Abstract
The purpose of this study was to determine the effect of soil contamination with tri- and hexavalent chromium and soil application of compost, zeolite, and CaO on the mass of oats and content of nitrogen compounds in different organs of oats. The oats mass and content of nitrogen compounds in the crop depended on the type and dose of chromium and alleviating substances incorporated to soil. In the series without neutralizing substances, Cr(VI), unlike Cr(III), had a negative effect on the growth and development of oats. The highest doses of Cr(VI) and Cr(III) stimulated the accumulation of total nitrogen but depressed the content of N-NO3 − in most of organs of oats. Among the substances added to soil in order to alleviate the negative impact of Cr (VI) on the mass of plants, compost had a particularly beneficial effect on the growth and development of oats. The application of compost, zeolite, and CaO to soil had a stronger effect on the content of nitrogen compounds in grain and straw than in roots. Soil enrichment with either of the above substances usually raised the content of nitrogen compounds in oats grain and straw, but decreased it in roots.
Keywords: Chromium contamination, Compost, Zeolite, CaO, Oats, Nitrogen content
Introduction
Once they enter the natural environment, heavy metals, including chromium, affect all elements of the food chain, from soil microorganisms to plants, animals, and humans (Bååth 1989; Jordao et al. 1999; Turkdogan et al. 2003; Liu et al. 2009). Chromium can have different oxidation numbers, from −2 to +6. Diagrams of the element’s oxidation state indicate that oxidation number 3 is the most stable one. However, the most widespread forms of chromium in the natural environment are the ones with the oxidation numbers +3 and +6 (Fendorf 1995), mainly two forms of trivalent chromium: cation Cr3+ and anion CrO2 −2 and two anion forms Cr2O7 2− and CrO4 2− (Chen and Hao 1998; Razić and Dogo 2010). The toxicity of trivalent chromium compounds is lower than that of hexavalent chromium and mutagenicity is almost exclusively a characteristic of Cr(VI) compounds (Banks et al. 2006; Kimbrough et al. 1999).
Chromium compounds found in the natural environment are emitted by different branches of industry in the form of waste, wastewater, or gases. Large quantities of chromium enter soil with fertilizers or waste material used for soil improvement (Ghosh et al. 2003; Dampare et al. 2006; Shams et al. 2010). Depending on the source, different chromium derivatives enter the natural environment, where they undergo further transformations conditioned by the soil pH, type, and abundance of inorganic and organic ions, presence of soil-borne organisms, etc. thus creating a wide range of chemical and physical forms (Richard and Bourg 1991). Solubility and plant availability of metals in soil increase rapidly as the soil pH declines to 3.3 (Chuan et al. 1996). Chromium is present in all plant tissues as an essential element, which means that both its deficiency and excess can cause negative consequences to plants (Shanker et al. 2005). Its deficit may lead to some disorder in the growth and photosynthesis of plants or can impair the tolerance of plants to pathogens (Bartlett and Klmble 1976). On the other hand, chromium added to soil may improve plant yields (Wyszkowski and Radziemska 2010). However, excess chromium causes toxic symptoms in plants, such as disturbed water balance (wilting of leaves), chlorosis of young leaves, and damage to the growth apex and roots (Pederno et al. 1997).
Reports published in recent years (Srivastava et al. 1999 ; Cervantez et al. 2001; Shanker et al. 2005; Banks et al. 2006; Wyszkowski and Radziemska 2010, 2013) confirm the negative influence of chromium compounds on plants, but they mostly deal with the effect of chromium (VI) compounds. The above studies prove that the two forms of chromium should be investigated separately because of the different effects they produce on plants and soil.
The purpose of this study has been to determine the effect of soil contamination with tri- and hexavalent chromium on mass of oats and content of nitrogen compounds in different organs of the crop (grain, straw, and roots) and the ability of added compost, zeolite, and calcium oxide to reduce the effect of chromium on plants.
Material and Methods
Pot Trial Methodology
A pot experiment was set up in 9.5 kg polyethylene pots kept in a greenhouse at the University of Warmia and Mazury in Olsztyn (Poland). Soil used for the trials was collected from the humic horizon. It was slightly acidic and had the texture of sand. The soil properties are given in Table 1. The tested plant was a Polish cultivar of oats (Avena sativa L) called Kasztan. Oats was grown until fully ripe and then divided into grain, straw and roots for analyses. Soil was artificially polluted with aqueous solutions of Cr(III) in the form of KCr(SO4)2 · 12H2O and Cr(VI) as K2Cr2O7. Three substances were applied to the soil: compost and zeolite (2 % relative to the dry mass of soil) and 50 % calcium oxide in a dose corresponding to one hydrolytic acidity (HAC), i.e., 1.25 g kg−1 of soil. The elemental composition of the substances applied to soil is given in Table 2. Control treatments were also established where neither neutralizing substances nor chromium (III) or chromium (VI) compounds were introduced. Aqueous solutions of mineral fertilizers were added to soil in each pot (in milligram per kilogram of soil): 110 N [CO(NH2)2 + (NH4)6 Mo7O24 · 4H2O + (NH4)2HO4], 50 P [(NH4)2HPO4], 110 K [KCl + KCr(SO4)2 · 12H2O + K2Cr2O7], 50 magnesium (Mg) [MgSO4 · 7H2O], 0.33 B [H3BO3], 5 manganese (Mn) [MnCl2 · 4H2O], and 5 Mo [(NH4)6Mo7O24 · 4H2O]. Oat was sown in pots filled with soil carefully mixed with substances according to the experiment’s design directly after mixing.
Table 1.
The properties of the soil and methods their estimation
| Property | Value and unit | Evaluation |
|---|---|---|
| Share of fractions [∅ mm] (%) | ||
| Soil texture | <0.002 (0.85) | |
| 0.002–0.005 (1.47) | ||
| 0.005–0.010 (2.06) | ||
| 0.010–0.020 (3.32) | ||
| 0.020–0.050 (7.19) | ||
| 0.050–0.100 (7.06) | ||
| 0.100–0.250 (34.63) | ||
| 0.250–0.500 (34.06) | ||
| 0.500–1.000 (9.36) | ||
| pH | 5.00 | Acid |
| Hydrolytic acidity (HAC) | 26.60 mmol(+) kg−1 | |
| Sum of exchangeable bases Ca++, Mg++, K+, and Na+ (TEB) | 100.00 mmol(+) kg−1 | |
| Cation exchange capacity (CEC) | 126.00 mmol(+) kg−1 | |
| Base saturation (BS) | 79.00 % | |
| Corg. | 7.87 g C kg−1 | |
| Total nitrogen | 1.01 g N kg−1 | |
| Ammonia | 21.29 mg N-NH4 + kg−1 | |
| Nitrate (V) | 2.95 mg N-NO3 − kg−1 | |
| Available forms of: | ||
| Phosphorus | 90.2 mg P kg−1 | High |
| Potassium | 37.9 mg K kg−1 | High |
| Magnesium | 77.0 mg Mg kg−1 | Very high |
| Total content of: | ||
| Chromium | 12.95 mg Cr kg−1 | |
| Manganese | 219.90 mg Mn kg−1 | |
| Copper | 9.01 mg Cu kg−1 | |
| Zinc | 24.25 mg Zn kg−1 | |
| Nickel | 3.99 mg Ni kg−1 | |
| Cobalt | 2.37 mg Co kg−1 | |
Table 2.
Content of trace elements in substances used in experiment
| Element | Unit | Compost | Zeolite | Calcium oxide |
|---|---|---|---|---|
| P | g kg−1 dm | 2.32 | 0.11 | 0.10 |
| K | 1.33 | 23.21 | 0.77 | |
| Mg | 1.47 | 0.31 | 2.65 | |
| Ca | 15.86 | 15.28 | 347.99 | |
| Na | 0.12 | 16.12 | 0.07 | |
| Cr | mg kg−1 dm | 3.48 | 1.81 | 2.70 |
| Mn | 208.73 | 2.04 | 295.02 | |
| Cu | 38.13 | 12.38 | 2.26 | |
| Zn | 31.80 | 14.68 | 5.14 | |
| Ni | 18.75 | 408.66 | 6.64 | |
| Co | 0.47 | 295.71 | 0.69 |
Laboratory Analyses
Plant Samples
The collected plant material was analyzed in order to determine yield of aerial parts, weight of grain, and mass of roots from each pot. Plant samples were fragmented, dried at 60 °C, ground, and mineralized. The following determinations were made on the plant material: total nitrogen content by Kjeldahl’s distillation method (Bremner 1965) after mineralization in concentrated sulphuric (VI) acid with hydrogen peroxide added as a catalyst; ammonia nitrogen (N-NH4 +) and nitrate nitrogen (N-NO3 −) by potentiometry using 2 % acetic acid as extraction solution (Ostrowska et al. 1991).
Soil Samples
Prior to the experiment, the following soil parameters were determined: the grain size composition of the soil with the laser method using a Mastersizer 2000 m, pHKCl by potentiometry in KCl aqueous solution of the concentration of 1 mol cubic dry meter (dm; ISO 10390 2005), HAC by Kappen’s method (Klute 1996), total exchangeable bases (TEB—K+, Na+, Ca2+, and Mg2+) by Kappen’s method (Klute 1996), cation exchange capacity (CEC) from the formula: CEC = HAC + TEB and percentage base saturation (V) from the formula: BS = 100 · TEB CEC−1. Concentrations of the following elements and compounds were determined: organic carbon content (Corg) by Tiurin’s method in potassium dichromate with diluted sulphuric (VI) acid (Kawada 1957), total nitrogen (Nog) by Kjedahl’s method after mineralization in concentrated sulphuric (VI) acid using hydrogen peroxide as a catalyst (Bremner 1965), N-NH4 + with Nessler’s reagent (Ostrowska et al. 1991), and N-NO3 − with phenoldisulphonic acid (Ostrowska et al. 1991). Besides, determinations of the available forms of phosphorus (P) and potassium (P) by Egner–Riehm’s method (Egner et al. 1960) and Mg by Schachtschabel’s method (Schlichting et al. 1995) were made. The total content of Cr, Mn, copper, zinc, nickel, and cobalt in soil (prior to the experiment), compost, zeolite, and calcium oxide was determined by flame atomic absorption spectrophotometry in air–acetylene flame on a SpectrAA 240FS spectrophotometer (VARIAN, Australia), using a Sample Introduction Pump System. The analysis was performed on extracts obtained after “wet” soil mineralization in nitric acid (analytically pure HNO3 in the concentration of 1.40 g cm−3), poured into Teflon™ vessels HP500 and placed a MARS 5 microwave oven (Microwave Accelerated Reaction System, manufactured by CEM Corporation, USA). All analytical parameters, such as the weight of samples, volume of nitric acid, and digestion temperature, adhered to the US-EPA 3051 method (1994). Certified reference material (Sigma Aldrich Chemie GmbH, No. BCR142R) was used for analyses.
Statistical Analysis of the Results
All the results were submitted to statistical processing using a three-factorial analysis of variance ANOVA and Duncan’s interval test. Statistica 9.1 software (StatSoft, Inc. 2010) was used for calculations. Pearson’s simple correlation coefficients (r) were also calculated between the analyzed variables.
Results and Discussion
Weight of Aerial Parts and Roots of Oats
Heavy metals may cause various morphological alterations in plants. Typical symptoms include chlorosis, necrotic spots, discoloration, rolling of leaves, and, consequently, depressed biomass of aerial parts of affected plants (Kahle 1993; MacFarlane and Burchett 2002; Han et al. 2004). When accumulated in soil, heavy metals, including chromium, can be easily absorbed by roots and accumulated in aerial parts of plants, unless certain measures are taken to control their phytoavailability. Otherwise, they can produce lasting influence on many biotic elements in the environment (Obata and Umebayashi 1997). Plants vary in their response to chromium (Zayed et al. 1998).
The growth and mass of oats were significantly affected by the type of contaminant and its dose as well as the applied neutralizing substances: compost, zeolite, or calcium oxide (Table 3, Figs. 1 and 2). In the control treatments (without alleviating substances), the average grain, straw, and root mass was higher in the treatments with trivalent chromium (CrIII) than in pots with its hexavalent form (CrVI). Among the analyzed plant parts, the biggest differences in weight were observed for straw. Oats grain in the control treatments was highly sensitive to soil contamination with chromium compounds, which was confirmed by the negative value (r = −0.904) of the correlation between the weight and the increasing rate of hexavalent chromium contamination (Table 3). However, up to the rate of 50 mg Cr(VI) kg−1 of soil, the weight of harvested oats grain continued to increase. In this treatments, 100 mg Cr(III) kg−1 of soil depressed the grain weight by nearly half and the rate of 150 mg Cr(VI) kg−1 of soil resulted in the complete inhibition of grain yielding. The successive rates of trivalent chromium had a positive effect on weight of grain and straw, but the highest dose of the contaminant (150 mg Cr(III) kg−1 of soil) depressing the mass of these components by a few percent (Table 3, Fig. 1). In the series without any neutralizing substances, only one rate of chromium (VI) (25 mg kg−1 of soil) increased the weight of straw by 18 % versus the control, whereas all the higher rates of the same contaminant considerably depressed the mass of oats, for example by as much as 98 % under the influence of 150 mg Cr(VI) kg−1 of soil (r = −0.953; Table 3, Fig. 1). The weight of roots of the tested plant in the treatments without alleviating substances depended on the effect of chromium (III). This dependence was confirmed by a significant positive correlation between increasing rates of contamination and harvested weight of roots. The highest dose of chromium (III) caused an increase in the weight of roots up to 149 % compared to the control. Reverse relationships were observed in pots contaminated with chromium (VI), although its lowest dose (25 mg Cr(VI) kg−1 of soil) stimulated the development of roots. Higher rates of the contaminant Cr(VI) had a negative effect on the weight of roots and the highest rate (150 mg Cr(VI) kg−1of soil nearly completely inhibited their development (r = −0.745).
Table 3.
Effect of chromium and various substances on the oats mass (A. sativa L.), in gram fresh mass per pot
| Dose of Cr mg kg−1 soil | Kind of contamination | |||||||
|---|---|---|---|---|---|---|---|---|
| Chromium (III) | Chromium (VI) | |||||||
| Kind of substance neutralizing effect of chromium | ||||||||
| Without substances | Compost | Zeolite | CaO | Without substances | Compost | Zeolite | CaO | |
| Grain | ||||||||
| 0 | 10.71 | 9.97 | 8.62 | 11.21 | 10.71 | 9.97 | 8.62 | 11.21 |
| 25 | 12.06 | 10.75 | 9.97 | 9.50 | 11.21 | 15.09 | 10.57 | 8.11 |
| 50 | 13.45 | 9.24 | 8.65 | 9.42 | 12.60 | 13.68 | 8.82 | 8.54 |
| 100 | 12.48 | 10.06 | 9.92 | 9.20 | 5.90 | 13.33 | 6.16 | 1.64 |
| 150 | 10.13 | 9.50 | 10.17 | 10.82 | 0.00 | 8.22 | 0.00 | 0.00 |
| Average | 11.77 | 9.90 | 9.47 | 10.03 | 8.08 | 12.06 | 6.83 | 5.90 |
| r | −0.253 | −0.408 | 0.668** | −0.036 | −0.904** | −0.424 | −0.905** | −0.968** |
| LSD | 0.41a**; 0.65b**; 0.58c, **; 0.92a,b**; 0.82ac**; 1.30bc**; 1.84a,b,c** | |||||||
| Straw | ||||||||
| 0 | 24.48 | 25.40 | 25.52 | 28.07 | 24.48 | 25.40 | 25.52 | 28.07 |
| 25 | 26.92 | 25.52 | 29.93 | 28.82 | 29.02 | 33.96 | 26.26 | 31.24 |
| 50 | 30.41 | 22.50 | 23.28 | 27.64 | 22.26 | 31.44 | 21.39 | 26.38 |
| 100 | 29.85 | 26.91 | 25.06 | 23.38 | 8.31 | 31.56 | 34.70 | 9.94 |
| 150 | 23.88 | 24.83 | 31.49 | 26.90 | 0.53 | 23.30 | 2.70 | 1.63 |
| Average | 27.11 | 25.03 | 27.06 | 26.96 | 16.92 | 29.13 | 22.11 | 19.45 |
| r | −0.069 | 0.112 | 0.416 | −0.563** | −0.953** | −0.361 | −0.554** | −0.958** |
| LSD | 1.48a**; 2.35b**; 2.10c**; 3.32a,b**; 2.97a,c**; 4.69b,c**; 6.64a,b,c** | |||||||
| Roots | ||||||||
| 0 | 16.17 | 26.35 | 32.48 | 24.52 | 16.17 | 26.35 | 32.48 | 24.52 |
| 25 | 17.79 | 32.07 | 52.33 | 36.06 | 32.71 | 29.78 | 34.04 | 13.24 |
| 50 | 26.74 | 52.61 | 50.08 | 25.66 | 21.55 | 31.79 | 32.91 | 12.99 |
| 100 | 29.84 | 42.89 | 35.48 | 34.99 | 16.15 | 36.38 | 14.94 | 0.00 |
| 150 | 40.34 | 41.60 | 42.63 | 22.06 | 0.00 | 29.57 | 0.00 | 0.00 |
| Average | 26.18 | 39.10 | 42.60 | 28.66 | 17.32 | 30.77 | 22.87 | 10.15 |
| r | 0.978** | 0.511* | −0.043 | −0.208 | −0.745** | 0.457* | −0.951** | −0.928** |
| LSD | 2.36a**; 3.74b**; 3.34c**; 5.29a,b**; 7.48a,c**; 7.48b,c**; 10.57a,b,c** | |||||||
LSD (least significant deviation), n.s. nonsignificant, r correlation coefficient
aKind of contamination
bChromium dose
cKind of substance
*P = 0.05; **P = 0.01
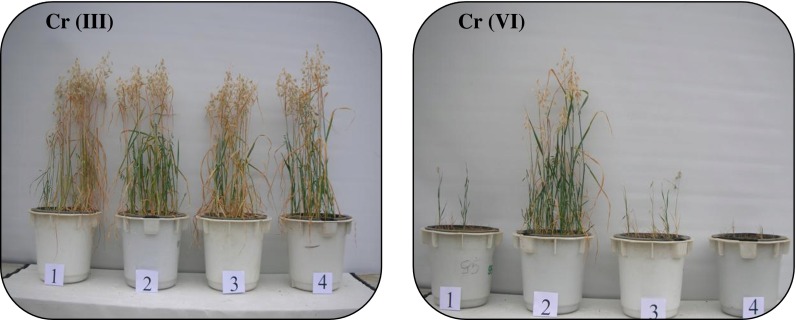
Fig. 1.
Effect of chromium (III) and chromium (VI) on the mass of above-ground parts of oat (Avena sativa L.) (1 0 mg kg−1 of soil, 2 25 mg kg−1 of soil, 3 50 mg kg−1 of soil, 4 150 mg kg−1 of soil)
Fig. 2.
Effect of various substances on the mass of above-ground parts of oats (Avena sativa L.) in treatments with 150 mg Cr(III) and Cr(VI) kg−1 of soil (average with all series; 1 without additions, 2 compost, 3 zeolite, 4 calcium oxide)
The application of compost, zeolite, and calcium oxide significantly affected the mass of particular organs of oats plants (Table 3, Fig. 2). The strongest negative impact on the average grain mass was produced by compost and zeolite in treatments with trivalent chromium as well as CaO and zeolite in soil contaminated with hexavalent chromium. Compost in soil with chromium (VI) was an exception as it helped to raise the grain mass by an average of 49 % compared to the treatments without substances. Compost, zeolite, and calcium oxide did not have any significant effect on the average straw mass in the treatments with trivalent chromium (Table 3, Fig. 2). In the treatments with chromium (VI), the application of compost and zeolite was the most favorable as these substances improved straw mass by 72 and 31 %, respectively, compared to the control (without soil amendments). Calcium oxide also produced a positive albeit much weaker effect (15 %). Among all the neutralizing substances, compost had a particularly positive influence on straw mass at the highest rate of Cr(VI) (Table 3, Fig. 2). Compost, zeolite, and CaO positively affected the weight of oats roots. Zeolite and compost introduced to soil led to an increase in the oats root weight by 63 and 49 %, respectively, in pots with chromium (III) and by 32 and 78 % in pots with chromium (VI) compared to the treatments without any neutralizing substances. The effect of CaO, although positive at low chromium rates, was much weaker than that produced by zeolite or compost.
The results reported by Sharma et al. (1995), Golovatyj et al. (1999), and Radha et al. (2000) provide firm evidence for the limiting effect of chromium (III) and chromium (VI) compounds on growth and mass of plants. Positive influence of small doses of chromium on plants has been observed by some other researchers, e.g., Bonet et al. (1991) and Poschenrieder et al. (1991). According to Chen and Hao (1998), as the acidity of soil decreases, the sorption of Cr(VI) increases while that of Cr(III) declines. Hexavalent chromium has a stronger negative effect on plants than Cr(III) (Singh and Oste 2001). Perlatam et al. (2001) demonstrated that a dose of 40 mg Cr(VI) kg−1 of soil depressed yields of alfalfa (Medicago sativa cv. Melone) by 23 %. In another study, completed by Wyszkowski and Wyszkowska (2004), chromium (VI) unlike chromium (III) depressed the mass of oats by as much as 95 % on soil contaminated with 80 mg Cr(VI) kg−1 of soil in comparison to the control. Comparable influence of chromium (VI) on spring barley yields was reported by Wyszkowski and Radziemska (2010) in an experiment in which also chromium (III) produced a negative but relatively weak effect on the growth and development of the tested plant. A contrary, positive effect of chromium as a soil contaminant was exerted on maize. Toxic influence of chromium (VI) on plants may manifest itself as depressed germination or even death of plant seedlings (Wyszkowski and Wyszkowska 2004, 2006).
The application of neutralizing substances to soil has a positive effect on plant yields (Wyszkowski and Wyszkowska 2006; Wyszkowski and Ziółkowska 2011). Organic matter added to soil can cause reduction of Cr(VI) (Kozuh et al. 2000; Bolan and Duraisamy 2003). By improving physical, chemical, and biological properties of soil, composts can stimulate yields of crops (Wyszkowski and Radziemska 2010) and enhance properties of soil polluted with tri- and hexavalent chromium compounds (Wyszkowski and Radziemska 2009, 2013). In another experiment conducted by Wyszkowski and Radziemska (2010), compost, zeolite and calcium oxide increased the average yield of aerial parts of maize grown on soil polluted with chromium (VI). Among some basic natural reducers of chromium (VI), there are numerous organic substances, reduced compounds of sulfur, and Fe(III) compounds, which play the most important role. Minerals such as biotite, hematite, magnetite, siderite, and pyrite are natural sources of the above compounds in soil and in natural water bodies (Buerge and Hug 1999). According to Wyszkowski and Ziółkowska (2009b), application of bentonite, calcium oxide, and compost to soil improve crop yields, with bentonite producing the best effect on spring rape and compost acting most beneficially on oats. In another trial by Wyszkowski and Ziółkowska (2011), compost and calcium oxide acted positively on yields of yellow lupine (main crop) but did not cause any considerable changes in yields of maize (after crop).
Concentration of Nitrogen in Grain, Straw, and Roots of Oats
The concentration of total nitrogen and its mineral forms in particular organs of oats was closely correlated with the type of contaminant and neutralizing substances introduced to soil (Tables 4, 5, and 6).
Table 4.
Effect of chromium and various substances on the nitrogen concentration in oats grain (Avena sativa L.)
| Dose of Cr mg kg−1 soil | Kind of contamination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chromium (III) | Chromium (VI) | |||||||||
| Kind of substance neutralizing effect of chromium | ||||||||||
| Without substances | Compost | Zeolite | CaO | Average | Without substances | Compost | Zeolite | CaO | Average | |
| Total-N in g kg−1 dry mass | ||||||||||
| 0 | 25.91 | 30.15 | 31.29 | 25.55 | 28.23 | 25.91 | 30.15 | 31.29 | 25.55 | 28.23 |
| 25 | 24.82 | 27.06 | 27.90 | 29.65 | 27.36 | 29.41 | 24.86 | 30.84 | 36.11 | 30.31 |
| 50 | 28.03 | 31.01 | 30.29 | 27.38 | 29.18 | 32.06 | 26.83 | 29.92 | 39.59 | 32.10 |
| 100 | 27.77 | 30.71 | 29.80 | 27.83 | 29.03 | 32.01 | 32.68 | 27.73 | 34.23 | 31.66 |
| 150 | 28.77 | 31.71 | 30.43 | 31.33 | 30.56 | n.a. | 33.01 | n.a. | n.a. | 33.01 |
| Average | 27.06 | 30.13 | 29.94 | 28.35 | 28.87 | 29.85 | 29.51 | 29.95 | 33.87 | 31.06 |
| r | 0.812** | 0.606* | 0.083 | 0.711* | 0.861** | 0.850** | 0.687* | −0.988** | 0.494 | 0.861** |
| LSD | 0.07a**; 0.12b**; 0.10c**; 0.16a,b**; 0.15a,c**; 0.23b,c**; 0.33a,b,c** | |||||||||
| N-NH4 + in mg kg−1 dry mass | ||||||||||
| 0 | 122.1 | 192.2 | 172.7 | 181.4 | 167.1 | 122.1 | 192.2 | 172.7 | 181.4 | 167.1 |
| 25 | 111.9 | 170.0 | 145.8 | 154.9 | 145.7 | 180.3 | 161.6 | 181.3 | 146.6 | 167.4 |
| 50 | 116.9 | 171.5 | 171.8 | 146.0 | 151.6 | 189.1 | 157.1 | 190.3 | 130.5 | 166.8 |
| 100 | 113.6 | 160.6 | 179.8 | 149.2 | 150.8 | 175.3 | 162.3 | 203.3 | 110.0 | 162.7 |
| 150 | 152.6 | 148.9 | 173.7 | 173.1 | 162.1 | n.a. | 129.2 | n.a. | n.a. | 129.2 |
| Average | 123.4 | 168.6 | 168.8 | 160.9 | 155.5 | 166.7 | 160.5 | 186.9 | 142.1 | 158.6 |
| r | 0.688* | −0.933** | 0.447 | −0.066 | 0.043 | 0.599 | −0.864** | 0.996** | −0.53** | −0.848** |
| LSD | 1.59a**; 2.51b**; 2.51c**; 3.55a,b**; 3.13a,c**; 5.02b,c**; 7.10a,b,c** | |||||||||
| N-NO3 − in mg kg−1 dry mass | ||||||||||
| 0 | 140.0 | 117.2 | 73.6 | 94.0 | 106.2 | 140.0 | 117.2 | 73.6 | 94.0 | 106.2 |
| 25 | 98.2 | 101.7 | 65.3 | 136.0 | 100.3 | 80.0 | 64.7 | 83.6 | 103.6 | 83.0 |
| 50 | 93.1 | 101.8 | 67.4 | 100.8 | 90.8 | 70.7 | 66.5 | 66.9 | 113.4 | 79.4 |
| 100 | 84.9 | 84.8 | 65.5 | 80.2 | 78.9 | 61.4 | 58.1 | 59.9 | 99.7 | 69.8 |
| 150 | 83.8 | 73.9 | 59.6 | 79.9 | 74.3 | n.a. | 47.1 | n.a. | n.a. | 47.1 |
| Average | 100.0 | 95.9 | 66.3 | 98.2 | 90.1 | 88.0 | 70.7 | 71.0 | 102.7 | 77.1 |
| r | −0.777** | −0.979** | −0.864** | −0.626* | −0.999** | −0.821 | −0.792** | −0.774** | 0.248 | −0.888** |
| LSD | 2.15a**; 3.40b**; 3.04c**; 4.80a,b**; 4.30a,c**; 6.79b,c; 9.61a,b,c** | |||||||||
LSD least significant deviation, n.s. nonsignificant, r correlation coefficient, n.a. not analyzed because of an insufficient amount of plant material
aKind of contamination
bChromium dose
cKind of substance
*P = 0.05; **P = 0.01
Table 5.
Effect of chromium and various substances on the nitrogen concentration in oats straw (Avena sativa L.)
| Dose of Cr mg kg−1 soil | Kind of contamination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chromium (III) | Chromium (VI) | |||||||||
| Kind of substance neutralizing effect of chromium | ||||||||||
| Without substances | Compost | Zeolite | CaO | Average | Without substances | Compost | Zeolite | CaO | Average | |
| Total-N in g kg−1 dry ;mass | ||||||||||
| 0 | 5.33 | 9.23 | 6.47 | 9.45 | 7.62 | 5.33 | 9.23 | 6.47 | 9.45 | 7.62 |
| 25 | 8.87 | 8.29 | 8.34 | 8.96 | 8.62 | 7.77 | 10.20 | 8.47 | 8.02 | 8.62 |
| 50 | 9.12 | 6.89 | 9.07 | 8.06 | 8.29 | 7.69 | 8.31 | 10.95 | 9.98 | 9.23 |
| 100 | 9.42 | 6.82 | 9.84 | 7.07 | 8.29 | 7.43 | 7.84 | 10.60 | 9.68 | 8.89 |
| 150 | 8.51 | 6.69 | 9.88 | 5.93 | 7.75 | 7.30 | 7.61 | 9.45 | 9.45 | 8.45 |
| Average | 8.25 | 7.58 | 8.72 | 7.89 | 8.11 | 7.10 | 8.64 | 9.19 | 9.32 | 8.56 |
| r | 0.536 | −0.840** | 0.866** | −0.997** | −0.152 | 0.445 | −0.819** | 0.577 | 0.314 | 0.352 |
| LSD | 0.09a**; 0.15b**; 0.13c**; 0.21a,b**; 0.19a,c**; 0.30b,c**; 0.42a,b,c** | |||||||||
| N-NH4 + in mg kg−1 dry mass | ||||||||||
| 0 | 127.0 | 135.3 | 131.7 | 151.4 | 136.4 | 127.0 | 135.3 | 131.7 | 151.4 | 136.4 |
| 25 | 119.9 | 135.9 | 136.7 | 147.8 | 135.1 | 135.9 | 129.8 | 144.8 | 140.8 | 137.8 |
| 50 | 115.8 | 131.7 | 140.6 | 139.5 | 131.9 | 142.6 | 136.9 | 144.0 | 144.6 | 142.0 |
| 100 | 127.5 | 129.3 | 149.4 | 143.1 | 137.4 | 145.6 | 150.3 | 149.9 | 156.1 | 150.5 |
| 150 | 139.9 | 133.1 | 151.7 | 147.0 | 142.9 | 149.2 | 163.6 | 151.3 | n.a. | 154.7 |
| Average | 126.0 | 133.1 | 142.0 | 145.8 | 136.7 | 140.1 | 143.2 | 144.3 | 148.2 | 144.3 |
| r | 0.709* | −0.569 | 0.977** | −0.314 | 0.732* | 0.922** | 0.949** | 0.854** | 0.481 | 0.990** |
| LSD | 1.03a**; 1.63b**; 1.45c**; 2.30a,b**; 2.06a,c**; 3.25b,c**; 4.60a,b,c** | |||||||||
| N-NO3 − in mg kg−1 dry mass | ||||||||||
| 0 | 193.6 | 217.8 | 118.4 | 172.7 | 175.6 | 193.6 | 217.8 | 118.4 | 172.7 | 175.6 |
| 25 | 153.4 | 191.3 | 105.6 | 166.6 | 154.2 | 102.0 | 188.9 | 64.9 | 194.0 | 137.4 |
| 50 | 127.3 | 199.9 | 95.2 | 148.9 | 142.8 | 68.4 | 172.5 | 82.0 | 148.3 | 117.8 |
| 100 | 114.3 | 211.2 | 89.6 | 185.8 | 150.2 | 106.7 | 155.8 | 86.0 | 102.3 | 112.7 |
| 150 | 138.8 | 261.9 | 83.7 | 205.3 | 172.4 | 197.7 | 141.5 | 157.3 | 102.4 | 149.7 |
| Average | 145.5 | 216.4 | 98.5 | 175.9 | 159.0 | 133.7 | 175.3 | 101.7 | 143.9 | 138.6 |
| r | −0.657* | 0.735* | −0.936** | 0.752** | 0.054 | 0.202 | −0.954** | 0.551 | −0.898** | −0.327 |
| LSD | 2.81a**; 4.44a**; 3.97a**; 6.28a,b**; 5.61a,c**; 8.88b,c**; 12.55a,b,c** | |||||||||
LSD least significant deviation, n.s. nonsignificant, r correlation coefficient, n.a. not analyzed because of an insufficient amount of plant material
aKind of contamination
bChromium dose
cKind of substance
*P = 0.05; **P = 0.01
Table 6.
Effect of chromium and various substances on the nitrogen concentration in oats roots (Avena sativa L.)
| Dose of Cr mg kg−1 soil | Kind of contamination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chromium (III) | Chromium (VI) | |||||||||
| Kind of substance neutralizing effect of chromium | ||||||||||
| Without substances | Compost | Zeolite | CaO | Average | Without substances | Compost | Zeolite | CaO | Average | |
| Total-N in g kg−1 dry mass | ||||||||||
| 0 | 14.94 | 16.62 | 10.01 | 14.95 | 14.13 | 14.94 | 16.62 | 10.01 | 14.95 | 14.13 |
| 25 | 17.28 | 15.46 | 14.56 | 15.32 | 15.66 | 15.03 | 16.84 | 13.97 | 15.21 | 15.26 |
| 50 | 18.56 | 17.67 | 17.90 | 15.98 | 17.53 | 17.05 | 17.59 | 14.25 | 17.26 | 16.54 |
| 100 | 20.23 | 19.32 | 19.27 | 15.99 | 18.70 | 18.15 | 17.88 | 14.95 | n. a. | 16.99 |
| 150 | 21.36 | 20.24 | 20.09 | 16.18 | 19.47 | n. a. | 18.41 | n. a. | n. a. | 18.41 |
| Average | 18.47 | 17.86 | 16.37 | 15.68 | 17.10 | 16.29 | 17.47 | 13.30 | 15.81 | 16.27 |
| r | 0.962** | 0.924** | 0.884** | 0.876** | 0.949** | 0.952** | 0.971** | 0.806** | 0.912** | 0.968** |
| LSD | 0.10a**; 0.16b**; 0.15c**; 0.23a,b**; 0.21a,c**; 0.33b,c**; 0.476a,b,c** | |||||||||
| N-NH4 + in mg kg−1 dry mass | ||||||||||
| 0 | 200.5 | 133.0 | 202.8 | 184.9 | 180.3 | 200.5 | 133.0 | 202.8 | 184.9 | 180.3 |
| 25 | 110.7 | 141.9 | 185.7 | 190.4 | 157.2 | 218.5 | 169.0 | 208.5 | 176.8 | 193.2 |
| 50 | 127.9 | 142.3 | 187.1 | 205.1 | 165.6 | 231.6 | 195.4 | 225.1 | n.a. | 217.4 |
| 100 | 136.8 | 151.5 | 165.7 | 193.7 | 161.9 | 237.1 | 161.2 | n.a. | n.a. | 199.2 |
| 150 | 149.9 | 160.0 | 146.2 | 179.7 | 158.9 | n.a. | 181.8 | n.a. | n.a. | 181.8 |
| Average | 145.2 | 145.7 | 177.5 | 190.8 | 164.8 | 221.9 | 168.1 | 212.1 | 180.9 | 194.4 |
| r | −0.226 | 0.985** | −0.984** | −0.285 | −0.590 | 0.919** | 0.486 | 0.962** | −1.000** | −0.080 |
| LSD | 1.48a**; 2.33b; 2.09c**; 3.30a,b**; 2.95a,c**; 4.67b,c**; 6.60a,b,c** | |||||||||
| N-NO3 − in mg kg−1 dry mass | ||||||||||
| 0 | 106.3 | 134.8 | 58.4 | 46.2 | 86.4 | 106.3 | 134.8 | 58.4 | 46.2 | 86.4 |
| 25 | 121.8 | 104.4 | 77.2 | 64.4 | 91.9 | 39.4 | 48.8 | 22.7 | 45.6 | 39.1 |
| 50 | 112.5 | 78.4 | 77.7 | 68.2 | 84.2 | 35.1 | 41.2 | 21.2 | n.a. | 32.5 |
| 100 | 93.9 | 63.9 | 84.3 | 71.7 | 78.5 | 25.4 | 47.7 | n.a. | n.a. | 36.6 |
| 150 | 79.2 | 58.7 | 91.0 | 87.8 | 79.1 | n.a. | 59.0 | n.a. | n.a. | 59.0 |
| Average | 102.7 | 88.0 | 77.7 | 67.7 | 84.0 | 51.6 | 66.3 | 34.1 | 45.9 | 50.7 |
| r | −0.862** | −0.906** | 0.899** | 0.937** | −0.812** | −0.790** | −0.497 | −0.883** | −1.000** | −0.255 |
| LSD | 1.99a**; 3.14b**; 2.81c**; 4.44a,b**; 3.97a,c**; 6.28b,c**; 8.88a,b,c** | |||||||||
LSD least significant deviation, n.s. nonsignificant, r correlation coefficient, n.a. not analyzed because of an insufficient amount of plant material
aKind of contamination
bChromium dose
cKind of substance
*P = 0.05; **P = 0.01
Grain: Total N, Ammonia, and Nitrate N
In the series without neutralizing substances, under the influence of soil contamination with the dose of Cr (VI) equal 100 mg kg−1 of soil, the content of total nitrogen rose by 23 %, while 150 mg of Cr (III) per kilogram of soil raised the total content of nitrogen in oats grain by mere 11 % (Table 4). In the same series, oats grain from pots polluted with 25 and 50 mg Cr(VI) per kilogram of soil was characterized by half as much N-NH4 + as in the control, contrary to the highest dose of Cr(VI) (150 mg kg−1 of soil; Table 4). In the analogous series with chromium (III), the highest dose (150 mg kg−1 of soil) raised the content of ammonia nitrogen by 25 % (r = 0.688) compared to the control. In the non-amended treatments, the increasing rates of chromium (III) and chromium (VI) had an explicitly negative (r = −0.777 and r = −0.821, respectively) effect on the content of nitrate nitrogen in oats grain (Table 4).
Changes in the content of total nitrogen in oats grain after application of alleviating substances were small (Table 4), with the exception of CaO added to soil polluted with hexavalent chromium, which raised the content of total nitrogen by an average of 14 % in grain compared to the control (without neutralizing substances). Compost and zeolite had a similar albeit much weaker effect on total nitrogen in oats grain in pots with Cr(III).
Compost, zeolite, and calcium oxide in treatments with both tri- and hexavalent chromium had a positive effect on the average content of ammonia nitrogen in oats grain versus the treatments without soil amendments (Table 4). The application of zeolite had the most profound effect, as it raised the content of nitrogen in oats grain from pots with Cr(III) by 37 % and from pots with Cr(VI) by 12 % compared to the treatments without neutralizing substances. In the pots with chromium (III), compost had the same influence, whereas calcium oxide produced a slightly weaker effect (+30 %). Calcium oxide had a negative effect on the average content of N-NH4 − in oats grain in pots with chromium (VI). Compost and zeolite reduced the content of nitrate nitrogen in oats grain in pots with Cr(VI), and zeolite had the same effect in pots with Cr(III) (Table 4).
Straw: Total N, Ammonia and Nitrate N
In the series without neutralizing substances, soil contamination with Cr(VI) increased the accumulation of total nitrogen in oats straw, with the smallest dose of Cr(VI) 25 mg kg−1 of soil producing the most beneficial effect, which then tended to weaken slightly under the subsequently higher doses (Table 5). Trivalent chromium applied in the doses of 50 and 100 mg kg−1 of soil led to a nearly double increase in the content of nitrogen in straw compared to the control, while the highest dose, i.e., 150 mg kg−1 of soil, produced a negative effect. Hexavalent chromium raised the content of N-NH4 + in oats straw, but the increase reached just 18 % (r = 0.922) under the growing rate of contamination (Table 5). In the analogous series with chromium (III), changes in the content of ammonia nitrogen were irregular but the highest rate of the contaminant raised the content of N-NH4 + by 10 % compared to the control. The rates between 25 and 150 mg Cr(III) kg−1 of soil depressed the content of nitrate nitrogen in oats straw (r = −0.657) compared to the control, i.e., without contamination (Table 5). When soil was polluted with chromium (VI), changes in the content of N-NO3 − in oats straw did not follow a unidirectional trend.
Soil application of CaO, zeolite and compost had a positive effect on the content of total nitrogen in oats straw (increased by 31, 29, and 22 %, respectively) but only in the pots contaminated with chromium (VI) compared to the control, i.e., without neutralizing substances (Table 5). In oats straw harvested from pots contaminated with chromium (VI), all the neutralizing substances helped to maintain the average ammonia nitrogen content on a similar level, whereas in pots with Cr(III), zeolite, and CaO favored accumulation of this form of nitrogen (Table 5). Compost and calcium oxide in pots with tri- and hexavalent chromium caused an increase in the content of nitrate nitrogen in oats straw, by 49 and 2 % and by 31 and 8 %, respectively, compared to the control treatments, i.e., without neutralizing substances (Table 5). In turn, zeolite limited its average content in oats straw by 32 % in treatments with Cr(III) and by 24 % in pots with Cr(VI) versus the series without neutralizing substances.
Roots: Total N, Ammonia, and Nitrate N
The increasing concentrations of tri- and hexavalent chromium in soil in the series without any alleviating substances had a positive effect on the content of total nitrogen in oats roots, raising it respectively by 43 % (r = 0.962) and 21 % (r = 0.952) compared to the control (Table 6). The higher rates of chromium (VI) increased the content of ammonia nitrogen in oats roots by 18 % (r = 0.919) relative to the control. Chromium (III) had a reverse effect, depressing the content of N-NH4 + in oats roots (Table 6). In the soil without any neutralizing substances, higher rates of trivalent chromium (r = −0.862) and specially hexavalent chromium (r = −0.790) depressed the content of nitrate nitrogen in oat roots, and the effect was more evident under the highest rates of the analyzed contaminants (100–150 mg kg−1 of soil; Table 6).
Compost and zeolite had a positive, but weaker effect on the content of total nitrogen in oats grain than in oats straw in pots with Cr(III) (Table 6). A more demonstrable impact of the neutralizing substances on the content of ammonia nitrogen found in oats roots rather than in straw (Table 6). In the series with Cr(III), the best effect on the average content of ammonia nitrogen in oats roots was produced by CaO (+31 %) and zeolite (+22 %) compared to the control treatment. In pots polluted with Cr(VI), calcium oxide and compost in particular reduced the content of N-NH4 + in oats roots, by 19 and 24 %, respectively. The content of nitrate nitrogen in oats roots was affected by compost added to soil contaminated with hexavalent chromium, which raised its accumulation by 29 % versus the control (Table 6). Zeolite and CaO reduced the content of nitrates (V) in oats roots by 34 and 11 % in treatments with Cr(VI). Compost, zeolite, and calcium oxide in the treatments with trivalent chromium reduced the average content of N-NO3 − in oats roots by 14, 24, and 34 %, respectively.
Depressed content of nitrogen in plants may be a consequence of the adverse effect of hexavalent chromium on the activity of key metabolic enzymes in particular organs of plants during the vegetative growth (Kumar and Joshi 2008). Moral et al. (1995) as well as Khan (2001) found significant changes in the content of nitrogen in organs of the analyzed plants (tomato and rice) in response to soil application of different doses of chromium. Low doses of chromium (VI) often raise the content of nitrogen while high ones reduce its level in aerial parts of plants (Singh and Oste 2001). In a study by Wyszkowski and Radziemska (2010), hexavalent chromium had a stronger effect than its trivalent form on the content of nitrogen in spring barley aerial parts. Chromium (III) and chromium (VI) also induced higher N-NH4 + accumulation and chromium (VI) reduced the accumulation of N-NO3 − in aerial organs of maize.
Soil application of organic matter usually causes higher accumulation of nitrogen in plants (Ciećko et al. 2001, 2004; Cox et al. 2001; Eghball et al. 2002). Incorporation of compost into soil improves the microbiological and biochemical soil properties (Wyszkowska and Wyszkowski 2006, 2010), availability of plant accessible forms of elements in soil (Eghball et al. 2002; Wyszkowski and Ziółkowska 2009b; Wyszkowski and Sivitskaya 2012, 2013), which encourages better yields (Wyszkowski and Wyszkowska 2006; Wyszkowski and Ziółkowska 2011) and improves chemical composition of plants (Wyszkowski and Ziółkowska 2009a, b). In response to soil enrichment with compost or compost earth, increased content of nitrogen has been observed in organs of many plant species, for example in triticale grain and straw (Ciećko et al. 2001), roots of yellow lupine and phacelia (Ciećko et al. 2004) and in aerial parts of oats (Wyszkowski and Ziółkowska 2009b), spring barley (Wyszkowski and Radziemska 2010), maize (Wyszkowski and Radziemska 2010) and spring rape (Wyszkowski and Ziółkowska 2009b), unlike yellow lupine (Wyszkowski and Ziółkowska 2011), or radish (Ciećko et al. 2004). In an experiment conducted by Wyszkowski and Radziemska (2010), the applied contamination alleviating substances stimulated an increase in the total nitrogen content, more evidently in maize than in spring barley, especially in treatments with calcium oxide. The accumulation of N-NH4 + in aerial parts of maize was positively affected by all the applied neutralizing substances whereas the content of N-NO3 − in aerial parts of spring barley was stimulated only by the application of compost and calcium oxide to soil. In another experiment by Wyszkowski and Ziółkowska (2009b) bentonite and zeolite added to soil also raised the content of nitrogen in aerial parts of spring rape and oats, with bentonite being more effective than calcium oxide or compost. In another study carried out by Wyszkowski and Ziółkowska (2011), bentonite and calcium oxide failed to produce an unambiguous effect on the content of nitrogen in yellow lupine and maize. Under the influence of CaO, the content of nitrogen may either increase or decrease depending on the plant species, for example its elevated accumulation has been observed in aerial parts and roots of maize (Ciećko et al. 2001, 2004) but less nitrogen has accumulated in aerial parts of spring triticale (Ciećko et al. 2001).
Conclusion
The mass of oats and the content of nitrogen compounds in particular organs of oats depended on the type and dose of a chromium contaminant and the contamination alleviating substances incorporated into soil. In the series without neutralizing substances, hexavalent chromium, unlike trivalent chromium, had a negative effect on the growth and development of oats. The phytotoxic effect was occurred in response to just 100 mg Cr(VI) kg−1 of soil, while the highest rate of Cr(VI) prohibited the germination of oats seeds. The content of nitrogen compounds in oats depended on the rate of chromium and plant organ. The most unambiguous changes were detected in the case of total nitrogen and N-NO3 −, where the highest doses of Cr(VI) and Cr(III) increased accumulation of total nitrogen and depressed the content of N-NO3 − in most of the examined organs of oats.
Among the tested substances added to soil in order to alleviate the negative impact of Cr(VI) on mass of plants, compost had a particularly beneficial effect on the growth and development of oats. In pots contaminated with chromium (VI), zeolite was less successful, as it had a positive effect only on straw and roots of oats plants. Calcium oxide, in turn, acted positively only on oats straw. Moreover, zeolite and compost were observed to have had a positive influence on the weight of roots in pots with Cr(III), which however did not have an adverse effect on the plants.
The application of compost, zeolite, and calcium oxide to soil had a stronger effect on the content of nitrogen compounds in grain and straw than in roots of oats. Following the soil enrichment with compost, zeolite, or calcium oxide, concentrations of nitrogen compounds tended to increase in oats grain and straw, in contrast to roots, where they were typically lower. Calcium oxide generated the strongest and most often negative effect on the content of nitrogen compounds in oats. Compost caused the highest increase in the content of nitrogen in the analyzed parts of oats.
Acknowledgments
The research was conducted as part of a project no N305 1059 33 and supported by Polish Ministry of Science and Higher Education.
References
- Bååth E. Effects of heavy metals in soil microbial processes and populations (a review) Water, Air, and Soil Pollution. 1989;47(3/4):335–379. doi: 10.1007/BF00279331. [DOI] [Google Scholar]
- Banks MK, Schwab AP, Henderson C. Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere. 2006;62:255–264. doi: 10.1016/j.chemosphere.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Bartlett RJ, Klmble JM. Behaviour of chromium in soils. I. Trivalent forms. Journal of Environmental Quality. 1976;5(4):383–389. doi: 10.2134/jeq1976.00472425000500040010x. [DOI] [Google Scholar]
- Bolan NS, Duraisamy VP. Role of inorganic and organic amendments on immobilization and phytoavailability of heavy metals: a review involving specific case studies. Australian Journal of Soil Research. 2003;41:533–555. doi: 10.1071/SR02122. [DOI] [Google Scholar]
- Bonet A, Poschenrieder C, Barcelo J. Chromium III-iron interaction in Fe-deficient and Fe-sufficient bean plants. I. Growth and nutrient content. Journal of Plant Nutrition. 1991;14(4):403–414. doi: 10.1080/01904169109364211. [DOI] [Google Scholar]
- Bremner, J.M. (1965). Total nitrogen. In: Methods of soil analysis, part 2. Chemical and microbiological properties. Black CA et al. (eds). American Society of Agronomy, Madison, WI. Agronomy vol. 9. pp 1149–1178
- Buerge IB, Hug SJ. Influence of mineral surfaces on chromium (VI) reduction by iron (II) Environmental Science and Technology. 1999;33:4285–4291. doi: 10.1021/es981297s. [DOI] [Google Scholar]
- Cervantez C, Campos-Garcia J, Devars S, Gutierrez-Corona F, Loza-Tavera H, Torres-Guzman JC, et al. Interreactions of chromium with microorganisms and plants. Microbiology Reviews. 2001;25:335–347. doi: 10.1111/j.1574-6976.2001.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Chen JM, Hao OJ. Microbial chromium (VI) reduction. Criticals Reviews Environmental Science and Technology. 1998;28(3):219–215. doi: 10.1080/10643389891254214. [DOI] [Google Scholar]
- Chuan MC, Shu GY, Liu JC. Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water, Air, and Soil Pollution. 1996;90(3–4):543–556. doi: 10.1007/BF00282668. [DOI] [Google Scholar]
- Ciećko Z, Wyszkowski M, Krajewski W, Zabielska J. Effect of organic matter and liming on the reduction of cadmium uptake from soil by triticale and spring oilseed rape. Science of the Total Environment. 2001;281(1–3):37–45. doi: 10.1016/S0048-9697(01)00800-2. [DOI] [PubMed] [Google Scholar]
- Ciećko, Z., Kalembasa, S., Wyszkowski, M., & Rolka, E. (2004). The effect of soil contamination with cadmium on the phosphorus content in plants. Electronic Journal of Polish Agricultural Universities, Environmental Development, 7(1), http://www.ejpau.media.pl/volume7/issue1/environment/art-05.html
- Cox D, Bezdieck D, Fauci M. Effects of compost, coal, and straw amendments on restoring the quality of eroded Palouse soil. Biology and Fertility of Soils. 2001;33:365–372. doi: 10.1007/s003740000335. [DOI] [Google Scholar]
- Dampare SB, Ameyaw Y, Adotey DK, Osae S, Serfor-Armah Y, Nyarko BJB, et al. Seasonal trend of potentially toxic trace elements in soils supporting medicinal plants in the eastern region of Ghana. Water, Air, and Soil Pollution. 2006;169:185–206. doi: 10.1007/s11270-006-2381-z. [DOI] [Google Scholar]
- Eghball B, Wienhold BJ, Gilley JE, Eigenberg RA. Nutrient management in the United States: a joint symposium. Journal of Soil and Water Conservation. 2002;57(6):470–473. [Google Scholar]
- Egner H, Riehm H, Domingo WR. Untersuchun-gen über die chemische Bodenanalyse als Grundlage für die Be-urteilung des Nährstoffzustandes der Böden. II. Chemische Extractionsmethoden zur Phospor- und Kaliumbestimmung. Ann. Royal Agricult. College Sweden. 1960;26:199–215. [Google Scholar]
- Fendorf SE. Surface reactions of chromium on soils and waters. Geoderma. 1995;67:55–71. doi: 10.1016/0016-7061(94)00062-F. [DOI] [Google Scholar]
- Ghosh UC, Dasgupta M, Debnath S, Bhat SC. Studies on management of chromium (VI)-contaminated industrial waste effluent using hydrous titanium oxide (HTO) Water, Air, and Soil Pollution. 2003;143:245–256. doi: 10.1023/A:1022814401404. [DOI] [Google Scholar]
- Golovatyj, S. E., Bogatyreva, E. N., & Golovatyi, S. E. (1999). Effect of leaves of chromium content in a soil on its distribution in organs of corn plants. Soil Resources and Fertilization, 197–204.
- Han FX, Maruthi, Sridhar BB, Monts DL, Su Y. Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea. New Phytologist. 2004;162:489–500. doi: 10.1111/j.1469-8137.2004.01027.x. [DOI] [Google Scholar]
- ISO 10390 (2005). Soil quality—determination of pH, International Standardization Organization.
- Jordao CP, Pereira JL, Jham GN, Bellato CR. Distribution of heavy metals in environmental samples near smelters and mining areas in Brazil. Environmental Technology. 1999;20:489–498. doi: 10.1080/09593332008616844. [DOI] [Google Scholar]
- Kahle H. Response of roots of trees to heavy metals. Environmental and Experimental Botany. 1993;33:99–119. doi: 10.1016/0098-8472(93)90059-O. [DOI] [Google Scholar]
- Kawada H. An examination of the Tiurin’s method for determination of soil organic carbon and a proposed modification of the chromic acid titration method. Forest Soils Japan Report. 1957;8:67–80. [Google Scholar]
- Khan AG. Relationships between chromium bio magnification ratio, accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Environment International. 2001;26:417–423. doi: 10.1016/S0160-4120(01)00022-8. [DOI] [PubMed] [Google Scholar]
- Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C. A critical assessment of chromium in the environment. Environmental Science and Technology. 1999;29:1–46. doi: 10.1080/10643389991259164. [DOI] [Google Scholar]
- Klute A. Methods of soil analysis. Madison: American Society of Agronomy; 1996. [Google Scholar]
- Kozuh N, Stupea J, Gorenc B. Reduction and oxidation processes of chromium in silos. Environmental Science and Technology. 2000;34:112–119. doi: 10.1021/es981162m. [DOI] [Google Scholar]
- Kumar S, Joshi UN. Nitrogen metabolism as affected by hexavalent chromium in sorghum (Sorghum bicolor L.) Environmental and Experimental Botany. 2008;64(2):135–144. doi: 10.1016/j.envexpbot.2008.02.005. [DOI] [Google Scholar]
- Liu H, Chen LP, Ai XY, Yu YH, Zuo YB. Heavy metal contamination in soil alongside mountain railway in Sichuan, China. Environmental Monitoring and Assessment. 2009;152:25–33. doi: 10.1007/s10661-008-0293-7. [DOI] [PubMed] [Google Scholar]
- MacFarlane GR, Burchett MD. Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Marine Environmental Research. 2002;54:65–84. doi: 10.1016/S0141-1136(02)00095-8. [DOI] [PubMed] [Google Scholar]
- Moral R, Pederno NJ, Gomez I, Mataix J. Effect of chromium on the nutrient element content and morphology of tomato. Journal of Plant Nutrition. 1995;18:815–822. doi: 10.1080/01904169509364940. [DOI] [Google Scholar]
- Obata H, Umebayashi M. Effect of cadmium on mineral nutrient concentration in plants differing in tolerance for cadmium. Journal of Plant Nutrition. 1997;20(1):97–105. doi: 10.1080/01904169709365236. [DOI] [Google Scholar]
- Ostrowska, A., Gawliński, S., & Szczubiałka, Z. (1991). Methods for analysis and evaluation of soil and plant properties. IOŚ Warszawa, 334 pp.
- Pederno NJI, Gomez R, Moral G, Palacios J, Mataix J. Heavy metals and plant nutrition and development. Recent Research Developments in Phytochemistry. 1997;1:173–179. [Google Scholar]
- Perlatam JR, Torresdey GJT, Tiemann KJ, Gomez E, Arteaga S, Rascon E. Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativia L.) Bulletin of Environmental Contamination and Toxicology. 2001;66:727–734. doi: 10.1007/s001280069. [DOI] [PubMed] [Google Scholar]
- Poschenrieder C, Vazquez MD, Bonet A, Barcelo J. Chromium-III-iron interaction in iron sufficient and iron deficient bean plants. 2. Ultrastructural aspects. Journal of Plant Nutrion. 1991;14(4):415–428. doi: 10.1080/01904169109364212. [DOI] [Google Scholar]
- Radha J, Srivastava S, Madan VK, Jain R. Influence of chromium on growth and cell division of sugarcane. Indian Journal of Plant Physiology. 2000;5(3):228–231. [Google Scholar]
- Razić S, Dogo S. Determination of chromium in Mentha piperita L. and soil by graphite furnace atomic absorption spectrometry after sequential extraction and microwave-assisted digestion to assess potential bioavailability. Chemosphere. 2010;78:451–456. doi: 10.1016/j.chemosphere.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Richard FC, Bourg ACM. Aqueous geochemistry of chromium: a review. Water Research. 1991;25(7):807–816. doi: 10.1016/0043-1354(91)90160-R. [DOI] [Google Scholar]
- Schlichting E, Blume HP, Stahr K. Boden-kundliches Praktikum, Pareys Studientexte 81. Berlin: Blackwell; 1995. [Google Scholar]
- Shams KM, Tichy G, Fischer A, Sager M, Peer T, Bashar A, et al. Aspects of phytoremediation for chromium contaminated sites using common plants Urtica dioica, Brassica napus and Zea mays. Plant and Soil. 2010;328:175–198. doi: 10.1007/s11104-009-0095-x. [DOI] [Google Scholar]
- Shanker AK, Cervantez C, Loza-Tavera H, Avudainayagam S. Chromium toxicity in plants. Environment International. 2005;31:739–753. doi: 10.1016/j.envint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Sharma DC, Chatterjee C, Sharma CP. Chromium accumulation and its effects on wheat (Triticum aestivum L. cv. HD 2204) metabolism. Plant Science. 1995;111(2):145–151. doi: 10.1016/0168-9452(95)04230-R. [DOI] [Google Scholar]
- Singh BR, Oste L. In situ immobilization of metals in contamined or naturally metal-rich soils. Environmental Review. 2001;9:81–97. doi: 10.1139/a01-002. [DOI] [Google Scholar]
- Srivastava S, Prakash S, Srivastava MM. Chromium mobilization and plant availability—the impact of organic complexing ligands. Plant and Soil. 1999;212:203–208. doi: 10.1023/A:1004691217480. [DOI] [Google Scholar]
- StatSoft, Inc. (2010). Statistica (data analysis software system), version 9.1. www.statsoft.com
- Turkdogan MK, Kilicel F, Kara K. Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environmental Toxicology and Pharmacology. 2003;13:175–179. doi: 10.1016/S1382-6689(02)00156-4. [DOI] [PubMed] [Google Scholar]
- US-EPA Method 3051 (1994). Microwave assisted acid digestion of sediment, sludges, soils and oils.
- Wyszkowska J, Wyszkowski M. Role of compost, bentonite and lime in recovering the biochemical equilibrium of diesel oil contaminated soil. Plant Soil and Environment. 2006;52(11):505–514. [Google Scholar]
- Wyszkowska J, Wyszkowski M. Activity of dehydrogenases, urease and phosphatases in soil polluted with petrol. Journal of Toxicology and Environmental Health. Part A. 2010;73(17):1202–1210. doi: 10.1080/15287394.2010.492004. [DOI] [PubMed] [Google Scholar]
- Wyszkowski M, Radziemska M. The effect of chromium content in soil on the concentration of some mineral elements in plants. Fresenius Environmental Bulletin. 2009;18(7):1039–1045. [Google Scholar]
- Wyszkowski M, Radziemska M. Effects of chromium (III and VI) on spring barley and mayze biomass yield and content of nitrogen compounds. Journal of Toxicology and Environment Health, Part A. 2010;73(17):1274–1282. doi: 10.1080/15287394.2010.492016. [DOI] [PubMed] [Google Scholar]
- Wyszkowski M, Radziemska M. Influence of chromium (III) and (VI) on the concentration of mineral elements in oats (Avena sativa L.) Fresenius Environmental Bulletin. 2013;22(4):979–986. [Google Scholar]
- Wyszkowski M, Sivitskaya V. Changes in the content of organic carbon and available forms of macronutrients in soil under the influence of soil contamination with fuel oil and application of different substances. Journal of Elementology. 2012;17(1):139–148. [Google Scholar]
- Wyszkowski M, Sivitskaya V. Effect of heating oil and neutralizing substances on the content of some trace elements in soil. Fresenius Environmental Bulletin. 2013;22(4):973–978. [Google Scholar]
- Wyszkowski M, Wyszkowska J. The effect of soil contamination with cadmium, chromium and mercury on the yield and content of macroelements in oats. Polish Journal of Natural Sciences. 2004;16(1):123–131. [Google Scholar]
- Wyszkowski M, Wyszkowska J. The content of macroelements in spring barley (Hordeum vulgare L.) and its correlations with the mass of aerial parts of plants and the enzymatic activity of heavy metal contaminated soil. Polish Journal of Environmental Studies. 2006;15(2a):212–221. [Google Scholar]
- Wyszkowski M, Ziółkowska A. Effect of compost, bentonite and calcium oxide on content of some macroelements in plants from soil contaminated by petrol and diesel oil. Journal of Elementology. 2009;14(2):405–418. [Google Scholar]
- Wyszkowski M, Ziółkowska A. Role of compost, bentonite and calcium oxide in restricting the effect of soil contamination with petrol and diesel oil on plants. Chemosphere. 2009;74:860–865. doi: 10.1016/j.chemosphere.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Wyszkowski M, Ziółkowska A. The importance of relieving substances in restricting the effect of soil contamination with oil derivatives on plants. Fresenius Environmental Bulletin. 2011;20(3a):711–719. [Google Scholar]
- Zayed A, Lytle CM, Qian JH, Terry N. Chromium accumulation and chemical speciation in vegetable crops. Planta. 1998;206:293–299. doi: 10.1007/s004250050403. [DOI] [Google Scholar]