 1200 MPa and 300 MPa, respectively) near that of trabecular bone.[22]
PPF undergoes bulk degradation through hydrolysis of the ester linkages yielding the non-toxic, non-immunogenic degradation products fumaric acid and propylene glycol.[23] Degradation time can be modified by modulating the molecular weight of the PPF macromer and the cross-linking density of the polymer network, as well as through the use of different cross-linking agents and the incorporation of other components to form PPF-based composites.[20],[22] This allows for degradation time to be tuned to match cell infiltration and bone deposition within the scaffold.
A variety of cross-linking agents have been used to cross-link PPF macromers. Propylene fumarate-diacrylate (PF-DA) and poly(ethylene glycol)-dimethacrylate (PEG-DMA) cross-links have been shown to improve the mechanical properties of the material with increased incorporation resulting in scaffolds that can be tuned to more closely match the mechanical properties of bone.[23],[24] Peter et al. demonstrated that unlike the previously mentioned cross-linking molecules, the compressive strength and compressive modulus of N-vinyl pyrrolidone (NVP)-cross-linked PPF decrease as the NVP/PPF ratio increases.[25] Additionally, Fisher et al. showed that diethyl fumarate (DEF)-cross-linked PPF presents an increase in sol fraction and swelling degree as the DEF/PPF ratio increases, while maintaining a compressive strength suitable for trabecular bone replacement.[26]
To characterize the biological properties of both PPF-based and non-PPF-based scaffolds, our laboratory has collaborated extensively with Dr. John Jansen of Radboud University Nijmegen Medical Center in the Netherlands. Although PPF alone has not been shown to be osteoinductive, it can be modified to contain osteoinductive factors. The transforming growth factor beta (TGF-β) superfamily is a large group of polypeptides that mediate multiple biological functions, including bone induction. Many of these growth factors are found in the ECM of bone, and some have been found to induce bone formation when delivered via scaffolds in vivo.[27] Vehof et al. have used TGF-β1-coated PPF to enhance bone formation in a rabbit cranial defect compared to PPF alone.[28]
The development and early investigations of PPF have been well chronicled by Kretlow and Mikos.[29] In particular, it should be noted that early work by Peter et al. and Fisher et al. demonstrated biocompatibility in rat and rabbit models, respectively.[30],[31] Additionally, an investigation of in vitro osteoconductivity of rat mesenchymal stem cells cultured on PPF/β-TCP composites showed increased osteoblastic differentiation over 4 weeks, as measured by alkaline phosphatase activity and osteocalcin production.[32] The osteoconductivity of PPF has also been established in vivo by Fisher et al. with a rabbit incisor extraction socket model, resulting in faster bone ingrowth for the PPF-filled socket than an empty socket at one week.[33]
In an effort to improve vascularization of PPF-based bone tissue grafts, both Young et al. and Patel et al. incorporated gelatin microparticles loaded with bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) into PPF scaffolds. BMP-2 is a cytokine that plays an important role in bone formation and healing, and its use in tissue engineering applications has been well described.[34] VEGF has also been shown to play a role in osteogenesis and angiogenesis and has potential for use in bone regeneration.[35] These dual growth factor-loaded composite scaffolds induced more bone formation at 4 weeks in rat critical-size cranial defects than composite scaffolds with only BMP-2 loaded microspheres, but no significant difference in bone growth was observed at 12 weeks (Figure 3).[36],[37] Possible explanations for this finding include the burst release of VEGF resulting in little effect at later time points, or that vascularity may not be a limiting factor for the rat cranial defect model due to the surrounding vasculature and thin cranium of the animal.[36],[37]
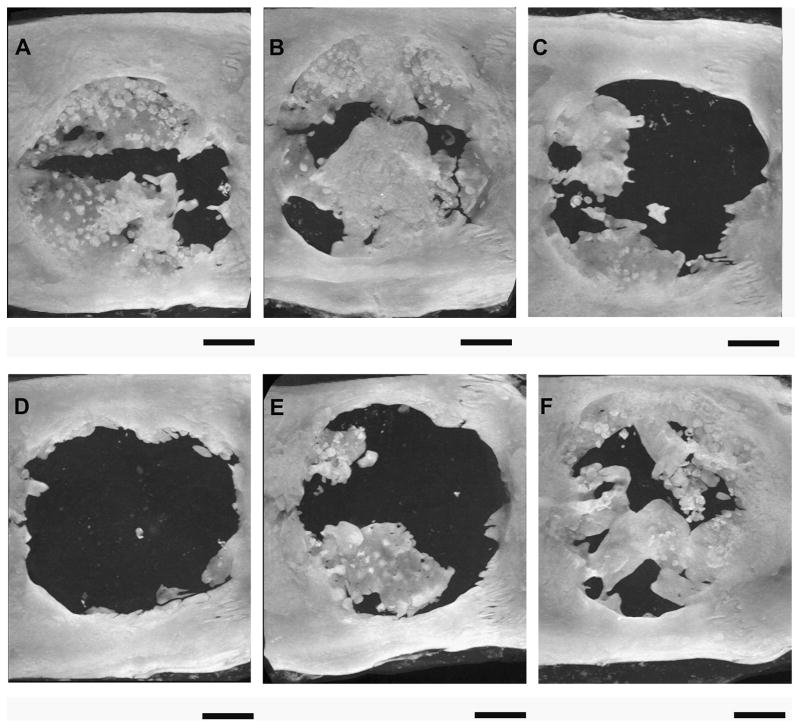
Figure 3.
MicroCT generated maximum intensity projections of rat cranial defects 12 weeks after implantation of various poly(propylene fumarate)/gelatin microparticle (GMP) composite scaffolds. (A) Scaffold loaded with 1.25 mg BMP-2-loaded 40 mM basic GMP demonstrated 28.5% bone fill. (B) Scaffold loaded with 1.25 mg BMP-2-loaded 40 mM basic GMPs and 1.25 mg VEGF-loaded 10 mM acidic GMPs demonstrated 40% bone fill. (C) Scaffold loaded with 1.25 mg BMP-2-loaded 40 mM basic GMP and 2.5 mg VEGF-loaded 10 mM acidic GMP demonstrated 10.9% bone fill. (D) Scaffold loaded with 0.63 mg BMP-2-loaded 40 mM basic GMP demonstrated 3.1% bone fill. (E) Scaffold loaded with 0.63 mg BMP-2-loaded 40 mM basic GMP and 1.25 mg VEGF-loaded 10 mM acidic GMP demonstrated 15.1% bone fill. (F) Scaffold loaded with 0.63 mg BMP-2-loaded 40 mM basic GMP and 2.5 mg VEGF-loaded 10 mM acidic GMP demonstrated 35% bone fill. Bars represent 2 mm. Reproduced with permission from [36].
The incorporation of alumoxane nanoparticles into PPF scaffolds has been shown to confer improved mechanical properties to the material.[38] These scaffolds were evaluated by Mistry et al. in a goat femoral condyle implantation model and demonstrated no difference in biocompatibility or degradation over 12 weeks compared to scaffolds comprised of PPF alone. A histological image illustrating PPF degradation is shown in Figure 4.[39] However, this degradation time may not have been sufficient for detectable differences between the pure polymer scaffolds and the composites. PPF/alumoxane nanoparticle (NP) composites have been shown to undergo more rapid degradation in an accelerated in vitro degradation study compared to the polymer alone, but predegraded composites did not show any signs of change in biocompatibility.[40]
Figure 4.
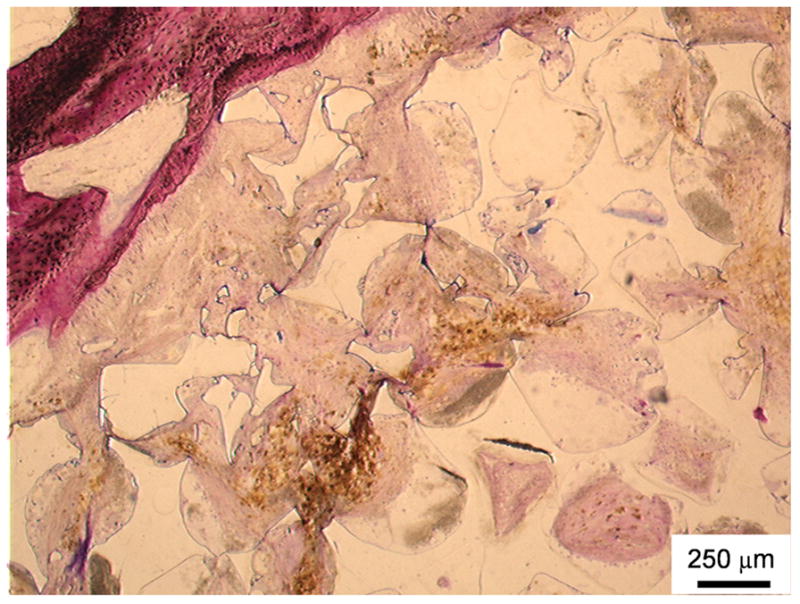
Histological section of a poly(propylene fumarate) scaffold stained using methylene blue and basic fuchsin (which stains nuclei purple, collagen and connective tissue blue, and cytoplasm and smooth muscle cells pink) 12 weeks after implantation into a goat femoral condyle. The top left area demonstates the in vivo breakdown of PPF into smaller fragments as well as soft tissue infiltration with minimal inflammation. Reproduced with permission from [39].
PPF/single-walled carbon nanotube (SWNT) nanocomposites have also been shown to possess improved mechanical properties compared to the polymer alone.[41] Furthermore, Shi et al. have demonstrated that SWNT functionalized with surfactant have improved dispersion within the nanocomposites,[42] resulting in enhanced mechanical properties.[43] The use of ultra-short single-walled carbon nanotubes (US-tubes) rather than SWNTs allowed for the development of injectable in situ cross-linkable nanocomposites,[41] as well as the fabrication of porous nanocomposite scaffolds for bone tissue engineering. [44] These PPF/US-tube nanocomposite scaffolds not only proved to be cytocompatible in vitro,[45] but also osteoinductive in vivo in a rabbit femoral condyle defect model. [46]
Kim et al. have shown that PPF/hydroxyapatite nanoparticle (HANP) composites result in improved surface properties due to increased roughness, hydrophilicity, protein adsorption, and initial cell attachment, as well as up-regulation of osteogenic growth factor expression, with corresponding osteoblastic differentiation of rat MSCs.[47]
Recently, Henslee et al. used a composite scaffold consisting of a solid PPF intramedullary rod surrounded by a porous PPF sleeve containing poly(DL-lactic-co-glycolic acid) (PLGA) microspheres with adsorbed recombinant human BMP-2, as shown in Figure 5, in a rat segmental femoral defect model. Though these combination scaffolds provided significant mechanical strength to help stabilize the defect, the porous outer scaffold may have impeded the migration of regenerative cells into the defect region, resulting in decreased bone formation.[48]
Figure 5.
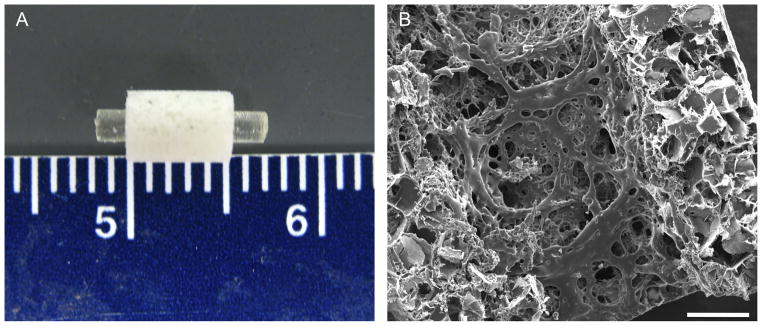
Combination scaffolds composed of a solid intramedullary poly(propylene fumarate) rod surrounded by a porous poly(propylene fumarate) sleeve to be used in a rat segmental femoral defect shown grossly (A) and microscopically through scanning electron microscopy (B). Scale bar in (B) represents 500 μm. Reproduced with permission from [48].
In summary, PPF is a biodegradable, cross-linkable synthetic macromer that has been developed in our laboratory for bone tissue engineering applications. It has been shown to degrade by hydrolysis of its ester bonds into non-toxic, non-immunogenic products that can be easily metabolized or removed from the body. PPF can be molded into a variety of shapes in order to fill bone defects before it is cross-linked. PPF has been combined with a variety of other materials to form composites that can not only modulate the overall mechanical and degradation properties, but also give rise to scaffolds with increased osteoconductivity and osteoinductivity, making PPF a favorable material for bone tissue regeneration.
2.3.2. Oligo(Poly(Ethylene Glycol) Fumarate) Hydrogels
Although oligo(poly(ethylene glycol) fumarate) (OPF) hydrogels have been more extensively used in cartilage tissue engineering (which will be discussed in detail in a later section), they were initially developed in our laboratory for use in bone tissue engineering. Early work by Temenoff et al. demonstrated the ability of OPF hydrogels to support the osteogenic differentiation of encapsulated rat MSCs for bone regeneration by modulating the swelling properties of the hydrogels.[49] Two OPF formulations with different swelling properties were used to encapsulate these cells in the presence and absence of osteogenic supplements, and the extent to which these cells underwent osteogenic differentiation was determined over a period of 28 days via histology and biochemical assays for osteogenic markers. It was found that the osteogenic differentiation of the encapsulated progenitor cells is dependent on the swelling properties of the OPF hydrogel, given that hydrogels with greater swelling promoted the osteogenic differentiation of MSCs over those that swelled less.[49]
In another approach, Shin et al. examined the potential of OPF hydrogels tethered with signaling peptides as instructive biomimetic substrates for the osteogenic differentiation and mineralization of cultured MSCs.[50],[51] It was hypothesized that by functionalizing the synthetic hydrogel with the tri-amino acid sequence, RGD (arginine-glycine-aspartic acid), which has been identified as the key integrin-binding domain in adhesive proteins in the ECM, cell adhesion can be improved and, therefore, better control over cell behavior can be achieved.[52] Specifically, it was found that hydrogels modified with the cell-binding peptide Gly-Arg-Gly-Asp-Ser (GRGDS) and an osteopontin (OPN)-derived peptide induced the differentiation and mineralization of MSCs, as indicated by phenotypic markers, including alkaline phosphatase activity, osteopontin secretion and calcium deposition.[51] In addition to RGD peptides, calcium phosphate nanocrystals and hydroxyapatite nanoparticles (HANP) have also been incorporated into OPF hydrogels to mimic the inorganic component of natural bone. [52],[53]
2.3.3. Poly(N-Isopropyl Acrylamide)-Based Hydrogels
One of the current focuses of our laboratory is the development of in situ forming, thermo-responsive, chemically cross-linkable hydrogels to be used for bone tissue regeneration. It should also be noted that although hydrogels do not possess the mechanical strength necessary to provide support for load-bearing functions in bone applications, they can be used to fill critical-size defects, in conjunction with mechanical supports where necessary, to promote bone regeneration that would not otherwise occur. Hydrogels are appealing for use in these defects because they can be formed in situ in a minimally-invasive manner and are highly hydrated. This hydrated environment allows them to not only deliver cells but also to support cell proliferation and differentiation throughout the scaffold by mimicking the in vivo tissue environment to allow for normal cellular function as well as vessel ingrowth.[54],[55]
Hacker et al. have developed novel poly(N-isopropyl acrylamide) (p(NiPAAm))-based macromers that are formed via free-radical co-polymerization of NiPAAm, pentaerythritol diacrylate monostearate (PEDAS), acrylamide (AAm), and hydroxyethyl acrylate (HEA). These co-polymers undergo a tandem gelation consisting first of a rapid thermally-induced physical gelation in the range of physiological temperatures, with the transition temperature dependent on the relative concentrations of the monomers. Subsequently, the side groups resulting from HEA incorporation can be chemically modified with methacrylate or acrylate groups to allow for in situ chemical cross-links to form between macromers, increasing the stability of the hydrogels, while the PEDAS group provides a hydrophobic domain that has the potential to improve protein and cellular binding.[56] Kretlow et al. have incorporated vinylphosphonic acid (VPA) into similar tandem gelling p(NiPAAm)-based polymers to allow for binding of calcium ions, which can be beneficial for mineralization and osteoblastic differentiation. Additionally, increasing VPA allowed for increased calcium ion binding to the macromers, resulting in decreases in transition temperature.[57]
Klouda et al. have evaluated the cytocompatability of p(NiPAAm)-based gels with rat fibroblasts placed in direct contact with the macromers in vitro. Before modification with the chemical cross-linkable methacrylate and acrylate groups, the macromers demonstrated over 60% cell viability at 24 hours. However, following modification, macromers showed a time- and methacrylate or acrylate dose-dependent effect on cell viability, with decreased cell viability seen as early as two hours in some heavily modified macromers. These studies suggested that the cell exposure time to unreacted crosslinking groups should be minimized when attempting cell encapsulation.[58] Additionally, some formulations of these thermoresponsive macromers with either methacrylate or acrylate cross-linking groups have been used to encapsulate rat MSCs. Qualitative detection of live cells within these hydrogels for up to three weeks indicated the suitability of these systems for cell encapsulation. Furthermore, significant mineralization of both cell laden and cell free hydrogels was seen after three weeks in osteogenic media, as shown in Figure 6. This was likely due to the ability of the hydrogels to bind proteins via their hydrophobic domains, which then facilitated mineralization.[59]
Figure 6.
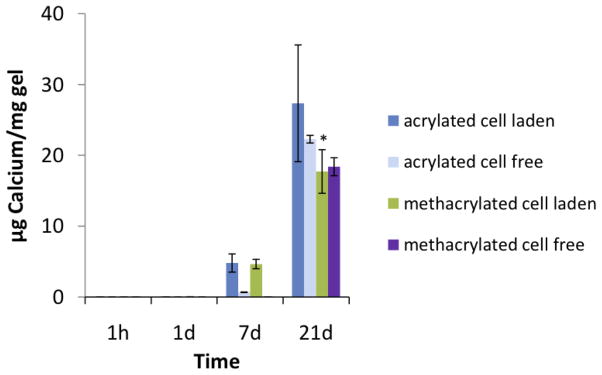
Calcium content of wet mesenchymal stem cell-laden and cell-free poly(N-isopropyl acrylamide)-based hydrogels containing pentaerythritol diacrylate monstearate hydrophobic domains after culture in osteogenic medium (n = 3–5). Calcium content was undetectable at the 1h and 1d time points. Reproduced with permission from [59].
2.3.4. Extracellular Matrix-Based Scaffolds
The current gold standard for treating skeletal defects employs autologous tissue, as it contains viable cells, components of the ECM and bioactive factors.[60],[61] These characteristics are pertinent components of an ideal bone graft material, one that is osteoconductive and osteoinductive.[61] While osteoconductivity may be imparted to scaffolding materials by modulating their composition, surface properties, and architecture, bioactive factors such as osteoinductive growth factors and multipotent MSCs may be necessary to instill scaffolds with osteoinductive properties. In situations where the inherent osteoinductivity of natural bone may be insufficient for spontaneous bone regeneration to occur, an osteoinductive scaffold would ideally serve to augment the osteogenic potential of the defect site and induce the differentiation of transplanted or host progenitor cells towards bone-forming cells.[62] One method to improve the osteoinductivity of scaffolds to enhance bone healing is the delivery of osteoinductive growth factors such as transforming growth factor (TGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and various types of BMPs.[63],[64] However, this method may be plagued by the drawbacks of possible growth factor-related side effects, high costs and desired release kinetics that may be difficult to achieve.[65]–[67]
For the past decade, our laboratory has established a cell-based approach to confer scaffolds with osteoinductive properties (Figure 7). Extensive studies have shown that a variety of synthetic scaffolds, such as titanium fiber meshes and electrospun poly(ε-caprolactone) (PCL) fiber meshes, can be coated with bone-like ECM using MSCs under flow perfusion conditions as well as static culture (Figure 8).[62],[68]–[72] In bone, interstitial fluid flow (and therefore, fluid shear stress) exists as a result of transcortical pressure gradients produced by vascular and hydrostatic pressure, and mechanical loading.[73] The rationale behind the use of flow perfusion culture conditions is not only to mitigate nutrient transport limitations associated with static culture but also to simulate the bone microenvironment and provide mechanical stimulation to cells to facilitate osteogenic differentiation.[62] Studies modulating flow perfusion culture parameters, such as medium flow rate, medium viscosity, and scaffold architecture (e.g., mesh size), have demonstrated that the extent of osteogenic differentiation of MSCs and corresponding bone-like ECM production is correlated to the magnitude of fluid shear stress.[68],[74]–[76] In vivo fluid shear forces during physiological loading have been estimated to be between 8 and 30 dynes cm−2.[68],[69] Although fluid shear forces on cells cultured within flow perfusion bioreactors are estimated to be lower and lie in the order of 0.1 dyne cm−2, shear forces within this order of magnitude have been found to be sufficient to augment the level of calcified matrix deposited in culture.[76] Furthermore, decellularization of these MSC-seeded constructs results in the generation of ECM-coated scaffolds with acquired osteogenic potential, as evidenced by in vitro studies which demonstrated that they support the osteogenic differentiation of fresh MSCs that were reseeded for a second culture duration.[69],[71],[72] The acquired ostegenicity of these ECM-coated scaffolds can in part be attributed to the presence of bioactive factors within the deposited ECM, which has been found to contain major bone components, including collagen, glycosaminosglycans and mineral.[71] Additionally, the ECM serves not only as a physical framework for adherent cells, but also as a local reservoir and modulator of growth factors.[79] Indeed, using immunohistochemistry, it has been shown that the bone-like ECM coating synthesized by MSCs cultured in a scaffold under flow perfusion conditions contains several bone-related growth factors such as BMP-2.[70]
Figure 7.
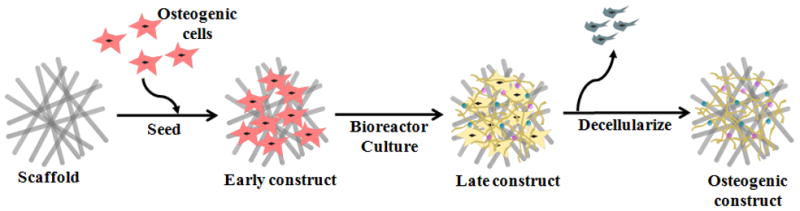
Generation of an extracellular matrix-scaffold construct for bone regeneration. A naked scaffold is first seeded with osteogenic progenitor cells. The cell/scaffold construct is then cultured in a bioreactor under flow perfusion conditions, where cells lay down extracellular matrix that coats the scaffold. By decellularizing the construct, an extracellular matrix-coated scaffold capable of supporting osteogenic differentiation of progenitor cells is obtained. Reproduced with permission from [171].
Figure 8.
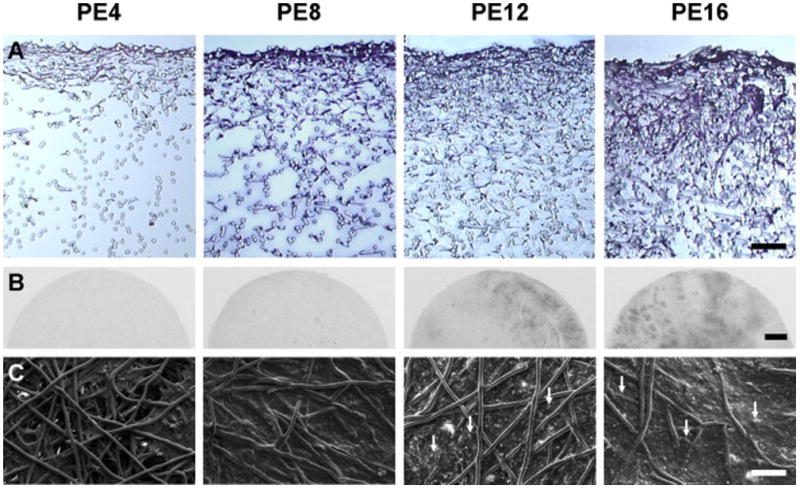
MSCs were cultured on poly(ε-caprolactone) microfiber scaffolds under flow perfusion conditions in a bioreactor to examine the effect of culture duration on mineralized extracellular matrix deposition. PE 4, PE 8, PE 12 and PE 16 represent the PCL/extracellular matrix (PE) constructs that were generated in flow perfusion culture of increasing durations (4, 8, 12 and 16 days, respectively). Flow perfusion conditions augmented the distribution of cells and extracellular matrix proteins over time, as observed via histological sections stained with hematoxylin and eosin, as shown in (A). Scale bar represents 100 μm. X-ray imaging indicated that radio-opaque regions of mineralized matrix increased over time, as shown in (B). Scale bar represents 1 mm. Scanning electron microscopy was used to visualize the surface morphology of constructs, as shown in (C). Arrows indicate mineral nodules and scale bar represents 100 μm. Reproduced with permission from [71].
Given the inherent complexity of the ECM milieu and difficulties associated with the delivery of growth factors from a scaffold, our laboratory has developed a novel tissue engineering strategy to address these challenges. As the examples above illustrate, under well-controlled engineering conditions, it is possible to harness cells to coat biologically inactive materials with an ECM containing bioactive factors capable of promoting osteogenesis. Looking forward, our laboratory is currently investigating the applicability of these established principles in bone tissue engineering to the regeneration of cartilage. Ultimately, we envision that these ECM-scaffold constructs will be translated in vivo, where they will provide a platform conducive for the recruitment of host progenitor cells, induce their differentiation, and facilitate bone and cartilage regeneration.
2.3.5. Poly(Methyl Methacrylate)-Based Implants
In addition to the development of novel biomaterials, another focus of our laboratory is the leverage of materials that are currently being used in the clinic in order to facilitate a timely clinical translation of new applications toward bone regeneration. For example, our laboratory is currently working with Dr. Mark Wong of the University of Texas School of Dentistry at Houston to develop poly(methyl methacrylate) (PMMA)-based space maintainers for use in a two-stage regenerative medicine approach to address composite tissue defects in the craniofacial region. The approach involves the application of a PMMA-based space maintainer in a bony defect to preserve the defect space and facilitate soft tissue healing prior to definitive repair. PMMA is a non-degradable polymer currently regulated by the Food and Drug Administration in the United States for human clinical use in the form of bone cement products. It is commonly used to fill bone defects, but does not facilitate fracture healing or integrate with host bone.[13] Additionally, two of the major complications that can arise when using the current PMMA-based bone cement products in space maintenance applications are wound opening, or dehiscence, and infection.[80]–[82]
In a study that was aimed at delivering antibiotics with space maintainers, Shi et al. combined PMMA bone cement with biodegradable carboxymethylcellulose (CMC) hydrogels to impart porosity and antibiotic-loaded microspheres of PLGA to facilitate controlled drug release. These composite materials demonstrated sustained antibiotic release over 5 weeks in vitro.[83] More recently, we have collaborated with Dr. Yasuhiko Tabata of Kyoto University in Japan who has extensive experience using gelatin microparticles for the delivery of bioactive factors. PMMA bone cement was combined with antibiotic-loaded gelatin microparticles, which served the dual function of imparting porosity into the construct and facilitating controlled drug release. These constructs demonstrated sustained antibiotic release over 10–14 days in vitro.[84] Soft tissue regeneration following the implantation of a space maintainer has been investigated in vivo using a non-healing rabbit mandibular defect model in conjunction with an oral mucosal defect, which allows for communication between the oral cavity and the mandibular defect. Kretlow et al. have demonstrated that preformed porous PMMA space maintainers show a trend of increased soft tissue healing compared to the solid PMMA implants, with those of higher porosity exhibiting increased inflammation. Cross sections of PMMA implants of varying porosity are shown in Figure 9.[85] When these implants were formed in situ by Spicer et al. in a rabbit mandibular defect model, the porous implants showed a trend of enhanced soft tissue healing relative to the solid implants, although no statistical difference in soft tissue healing was observed between the in situ formed groups.[86]
Figure 9.
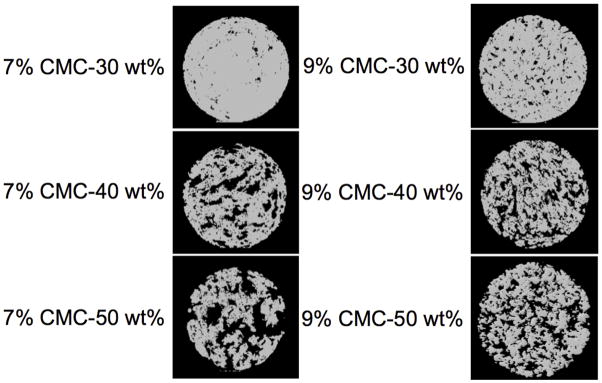
MicroCT images of cross-sections of cylindrical poly(methyl methacrylate) implants (10 mm diameter × 6 mm height) of varying porosity. Either 7 or 9 wt% carboxymethylcellulose hydrogels were incorporated at 30, 40, or 50 wt% with poly(methyl methacrylate) cement to form the above porous implants. Digital cross sections of the implants were made by slicing through the center of the axially oriented implant. Reproduced with permission from [85].
3. Cartilage Tissue Engineering
3.1. Clinical Need
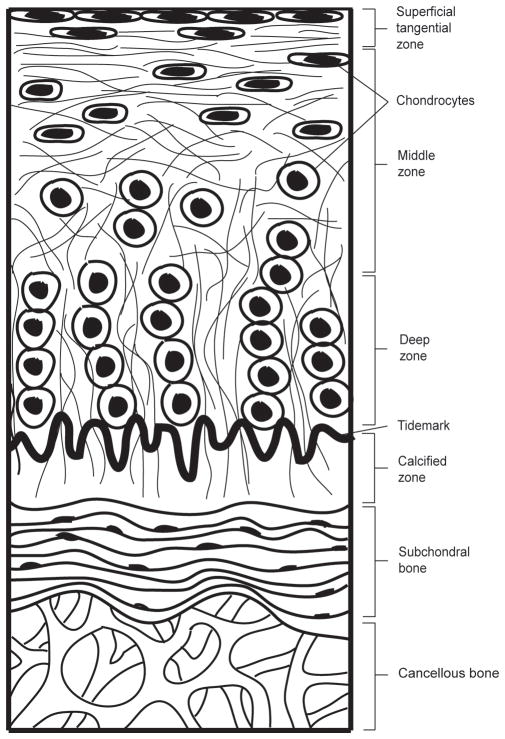
Articular cartilage is a highly specialized and complex connective tissue that lines the end surfaces of articulating bones. A unique tissue composition and structure (Figure 10) confers the tissue with the ability to fulfill its primary function of providing a low-friction surface and facilitating load transfer between bones in joints.[87] Compositionally, articular cartilage has an extremely high matrix to cell ratio, as it is composed predominantly of ECM, with only 2–3% of its mass consisting of embedded chondrocytes.[88] These resident cells synthesize and maintain this ECM, which consists of a reinforcing network of collagen fibrils within a hydrated proteoglycan gel that resists compressive forces.[89] Structurally, articular cartilage can be described as a non-homogeneous, multi-layered tissue with zonal organization, identified as the superficial, middle, deep, and calcified zones.[90] Each of these zones varies with regard to matrix composition and morphology, and cellular, mechanical, and metabolic properties.[91]
Figure 10.
Structure of articular cartilage. The articular cartilage is divided into four distinct zones – superficial tangential zone, middle zone, deep zone and calcified zone. Within each zone, chondrocytes and collagen fibers are uniquely organized. The underlying subchondral bone and cancellous bone provide support to the articular cartilage layer.
As a result of sports injuries, trauma, osteoarthritis or osteochondritis, damage to the articular cartilage can occur.[92] However, unlike bone, articular cartilage lacks the intrinsic ability to naturally regenerate because of its avascularity, and lack of mobility of the chondrocytes that reside within the dense cartilaginous matrix.[93] As such, physicians have long faced the challenge of treating articular cartilage defects. This was recognized as early as 1743, when the famous anatomist William Hunter stated, “an ulcerated cartilage is a troublesome problem and once destroyed, it never repairs.”[94] Despite centuries of progress in medicine and science, this clinical observation has remained unchanged, and there is currently no successful and universally accepted approach for the treatment of damaged articular cartilage.[95]
Treatment strategies for articular cartilage defects are currently limited to surgical procedures that seek to either encourage the intrinsic capacity of cartilage and the subchondral bone to self-heal by creating access to the marrow or fill the lesion with replacement tissue grafts or cells capable of chondrogenesis.[93],[96] The former strategy includes techniques such as abrasion arthroplasty, Pridie drilling, and microfracture.[97] These techniques were developed based on the observation that, while partial-thickness defects do not heal spontaneously, defects that do penetrate into the subchondral bone evoke an intrinsic repair response that generates a fibrocartilaginous repair tissue.[97] The aim of drilling and microfracturing is to create perforations into the bone marrow space underlying regions of damaged cartilage in order to release bioactive factors and progenitor cells, which may stimulate cartilage repair.
However, there is large variability in the clinical outcome of these procedures as the quality of repair tissue formed is unpredictable, ranging from no cartilage to fibrocartilage to hyaline cartilage, depending on the patient.[98] Another approach, tissue grafting, requires inflicting damage to healthy tissue so that the desired cell type or tissue can be harvested for transplantation into the defect site. For example, osteochondral transfer, or mosaicplasty, is a common technique used to treat small, full-thickness defects, whereby a cylindrical plug of healthy autologous osteochondral tissue from a low-load-bearing region of the articular cartilage is removed and press-fit into the defect site.[97] Like-wise, traditional autologous chondrocyte transplantation requires the excision of cartilage from an uninjured region of the joint to harvest healthy chondrocytes, which are cultured and expanded ex vivo, and subsequently re-injected back into the defect site.[99] At present, it appears that current treatment approaches for articular cartilage injuries require the infliction of tissue damage before any desired therapeutic effect (which is not guaranteed) can be achieved.
Although current approaches are reasonably effective in achieving the clinical endpoints of symptomatic relief and improved joint function, they have not been successful at averting the future degeneration of repair tissue and surrounding host tissue and therefore, ensuring long-term efficacy. This is largely because the repair tissue that arises typically does not possess the same mechanical properties as native articular cartilage, nor does it successfully integrate with surrounding host tissue.[97] As such, these concerns necessitate the development of improved strategies. As previously discussed, within the tissue engineering paradigm, scaffolds are typically an indispensable component; besides serving as a delivery vehicle for cells and bioactive factors to the defect site, they can potentially be engineered with appropriate cellular cues and regulators to provide an instructive environment that can direct the behavior of transplanted cells or evoke desired host responses in vivo.[100]
3.2. Scaffold Criteria for Cartilage Tissue Engineering
In designing a scaffold for cartilage tissue engineering, there are several fundamental criteria that have to be addressed. Ideally, the scaffold should not only support the growth and expansion of transplanted cells or induce the in-growth of host cell populations, but also possess an adequate degree of porosity to allow for cell migration and diffusion of nutrients and waste products.[101],[102] Another important criterion for an optimal scaffold is the ability of the scaffold to integrate well with surrounding cartilage tissue and degrade at a rate that matches the rate of neocartilage tissue formation.[103] Additionally, the biomaterial must be biocompatible; neither the intact material or its degradation products should elicit any prolonged inflammatory response nor exhibit severe immunogenicity or cytotoxicity.[104] To date, a wide variety of scaffolding materials have been investigated for use in cartilage tissue engineering, including both natural materials[105]–[107] and synthetic materials.[108],[109] As an extensive discussion of scaffolding materials for cartilage tissue engineering is not within the scope of this review, the reader is encouraged to refer to review articles by Temenoff and Mikos[98] and Seifalian et al.[103]
3.3. Cartilage Tissue Engineering Materials
Over the past decade, our laboratory has developed novel, injectable scaffolding materials for the delivery of bioactive factors and cells to cartilage defects to promote tissue regeneration. These biomaterials are based on poly(propylene fumarate-co-ethylene glycol) (P(PF-co-EG)) and oligo(poly(ethylene glycol) fumarate) (OPF), both of which can be used to engineer biodegradable and biocompatible hydrogels.[33],[110]–[112] Since cartilage is a tissue that has an exceptionally high water content of about 65 to 80% of its wet weight, hydrophilic materials, such as these poly(ethylene glycol) (PEG)-based materials, that can be manipulated to form hydrogels may be ideal as scaffolding materials for cartilage repair, as they have the capacity to mimic aspects of the hydrated cartilaginous matrix.[113]
3.3.1. Poly(Propylene Fumarate-co-Ethylene Glycol) Hydrogels
P(PF-co-EG)-based hydrogels developed in our laboratory were initially investigated for use in cartilage tissue engineering applications. This hydrophilic, biodegradable, biocompatible, and in situ cross-linkable block copolymer is synthesized by copolymerizing PPF with PEG via a transesterification reaction.[111],[112],[114]
PEG-based copolymers such as P(PF-co-EG) are typically thermoreversible due to the hydrogen bonding interactions between PEG and water molecules.[115] Behravesh et al. synthesized ABA-type block copolymers of PPF and methoxy PEG (mPEG) and investigated the thermoreversible properties of these block copolymers with varying mPEG molecular weight.[116] Notably, critical solution temperatures and sol-gel transition temperatures can be controlled by modulating the block length of mPEG; these copolymers have been formulated to exist as a liquid below 25°C and a gel above 35°C, enabling cells within the polymer liquid at room temperature to be hydrogel-encapsulated at physiological temperature.[116]–[118] Leveraging this thermo-responsive property of the P(PF-co-EG) polymer system, Fisher et al. explored the use of this material for chondrocyte delivery to articular cartilage defects.[118] Using bovine articular chondrocytes as an experimental model, it was demonstrated that the P(PF-co-EG) polymer system supported the viability of encapsulated chondrocytes and the production of proteoglycans and type II collagen.[118]
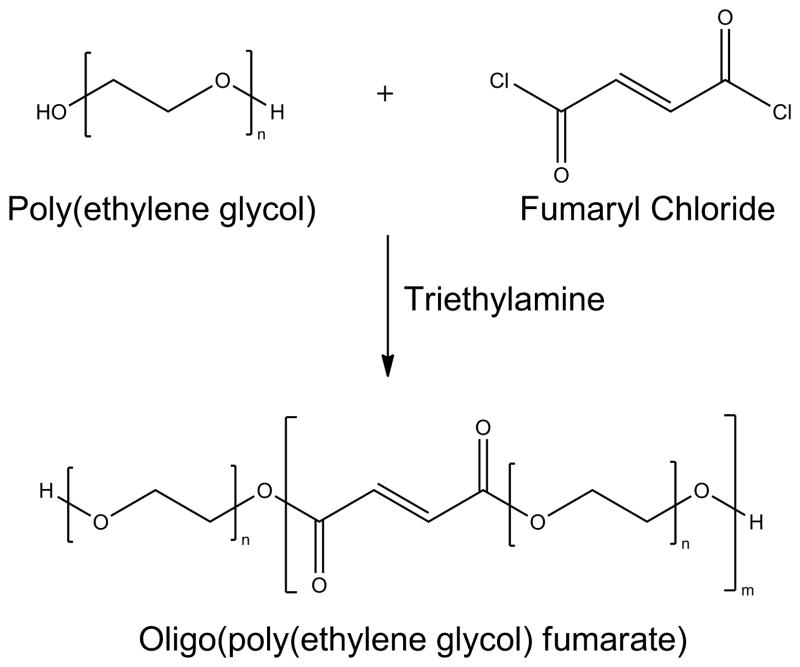
3.3.2. Oligo(Poly(Ethylene Glycol) Fumarate Hydrogels
While P(PF-co-EG) copolymer systems have the potential to serve as hydrogels for tissue engineering applications, the multiple fumarate groups within PPF blocks in the copolymer result in hydrogel networks with varying molecular weight between cross-links and therefore varying mesh sizes.[114],[119] To confer a greater degree of control over hydrogel parameters, Jo et al. pioneered the synthesis and characterization of the OPF macromer (Figure 11), which consists of alternating units of fumaric acid and PEG linked together by ester bonds.[120] If PEG of higher molecular weight is used to synthesize the OPF macromer, the increased distance between cross-links will result in a hydrogel with a larger mesh size and hence, a higher swelling ratio.[121] This unique feature confers the user with the flexibility to tailor hydrogels of different material properties simply by altering the molecular weight of PEG used in the formulation of the macromer.[110],[111] Additionally, the OPF macromer possesses unsaturated double bonds along its chain that allow for the synthesis of hydrogels with tunable structure and properties.[120]
Figure 11.
The oligo(poly(ethylene glycol) fumarate) macromer is synthesized by reacting poly(ethylene glycol) with fumaryl chloride in the presence of triethylene amine.
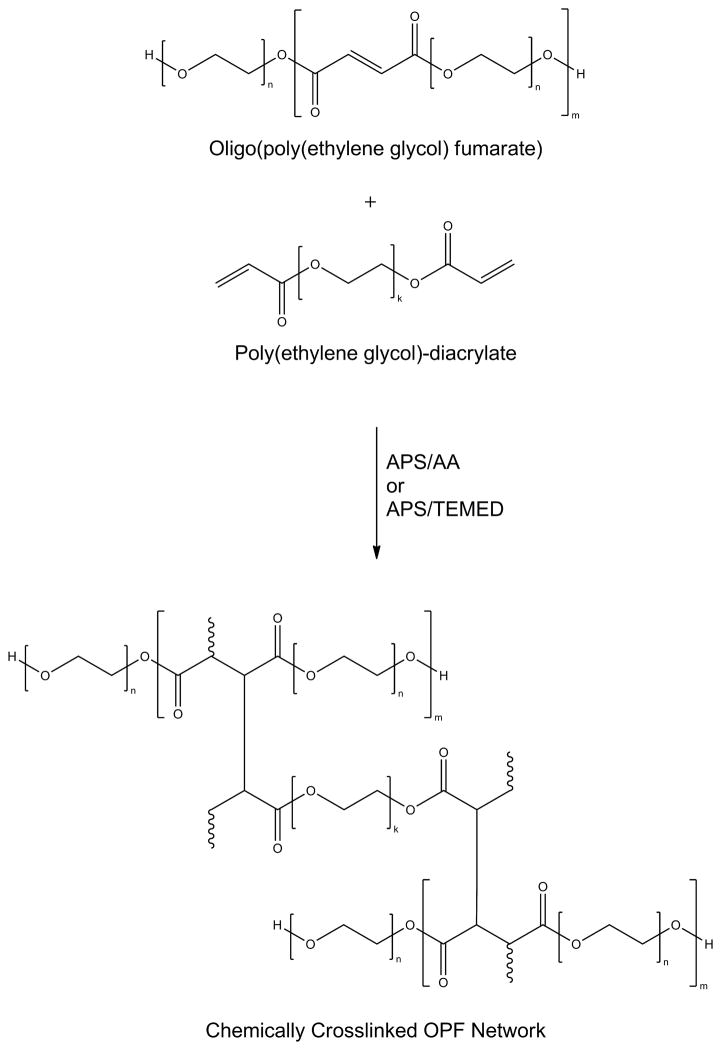
Cross-linking of the OPF macromer occurs via radical polymerization[120], either by photoinitiation[112],[120] or thermal initiation in the presence of a suitable radical initiator system, such as ammonium persulfate and ascorbic acid (APS/AA).[120] These cross-linking mechanisms were employed to allow the macromer to be injected into a defect site in a minimally invasive manner and cross-linked under physiological conditions. Of the two cross-linking methods, a thermal initiation system would be more advantageous under circumstances where there is limited light penetration.[121] To decrease cross-linking time and increase the strength of the cross-linked hydrogels, subsequent studies in our laboratory have employed the use of PEG-diacrylate (Figure 12) as a cross-linker in the presence of radical initiator systems APS/AA[111] or APS/N,N,N′,N′-tetramethylethylenediamine.[122]
Figure 12.
Poly(ethylene glycol)-diacrylate can be used to cross-link oligo(poly(ethylene glycol) fumarate) in the presence of ammonium persulfate/ascorbic acid (APS/AA) or ammonium persulfate/tetramethylethylenediamine (APS/TEMED) to form a hydrogel.
Like most well-designed biomaterials, the OPF macromer was designed based on the fundamental requirements for biocompatibility and biodegradability. OPF is composed of biocompatible PEG and fumaric acid, which is a non-toxic carboxylic acid that is part of the Krebs cycle.[112] The cytocompatibility of each constituent of the hydrogel and its leachable by-products has been demonstrated by an in vitro study using rat MSCs as the model cell type,[114] while an in vivo study in a rabbit model has shown that OPF hydrogels only elicit a mild soft tissue and bone tissue response.[123] In addition to being biocompatible, the numerous ester bonds in the fumarate groups along the macromolecular OPF chain can be hydrolytically cleaved, which allows for degradation in an aqueous environment.[120] Additionally, the OPF macromer was also designed to be end-capped with PEG blocks to allow for covalent coupling of bioactive molecules to the hydrogel. The ability to conjugate proteins, peptides and specific growth factors of interest to the macromer allows for the incorporation of biological cues to guide tissue regeneration. Jo et al. have shown that the OPF macromer can be functionalized with Gly-Arg-Gly-Asp (GRGD), a model cell-modulating peptide, after being activated with 4-nitrophenyl chloroformate.[124]
As discussed, despite progress made in surgical procedures and techniques, the treatment of injured articular cartilage still poses a major challenge to clinicians today. To augment healing of chondral injuries, the use of bioactive factors is currently being investigated as a potential therapeutic strategy.[125] Shortly after the development of the OPF macromer, Holland et al.[110] initiated studies that investigated the potential of the OPF hydrogel system as an injectable drug delivery vehicle to encourage cartilage repair via the controlled release of TGF-β1, a 25-kDa protein which has the ability to encourage the chondrogenic differentiation of progenitor cells[119],[126],[127] and to increase the synthesis of cartilage ECM.[128]–[130] Early work demonstrated that by varying hydrogel mesh size, a material parameter that is dependent on the parent PEG molecular weight used to synthesize the OPF macromer, in vitro release of TGF-β1 from OPF hydrogels can be diffusionally controlled.[110] However, the main drawback that was observed was burst release of the growth factor, a typical phenomenon with hydrogel delivery systems. This paved the way for encapsulation of TGF-β1-loaded gelatin microparticles within an OPF hydrogel network, which was demonstrated to be effective in minimizing burst release and providing better control over the release kinetics of the growth factor.[110] Since gelatin can be enzymatically degraded by a number of matrix metalloproteinases, which are upregulated in injured cartilage, subsequent studies have leveraged this property to use gelatin microparticles as digestible porogens, not only to modulate the degradability of the OPF network and release kinetics of TGF-β1, but also to create space for tissue ingrowth.[131]
However, given the necessity for a multitude of growth factors and their interactions for proper cartilage development and homeostasis, it is improbable that complete articular cartilage repair can solely be achieved by a single growth factor.[125] Besides TGF-β1, insulin-like growth factor-1 (IGF-1) is known to play a role in stimulating the synthesis of ECM. Specifically, it has been shown to exert an anabolic effect to increase proteoglycan and type II collagen synthesis.[132],[133] Therefore, sustained delivery of IGF-1 could potentially augment the biomechanical and biochemical properties of repaired cartilage tissue by stimulating ECM synthesis.[132],[133] Holland et al. demonstrated that highly cross-linked gelatin microparticles serve as an effective carrier to sustain the release of IGF-1 over a period of 4 weeks in vitro.[134] In addition, it was found that release of the growth factor could be further controlled by encapsulating these IGF-1-loaded microparticles within an OPF hydrogel network.[134] Drawing from the potential of the OPF-gelatin system in delivering single growth factors, the dual delivery of TGF-β1 and IGF-1 has also been investigated, where it was shown that the release profiles of each growth factor can be modulated by varying the extent of microparticle cross-linking and phase of growth factor loading (into either the OPF hydrogel phase or gelatin microparticle phase).[134] As these studies illustrate, the design flexibility of OPF-based systems allows for the precise tailoring of growth factor release rates to explore how healing is affected by the release kinetics of single or multiple growth factors in vivo.[135]
One of the most important criteria for success in an implanted tissue engineered substitute is the presence of an adequate number of viable cells in the defect area for tissue synthesis.[136] Therefore, in addition to growth factors, transplanting cells to stimulate repair is a widely employed strategy in tissue engineering. This is especially critical for cartilage repair, as the tissue itself has very low cellularity. In some clinical cases, there may be sufficient numbers of cartilage-forming cells in the healthy tissue around the defect site to promote tissue regeneration. However, in many other clinical settings, such as situations where the surrounding tissue is a site of previous surgery or infection, there may be a scarcity of these viable tissue-forming cells.[136] Based on the need to transplant cells to facilitate cartilage regeneration, the potential of OPF–based hydrogels as a cell delivery vehicle for cartilage regeneration was explored by Park et al.[137] In this study, bovine chondrocytes were embedded in hydrogels co-encapsulating gelatin microparticles loaded with TGF-β1 and cultured up to 28 days in vitro.[137] Histological images and biochemical analyses indicated that chondrocytes encapsulated within OPF hydrogels remained viable throughout the 28-day period, demonstrating the potential of OPF-based hydrogels as carriers to deliver therapeutic cells to cartilage defects.[137]
While the use of chondrocytes is a potential therapeutic approach, one major drawback is the need to harvest autologous chondrocytes for ex vivo expansion. In such an approach, not only is there a risk for donor site morbidity, it is also a challenging task to obtain sufficient cells for expansion, as only a small number of cells can typically be harvested.[138] Furthermore, the expansion of chondrocytes ex vivo is hindered by the relatively low expansion rates of these cells, which is also compounded by the propensity for these cells to de-differentiate in culture.[138] An alternative candidate are MSCs, whose potential to undergo osteochondral differentiation when implanted in vivo has long been established.[139]–[141] These cells can easily be isolated from the bone marrow, and still retain their ability to differentiate into connective tissue cell types such as chondrocytes and osteoblasts.[142] Recognizing the limitations associated with the use of chondrocytes, Park et al. investigated the use of rabbit MSCs as an alternative cell source, where the effects of the presence of gelatin microparticles alone or TGF-β1-loaded gelatin microparticles on the chondrogenic differentiation of these cells (encapsulated within OPF hydrogels) were elucidated.[143] By analyzing the expression levels of cartilage-associated genes, it was found that when MSCs were encapsulated together with TGF-β1-loaded gelatin microparticles, the expression of collagen type II and aggrecan was upregulated, suggesting the potential of OPF hydrogel composites as supportive materials for the delivery of chondrogenic progenitor cells in conjunction with growth factors.[143]
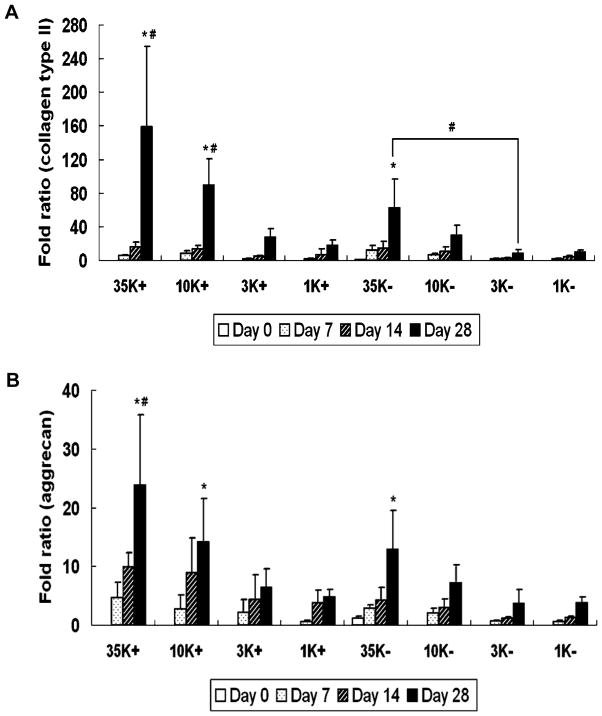
Years of extensive characterization have clearly established the potential of OPF-based hydrogels as carriers for cells and growth factors. In recent years, research in our laboratory has focused on developing strategies to promote the chondrogenic differentiation of encapsulated progenitor cells. One such approach, explored by Park et al., is to modulate the swelling properties of OPF-based hydrogels.[121] Previous studies in our laboratory have demonstrated the influence of swelling properties of OPF hydrogels on the osteogenic differentiation of rat MSCs in vitro.[49],[122] Based on this study, Park et al., in collaboration with Dr. Arnold Caplan of Case Western Reserve University, investigated the effect of hydrogel swelling on the chondrogenic differentiation of rabbit MSCs.[121] Rabbit MSCs were encapsulated together with TGF-β1-loaded gelatin microparticles within OPF hydrogel composites with different swelling ratios and cultured for four weeks. Consistent with the aforementioned study that demonstrated the effect of hydrogel swelling ratio on the osteogenic differentiation of rat MSCs, hydrogel composites of higher swelling ratio fostered chondrogenic differentiation of the encapsulated cells, as measured by collagen type II (Figure 13A) and aggrecan gene expression (Figure 13B).[121] Based on the consistency of results from these two studies, it can be inferred that proliferation and differentiation of encapsulated progenitor cells may be influenced by the availability of nutrients and effectiveness of growth factor delivery, both of which can be addressed by adjusting the mesh size and hence swelling properties of the OPF-based hydrogel employed.
Figure 13.
Collagen type II and aggregan gene expression over time. Poly(ethylene glycol) of four different molecular weights (35000 g mol−1, 10000 g mol−1, 3300 g mol−1 and 1000 g mol−1) were used to prepare oligo(poly(ethylene glycol) fumarate) macromers of four repeating units (OPF 35K, OPF 10K, OPF 3K and OPF 1K, respectively). (A) depicts collagen type II gene expression and (B) depicts aggrecan gene expression for OPF 35K, 10K, 3K and 1K hydrogel composites encapsulating rabbit MSCs and TGF-β1-loaded microparticles (+) or rabbit MSCs and blank microparticles (−). Results are presented as a fold ratio after being normalized to GAPDH values. The OPF 35K- group shows the average expression level of controls (Day 0), represented as one. (*) indicates that within a given hydrogel formulation, a significantly higher (p < 0.05) gene expression than the Day 0 value (control) was observed. (#) indicates samples which had significantly higher (p < 0.05) gene expression than other OPF formulations at the same time point. Error bars represent means ± standard deviation for n=4. Reproduced with permission from [121].
In partial-thickness defects where the lesion is limited to the chondral layer, the use of scaffolds that are designed to regenerate cartilage tissue only may not be the most effective strategy, as it is typically difficult to achieve good integration of the implant with the surrounding tissue.[138] Given the observation that a bone-to-bone interface integrates more quickly and effectively than a cartilage-to-cartilage interface, a potential strategy to improve fixation is to surgically create a full-thickness defect that penetrates from the cartilage layer into the underlying subchondral bone tissue for the subsequent implantation of an osteochondral tissue substitute - a dual-layer scaffold consisting of a cartilaginous layer and a calcified tissue layer that is designed to regenerate simultaneously both cartilage and subchondral bone, respectively.[138],[144] Additionally, by creating an osteochondral defect that penetrates into the bone marrow, the intrinsic healing potential of cartilage can be enhanced via the recruitment of pluripotent stem cells and bioactive factors from the bone marrow into the implanted scaffold, which is the rationale behind current surgical techniques such as drilling and microfracturing.[96]
For true osteochondral defects, which extend beyond the superficial articular cartilage layer and affect the underlying subchondral bone, engineered osteochondral tissue composites also represent a promising alternative to current treatment approaches. In the natural joint, the articular cartilage and underlying subchondral bone constitute a functional unit, where each play a unique role in load-bearing, allowing for a wide range of joint motion with good lubrication and stability.[145] While the subchondral bone provides mechanical support for the articular cartilage, the latter protects the former from high stresses and also facilitates low-friction movements within the joint.[145] Frequently as a result of osteoarthritis and related joint disorders, degenerative changes that affect both tissues can occur, resulting in severe pain, joint deformity and limited joint motion.[145] At present, there are two main approaches proposed for the treatment of osteochondral defects, which include osteochondral autografts and allografts.[146] While the use of autologous osteochondral grafts in the technique commonly referred to as mosaicplasty has produced encouraging results, there are several drawbacks associated with this strategy, including limited availability, donor site morbidity and the challenge of ensuring that the geometry of the graft is complementary with the defect site.[147] The use of osteochondral allografts is also restricted by a limited supply relative to an increasing demand for osteochondral tissue.[146] By taking a tissue engineering approach to fabricate osteochondral composite constructs to fill osteochondral defects, these limitations could potentially be addressed.[147]
Compared to bone tissue engineering or cartilage tissue engineering, where the goal is to regenerate only a single tissue type, the scaffold design criteria for osteochondral tissue engineering is much more demanding, given that the ultimate goal in employing osteochondral scaffolds is to guide the simultaneous growth of two uniquely different tissues, each with vastly different biological properties.[148] Such an end-goal can potentially be achieved via the use of bilayered scaffolds, where the mechanical, structural and chemical properties in each layer are optimized to generate relevant biological environments that are specific to the two tissue types.[148] For the past decade, our laboratory has been working on designing a bilayered scaffolding system based on OPF for the repair of osteochondral defects. Shortly after the development of the OPF macromer, Temenoff et al. demonstrated the feasibility of creating biphasic OPF-based hydrogels with independently controlled material properties in each layer via a multi-step cross-linking procedure.[111] It was found that the presence of an interfacial area in these laminated gels did not significantly affect their mechanical properties, suggesting good integration of the two scaffold layers - a critical osteochondral scaffold design requirement. In a subsequent in vivo study by Holland et al., the potential of these bilayered OPF-based hydrogel scaffolds to support cartilage and bone growth was examined in a rabbit osteochondral defect model.[149] Hydrogel composites of 3-mm diameter and 3-mm thickness, comprising an OPF matrix and TGF-β1-incorporating gelatin microparticles localized to the chondral layer, were implanted in full-thickness defects created in a rabbit knee joint. Histological evaluation of tissue in both the chondral and subchondral regions of the defect was performed, and it was found that tissue quality improved over time, with hyaline cartilage filling the chondral region predominantly and a mixture of trabecular and compact bone occupying the subchondral region at 14 weeks.[149] Typically, incomplete integration with surrounding tissue is observed in the long term when grafts or degradable scaffolds are employed in cartilage repair, which may result in displacement of the implant and further joint pain.[149] Therefore, the complete integration of regenerated subchondral bone in the bottom layer of the OPF bilayered scaffold with surrounding bone that was observed in this study provided a strong impetus for the continued development of this system for osteochondral repair.
Building upon encouraging results from previous studies in our laboratory, Guo et al. explored the fabrication of a bilayered OPF-gelatin microparticle composite comprising a chondrogenic layer and an osteogenic layer, each with encapsulated rabbit MSCs.[150] The aim of this in vitro study was to establish the feasibility of promoting chondrogenic differentiation of MSCs in one layer while maintaining the osteoblastic phenotype of pre-differentiated MSCs in the other layer of a bilayered scaffold under the same culture conditions, consisting of chondrogenic medium supplemented with β-glycerophosphate. By evaluating the gene and biochemical expression of chondrogenic and osteogenic markers, it was found that, while the osteogenically predifferentiated MSCs in the bottom osteogenic layer maintained their differentiated phenotype, undifferentiated MSCs in the top chondrogenic layer underwent chondrogenic differentiation in the osteochondral bilayered construct.[150] Additionally, an encouraging finding was that in this co-culture system, the presence of osteogenic cells in the bottom layer of the composite augmented the chondrogenic differentiation of MSCs in the top layer, likely via the production of chondro-inductive signals.[150] Also, together with TGF-β1-loaded gelatin microparticles in the chondrogenic layer, these osteogenic cells further promoted chondrogenesis. In an extension of this study, Guo et al. further demonstrated that osteogenic cells at varying stages of differentiation in the osteogenic layer, together with TGF-β3, augmented the gene expression of chondrogenic markers of MSCs in the chondrogenic layer to different degrees.[151] These studies suggest that, beyond functioning as a vehicle for the co-delivery of growth factors and MSCs for osteochondral regeneration, OPF-based hydrogels may also serve as a model to study the biology behind the crosstalk that exists between articular cartilage and subchondral bone.[152],[153]
4. Other Tissue Engineering Applications
4.1. Lens Tissue Engineering
Although PPF and OPF were initially developed for applications in orthopedic tissue regeneration, the use of these materials in other tissue-engineering applications has also been investigated. The ability of the OPF macromer to cross-link under physiological conditions in the presence of radical initiators to form hydrogels suggests the feasibility of fabricating implants of various shapes appropriate for different tissue engineering applications.[154] Such a concept was explored via a collaboration between our laboratory and Dr. Panagiotis Tsonis of the University of Dayton. Through this collaboration, the use of OPF hydrogel beads for the encapsulation and transplantation of iris pigment epithelial cells (PECs) for lens regeneration was investigated.[154] Previous studies involving OPF hydrogels for bone and cartilage tissue engineering have used the hydrogel in the form of disks or cylinders. In this study, in order to approximate the dimensions of newt lens for use in a newt model, OPF hydrogel spheres of diameter 1 mm were fabricated from OPF macromers cross-linked with PEG-DA, using silicone molds.[154] Unimpeded by the presence of these OPF hydrogel beads, host lens regeneration was observed when beads of optimized degradation rate were implanted into newt lentectomized eyes with or without encapsulated PECs, therefore underscoring the potential of these OPF hydrogel beads for extrapolated use in mammalian lens tissue engineering.[154]
4.2. Ocular Drug Delivery
One potential use of PPF matrices is as injectable, in situ forming, controlled-release drug delivery vehicles to treat chronic ophthalmic disorders, while avoiding the complications that are associated with repeated injections into the eye. Specifically, Ueda et al. and Hacker et al. have demonstrated that anti-inflammatory and model ophthalmic drugs, respectively, underwent sustained release over 200 days in vitro, as shown in Figure 14, while in vivo work using drug-free PPF demonstrated no significant inflammation and minimal fibrous capsule formation two weeks after intra-scleral and intra-vitreal injections.[155],[156] Additionally, since the drug release kinetics can be varied by changing the PPF macromer molecular weight, PPF has also been investigated for use in other drug delivery applications, such as sustained anti-cancer therapeutic drug release by Choi et al.[157]
Figure 14.
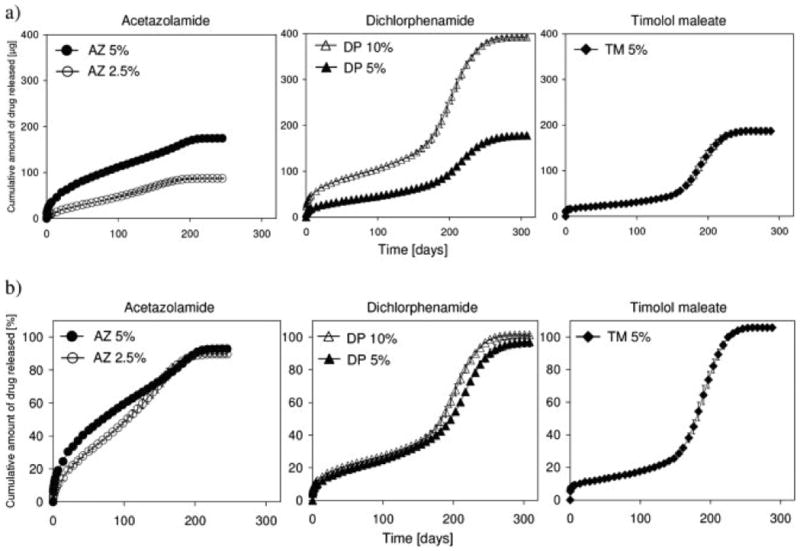
Cumulative amount of model ophthalmic drug release over time from photo-cross-linked poly(propylene fumarate)/poly(N-vinyl pyrrolidone) (PPF/PVP) (3:2 ratio) matrices in phosphate buffered saline at 37°C measured (a) absolutely (μg) and (b) relatively (%). The drugs acetazolamide (AZ) (2.5 and 5 wt%), dichlorphenamide (DP) (5 and 10 wt%), and timolol maleate (TM) (5 wt%) were incorporated into the PPF/PVP matricies. Data represent means ± standard deviation for n = 3. Reproduced with permission from [156].
4.3. Cardiac Tissue Engineering
Perhaps the most advantageous feature of OPF-based formulations is their injectability and ability to gel in situ within a short time frame to entrap cells and growth factors within the defect site. In collaboration with Dr. Changyong Wang of the Academy of Military Medical Sciences, Beijing, China, our laboratories harnessed these properties for cardiac regeneration and investigated the ability of OPF hydrogels to support the retention and survival of encapsulated mouse embryonic stem cells (mESCs) when injected into a site of myocardial infarction.[158] Evaluation of 24-hour cell retention and 4-week graft size indicated that these parameters were significantly greater in the OPF + mESCs group relative to the control group where mESCs were delivered in phosphate buffered saline solution.[158] Additionally, it was observed that the OPF + mESCs group had the best improvement in left ventricular function as well as the smallest infarct size and fibrotic area as compared to the other groups.[158] This study suggests the feasibility of using injectable OPF hydrogels not only as a delivery vehicle for therapeutic stem cells to the ischemic myocardium, but also as a temporary matrix to promote the retention and viability of these cells.
4.4. Gene Delivery
In addition to scaffold development, our laboratory also has a vested interest in developing methods for non-viral delivery of nucleotides that encode for cell-modulating substances, such as growth factors, to improve tissue regeneration. Saraf et al. developed a novel gene delivery vector that was fabricated by conjugating a branched form of the well-characterized gene delivery vector polyethylenimine (bPEI) with hyaluronic acid (HA). This gene delivery vector has been shown to improve viability and transfection efficiency of human MSCs when the combined bPEI-HA is used compared to bPEI alone.[159] Although this study used these vectors to upregulate enhanced green fluorescent protein (eGFP) as proof-of-concept, the technology can potentially also be applied to upregulate therapeutic proteins of interest. These vectors have been incorporated into coaxial electrospun scaffolds with PEI-HA in the sheath and plasmid DNA (pDNA) within the core of the fiber. By varying the PCL sheath polymer concentration, the PEG core polymer molecular weight and concentration, and pDNA concentration within the core, the release time of the pDNA was shown to be tunable, with transfection sustained for up to 60 days in scaffolds that had PEI-HA incorporated into the sheath. These scaffolds demonstrate the potential for sustained gene delivery for tissue engineering applications.[160]
Recently, Needham et al. have shown that both the cytotoxicity and the transfection efficiency of these gene delivery vectors in human MSCs is heavily dependent on HA length, with the use of 10-saccharide HA demonstrating the best result.[161] Other gene delivery vectors that have resulted in efficient in vitro transfection are positively charged C60 transfecting agents generated by Sitharaman et al.[162] These were successfully used to deliver DNA carrying the GFP reporter gene to mouse fibroblasts. However, a dose-dependent increase in cytotoxicity was observed, as is typical with non-viral transfection agents, and further modification is likely necessary before it can be used for therapeutic and diagnostic purposes.[162]
Another technique to improve gene delivery involves the use of hydrophilic amine spacers in branched polycationic polymers to reduce cytotoxicity and increase degradation rates while maintaining effective delivery of gene vectors.[163] Amine monomers that were able to dissociate at physiologic pH were shown to more effectively complex pDNA, leading to improved transfection efficiency.[164] Some of these vectors have been complexed with gelatin microparticles and used in a PPF scaffold in an attempt to deliver pDNA encoding for BMP-2 to a critical-size rat cranial defect. However, the results suggest that the transfection efficiency of the pDNA depends not only on the vector’s degradation rate, but also on that of the gelatin microparticles.[165]
Although OPF-based hydrogels were originally developed to function as a carrier for cells and growth factors, our laboratory has also evaluated the potential of this hydrogel system in controlled gene delivery applications. Delivering bioactive molecules with a scaffold to guide tissue development towards a desired pathway is a commonly employed strategy in tissue engineering. However, protein instability poses a major challenge if long-term controlled release of the therapeutic protein is warranted.[166] An alternative approach to overcome this issue is the delivery of pDNA that encodes the desired protein. In the first of a series of studies, Kasper et al. demonstrated that pDNA physically entrapped within OPF hydrogels can be released in a sustained, linear manner over a duration of 45–62 days in vitro, the release kinetics of which can be controlled by varying the molecular weight of the PEG used to synthesize the OPF macromer.[167] Subsequent studies incorporated cationized gelatin microspheres into OPF hydrogels to generate porosity necessary for tissue infiltration.[168] Additionally, besides serving as a porogen, cationized gelatin microspheres enable the controlled release of DNA via electrostatic complexation since DNA is negatively charged.[169],[170] Indeed, a smaller burst release and slower DNA release rate was observed when pDNA was loaded into cationized gelatin microspheres embedded within OPF hydrogels, relative to samples where DNA was directly loaded into OPF hydrogels in the absence of these gelatin microspheres.[169]
5. Outlook and Conclusions
The studies presented herein collectively exemplify the far-reaching potential of biomaterials, such as fumarate-based systems, to meet numerous clinical needs, including bone and cartilage repair. By appropriate modulation of material properties, it is possible to harness the great versatility of these materials to advance current tissue engineering strategies. In an effort to more closely recreate the cellular microenvironment in these versatile scaffolds, engineered culture conditions can be leveraged to coat the scaffolds with ECM to present growth factors in a more biomimetic fashion. As more sophisticated tissue engineering strategies such as these are developed for clinical use, an increased emphasis should be placed on regenerating tissue within the context of the injury to help address clinical challenges associated with the cause of the defect. For instance, staged strategies to first improve healing of surrounding tissues can facilitate repair. In traumatic injuries, priming of wounds to direct soft tissue growth and prevent infection prior to implementation of bone regenerating materials can lead to improved multiple tissue reconstruction. Regeneration of tissue within the context of the injury will require continued interactions between clinicians and those developing tissue engineering strategies.
As highlighted through the various examples presented, the development of biomaterials is a laborious process that requires years of in vitro characterization and in vivo validation. Though this review focused specifically on the collaborative materials research in the Mikos laboratory, it should be noted that interdisciplinary interactions are being fostered by many others in the field. Through leverage of these interactions between materials scientists and engineers and clinicians toward the cooperative development of accurately formulated design criteria, well-informed collaborative studies can be carried out to efficiently develop biomaterials to meet clinical needs.
Acknowledgments
The authors acknowledge support from the National Institutes of Health for work in the areas of bone and cartilage tissue engineering (R01 AR048756, R01 AR057083, and R01 DE017441), as well as from the Armed Forces Institute of Regenerative Medicine (W81XWH-08-2-0032). The authors thank their colleagues at Rice University and their collaborators for their significant contributions to these research efforts. The authors also acknowledge the indispensable contributions of past and current students, trainees and fellows in the laboratory. E.F. acknowledges funding from the National University of Singapore - Overseas Graduate Scholarship. B.M.W. acknowledges support from the Baylor College of Medicine Medical Scientist Training Program (NIH T32 GM007330) and a training fellowship from the Keck Center of the Gulf Coast Consortia in the Nanobiology Interdisciplinary Graduate Training Program (NIH T32 EB009379-03).
List of Abbreviations
- AAm
Acrylamide
- APS
Ammonium Persulfate
- AA
Ascorbic Acid
- BMP
Bone Morphogenetic Protein
- bPEI
Branched Poly(Ethylenimine)
- CMC
Carboxymethylcellulose
- DEF
Diethyl Fumarate
- eGFP
Enhanced Green Fluorescent Protein
- ECM
Extracellular Matrix
- FGF
Fibroblast Growth Factor
- GMP
Gelatin Microparticle
- GRGDS
Glycine-Arginine-Glycine-Aspartic Acid-Serine
- GFP
Green Fluorescent Protein
- HA
Hyaluronic Acid
- HANP
Hydroxyapatite Nanoparticle
- HEA
Hydroxyethyl Acrylate
- IGF-1
Insulin-Like Growth Factor-1
- MSC
Mesenchymal Stem Cell
- MicroCT
Micro-Computer Tomography
- mESC
Mouse Embryonic Stem Cell
- NVP
N-Vinyl Pyrrolidone
- NP
Nanoparticle
- NiPAAm
N-Isopropyl Acrylamide
- OPF
Oligo(Poly(Ethylene Glycol) Fumarate)
- OPN
Osteopontin
- PDGF
Platelet-Derived Growth Factor
- PEDAS
Pentaerythritol Diacrylate Monostearate
- PEC
Pigment Epithelial Cell
- pDNA
Plasmid DNA
- PCL
Poly(ε-Caprolactone)
- PEG
Poly(Ethylene Glycol)
- PEG-DMA
Poly(Ethylene Glycol)-Dimethacrylate
- PMMA
Poly(Methyl Methacrylate)
- PEI
Poly(Ethylenimine)
- PPF
Poly(Propylene Fumarate)
- P(PF-co-EG)
Poly(Propylene Fumarate-Co-Ethylene Glycol)
- PF-DA
Propylene Fumarate-Diacrylate
- p(NiPAAm)
Poly(N-Isopropyl Acrylamide)
- RGD
Arginine-Glycine-Aspartic Acid
- SWNT
Single-Walled Carbon Nanotube
- TGF-β
Transforming Growth Factor Beta
- TCP
Tricalcium Phosphate
- US-Tube
Ultra-Short Single-Walled Carbon Nanotube
- VEGF
Vascular Endothelial Growth Factor
- VPA
inylphosphonic Acid
Bibliography
- 1.Myeroff C, Archdeacon M. J Bone Joint Surg Am. 2011;93:2227. doi: 10.2106/JBJS.J.01513. [DOI] [PubMed] [Google Scholar]
- 2.Sawin PD, Traynelis VC, Menezes aH. J Neurosurg. 1998;88:255. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 3.Laurencin C, Khan Y, El-Amin SF. Expert Rev Med Devices. 2006;3:49. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald aS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. J Bone Joint Surg Am. 2001;83-A(Suppl):98. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 5.Polak JM, Bishop AE. Ann N Y Acad Sci. 2006;1068:352. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- 6.Vo TN, Kasper FK, Mikos AG. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mescher A. Junqueira’s Basic Histology: Text and Atlas. Mcgraw-hill; 2009. [Google Scholar]
- 8.Liebschner MAK. Biomaterials. 2004;25:1697. doi: 10.1016/s0142-9612(03)00515-5. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Leong KF, Du Z, Chua CK. Tissue Eng. 2001;7:679. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein SA. J Biomech. 1987;20:1055. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 11.Wolff J. The Law of Bone Remodelling. Berlin: Springer-verlag; 1986. [Google Scholar]
- 12.McCarthy I, Goodship A, Herzog R, Oganov V, Stussi E, Vahlensieck M. Eur J Clin Invest. 2000;30:1044. doi: 10.1046/j.1365-2362.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 13.Thomson R, Wake M, Yaszemski M, Mikos AG. Adv Polym Sci. 1995;122:245. [Google Scholar]
- 14.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. J Bone Joint Surg Am. 2008;90(Suppl 1):36. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 15.Ryan G, Pandit A, Apatsidis DP. Biomaterials. 2006;27:2651. doi: 10.1016/j.biomaterials.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Orban JM, Marra KG, Hollinger JO. Tissue Eng. 2002;8:529. doi: 10.1089/107632702760240454. [DOI] [PubMed] [Google Scholar]
- 17.Kasper FK, Tanahashi K, Fisher JP, Mikos AG. Nat Protoc. 2009;4:518. doi: 10.1038/nprot.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peter SJ, Yaszemski MJ, Suggs LJ, Payne RG, Langer R, Hayes WC, Unroe MR, Alemany LB, Engel PS, Mikos AG. J Biomater Sci Polym Ed. 1997;8:893. doi: 10.1163/156856297x00074. [DOI] [PubMed] [Google Scholar]
- 19.Peter SJ, Suggs LJ, Yaszemski MJ, Engel PS, Mikos AG. J Biomater Sci Polym Ed. 1999;10:363. doi: 10.1163/156856299x00423. [DOI] [PubMed] [Google Scholar]
- 20.Peter SJ, Nolley JA, Widmer MS, Merwin JE, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Tissue Eng. 1997;3:207. [Google Scholar]
- 21.Timmer MD, Carter C, Ambrose CG, Mikos AG. Biomaterials. 2003;24:4707. doi: 10.1016/s0142-9612(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 22.Timmer MD, Ambrose CG, Mikos AG. Biomaterials. 2003;24:571. doi: 10.1016/s0142-9612(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 23.He S, Timmer MD, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Polymer. 2001;42:1251. [Google Scholar]
- 24.He S, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Biomaterials. 2000;21:2389. doi: 10.1016/s0142-9612(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 25.Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos AG. J Biomed Mater Res. 1999;44:314. doi: 10.1002/(sici)1097-4636(19990305)44:3<314::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Fisher JP, Dean D, Mikos AG. Biomaterials. 2002;23:4333. doi: 10.1016/s0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 27.Ramoshebi LN, Matsaba TN, Teare J, Renton L, Patton J, Ripamonti U. Expert Rev Mol Med. 2002;4:1. doi: 10.1017/S1462399402004969. [DOI] [PubMed] [Google Scholar]
- 28.Vehof JWM, Fisher JP, Dean D, van der Waerden JPCM, Spauwen PHM, Mikos AG, Jansen JA. J Biomed Mater Res. 2002;60:241. doi: 10.1002/jbm.10073. [DOI] [PubMed] [Google Scholar]
- 29.Kretlow JD, Mikos AG. AIChE J. 2008;54:3048. doi: 10.1002/aic.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peter SJ, Miller ST, Zhu G, Yasko AW, Mikos AG. J Biomed Mater Res. 1998;41:1. doi: 10.1002/(sici)1097-4636(199807)41:1<1::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Fisher JP, Vehof JWM, Dean D, van der Waerden JPCM, Holland TA, Mikos AG, Jansen JA. J Biomed Mater Res. 2002;59:547. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 32.Peter SJ, Lu L, Kim DJ, Mikos AG. Biomaterials. 2000;21:1207. doi: 10.1016/s0142-9612(99)00254-9. [DOI] [PubMed] [Google Scholar]
- 33.Fisher JP, Lalani Z, Bossano CM, Brey EM, Demian N, Johnston CM, Dean D, Jansen JA, Wong MEK, Mikos AG. J Biomed Mater Res A. 2004;68:428. doi: 10.1002/jbm.a.20073. [DOI] [PubMed] [Google Scholar]
- 34.Bessa PC, Casal M, Reis RL. J Tissue Eng Regen Med. 2008;2:81. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 35.Rose FRAJ, Hou Q, Oreffo ROC. J Pharm Pharmacol. 2004;56:415. doi: 10.1211/0022357023312. [DOI] [PubMed] [Google Scholar]
- 36.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M, Mikos AG. Tissue Eng Part A. 2009;15:2347. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Bone. 2008;43:931. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horch RA, Shahid N, Mistry AS, Timmer MD, Mikos AG, Barron AR. Biomacromolecules. 2004;5:1990. doi: 10.1021/bm049768s. [DOI] [PubMed] [Google Scholar]
- 39.Mistry AS, Pham QP, Schouten C, Yeh T, Christenson EM, Mikos AG, Jansen JA. J Biomed Mater Res A. 2010;92:451. doi: 10.1002/jbm.a.32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mistry AS, Mikos AG, Jansen JA. J Biomed Mater Res A. 2007;83:940. doi: 10.1002/jbm.a.31280. [DOI] [PubMed] [Google Scholar]
- 41.Sitharaman B, Shi X, Tran La, Spicer PP, Rusakova I, Wilson LJ, Mikos AG. J Biomater Sci Polym Ed. 2007;18:655. doi: 10.1163/156856207781034133. [DOI] [PubMed] [Google Scholar]
- 42.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Nanotechnology. 2005;16:S531. doi: 10.1088/0957-4484/16/7/030. [DOI] [PubMed] [Google Scholar]
- 43.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Biomacromolecules. 2006;7:2237. doi: 10.1021/bm060391v. [DOI] [PubMed] [Google Scholar]
- 44.Shi X, Sitharaman B, Pham QP, Liang F, Wu K, Edward Billups W, Wilson LJ, Mikos AG. Biomaterials. 2007;28:4078. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X, Sitharaman B, Pham QP, Spicer PP, Hudson JL, Wilson LJ, Tour JM, Raphael RM, Mikos AG. J Biomed Mater Res A. 2008;86:813. doi: 10.1002/jbm.a.31671. [DOI] [PubMed] [Google Scholar]
- 46.Sitharaman B, Shi X, Walboomers XF, Liao H, Cuijpers V, Wilson LJ, Mikos AG, Jansen Ja. Bone. 2008;43:362. doi: 10.1016/j.bone.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Kim K, Dean D, Lu A, Mikos AG, Fisher JP. Acta Biomater. 2011;7:1249. doi: 10.1016/j.actbio.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henslee AM, Spicer PP, Yoon DM, Nair MB, Meretoja VV, Witherel KE, Jansen JA, Mikos AG, Kasper FK. Acta Biomater. 2011;7:3627. doi: 10.1016/j.actbio.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. J Biomed Mater Res A. 2004;70:235. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 50.Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Biomaterials. 2005;26:3645. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 51.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. J Biomed Mater Res A. 2004;69:535. doi: 10.1002/jbm.a.30027. [DOI] [PubMed] [Google Scholar]
- 52.Bongio M, van den Beucken JJJP, Nejadnik MR, Leeuwenburgh SCG, Kinard LA, Kasper FK, Mikos AG, Jansen JA. Eur Cell Mater. 2011;22:359. doi: 10.22203/ecm.v022a27. [DOI] [PubMed] [Google Scholar]
- 53.Nejadnik MR, Mikos AG, Jansen Ja, Leeuwenburgh SCG. J Biomed Mater Res A. 2012;100:1316. doi: 10.1002/jbm.a.34071. [DOI] [PubMed] [Google Scholar]
- 54.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 55.Hunt NC, Grover LM. Biotechnol Lett. 2010;32:733. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 56.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Biomacromolecules. 2008;9:1558. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 57.Kretlow JD, Hacker MC, Klouda L, Ma BB, Mikos AG. Biomacromolecules. 2010;11:797. doi: 10.1021/bm9014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klouda L, Hacker MC, Kretlow JD, Mikos AG. Biomaterials. 2009;30:4558. doi: 10.1016/j.biomaterials.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klouda L, Perkins KR, Watson BM, Hacker MC, Bryant SJ, Raphael RM, Kasper FK, Mikos AG. Acta Biomater. 2011;7:1460. doi: 10.1016/j.actbio.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Amin SF, Lu HH, Khan Y, Burems J, Mitchell J, Tuan RS, Laurencin CT. Biomaterials. 2003;24:1213. doi: 10.1016/s0142-9612(02)00451-9. [DOI] [PubMed] [Google Scholar]
- 61.Pham QP, Kasper FK, Mistry AS, Sharma U, Yasko AW, Jansen JA, Mikos AG. J Biomed Mater Res A. 2009;88:295. doi: 10.1002/jbm.a.31875. [DOI] [PubMed] [Google Scholar]
- 62.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. Proc Natl Acad Sci U S A. 2006;103:2488. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janicki P, Schmidmaier G. Injury. 2011;42(Suppl 2):S77. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 64.Lieberman JR, Daluiski A, Einhorn TA. J Bone Joint Surg Am. 2002;84-A:1032. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 65.Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, Alder M, Nummikoski P. Int J Periodontics Restorative Dent. 1997;17:11. [PubMed] [Google Scholar]
- 66.Geesink RG, Hoefnagels NH, Bulstra SK. J Bone Joint Surg Br. 1999;81:710. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 67.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. PLoS Med. 2007;4:e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Proc Natl Acad Sci U S A. 2002;99:12600. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Biomaterials. 2005;26:971. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Gomes ME, Bossano CM, Johnston CM, Reis RL, Mikos AG. Tissue Eng. 2006;12:177. doi: 10.1089/ten.2006.12.177. [DOI] [PubMed] [Google Scholar]
- 71.Liao J, Guo X, Nelson D, Kasper FK, Mikos AG. Acta Biomater. 2010;6:2386. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Tissue Eng Part A. 2010;16:431. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hillsley MV, Frangos JA. Biotechnol Bioeng. 1994;43:573. doi: 10.1002/bit.260430706. [DOI] [PubMed] [Google Scholar]
- 74.Holtorf HL, Datta N, Jansen JA, Mikos AG. J Biomed Mater Res A. 2005;74:171. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 75.Kasper FK, Liao J, Kretlow JD, Sikavitsas VI, Mikos AG StemBook, editor. The Stem Cell Research Community, StemBook. Harvard Stem Cell Institute; 2008. [PubMed] [Google Scholar]
- 76.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Proc Natl Acad Sci U S A. 2003;100:14683. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burger EH, Klein-Nulend J. FASEB Journal3: Official Publication of the Federation of American Societies for Experimental Biology. 1999;13(Suppl):S101. [PubMed] [Google Scholar]
- 78.Weinbaum S, Cowin SC, Zeng Y. J Biomech. 1994;27:339. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Zhang YP, Kirsner RS. Microsc Res Tech. 2003;60:107. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 80.Chiarini L, Figurelli S, Pollastri G, Torcia E, Ferrari F, Albanese M, Nocini PF. J Craniomaxillofac Surg. 2004;32:5. doi: 10.1016/j.jcms.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Chaushu G, Mardinger O, Peleg M, Ghelfan O, Nissan J. J Periodontol. 2010;81:1759. doi: 10.1902/jop.2010.100235. [DOI] [PubMed] [Google Scholar]
- 82.Gooch MR, Gin GE, Kenning TJ, German JW. Neurosurg Focus. 2009;26:E9. doi: 10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 83.Shi M, Kretlow JD, Nguyen A, Young S, Scott Baggett L, Wong ME, Kasper FK, Mikos AG. Biomaterials. 2010;31:4146. doi: 10.1016/j.biomaterials.2010.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi M, Kretlow JD, Spicer PP, Tabata Y, Demian N, Wong ME, Kasper FK, Mikos AG. J Control Release. 2011;152:196. doi: 10.1016/j.jconrel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kretlow JD, Shi M, Young S, Spicer PP, Demian N, Jansen JA, Wong ME, Kasper FK, Mikos AG. Tissue Eng Part C Methods. 2010;16:1427. doi: 10.1089/ten.tec.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spicer PP, Kretlow JD, Henslee AM, Shi M, Young S, Demian N, Jansen JA, Wong ME, Mikos AG, Kasper FK. J Biomed Mater Res A. 2012;100:827. doi: 10.1002/jbm.a.34016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cancedda R, Dozin B, Giannoni P, Quarto R. Matrix Biol. 2003;22:81. doi: 10.1016/s0945-053x(03)00012-x. [DOI] [PubMed] [Google Scholar]
- 88.Moreira-Teixeira LS, Georgi N, Leijten J, Wu L, Karperien M. Endocr Dev. 2011;21:102. doi: 10.1159/000328140. [DOI] [PubMed] [Google Scholar]
- 89.Becerra J, Andrades JA, Guerado E, Zamora-Navas P, López-Puertas JM, Reddi AH. Tissue Eng Part B Rev. 2010;16:617. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed TAE, Hincke MT. Tissue Eng Part B Rev. 2010;16:305. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 91.Ofek G, Athanasiou KA. J Mech Mater Struct. 2007;2:1059. [Google Scholar]
- 92.Sellards RA, Nho SJ, Cole BJ. Curr Opin Rheumatol. 2002;14:134. doi: 10.1097/00002281-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Newman AP. Am J Sports Med. 1998;26:309. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 94.Hunter W. Philos Trans R Soc Lond. 1742;42:514. [Google Scholar]
- 95.Kalson NS, Gikas PD, Briggs TWR. Int J Clin Pract. 2010;64:1444. doi: 10.1111/j.1742-1241.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 96.O’Driscoll SW. J Bone Joint Surg Am. 1998;80:1795. [PubMed] [Google Scholar]
- 97.Redman SN, Oldfield SF, Archer CW. Eur Cell Mater. 2005;9:23. doi: 10.22203/ecm.v009a04. [DOI] [PubMed] [Google Scholar]
- 98.Temenoff JS, Mikos AG. Biomaterials. 2000;21:431. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 99.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 100.Danisovic L, Varga I, Zamborsky R, Böhmer D. Exp Biol Med (Maywood) 2012;237:10. doi: 10.1258/ebm.2011.011229. [DOI] [PubMed] [Google Scholar]
- 101.Kessler MW, Grande DA. Organogenesis. 2008;4:28. doi: 10.4161/org.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vinatier C, Bouffi C, Merceron C, Gordeladze J, Brondello JM, Jorgensen C, Weiss P, Guicheux J, Noël D. Curr Stem Cell Res Ther. 2009;4:318. doi: 10.2174/157488809789649205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raghunath J, Rollo J, Sales KM, Butler PE, Seifalian AM. Biotechnol Appl Biochem. 2007;46:73. doi: 10.1042/BA20060134. [DOI] [PubMed] [Google Scholar]
- 104.Temenoff JS, Mikos AG. Biomaterials. 2000;21:2405. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 105.Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, Owaki H, Ono K. J Bone Joint Surg Br. 1989;71:74. doi: 10.1302/0301-620X.71B1.2915011. [DOI] [PubMed] [Google Scholar]
- 106.Sims CD, Butler PE, Cao YL, Casanova R, Randolph MA, Black A, Vacanti CA, Yaremchuk MJ. Plast Reconstr Surg. 1998;101:1580. doi: 10.1097/00006534-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 107.Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, Giardino R, Facchini A. Biomaterials. 2001;22:2417. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 108.Rahman MS, Tsuchiya T. Tissue Eng. 2001;7:781. doi: 10.1089/107632701753337726. [DOI] [PubMed] [Google Scholar]
- 109.Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. J Biomed Mater Res. 1993;27:11. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 110.Holland TA, Tabata Y, Mikos AG. J Control Release. 2003;91:299. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 111.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. J Biomed Mater Res. 2002;59:429. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 112.Dadsetan M, Szatkowski JP, Yaszemski MJ, Lu L. Biomacromolecules. 2007;8:1702. doi: 10.1021/bm070052h. [DOI] [PubMed] [Google Scholar]
- 113.Bhosale AM, Richardson JB. Br Med Bull. 2008;87:77. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 114.Shin H, Temenoff JS, Mikos AG. Biomacromolecules. 2003;4:552. doi: 10.1021/bm020121m. [DOI] [PubMed] [Google Scholar]
- 115.Kjellander R, Florin E. J Chem Soc, Faraday Trans 1. 1981;77:2053. [Google Scholar]
- 116.Behravesh E, Shung AK, Jo S, Mikos AG. Biomacromolecules. 2002;3:153. doi: 10.1021/bm010137x. [DOI] [PubMed] [Google Scholar]
- 117.Behravesh E, Jo S, Zygourakis K, Mikos AG. Biomacromolecules. 2002;3:374. doi: 10.1021/bm010158r. [DOI] [PubMed] [Google Scholar]
- 118.Fisher JP, Jo S, Mikos AG, Reddi aH. J Biomed Mater Res A. 2004;71:268. doi: 10.1002/jbm.a.30148. [DOI] [PubMed] [Google Scholar]
- 119.Seyedin SM, Rosen DM, Segarini PR. Pathol Immunopathol Res. 1988;7:38. doi: 10.1159/000157090. [DOI] [PubMed] [Google Scholar]
- 120.Jo S, Shin H, Shung AK, Fisher JP, Mikos AG. Macromolecules. 2001;34:2839. [Google Scholar]
- 121.Park H, Guo X, Temenoff JS, Tabata Y, Caplan AI, Kasper FK, Mikos AG. Biomacromolecules. 2009;10:541. doi: 10.1021/bm801197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Temenoff JS, Park H, Jabbari E, Conway DE, Sheffield TL, Ambrose CG, Mikos AG. Biomacromolecules. 2004;5:5. doi: 10.1021/bm030067p. [DOI] [PubMed] [Google Scholar]
- 123.Shin H, Quinten Ruhé P, Mikos AG, Jansen JA. Biomaterials. 2003;24:3201. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 124.Jo S, Shin H, Mikos AG. Biomacromolecules. 2001;2:255. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 125.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. Clin Orthop Relat Res. 2011;469:2706. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, Caplan AI, Ono K. Endocrinology. 1993;132:1603. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- 127.Majumdar MK, Banks V, Peluso DP, Morris EA. J Cell Physiol. 2000;185:98. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 128.Morales TI. Arch Biochem Biophys. 1991;286:99. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- 129.Venn G, Lauder RM, Hardingham TE, Muir H. Biochem Soc Trans. 1990;18:973. doi: 10.1042/bst0180973. [DOI] [PubMed] [Google Scholar]
- 130.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Lab Invest. 1994;71:279. [PubMed] [Google Scholar]
- 131.Holland TA, Tessmar JKV, Tabata Y, Mikos AG. J Control Release. 2004;94:101. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 132.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Arch Biochem Biophys. 1988;267:416. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 133.Xu C, Oyajobi BO, Frazer A, Kozaci LD, Russell RG, Hollander AP. Endocrinology. 1996;137:3557. doi: 10.1210/endo.137.8.8754787. [DOI] [PubMed] [Google Scholar]
- 134.Holland TA, Tabata Y, Mikos AG. J Control Release. 2005;101:111. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 135.Holland TA, Bodde EWH, Cuijpers VMJI, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteoarthritis Cartilage. 2007;15:187. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 136.Panseri S, Russo A, Cunha C, Bondi A, Di Martino A, Patella S, Kon E. Knee Surg Sports Traumatol Arthrosc. 2011;20:1182. doi: 10.1007/s00167-011-1655-1. [DOI] [PubMed] [Google Scholar]
- 137.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Biomaterials. 2005;26:7095. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 138.Mano JF, Reis RL. J Tissue Eng Regen Med. 2007;1:261. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 139.Ashhurst DE, Ashton BA, Owen ME. Journal of Orthopaedic Research3: Official Publication of the Orthopaedic Research Society. 1990;8:741. doi: 10.1002/jor.1100080516. [DOI] [PubMed] [Google Scholar]
- 140.Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Clin Orthop Relat Res. 1980:294. [PubMed] [Google Scholar]
- 141.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Cell Tissue Kinet. 1987;20:263. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 142.Caplan AI. Tissue Eng. 2005;11:1198. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 143.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Biomaterials. 2007;28:3217. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goldberg VM, Caplan AI. Instructional Course Lectures. 1999;48:623. [PubMed] [Google Scholar]
- 145.Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Biomol Eng. 2007;24:489. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 146.Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E. Knee Surg Sports Traumatol Arthrosc. 2010;18:434. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Journal of Biomechanics. 2007;40:750. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 148.O’Shea TM, Miao X. Tissue Eng Part B Rev. 2008;14:447. doi: 10.1089/ten.teb.2008.0327. [DOI] [PubMed] [Google Scholar]
- 149.Holland TA, Bodde EWH, Baggett LS, Tabata Y, Mikos AG, Jansen JA. J Biomed Mater Res A. 2005;75:156. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 150.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, Kasper FK, Mikos AG. Biomaterials. 2009;30:2741. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guo X, Liao J, Park H, Saraf A, Raphael RM, Tabata Y, Kasper FK, Mikos AG. Acta Biomater. 2010;6:2920. doi: 10.1016/j.actbio.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amin AK, Huntley JS, Simpson AHRW, Hall AC. J Bone Joint Surg Br. 2009;91:691. doi: 10.1302/0301-620X.91B5.21544. [DOI] [PubMed] [Google Scholar]
- 153.Zhang LZ, Zheng HA, Jiang Y, Tu YH, Jiang PH, Yang AL. Arthritis Care Res (Hoboken) 2012;64:960. doi: 10.1002/acr.21640. [DOI] [PubMed] [Google Scholar]
- 154.Zhang MW, Park H, Guo X, Nakamura K, Raphael RM, Kasper FK, Mikos AG, Tsonis PA. Tissue Eng Part C Methods. 2010;16:261. doi: 10.1089/ten.tec.2009.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ueda H, Hacker MC, Haesslein A, Jo S, Ammon DM, Borazjani RN, Kunzler JF, Salamone JC, Mikos AG. J Biomed Mater Res A. 2007;83:656. doi: 10.1002/jbm.a.31226. [DOI] [PubMed] [Google Scholar]
- 156.Hacker MC, Haesslein A, Ueda H, Foster WJ, Garcia CA, Ammon DM, Borazjani RN, Kunzler JF, Salamone JC, Mikos aG. J Biomed Mater Res A. 2009;88:976. doi: 10.1002/jbm.a.31942. [DOI] [PubMed] [Google Scholar]
- 157.Choi J, Kim K, Kim T, Liu G, Bar-Shir A, Hyeon T, McMahon MT, Bulte JWM, Fisher JP, Gilad AA. J Control Release. 2011;156:239. doi: 10.1016/j.jconrel.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang H, Liu Z, Li D, Guo X, Kasper FK, Duan C, Zhou J, Mikos AG, Wang C. J Cell Mol Med. 2011;16:1310. doi: 10.1111/j.1582-4934.2011.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saraf A, Hacker MC, Sitharaman B, Grande-Allen KJ, Barry MA, Mikos AG. Biomacromolecules. 2008;9:818. doi: 10.1021/bm701146f. [DOI] [PubMed] [Google Scholar]
- 160.Saraf A, Baggett LS, Raphael RM, Kasper FK, Mikos AG. J Control Release. 2010;143:95. doi: 10.1016/j.jconrel.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Needham CJ, Williams AK, Chew SA, Kasper FK, Mikos AG. Biomacromolecules. 2012;13:1429. doi: 10.1021/bm300145q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sitharaman B, Zakharian TY, Saraf A, Misra P, Ashcroft J, Pan S, Pham QP, Mikos AG, Wilson LJ, Engler DA. Mol Pharm. 2008;5:567. doi: 10.1021/mp700106w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chew SA, Hacker MC, Saraf A, Raphael RM, Kasper FK, Mikos AG. Biomacromolecules. 2009;10:2436. doi: 10.1021/bm9003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chew SA, Hacker MC, Saraf A, Raphael RM, Kasper FK, Mikos AG. Biomacromolecules. 2010;11:600. doi: 10.1021/bm901147k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chew SA, Kretlow JD, Spicer PP, Edwards AW, Baggett LS, Tabata Y, Kasper FK, Mikos AG. Tissue Eng Part A. 2011;17:751. doi: 10.1089/ten.tea.2010.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fu K, Klibanov AM, Langer R. Nat Biotechnol. 2000;18:24. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 167.Kasper FK, Seidlits SK, Tang A, Crowther RS, Carney DH, Barry MA, Mikos AG. J Control Release. 2005;104:521. doi: 10.1016/j.jconrel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 168.Kasper FK, Young S, Tanahashi K, Barry MA, Tabata Y, Jansen JA, Mikos AG. J Biomed Mater Res A. 2006;78:335. doi: 10.1002/jbm.a.30698. [DOI] [PubMed] [Google Scholar]
- 169.Kasper FK, Jerkins E, Tanahashi K, Barry MA, Tabata Y, Mikos AG. J Biomed Mater Res A. 2006;78:823. doi: 10.1002/jbm.a.30736. [DOI] [PubMed] [Google Scholar]
- 170.Kasper FK, Kushibiki T, Kimura Y, Mikos AG, Tabata Y. J Control Release. 2005;107:547. doi: 10.1016/j.jconrel.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 171.Thibault RA, Mikos AG, Kasper FK. Biomacromolecules. 2011;12:4204. doi: 10.1021/bm200975a. [DOI] [PMC free article] [PubMed] [Google Scholar]