Abstract
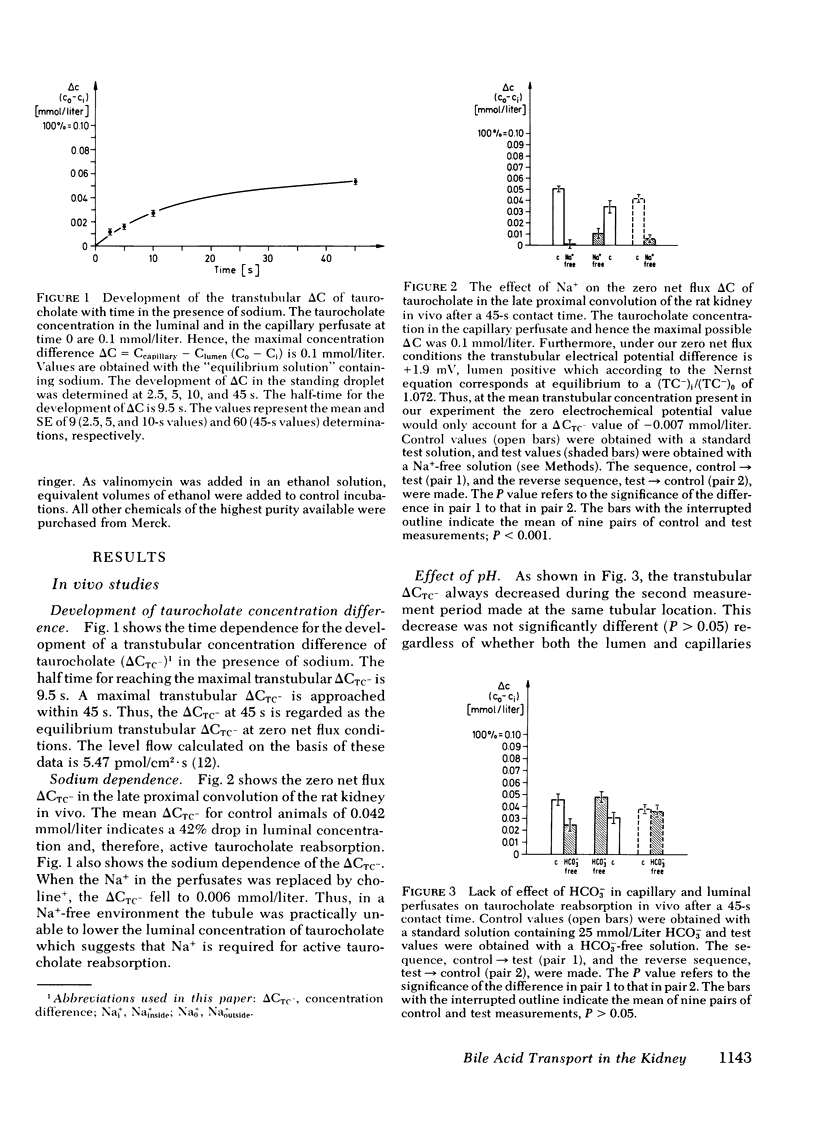
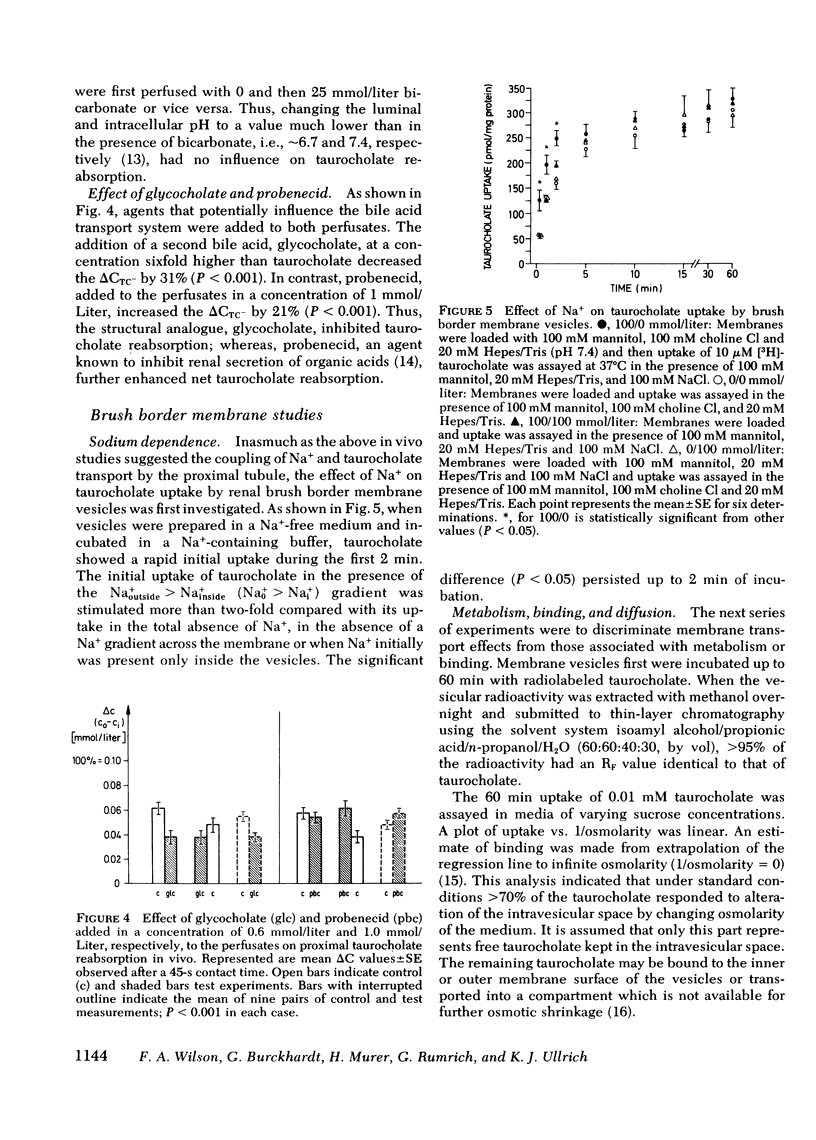
Using the standing droplet technique in the renal proximal convolution and simultaneous microperfusion of the peritubular capillaries, the zero net flux transtubular concentration difference of taurocholate (ΔCTC−) at 45 s was determined as a measure of active bile acid reabsorption in vivo. Starting with 0.1 mmol/liter taurocholate in both perfusates the control ΔCTC− of 0.042 mmol/liter fell to 0.006 mmol/liter (P < 0.001) when the Na+ concentration in the perfusates was reduced to zero. Removal of bicarbonate from the perfusates to alter pH had no influence on ΔCTC−. When glycocholate was added to the perfusates ΔCTC− was decreased, while probenecid increased ΔCTC−.
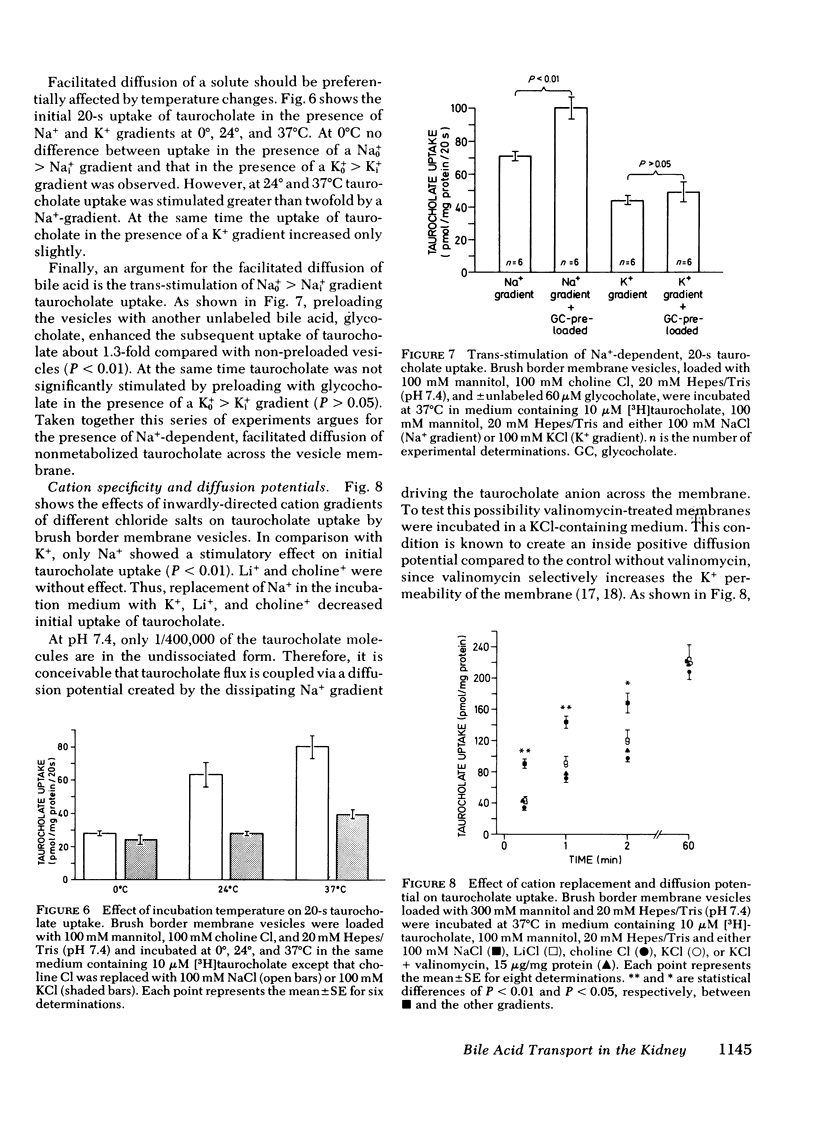
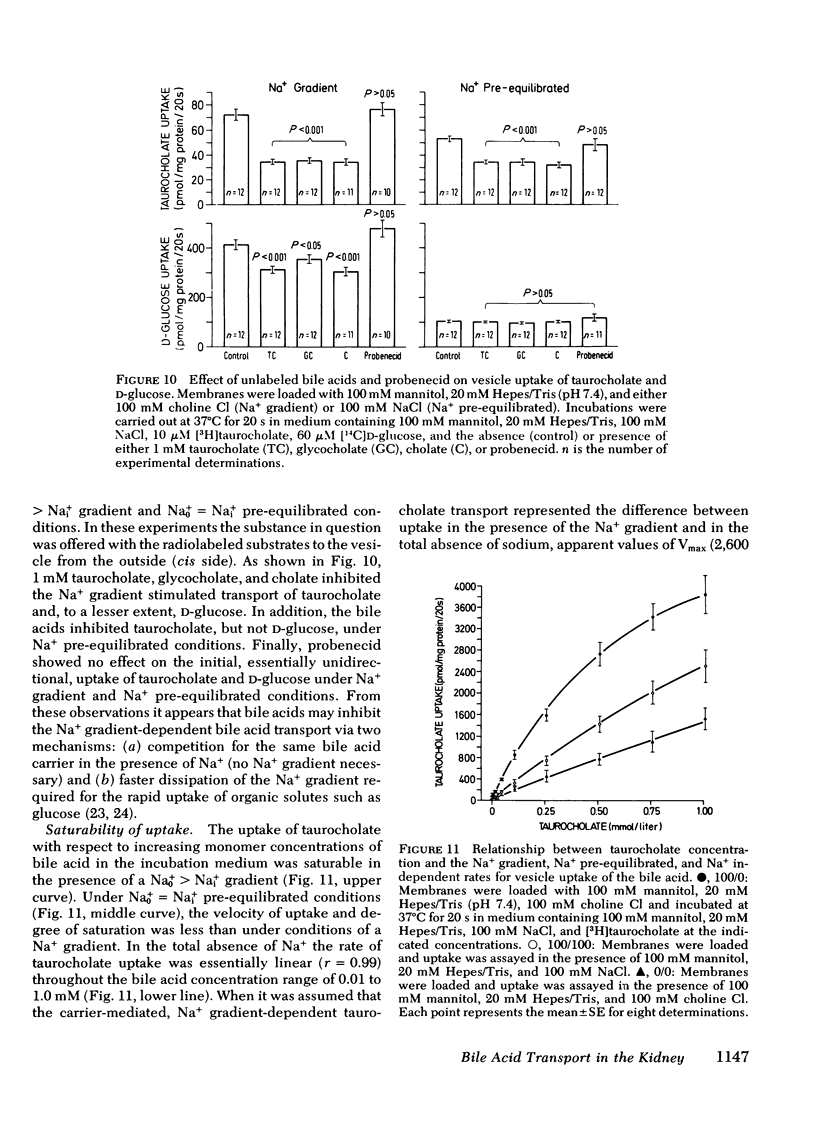
These observations were extended by studies performed with brush border membrane vesicles derived from renal cortex. The initial (20 s) uptake of 0.01 mmol/liter taurocholate in the presence of a Nao+ > Nai+ gradient was stimulated twofold compared with its uptake in the absence of a Na+ gradient. Uptake of taurocholate was osmotically and temperature sensitive. Membranes preloaded with unlabeled glycocholate showed accelerated entry of labeled taurocholate (trans-stimulation) only in the presence of Na+. Replacement of Na+ in the media with K+, Li+, and choline+ decreased initial taurocholate uptake by 49, 53, and 62%, respectively. Stimulation of taurocholate transport by cation gradient diffusion potentials was unlikely inasmuch as the addition of valinomycin under K+ gradient conditions had no effect. A transmembrane pH gradient (pHo < pHi) did not influence initial uptake of taurocholate. Finally, in the presence of Na+ taurocholate transport showed cis-inhibition with unlabeled bile acids and saturation kinetics with respect to increasing taurocholate concentrations. The micropuncture and vesicle data indicate that the net transport of taurocholate in the proximal tubule is the result of an electroneutral Na+-taurocholate cotransport across the brush border membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Beesley R. C., Faust R. G. Sodium ion-coupled uptake of taurocholate by intestinal brush-border membrane vesicles. Biochem J. 1979 Feb 15;178(2):299–303. doi: 10.1042/bj1780299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner W., Kinne R., Murer H. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem J. 1976 Dec 15;160(3):467–474. doi: 10.1042/bj1600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstedt J. W., Aronson P. S. pH gradient-stimulated transport of urate and p-aminohippurate in dog renal microvillus membrane vesicles. J Clin Invest. 1980 Apr;65(4):931–934. doi: 10.1172/JCI109748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy R., Mauskopf J., Walker J. T., Lack L. Interaction of uncharged bile salt derivatives with the ileal bile salt transport system. J Lipid Res. 1977 May;18(3):389–395. [PubMed] [Google Scholar]
- Evers C., Haase W., Murer H., Kinne R. Properties of brush border vesicles isolated from rat kidney cortex by calcium precipitation. Membr Biochem. 1978;1(3-4):203–219. doi: 10.3109/09687687809063848. [DOI] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Gallagher K., Mauskopf J., Walker J. T., Lack L. Ionic requirements for the active ileal bile salt transport system. J Lipid Res. 1976 Nov;17(6):572–577. [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildmann B., Storelli C., Haase W., Barac-Nieto M., Murer H. Sodium ion/L-lactate co-transport in rabbit small-intestinal brush-border-membrane vesicles. Biochem J. 1980 Jan 15;186(1):169–176. doi: 10.1042/bj1860169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt N. B. Diagnostic value of serum bile acids. Clin Gastroenterol. 1977 Jan;6(1):219–226. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lack L., Walker J. T., Hsu C. Y. Taurocholate uptake by membrane vesicles prepared from ileal brush borders. Life Sci. 1977 May 1;20(9):1607–1611. doi: 10.1016/0024-3205(77)90455-6. [DOI] [PubMed] [Google Scholar]
- Lücke H., Stange G., Kinne R., Murer H. Taurocholate--sodium co-transport by brush-border membrane vesicles isolated from rat ileum. Biochem J. 1978 Sep 15;174(3):951–958. doi: 10.1042/bj1740951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U. Demonstration of electrogenic Na+-dependent D-glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):484–488. doi: 10.1073/pnas.71.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Sigrist-Nelson K., Hopfer U. On the mechanism of sugar and amino acid interaction in intestinal transport. J Biol Chem. 1975 Sep 25;250(18):7392–7396. [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. I. Serum bile acids in patients with hepatic disease. J Clin Invest. 1957 Apr;36(4):530–537. doi: 10.1172/JCI103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. II. The binding of cholanic acids by human plasma proteins. J Clin Invest. 1957 Apr;36(4):538–542. doi: 10.1172/JCI103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D. J., Lack L. Ion requirements for taurocholate transport by ileal brush border membrane vesicles. Life Sci. 1979 Jul 2;25(1):45–52. doi: 10.1016/0024-3205(79)90488-0. [DOI] [PubMed] [Google Scholar]
- Sheikh M. I., Møller J. V., Jacobsen C., Maxild J. Effect of hydrocarbon chain length on renal transport of monosubstituted sulfamyl benzoic acid derivatives and probenecid. Biochem Pharmacol. 1979 Aug 15;28(16):2451–2456. doi: 10.1016/0006-2952(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Silverman M. Sugar uptake into brush border vesicles from normal human kidney. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2825–2829. doi: 10.1073/pnas.74.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Phosphate transport in the proximal convolution of the rat kidney II. Effect of extracellular Ca2+ and application of the Ca2+ ionophore A 23187 in chronic PTX animals. Pflugers Arch. 1978 Jun 21;375(1):97–103. doi: 10.1007/BF00584153. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Phosphate transport in the proximal convolution of the rat kidney. I. Tubular heterogeneity, effect of parathyroid hormone in acute and chronic parathyroidectomized animals and effect of phosphate diet. Pflugers Arch. 1977;372(3):269–274. doi: 10.1007/BF01063862. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Phosphate transport in the proximal convolution of the rat kidney. III. Effect of extracellular and intracellular pH. Pflugers Arch. 1978 Oct 18;377(1):33–42. doi: 10.1007/BF00584371. [DOI] [PubMed] [Google Scholar]
- WEINER I. M., GLASSER J. E., LACK L. RENAL EXCRETION OF BILE ACIDS: TAUROCHOLIC, GLYCOCHOLIC, AND COLIC ACIDS. Am J Physiol. 1964 Nov;207:964–970. doi: 10.1152/ajplegacy.1964.207.5.964. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Treanor L. L. Characterization of bile acid binding to rat intestinal brush border membranes. J Membr Biol. 1977 May 12;33(3-4):213–230. doi: 10.1007/BF01869517. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Treanor L. L. Glycodeoxycholate transport in brush border membrane vesicles isolated from rat jejunum and ileum. Biochim Biophys Acta. 1979 Jul 5;554(2):430–440. doi: 10.1016/0005-2736(79)90382-1. [DOI] [PubMed] [Google Scholar]
- Zins G. R., Weiner I. M. Bidirectional transport of taurocholate by the proximal tubule of the dog. Am J Physiol. 1968 Oct;215(4):840–845. doi: 10.1152/ajplegacy.1968.215.4.840. [DOI] [PubMed] [Google Scholar]



