Abstract
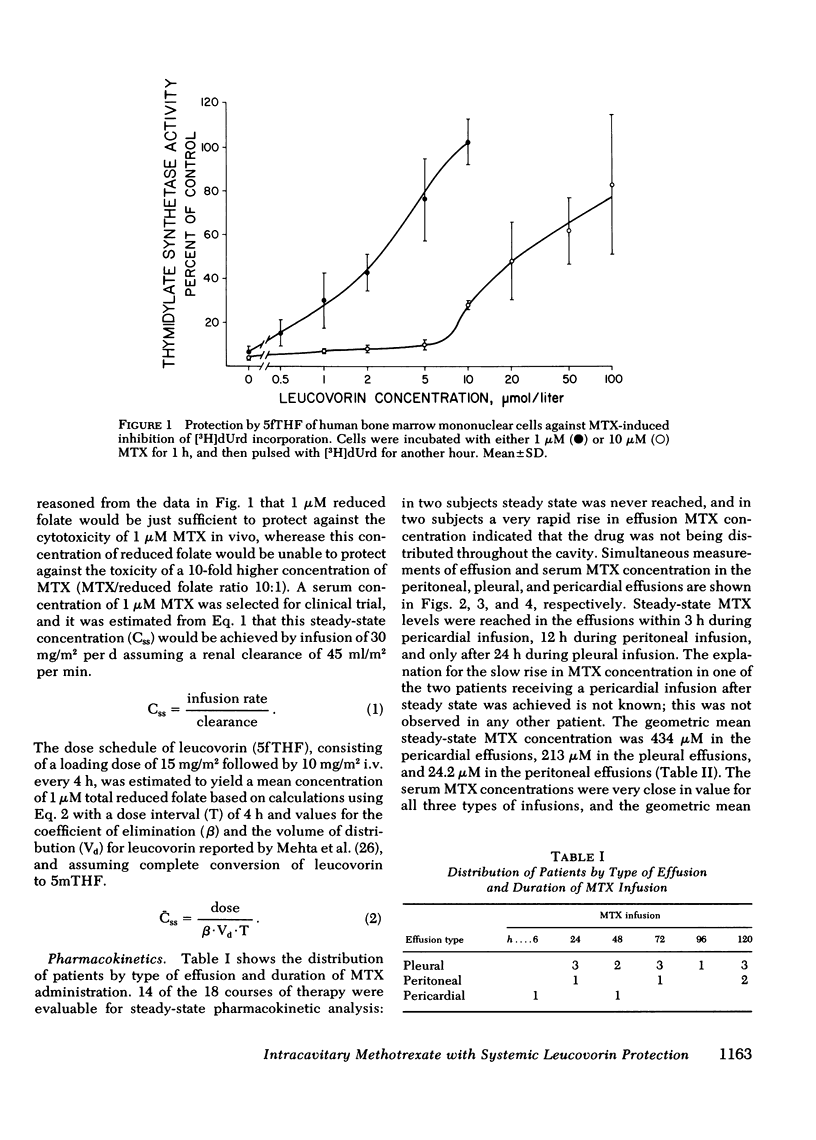
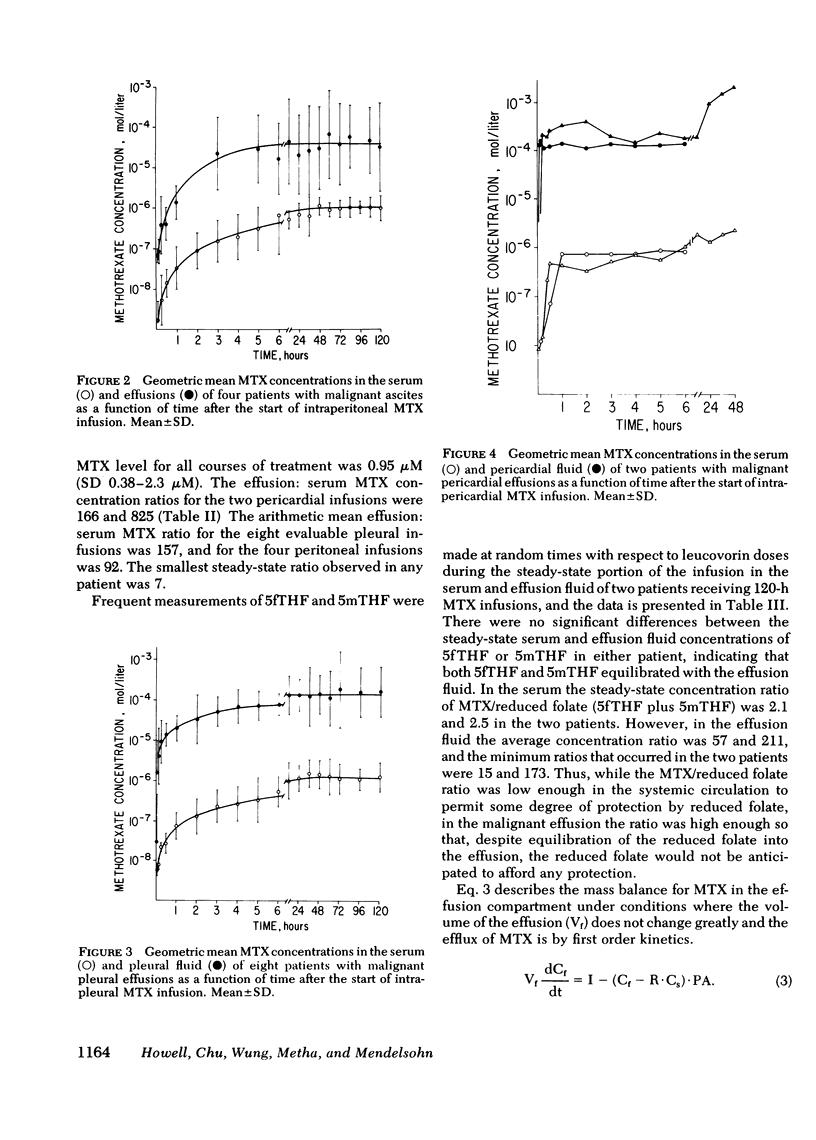
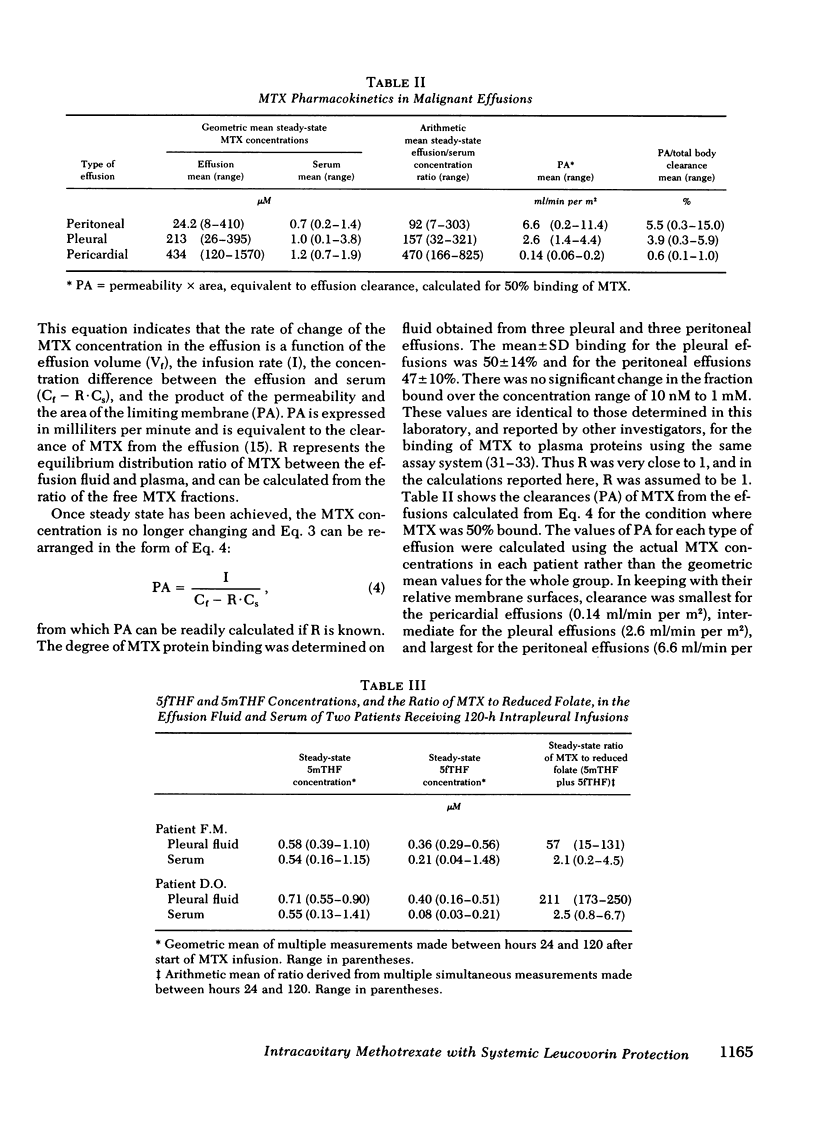
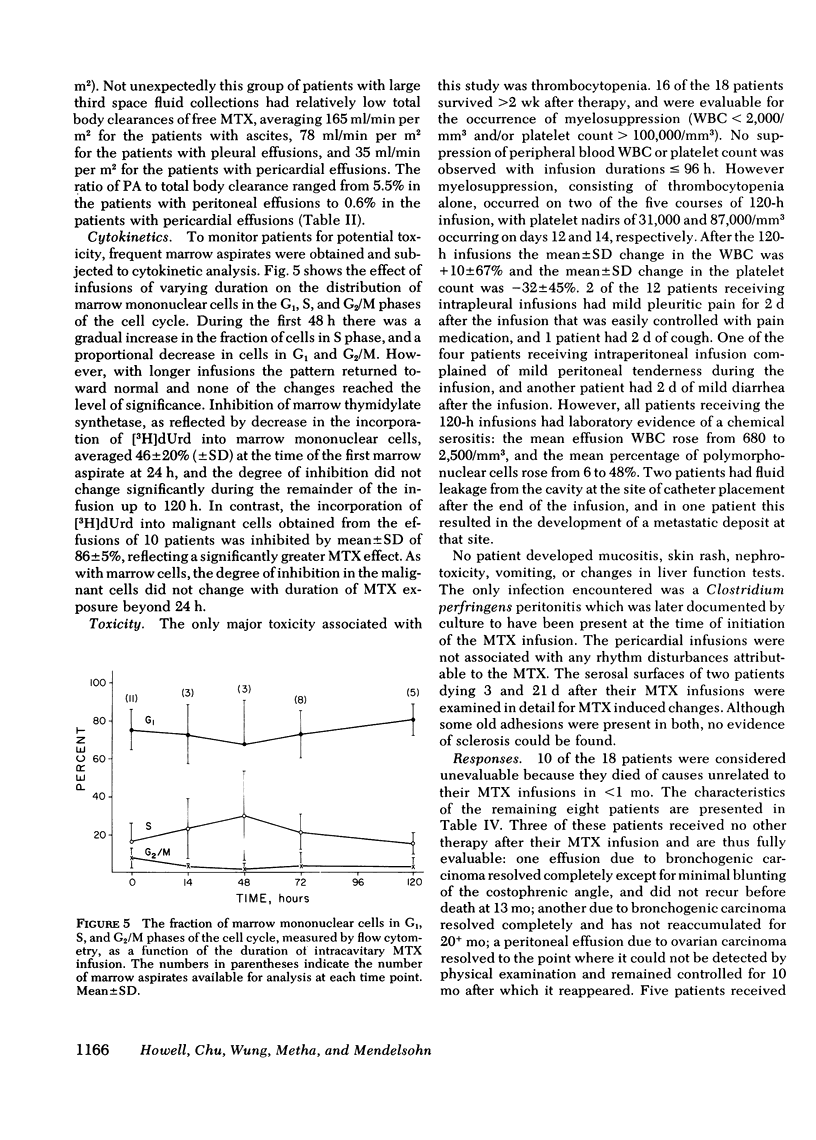
18 patients with malignant effusions were treated with continuous intraperitoneal, intrapleural, or intrapericardial infusion of methotrexate (MTX) 30 mg/m2 per d combined with simultaneous intravenous administration of leucovorin at a dose rate calculated to yield an equimolar concentration in the serum. In the serum the geometric mean steady-state MTX concentration was 0.95 microM, whereas it was 24 microM in the peritoneal, 213 microM in the pleural, and 434 microM in the pericardial cavities. Mean clearance was 6.6 ml/min from the peritoneal cavity, 2.6 ml/min from the pleural cavity, and 0.14 ml/min from the pericardial cavity. Leucovorin provided sufficient protection to allow the duration of infusion to be escalated from 24 to 120 h before myelosuppression was encountered. Marrow thymidylate synthetase activity was inhibited by an average of 46% compared to 86% inhibition in malignant cells in the effusions. Flow cytometric analysis showed no perturbation of the cell cycle phase distribution of marrow cells. All eight of the evaluable patients have responded: three received no other form of therapy, five also received systemic hormonal or chemotherapy. This study demonstrated that tumors confined to third space body fluids can be given very high concentration x time exposures to MTX with minimal systemic toxicity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alabaster O., Tannenbaum E., Habbersett M. C., Magrath I., Herman C. Drug-induced changes in DNA fluorescence intensity detected by flow microfluorometry and their implications for analysis of DNA content distributions. Cancer Res. 1978 Apr;38(4):1031–1035. [PubMed] [Google Scholar]
- Bertino J. R. "Rescue" techniques in cancer chemotherapy: use of leucovorin and other rescue agents after methotrexate treatment. Semin Oncol. 1977 Jun;4(2):203–216. [PubMed] [Google Scholar]
- Bertino J. R., Levitt M., McCullough J. L., Chabner B. New approaches to chemotherapy with folate antagonists: use of leucovorin "rescue" and enzymic folate depletion. Ann N Y Acad Sci. 1971 Nov 30;186:486–495. [PubMed] [Google Scholar]
- Bleyer W. A., Dedrick R. L. Clinical pharmacology of intrathecal methotrexate. I. Pharmacokinetics in nontoxic patients after lumbar injection. Cancer Treat Rep. 1977 Jul;61(4):703–708. [PubMed] [Google Scholar]
- Bleyer W. A., Drake J. C., Chabner B. A. Neurotoxicity and elevated cerebrospinal-fluid methotrexate concentration in meningeal leukemia. N Engl J Med. 1973 Oct 11;289(15):770–773. doi: 10.1056/NEJM197310112891503. [DOI] [PubMed] [Google Scholar]
- Bleyer W. A., Poplack D. G., Simon R. M. "Concentration x time" methotrexate via a subcutaneous reservoir: a less toxic regimen for intraventricular chemotherapy of central nervous system neoplasms. Blood. 1978 May;51(5):835–842. [PubMed] [Google Scholar]
- Bogyo D., Mihich E. Reversal of the in vitro methotrexate suppression of cell-mediated immune response by folinic acid and thymidine plus hypoxanthine. Cancer Res. 1980 Mar;40(3):650–654. [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Studies relating to the mode of action of methotrexate. II. Studies on sites of action in L-cells in vitro. Mol Pharmacol. 1969 Jul;5(4):303–317. [PubMed] [Google Scholar]
- Dedrick R. L., Myers C. E., Bungay P. M., DeVita V. T., Jr Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978 Jan;62(1):1–11. [PubMed] [Google Scholar]
- Eichholtz H., Trott K. R. Effect of methotrexate concentration and exposure time on mammalian cell survival in vitro. Br J Cancer. 1980 Feb;41(2):277–284. doi: 10.1038/bjc.1980.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. M., Woods R. L., Tattersall M. H., Brodie G. M. Allopurinol modulation of high-dose fluorouracil toxicity. Lancet. 1979 Mar 24;1(8117):677–677. doi: 10.1016/s0140-6736(79)91131-0. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Bickers J. N., Hewlett J. S., Lane M., Leary W. V., Talley R. W. Dose schedule and antitumor studies of arabinosyl cytosine (NSC 63878). Cancer Res. 1969 Jul;29(7):1325–1332. [PubMed] [Google Scholar]
- Goldie J. H., Price L. A., Harrap K. R. Methotrexate toxicity: correlation with duration of administration, plasma levels, dose and excretion pattern. Eur J Cancer. 1972 Aug;8(4):409–414. doi: 10.1016/0014-2964(72)90125-9. [DOI] [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- Goldman I. D. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Ann N Y Acad Sci. 1971 Nov 30;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- Groff J. P., Blakley R. L. Rescue of human lymphoid cells from the effects of methotrexate in vitro. Cancer Res. 1978 Nov;38(11 Pt 1):3847–3853. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. B., Chu B., Mendelsohn J., Carson D. A., Kung F. H., Seegmiller J. E. Thymidine as a chemotherapeutic agent: pharmacologic, cytokinetic, and biochemical studies in a patient with T-cell acute lymphocytic leukemia. J Natl Cancer Inst. 1980 Aug;65(2):277–284. [PubMed] [Google Scholar]
- Howell S. B., Herbst K., Boss G. R., Frei E., 3rd Thymidine requirements for the rescue of patients treated with high-dose methotrexate. Cancer Res. 1980 Jun;40(6):1824–1829. [PubMed] [Google Scholar]
- Howell S. B., Krishan A., Frei E., 3rd Cytokinetic comparison of thymidine and leucovorin rescue of marrow in humans after exposure to high-dose methotrexate. Cancer Res. 1979 Apr;39(4):1315–1320. [PubMed] [Google Scholar]
- Hryniuk W. M., Fischer G. A., Bertino J. R. S-phase cells of rapidly growing and resting populations. Differences in response to methotrexate. Mol Pharmacol. 1969 Nov;5(6):557–564. [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Abelson H. T. Resistance of resting 3T6 mouse fibroblasts to methotrexate cytotoxicity. Cancer Res. 1978 Aug;38(8):2408–2412. [PubMed] [Google Scholar]
- Jones R. B., Myers C. E., Guarino A. M., Dedrick R. L., Hubbard S. M., DeVita V. T. High volume intraperitoneal chemotherapy ("belly bath") for ovarian cancer. Pharmacologic basis and early results. Cancer Chemother Pharmacol. 1978;1(3):161–166. doi: 10.1007/BF00253116. [DOI] [PubMed] [Google Scholar]
- Liegler D. G., Henderson E. S., Hahn M. A., Oliverio V. T. The effect of organic acids on renal clearance of methotrexate in man. Clin Pharmacol Ther. 1969 Nov-Dec;10(6):849–857. doi: 10.1002/cpt1969106849. [DOI] [PubMed] [Google Scholar]
- Mehta B. M., Gisolfi A. L., Hutchison D. J., Nirenberg A., Kellick M. G., Rosen G. Serum distribution of citrovorum factor and 5-methyltetrahydrofolate following oral and im administration of calcium leucovorin in normal adults. Cancer Treat Rep. 1978 Mar;62(3):345–350. [PubMed] [Google Scholar]
- Pinedo H. M., Chabner B. A. Role of drug concentration, duration of exposure, and endogenous metabolites in determining methotrexate cytotoxicity. Cancer Treat Rep. 1977 Jul;61(4):709–715. [PubMed] [Google Scholar]
- Pinedo H. M., Zaharko D. S., Bull J. M., Chabner B. A. The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res. 1976 Dec;36(12):4418–4424. [PubMed] [Google Scholar]
- Schwartz P. M., Handschumacher R. E. Selective antagonism of 5-fluorouracil cytotoxicity by 4-hydroxypyrazolopyrimidine (allopurinol) in vitro. Cancer Res. 1979 Aug;39(8):3095–3101. [PubMed] [Google Scholar]
- Shackney S. E., McCormack G. W., Cuchural G. J., Jr Growth rate patterns of solid tumors and their relation to responsiveness to therapy: an analytical review. Ann Intern Med. 1978 Jul;89(1):107–121. doi: 10.7326/0003-4819-89-1-107. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Dorick D. M. Optimization of high-dose methotrexate with leucovorin rescue therapy in the L1210 leukemia and sarcoma 180 murine tumor models. Cancer Res. 1978 Feb;38(2):345–353. [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Wilcox W. S. Experimental evaluation of potential anticancer agents. XXI. Scheduling of arabinosylcytosine to take advantage of its S-phase specificity against leukemia cells. Cancer Chemother Rep. 1967 Jun;51(3):125–165. [PubMed] [Google Scholar]
- Speyer J. L., Collins J. M., Dedrick R. L., Brennan M. F., Buckpitt A. R., Londer H., DeVita V. T., Jr, Myers C. E. Phase I and pharmacological studies of 5-fluorouracil administered intraperitoneally. Cancer Res. 1980 Mar;40(3):567–572. [PubMed] [Google Scholar]
- Straus M. J., Moran R. E. Cell cycle parameters in human solid tumors. Cancer. 1977 Oct;40(4):1453–1461. doi: 10.1002/1097-0142(197710)40:4<1453::aid-cncr2820400416>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Ohnuma T., Holland J. F. A comparison of the biological effects of dichloromethotrexate and methotrexate on human leukemic cells in culture. Cancer Res. 1979 Apr;39(4):1264–1268. [PubMed] [Google Scholar]
- Tannenbaum E., Cassidy M., Alabaster O., Herman C. Measurement of cellular DNA mass by flow microfluorometry with use of a biological internal standard. J Histochem Cytochem. 1978 Feb;26(2):145–148. doi: 10.1177/26.2.624835. [DOI] [PubMed] [Google Scholar]
- Taylor J. R., Halprin K. M. Effect of sodium salicylate and indomethacin on methotrexate-serum albumin binding. Arch Dermatol. 1977 May;113(5):588–591. [PubMed] [Google Scholar]
- Terz J. J., Lawrence W., Jr, Cox B. Analysis of the cycling and noncycling cell population of human solid tumors. Cancer. 1977 Oct;40(4):1462–1470. doi: 10.1002/1097-0142(197710)40:4<1462::aid-cncr2820400417>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]



