Abstract
Although the liver is the major site of erythropoietin (Ep) production in the fetus, this function is assumed by kidneys in the adult. The mechanisms underlying the liver to kidney switch of Ep formation are not understood. We studied the natural progression of this transition in sheep by measuring Ep production in response to anemia in normal and bilaterally nephrectomized fetal and newborn sheep beginning at about 80 d gestation (normal gestation: 140 d). Removal of both kidneys before induction of anemia did not affect Ep formation up to about 120 d of gestation. A significant reduction (29%, P < 0.02) in Ep synthesis was first noted at about 130 d of gestation (initiation of switch). This level of nephrectomy-induced reduction of Ep formation persisted until about 15 d after birth. Thereafter, bilateral nephrectomy caused further significant decreases (P < 0.05) in Ep production, gradually resulting in near total absence of Ep production at about day 40 postpartum (completion of switch). Chronic administration of testosterone (12 mg/wk) or estradiole benzoate (1.5 mg/d, 5 d/wk) to the fetus/newborn beginning at 85-90 d of gestation enhanced or suppressed erythropoiesis, respectively, but failed to affect the time at which the liver to kidney switch was initiated and/or completed. By contrast, a significant delay (P < 0.001) in the onset, but not completion of the switch occurred in animals that were either thyroidectomized or rendered chronically anemic beginning in the second third of the gestation period. Administration of thyroxin (1.2 mg/d, 5 d/wk) to thyroidectomized fetus/newborns not only prevented the delay in the initiation of the switch, but also accelerated the rate at which the switch was completed. These results demonstrate that in sheep (a) the liver to kidney switch of Ep production is initiated in utero during the last third of the gestation period, but is completed after birth, (b) this transition occurs gradually; the assumption of Ep producing capacity by the kidney is not preceded by an abrupt loss of hepatic Ep formation; and (c) the switch is not affected by changes in sex hormone levels during the prenatal-postnatal growth periods, but is profoundly influenced by alterations in thyroid hormone and oxygen supply-demand levels.
Full text
PDF

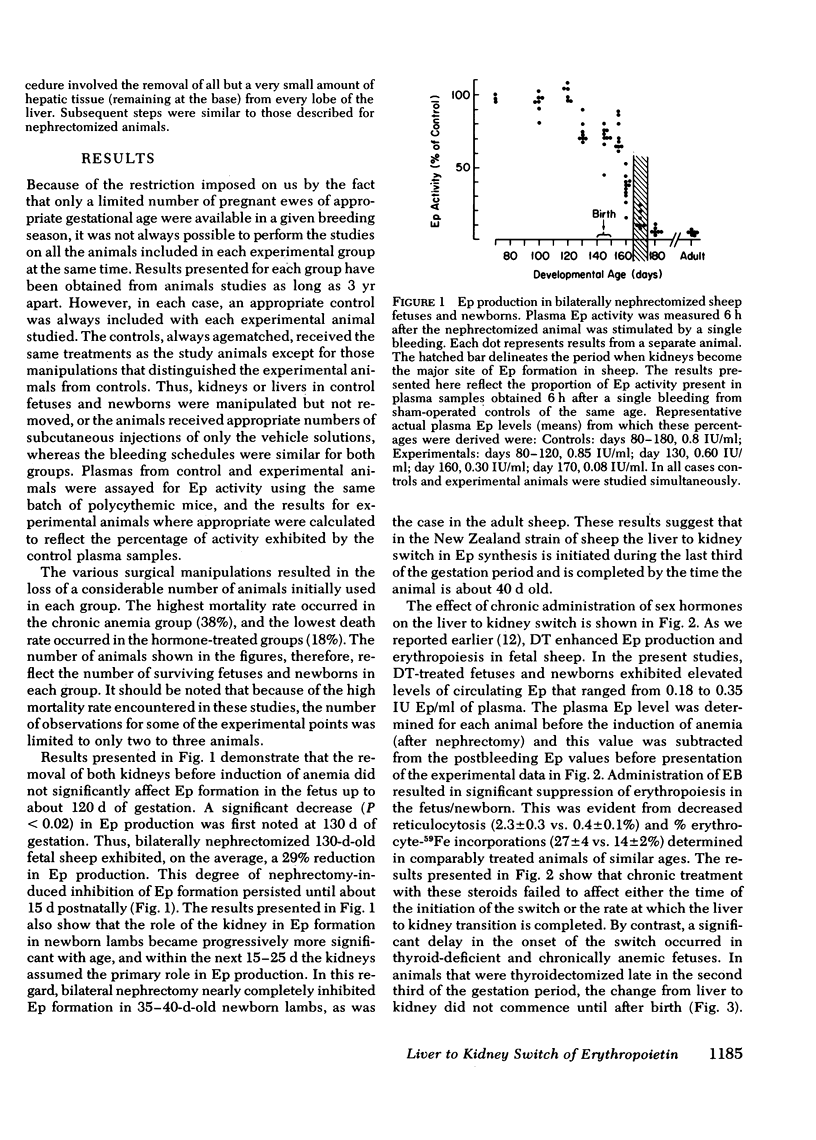
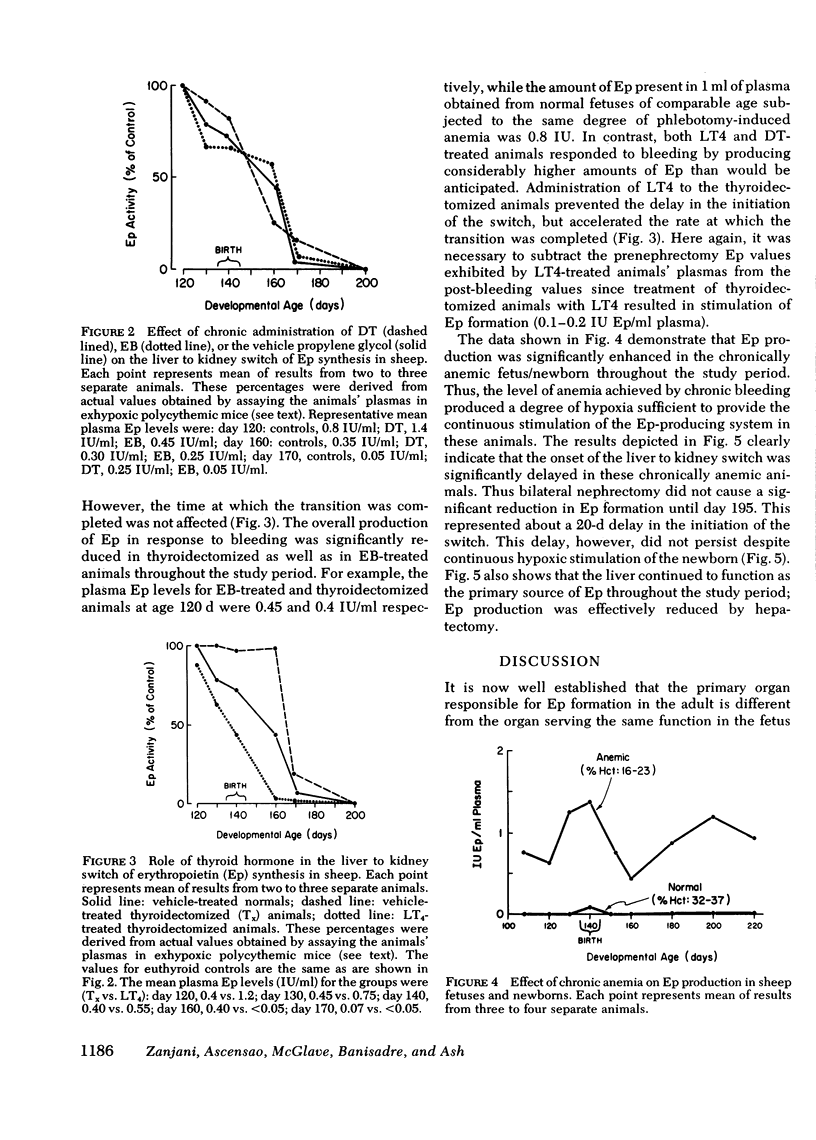
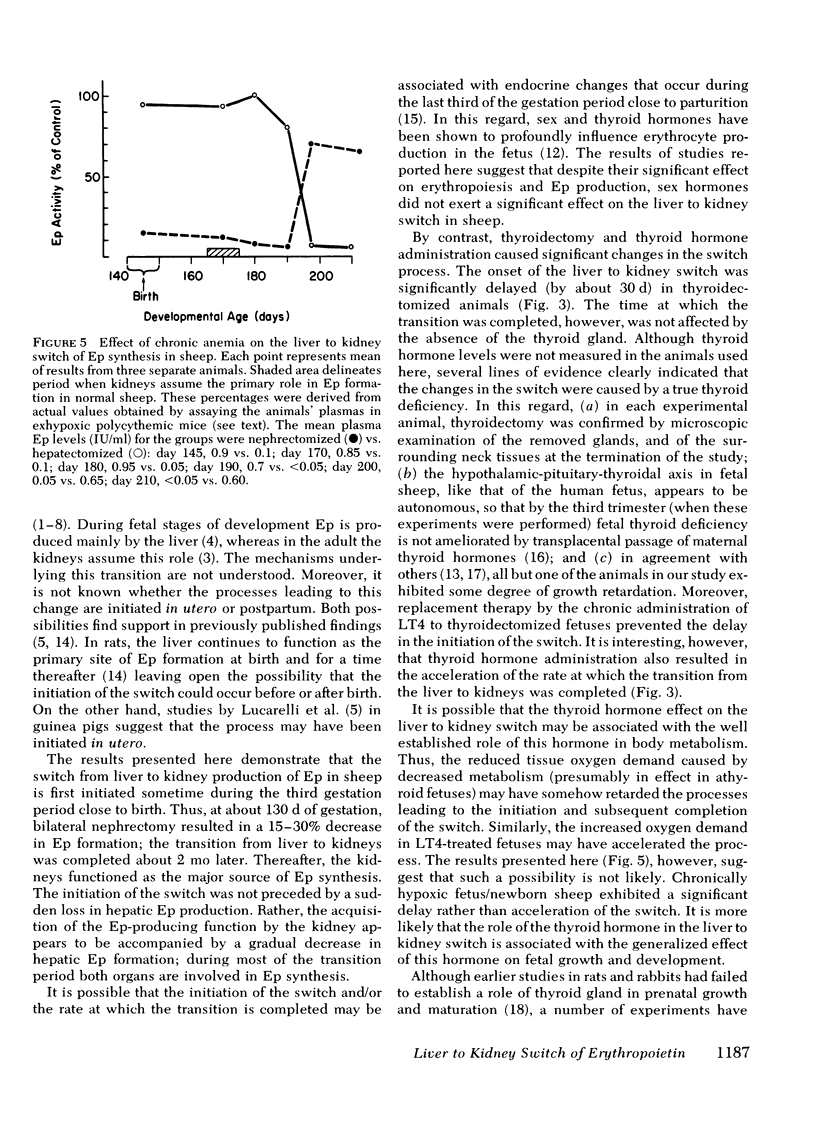

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakthavathsalan A., Mann L. I., Ayromlooi J., Kunzel W., Liu M. The effects of fetal thyroidectomy in the ovine fetus. Am J Obstet Gynecol. 1977 Feb 1;127(3):278–284. doi: 10.1016/0002-9378(77)90469-0. [DOI] [PubMed] [Google Scholar]
- Challis J. R., Thorburn G. D. Prenatal endocrine function and the initiation of parturition. Br Med Bull. 1975 Jan;31(1):57–61. doi: 10.1093/oxfordjournals.bmb.a071242. [DOI] [PubMed] [Google Scholar]
- Creasy R. K., Drost M., Green M. V., Morris J. A. Determination of fetal, placental and neonatal blood volumes in the sheep. Circ Res. 1970 Oct;27(4):487–494. doi: 10.1161/01.res.27.4.487. [DOI] [PubMed] [Google Scholar]
- Erslev A. J., Caro J., Kansu E., Silver R. Renal and extrarenal erythropoietin production in anaemic rats. Br J Haematol. 1980 May;45(1):65–72. doi: 10.1111/j.1365-2141.1980.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Fried W. The liver as a source of extrarenal erythropoietin production. Blood. 1972 Nov;40(5):671–677. [PubMed] [Google Scholar]
- Gordon A. S. Erythropoietin. Vitam Horm. 1973;31:105–174. doi: 10.1016/s0083-6729(08)60997-8. [DOI] [PubMed] [Google Scholar]
- JACOBSON L. O., GOLDWASSER E., FRIED W., PLZAK L. Role of the kidney in erythropoiesis. Nature. 1957 Mar 23;179(4560):633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- LUCARELLI G., HOWARD D., STOHLMAN F., Jr REGULATION OF ERYTHROPOIESIS. XV. NEONATAL ERYTHROPOIESIS AND THE EFFECT OF NEPHRECTOMY. J Clin Invest. 1964 Nov;43:2195–2203. doi: 10.1172/JCI105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli G., Porcellini A., Carnevali C., Carmena A., Stohlman F., Jr Fetal and neonatal erythropoiesis. Ann N Y Acad Sci. 1968 Mar 29;149(1):544–559. doi: 10.1111/j.1749-6632.1968.tb15194.x. [DOI] [PubMed] [Google Scholar]
- Schooley J. C., Mahlmann L. J. Erythropoietin production in the anephric rat. I. Relationship between nephrectomy, time of hypoxic exposure, and erythropoietin production. Blood. 1972 Jan;39(1):31–38. [PubMed] [Google Scholar]
- Zanjani E. D., Banisadre M. Hormonal stimulation of erythropoietin production and erythropoiesis in anephric sheep fetuses. J Clin Invest. 1979 Nov;64(5):1181–1187. doi: 10.1172/JCI109571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani E. D., Horger E. O., 3rd, Gordon A. S., Cantor L. N., Hutchinson D. L. Erythropoietin production in the fetal lamb. J Lab Clin Med. 1969 Nov;74(5):782–788. [PubMed] [Google Scholar]
- Zanjani E. D., Lutton J. D., Hoffman R., Wasserman L. R. Erythroid colony formation by polycythemia vera bone marrow in vitro. Dependence on erythropoietin. J Clin Invest. 1977 May;59(5):841–848. doi: 10.1172/JCI108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani E. D., Mann L. I., Burlington H., Gordon A. S., Wasserman L. R. Evidence for a physiologic role of erythropoietin in fetal erythropoiesis. Blood. 1974 Aug;44(2):285–290. [PubMed] [Google Scholar]
- Zanjani E. D., Peterson E. N., Gordon A. S., Wasserman L. R. Erythropoietin production in the fetus: role of the kidney and maternal anemia. J Lab Clin Med. 1974 Feb;83(2):281–287. [PubMed] [Google Scholar]
- Zanjani E. D., Poster J., Burlington H., Mann L. I., Wasserman L. R. Liver as the primary site of erythropoietin formation in the fetus. J Lab Clin Med. 1977 Mar;89(3):640–644. [PubMed] [Google Scholar]



