Abstract
The reduction in the expression of glucose-responsive insulin gene transcription factor MafA accompanies the development of β-cell dysfunction under oxidative stress/diabetic milieu. Humans with type 2 diabetes have reduced MafA expression, and thus preventing this reduction could overcome β-cell dysfunction and diabetes. We previously showed that p38 MAPK, but not glycogen synthase kinase 3 (GSK3), is a major regulator of MafA degradation under oxidative stress. Here, we examined the mechanisms of this degradation and whether preventing MafA degradation under oxidative stress will overcome β-cell dysfunction. We show that under oxidative and nonoxidative conditions p38 MAPK directly binds to MafA and triggers MafA degradation via ubiquitin proteasomal pathway. However, unlike nonoxidative conditions, MafA degradation under oxidative stress depended on p38 MAPK-mediated phosphorylation at threonine (T) 134, and not T57. Furthermore the expression of alanine (A) 134-MafA, but not A57-MafA, reduced the oxidative stress-mediated loss of glucose-stimulated insulin secretion, which was independent of p38 MAPK action on protein kinase D, a regulator of insulin secretion. Interestingly, the expression of proteasomal activator PA28γ that degrades GSK3-phosphorylated (including T57) MafA was reduced under oxidative stress, explaining the dominance of p38 MAPK over the GSK3 pathway in regulating MafA stability under oxidative stress. These results identify two distinct pathways mediating p38 MAPK-dependent MafA degradation under oxidative and nonoxidative conditions and show that inhibiting MafA degradation under oxidative stress ameliorates β-cell dysfunction and could lead to novel therapies for diabetes.
Cloning of the glucose-responsive insulin gene transcription factor RIPE3b1 as MafA (1) initiated a series of studies on the role of MafA and Maf factors in the differentiation and function of pancreatic endocrine cells. As a result, it became increasingly evident that MafA regulates function, but not the specification, of β-cells (2–5). MafA-deficient mice were born with normal-looking pancreatic islets, but the ratio of β- to α-cells gradually decreased, resulting in glucose intolerance and diabetes by 8–12 weeks (4). A similar loss of β-cell function and altered islet architecture was reported for pancreas-specific MafA knockout mice (5). Reduced MafA expression was found after 90% pancreatectomy and in the db/db mice, the animal models of oxidative stress mediated β-cell dysfunction, and this expression was rescued upon reversal of diabetes (6, 7). It is important to note that a recent study demonstrated a similar reduction in MafA staining in pancreatic β-cells from type 2 diabetic individuals (8). Similarly, MafA expression is also associated with maturity of β-cells. Immature β-cells derived from human embryonic stem cells lacked MafA expression (9), but after transplantation in mice they acquired both glucose responsiveness and MafA expression (10). Increasing MafA expression in insulinoma cell line INS1 enhanced insulin synthesis and secretion as well as the expression of several key regulators of glucose-stimulated insulin secretion (GSIS) (11). Furthermore, MafA expression is low during the early neonatal period (12) when β-cells have not yet acquired normal GSIS (13), and its expression increases as β-cells acquire glucose responsiveness (12). Enhancing MafA expression in immature neonatal β-cells increased the amount of insulin secreted by individual β-cells in response to glucose (12), further demonstrating a role of MafA in their maturation and function. Together these results support the concept that MafA is an important regulator of β-cell maturation and function, and its loss accompanies impaired β-cell function and diabetes. Thus, developing strategies to enhance MafA expression in β-cells would have potential therapeutic benefits for the treatment of diabetes.
MafA is a highly phosphorylated protein, and kinases, such as p38 MAPK, ERK1/2, and glycogen synthase kinase 3 (GSK3), regulate its phosphorylation and degradation (6, 14–19). Previously, we showed that MafA stability was regulated by both p38 MAPK and GSK3 under nonoxidative conditions in insulin-producing MIN6 cells as well as in mouse islets (6). Furthermore, the presence of either threonine (T) 57 or T134 was sufficient for p38 MAPK-mediated MafA degradation under nonoxidative conditions, and only simultaneously substituting both threonines to alanines (A) prevented this degradation (6). Additionally, in animal models of hyperglycemia and oxidative stress, we showed that MafA protein and RNA were reduced in the pancreatic islets. Because it is difficult to perform a detailed mechanistic study in pancreatic islets, we used an established β-cell culture system to characterize the molecular mechanisms underlying the degradation of MafA under oxidative stress and determined the consequences of preventing this degradation on β-cell function. In this study, we show that p38 MAPK binds to MafA protein and regulates its degradation via the ubiquitin proteasomal pathway (UPP). We also demonstrate that p38 MAPK differentially regulates MafA stability in the absence and presence of oxidative stress. In contrast to nonoxidative conditions in which phosphorylation of either T57 or T134 triggered MafA degradation, in the presence of oxidative stress, phosphorylation of only T134, and not T57, regulated p38 MAPK-dependent MafA degradation. Furthermore, MafA with T134 to A134 substitution, and not T57 to A57, reduced the oxidative stress-dependent inhibition of GSIS, independent of protein kinase D (PKD) activation. Thus, our results demonstrate the presence of multiple pathways that regulate MafA stability and provide a novel approach for enhancing MafA expression to overcome β-cell dysfunction in the presence of oxidative stress.
Results
Threonine 57 (T57) in MafA is phosphorylated by p38 MAPK in insulin-producing cells
Overexpression of avian MafA and p38 MAPK in human embryonic kidney 293T cells resulted in phosphorylation of MafA at T57, T113 (mammalian equivalent of T134), and S272 (18). Previously, we showed that p38 MAPK regulates mammalian MafA protein stability through phosphorylation of T57 and T134 under nonoxidative conditions (6). However, under this condition MafA is also phosphorylated at T57 by GSK3, a major regulator of MafA stability under nonoxidative conditions (14, 15, 19) that might primarily be responsible for T57 phosphorylation under nonoxidative conditions. To test whether, independent of GSK3 activity, p38 MAPK can phosphorylates T57 in β-cells under nonoxidative conditions, we generated MafA with mutation in the GSK3 priming kinase site [serine (S)65 to alanine (A)65] that inhibits the ability of GSK3 to phosphorylate S61, T57, T53, and S49 in MafA, and the subsequent MafA degradation (14, 15).
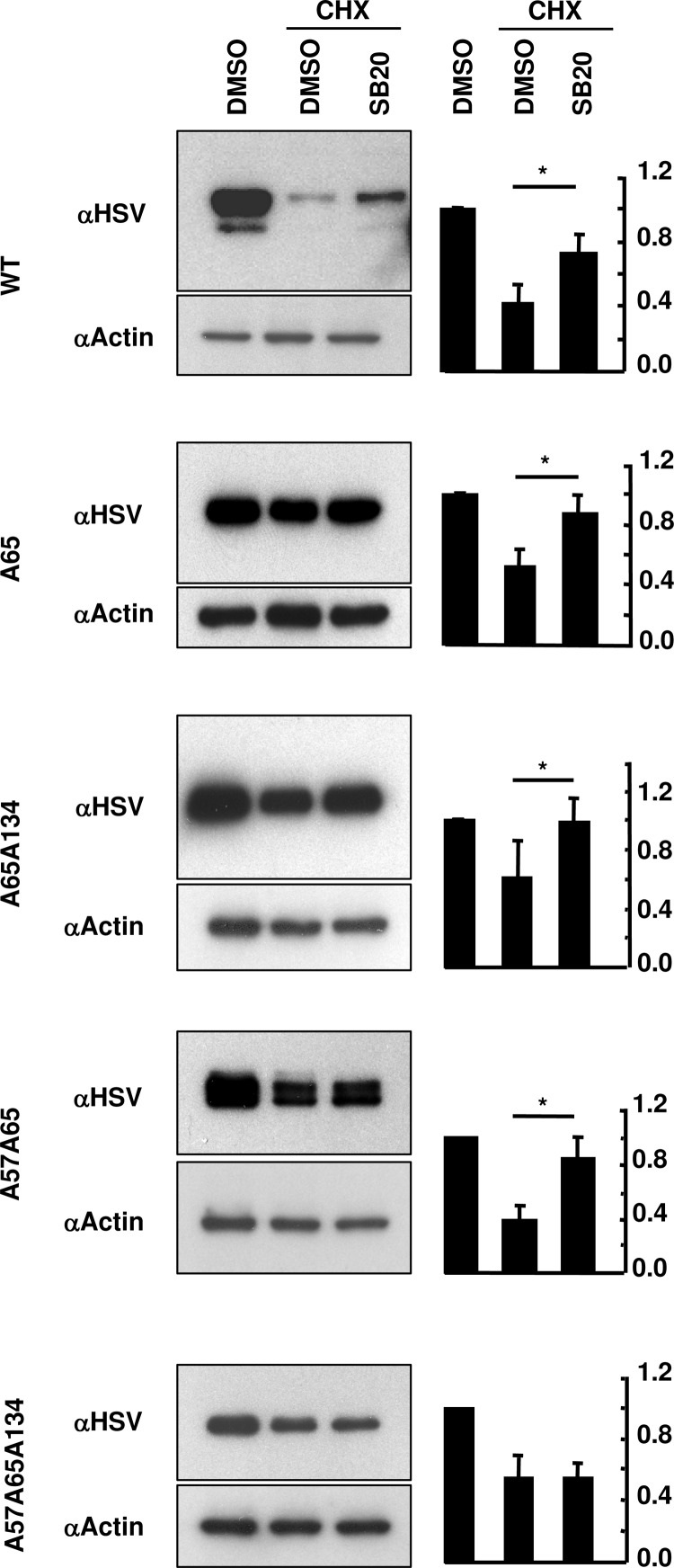
MIN6 cells transfected with Herpes simplex virus (HSV)-tagged wild-type (WT) MafA, MafA with mutations in priming kinase site alone (A65), or with the p38 MAPK targets T57 and T134 (A65A134, A57A65, or A57A65A134), were cultured in the presence or absence of cycloheximide (CHX), a protein synthesis inhibitor, and p38 MAPK inhibitor SB203580 (SB20) for the last 16 hours. Cell extracts were immunoblotted using anti-HSV antibody to determine MafA protein stability (Figure 1). If phosphorylation of T57MafA was entirely dependent on GSK3 or on the phosphorylation of S61 or S65, then A65 mutation should prevent phosphorylation at T57. In such a case, cells transfected with A65A134-MafA would have both T57 and A134 amino acids unavailable for phosphorylation, making A65A134-MafA resistant to p38 MAPK-mediated degradation. However, if p38 MAPK phosphorylates T57 independent of GSK3 or its priming kinase activity, cells transfected with A65A134-MafA would have unphosphorylated A134 but still have T57 available for p38 MAPK-dependent phosphorylation and degradation. Inhibition of p38 MAPK activity rescued degradation of WT-, A65-, and A65A57-, but it did not rescue A57A65A134-MafA (Figure 1). Inhibition of p38 MAPK also rescued degradation of A65A134-MafA (Figure 1) showing that in insulin-producing cells T57 is phosphorylated by p38 MAPK independent of GSK3 or its priming kinase activity.
Figure 1.
p38 MAPK mediates MafA degradation via threonine 57 independent of GSK3 priming kinase activity. MIN6 cells transfected with WT-MafA, or MafA expression plasmids with mutations in priming kinase (S65) and p38 MAPK (T57, T134) target sites (A65, A57A65, A65A134, or A57A65A134) were cultured in 0.8 mM glucose for the last 16 hours in the presence of indicated combination of 50 μM CHX, DMSO, and 20 μM SB203580 (SB20). Representative gels immunoblotted with α-HSV and α-actin antibodies are shown for each MafA:HSV expression plasmid. Graphs on the right present results from quantification of band intensities from at least 3 independent experiments. Results are presented relative to the expression of respective MafA derivative in the presence of DMSO alone as 1 ± SEM. *, P < .05.
p38 MAPK-mediated degradation of MafA occurs via ubiquitin proteasomal pathway
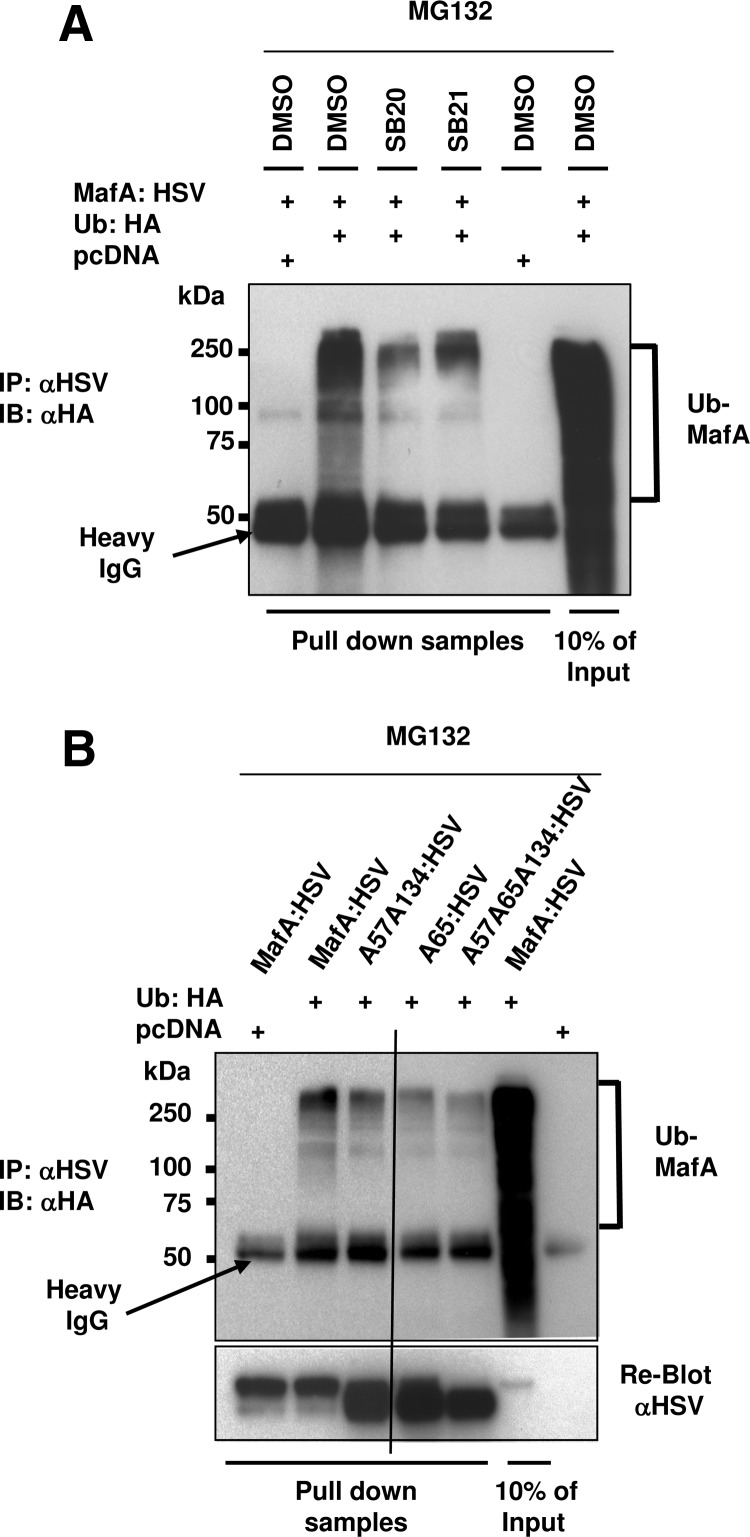
Because phosphorylation of MafA by GSK3 triggers its degradation via the ubiquitin proteasomal pathway (UPP) (15), we examined whether p38 MAPK also activates MafA degradation through this pathway. MIN6 cells cotransfected with MafA:HSV and either hemagglutinin (HA)-tagged Ubiquitin (Ub:HA) or pcDNA (control) plasmids were treated with dimethylsulfoxide (DMSO), p38 MAPK inhibitor SB20, or GSK3 inhibitor SB216763 (SB21) in the presence of proteasomal inhibitor MG132 to increase the accumulation of ubiquitinated proteins. Ubiquitinated MafA was detected by immunoprecipitating whole-cell extracts with anti-HSV antibody, followed by immunoblotting with anti-HA antibody (Figure 2A). As reported earlier (15), in the presence of MG132, a significant amount of ubiquitinated-MafA (Ub-MafA) was present in DMSO-treated MIN6 cells, and the presence of GSK3 inhibitor reduced this accumulation. We observed that SB20 also drastically reduced the Ub-MafA accumulation (Figure 2A), demonstrating that p38 MAPK-mediates MafA degradation via the UPP.
Figure 2.
Under nonoxidative conditions p38 MAPK mediates MafA degradation through the proteasomal pathway. A, MIN6 cells cotransfected with pcDNA or WT-MafA:HSV along with Ub:HA expression plasmids were cultured in 0.8 mM glucose for the last 16 hours in the presence of indicated combination of DMSO, 20 μM SB203580 (SB20), 20 μM SB216763 (SB21), and 10 μM MG132. Whole-cell extracts were immunoprecipitated (IP) with α-HSV antibody followed by immunoblotting (IB) with α-HA antibody. A representative gel from at least 3 independent experiments is shown. B, MIN6 cells cotransfected with pcDNA, WT-MafA:HSV, A57A134-, A65-, or A57A65A134-HSV-tagged MafA and Ub:HA expression plasmids were cultured in 0.8 mM glucose for the last 16 hours in the presence of 10 μM MG132. Whole-cell extracts were immunoprecipitated with α-HSV antibody followed by immunoblotting with α-HA antibody. The reblot with α-HSV shows correct mobility and levels of different MafA derivatives. A representative gel from at least 3 independent experiments is shown.
To confirm that phosphorylation at T57 and T134 was involved in UPP-mediated MafA degradation, MIN6 cells cotransfected with HSV-tagged WT-, A57A134-, A65-, or A57A65A134-MafA expression plasmids and either pcDNA or Ub:HA plasmids were treated and analyzed as above. As in Figure 2A, inhibition of the proteasomal activity led to accumulation of ubiquitinated WT-MafA (Figure 2B). However, the proportion of Ub-MafA was drastically reduced for MafA with mutations in p38 MAPK targets (A57A134), in GSK3 priming kinase site (S65A), and in those that blocked both priming kinase and p38 MAPK activities (A57A65A134) and thus are resistant to both GSK3- and p38 MAPK-mediated degradation. The reblot with anti-HSV antibody indicated appropriate mobility and accumulation of MafA derivatives in the immunoprecipitated samples. This series of experiments combined with results in Figure 1 demonstrates that p38 MAPK-mediated phosphorylation of MafA at T57 and T134 triggers its degradation via the UPP.
p38 MAPK physically interacts with MafA
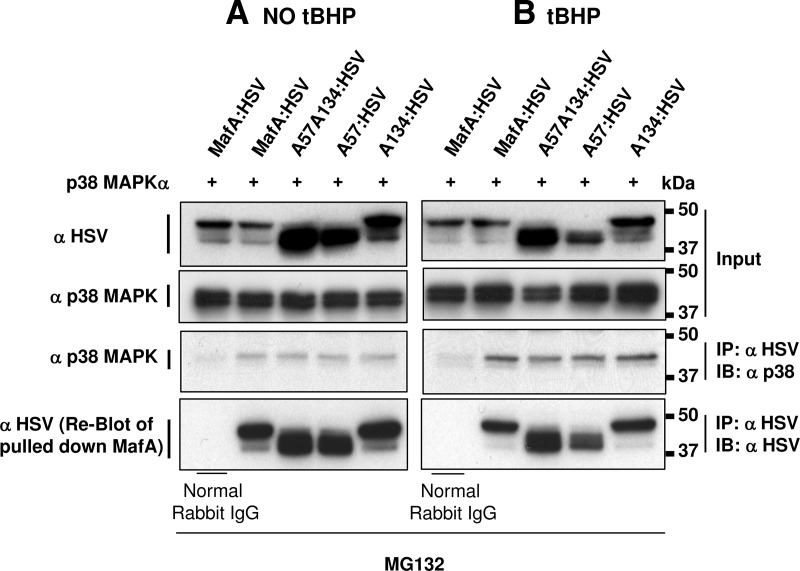
MIN6 cells cotransfected with p38 MAPKα expression plasmid and either WT-MafA or MafA plasmids with mutation(s) in p38 MAPK phosphorylation site(s) (A57A134, A57, or A134) were cultured in presence of MG132, and the coimmunoprecipitation of p38 MAPK with MafA was examined. No band corresponding to p38 MAPK was pulled down in control rabbit IgG immunoprecipitate when immunoblotted with anti-p38 MAPK antibody. However, a 41-kDa band, corresponding to p38 MAPK, was detected in extracts of cells transfected with WT- and mutant-MafA derivatives immunoprecipitated with anti-HSV antibody, supporting a direct interaction between p38 MAPK and MafA (Figure 3A).
Figure 3.
p38 MAPK directly interacts with MafA. MIN6 cells cotransfected with recombinant p38 MAPKα and each of the following HSV-tagged WT-, A57-, A134-, or A57A134-MafA expression plasmids were cultured in the absence (A) or presence of 100 μM tBHP (B) and 10 μM of MG132 during the last 6 hours. Whole-cell extracts were immunoprecipitated with α-HSV or normal rabbit IgG antibodies followed by immunoblotting with αp38 MAPK antibody (third row). The bottom row shows the reblots using α-HSV antibody. The first and second rows represent 10% of input samples used for immunoprecipitation (IP) experiments, immunoblotted (IB) with α-HSV and αp38 MAPK antibody, respectively, to detect the amount of MafA and p38 MAPK in input. Representative gels from 2–3 independent experiments are shown.
Next, using the same approach, we examined whether p38 MAPK directly interacts with MafA in the presence of oxidant tert-butyl hydroperoxide (tBHP). Under oxidative stress, p38 MAPK (41-kDa band) was coprecipitated with WT and all MafA derivatives (Figure 3B). Although, immunoprecipitation results are not quantitative in nature, the results clearly show a slightly higher level of p38 MAPK input, and immunoprecipitated p38 MAPK (row 3) in the presence of oxidative stress. However, slightly lower amounts of MafA derivatives (row 4) were pulled down under oxidative (Figure 3B) compared with nonoxidative conditions (Figure 3A). These observations suggest that a higher proportion of MafA may bind to p38 MAPK under oxidative stress.
p38 MAPK regulates degradation of endogenous MafA under oxidative stress
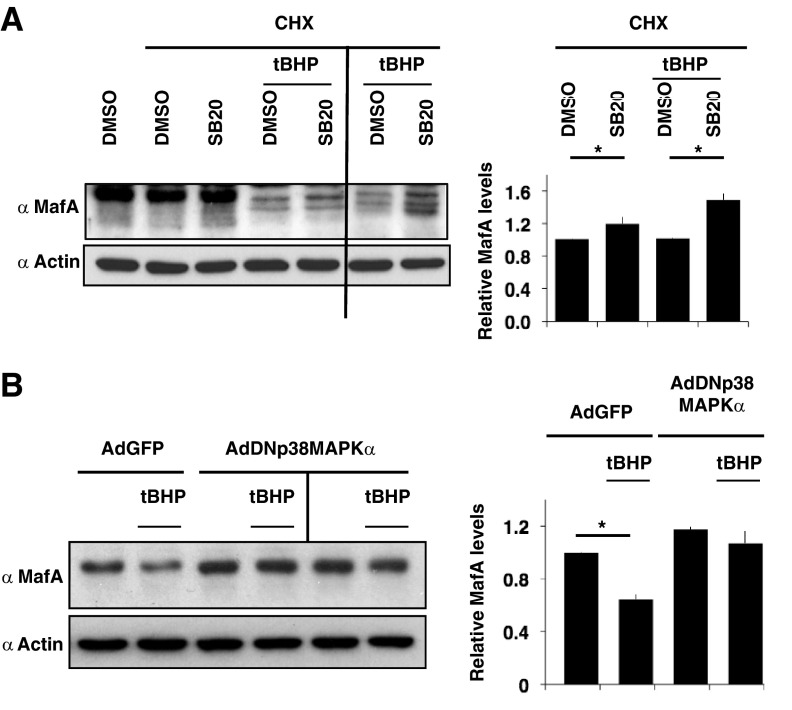
To confirm that oxidative stress triggers degradation of MafA in pancreatic β-cells, isolated rat islets were cultured for the last 5 hours in the presence of CHX, and islets were treated with either DMSO or SB20 in the presence or absence of 100 μM tBHP (Figure 4A). Treating pancreatic islets with tBHP resulted in a greater reduction in the endogenous MafA protein in the absence compared with the presence of pharmacologic p38 MAPK inhibitor (SB20). Degradation of endogenous MafA in rat islets was very sensitive to 100 μM tBHP. We observed some variation in the sensitivity of MafA to tBHP-mediated degradation from different batches of islet preparation. To minimize the variability in the results due to islet isolations and differences in the sensitivity of islet batches to oxidative stress, the effects of p38 MAPK inhibitor were quantified for individual islet preparations. In the presence of tBHP, endogenous MafA levels in rat islets were reduced to 0.47 ± 0.08 of the control (in the absence of tBHP), and p38 MAPK inhibitor SB20 consistently prevented this degradation (0.68 ± 0.09) by 40%. Quantifying the effect of SB20 on MafA degradation in the presence and the absence of oxidative stress (Figure 4A) confirms that, under oxidative stress, some of the endogenous MafA in pancreatic islets is degraded via p38 MAPK.
Figure 4.
p38 MAPK mediates degradation of endogenous MafA under oxidative stress. A, Inhibitor of p38 MAPK, SB203580, rescues oxidative stress-mediated degradation of endogenous MafA in isolated pancreatic islets. Adult rat islets were cultured for the last 5 hours in the presence of DMSO or 20 μM SB203580 (SB20) in the presence or absence of CHX and 100 μM tBHP. Whole-cell extracts were subjected to immunoblots with α-MafA and α-actin antibodies. An image of a representative gel from 3 independent experiments is shown. Immunoblot of extracts from a second independent islet preparation for the effects of tBHP and SB20 is also shown. Graph on the right presents quantification of MafA band intensity normalized to the loading control (actin) from 3 independent experiments. Results are presented relative to the expression of MafA in CHX+DMSO as 1 ± SEM both in the presence and absence of tBHP. *, P < .01. B, p38 MAPK mediates degradation of MafA in the presence of oxidative stress. MIN6 cells infected with either GFP or dominant-negative (DN)p38 MAPK adenoviruses cultured for 24 hours were split into 6-well plates and cultured for an additional 24 hours with the last 5 hours in the presence or absence of 100 μM tBHP. Whole-cell extracts were subjected to immunoblots with α-MafA and α-actin antibodies. An image of a representative gel from 3 independent experiments is shown. Immunoblot from a second independent set of extracts from MIN6 cells infected with AdDNp38 MAPK is also shown. Graph on the right presents quantification of band intensity of endogenous MafA from 3 independent experiments. The results are presented relative to the expression of MafA (normalized to the loading control, actin) in MIN6 cells infected with GFP adenovirus (AdGFP) and treated with DMSO in the absence of tBHP as 1 ± SEM. *, P < .01. MIN6 cells infected with DNp38 MAPK adenovirus (AdDNp38 MAPK) had no significant difference in MafA protein levels in the presence or absence of tBHP.
To confirm that in the presence of oxidative stress, the increase in MafA levels mediated by SB20 results from inhibition of p38 MAPK and not off-target effects of SB20, we examined the ability of dominant-negative (DN) p38 MAPK (6, 20) to prevent degradation of MafA in the presence of tBHP. MIN6 cells infected with either green fluorescent protein (GFP) or DNp38 MAPK adenoviruses were cultured in the presence or absence of 100 μM tBHP for last 5h (Figure 4B). Endogenous MafA in MIN6 cells infected with DNp38 MAPK adenovirus was more resistant to tBHP-mediated degradation than the MafA in cells infected with AdGFP. These results support that SB20 prevents MafA degradation under oxidative stress by inhibiting p38 MAPK activity and not by its off-target action. Together, the findings from pancreatic islets and the DNp38 MAPK study demonstrate the role of p38 MAPK in mediating degradation of endogenous MafA under oxidative stress. At present, it is unclear why endogenous MafA in rat pancreatic islets is more sensitive to degradation in the presence of 100 μM tBHP than the MafA in MIN6 cells. It is possible that in the pancreatic islets, the cells are more sensitive to oxidative stress, or pathways other than p38 MAPK also play a role in MafA degradation in such conditions.
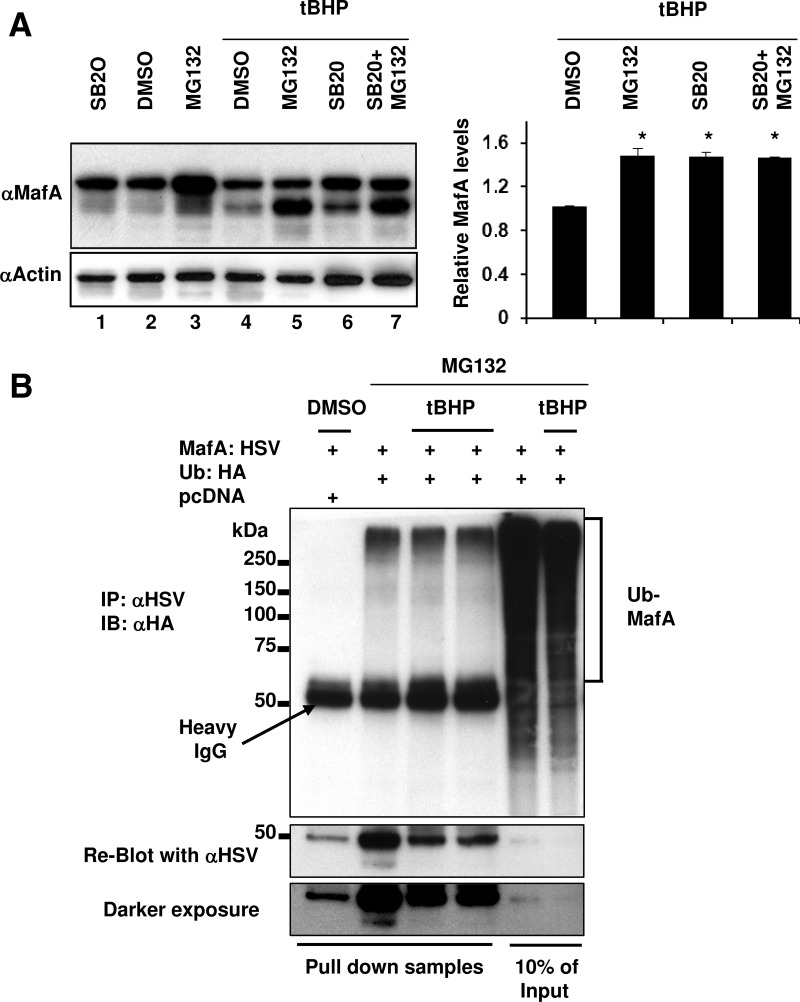
Under oxidative stress, p38 MAPK mediates MafA degradation via the proteasomal pathway
To test whether under oxidative stress p38 MAPK mediates endogenous MafA degradation via the proteasomal pathway, MIN6 cells were treated with combinations of MG132 and SB20 in the absence or presence of tBHP for the last 6 hours (Figure 5A). Although treating cells with MG132 itself can enhance oxidative stress, a 6-hour treatment had no effect on cell growth. Similarly, 6-hour CHX treatment had no detrimental effects on cell survival. Cells incubated with tBHP alone had significantly reduced MafA protein levels, but addition of MG132 resulted in more MafA than tBHP alone (compare lanes 4 and 5), demonstrating that MafA is degraded through UPP under oxidative stress. Culturing MIN6 cells in the presence of tBHP and SB20 also enhanced MafA protein levels compared with tBHP alone (compare lanes 4 and 6). The presence of MG132 and SB20 together did not show a further increase in MafA protein levels than either of these inhibitors alone (compare lane 7 with lanes 5 and 6). Quantification of MafA levels from lanes 4–7 confirmed that, in the presence of both MG132 and SB20, MafA levels were not different than in the presence of individual inhibitors. These observations strongly indicate that, in the presence of oxidative stress, SB20 and MG132 inhibit different steps of the same degradation pathway.
Figure 5.
Under oxidative stress p38 MAPK mediates MafA degradation through the proteasomal pathway. A, MIN6 cells were cultured in 0.8 mM glucose for the last 6 hours in the presence of indicated combination of DMSO, 20 μM SB203580 (SB20), and 10 μM MG132 in the presence or absence of 100 μM tBHP. Whole-cell extracts were subjected to immunoblots (IB) with α-MafA and α-actin antibodies. A representative gel from 3 independent experiments is shown. Graph on the right presents quantification of band intensity of endogenous MafA corresponding to lanes 4–7 from 3 independent experiments. The results are presented relative to the expression of MafA (normalized to the loading control, actin) as 1 ± SEM in MIN6 cells treated with DMSO in the presence of tBHP. *, P < .01. B, MIN6 cells cotransfected with pcDNA or MafA:HSV and Ub:HA expression plasmids were treated with a combination of DMSO, 10 μM MG132, and 100 μM tBHP for the last 6 hours in the presence of 0.8 mM glucose. Whole-cell extracts were immunoprecipitated (IP) with α-HSV antibody followed by immunoblotting with α-HA antibody. Whole-cell extracts from 2 independent experiments of MIN6 cells cotransfected with MafA:HSV and Ub:HA and treated with tBHP is shown. Reblot using α-HSV antibody is shown. A representative gel from at least 3 independent experiments is shown.
To examine the effects of oxidative stress on the ubiquitination of MafA, we cotransfected MIN6 cells with Ub:HA and MafA:HSV or control pcDNA plasmids and cultured them for the last 6 hours in the presence of MG132 with or without tBHP. Immunoprecipitation with anti-HSV antibody followed by immunoblotting with anti-HA antibody, revealed slower migrating Ub-MafA (Figure 5B). The amount of Ub-MafA was comparable in the presence and absence of tBHP, whereas the amount of pulled-down MafA was significantly less in the presence of tBHP (see reblot with anti-HSV antibody). These observations suggest that in MIN6 cells MafA is ubiquitinated under oxidative stress and that a greater proportion of MafA may be present as Ub-MafA under oxidative as compared with nonoxidative conditions.
Oxidative stress reduces PA28γ expression and triggers MafA degradation only via T134 and not T57
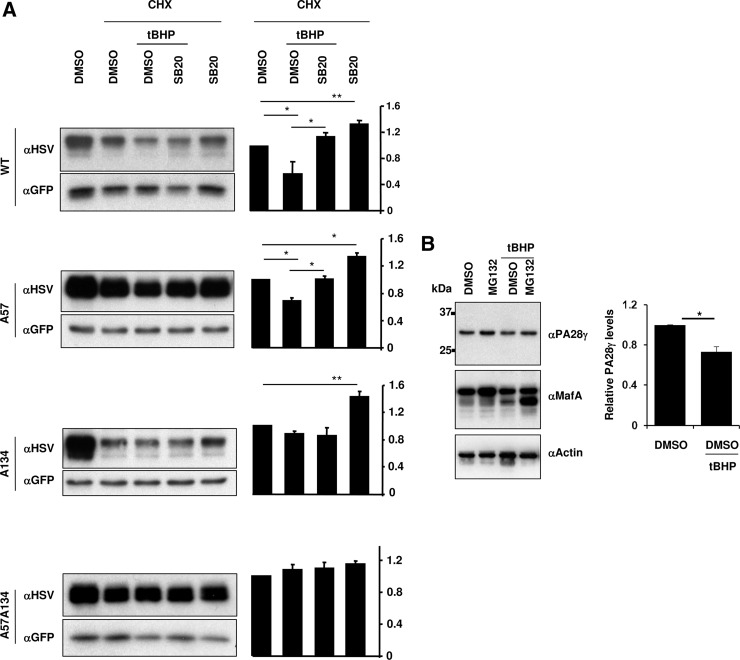
We next examined how p38 MAPK mediates degradation of MafA under oxidative stress conditions. MIN6 cells cotransfected with pEGFP-N1 and WT-, A57-, A134-, or A57A134:HSV-MafA expression plasmids were cultured for the last 6 hours in the presence of CHX, with or without tBHP, and p38 MAPK inhibitor SB20. Quantification of MafA protein levels (anti-HSV immunoblot) and GFP loading control for transfection efficiency (Figure 6A) showed that both in the absence and presence of oxidative stress, inhibition of p38 MAPK activity significantly rescued the degradation of WT- and A57-MafA, whereas A57A134-MafA with mutations in both p38 MAPK targets did not show p38 MAPK-dependent degradation. In the absence of oxidative stress, increased levels of A134-MafA in the presence of SB20 demonstrate that the phosphorylation at T57 triggered a p38 MAPK-dependent A134-MafA degradation. However, under oxidative stress, the presence of SB20 did not enhance the levels of A134-MafA. These findings demonstrate that p38 MAPK-mediated phosphorylation of either T57 or T134 under nonoxidative conditions is sufficient to trigger MafA degradation, whereas, under oxidative stress, only the phosphorylation at T134 triggers such MafA degradation.
Figure 6.
Oxidative stress reduces PA28γ expression and triggers MafA degradation only via T134 and not T57. A, p38 MAPK mediates MafA degradation via T134 and not T57 under oxidative stress: MIN6 cells cotransfected with WT, A57-, A134-, or A57A134-MafA expression plasmids and pEGPN1 were cultured in 0.8 mM glucose for the last 6 hours in the presence of indicated combination of 50 μM CHX, DMSO, and 20 μM SB203580 (SB20) in the presence or absence of 100 μM tBHP. Representative gels immunoblotted with α-HSV and α-GFP antibodies are shown for each MafA:HSV expression plasmid. Graphs on the right present results from quantification of band intensities from at least 3 independent experiments. Results are presented relative to the expression of respective MafA derivative in the presence of CHX and DMSO as 1 ± SEM. *, P < .05; **, P < .01. B, Oxidative stress reduces PA28γ expression: MIN6 cells were cultured in 0.8 mM glucose for the last 6 hours in the presence of the indicated combination of DMSO and 10 μM MG132 in the presence or absence of 100 μM tBHP. Whole-cell extracts were subjected to immunoblots with αPA28γ, α-MafA, and α-actin antibodies. A representative gel from 3 independent experiments is shown. Graph on the right presents quantification of band intensity of PA28γ in MIN6 cells in the presence or absence of tBHP from 3 independent experiments. Results are presented relative to the expression of PA28γ (normalized to the loading control, actin) in cells treated with DMSO and in the absence of tBHP as 1 ± SEM. *, P < .01.
A proteasomal activator PA28γ binds both the proteasome and MafA phosphorylated by GSK3, including phosphorylation at T57, and enhances the proteasomal degradation of phospho-MafA (21). Hence, we examined whether oxidative stress prevented phospho-T57-mediated MafA degradation by reducing PA28γ expression. MIN6 cells cultured in the absence or presence of tBHP and MG132 were analyzed for the expression of PA28γ, MafA, and actin, and the results demonstrated a modest (0.73 ± 0.05) but significant reduction in PA28γ expression in the presence of tBHP compared with control (Figure 6B). The reduction in the expression of both PA28γ and MafA in the presence of oxidative stress suggests that PA28γ may not regulate MafA degradation under this condition, likely explaining the lack of phospho-T57-mediated degradation of MafA under oxidative stress (Figure 6A).
Under oxidative stress, substitution of A134 for T134 in MafA improves insulin secretion independent of PKD activation
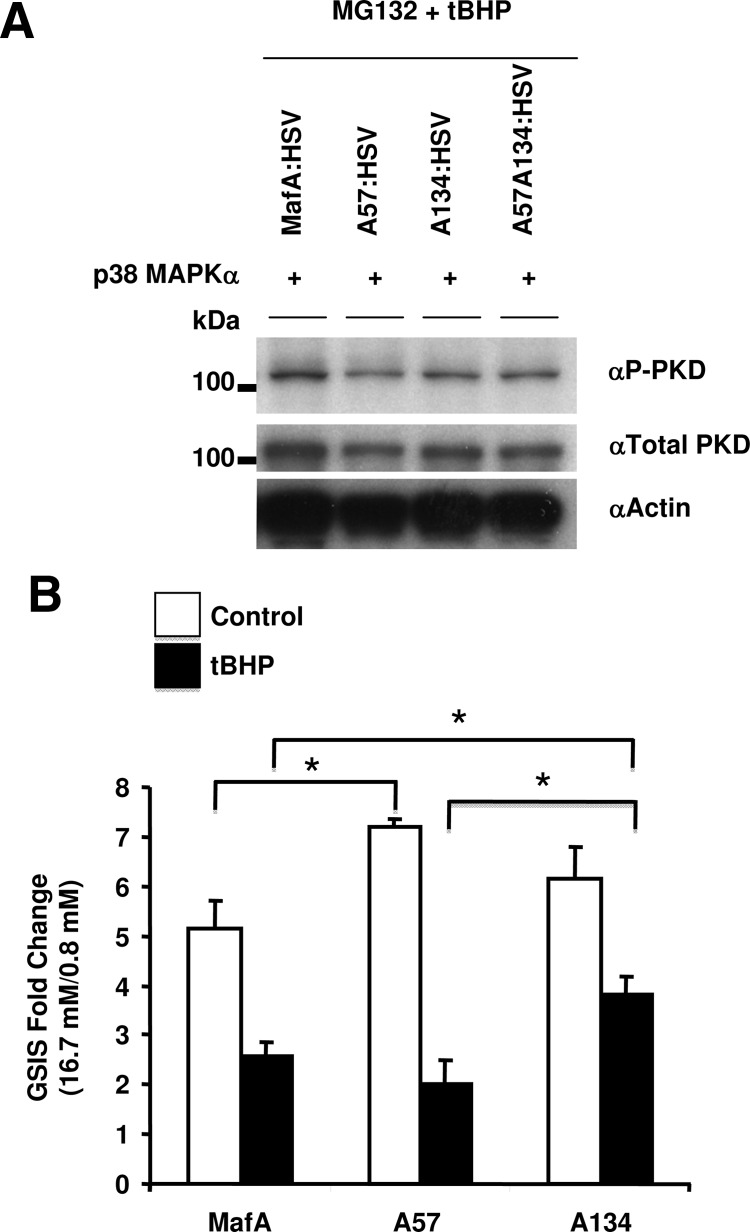
p38 MAPKδ-null mice have improved glucose tolerance and enhanced GSIS due to activation of PKD, suggesting that p38 MAPK regulates insulin secretion through inhibition of PKD (22). Increasing evidence suggests that MafA is a master regulator of β-cell maturation and GSIS (2, 4, 5, 11, 12), which raises the possibility that the inhibition of PKD and GSIS by p38 MAPK may depend on the p38 MAPK down-regulating MafA expression. Hence, we examined whether a MafA derivative (A134-MafA) resistant to p38 MAPK-dependent degradation would enhance PKD activation in the presence of oxidative stress. MIN6 cells cotransfected with WT, A57-, A134-, or A57A134-MafA expression plasmids and recombinant p38 MAPKα were cultured in the presence of MG132 and tBHP. Immunoblots using anti-phospho-PKD (activating phosphorylation at serines 774 and 778), antitotal-PKD and antiactin antibodies showed no difference in the expression levels of active PKD in presence of different MafA derivatives (Figure 7A). The inability of A134-MafA to increase the levels of active phospho-PKD in the presence of tBHP suggests that the p38 MAPK-mediated inhibition of PKD activation is independent of p38 MAPK-mediated MafA degradation under oxidative stress. This observation suggests that p38 MAPK can regulate GSIS by at least 2 independent pathways: MafA and PKD.
Figure 7.
Under oxidative stress, substitution of A134 for T134 in MafA improves insulin secretion independent of PKD activation. A, Expression of MafA under oxidative stress does not affect PKD activity. MIN6 cells cotransfected with recombinant p38 MAPKα and each of the indicated MafA expression plasmids including oxidative stress-resistant A134-MafA were cultured in the presence of 100 μM tBHP and 10 μM MG132 during the last 6 hours. Whole-cell extracts were subjected to immunoblotting with the following antibodies αP-PKD to detect active PKD, αtotal-PKD or α-actin. Representative gels from 3 independent experiments are shown. B, Oxidative stress-resistant A134-MafA, but not A57-MafA, retains greater GSIS in the presence of oxidative stress. MIN6 cells were transfected with the indicated MafA expression plasmids followed by treatment with or without 75 μM tBHP during the last 6 hours, and GSIS was determined by ratio of insulin secreted in response to 16.7 and 0.8 mM glucose during 60 minutes. Data represent mean ± SEM of insulin secretion expressed as fold induction from 3–6 independent experiments. *, P < .05.
Because oxidative stress impairs β-cell function (23, 24), we tested whether A134-MafA could rescue the inhibition of GSIS from β-cells exposed to oxidative stress. Caution was taken to minimize the variability in the results due to differences in transfection efficiency, as described in Materials and Methods. MIN6 cells transfected with WT-MafA or different MafA derivatives were cultured for the last 6 hours in the absence or presence of tBHP (75 μM) and then their capacity to secrete insulin was determined. Immediately at the end of treatment, GSIS was performed and fold induction in insulin secretion between low (0.8 mM) and high (16.7 mM) glucose concentrations was calculated. In the absence of oxidative stress, MIN6 cells expressing WT, A57-, or A134-MafA all had excellent GSIS of 4- to 7-fold stimulation (Figure 7B). tBHP treatment inhibited GSIS from cells transfected with any of the 3 MafA derivatives, with cells expressing A57- or WT-MafA showing more inhibition of GSIS than those expressing A134-MafA. These results demonstrate that the mutation in MafA that resists degradation under oxidative stress overcomes β-cell dysfunction, most likely via a PKD-independent pathway.
Discussion
Chronic hyperglycemia and accompanying oxidative stress play a major role in the development of β-cell dysfunction and diabetes (23–26). The transcription factor MafA is a critical regulator of β-cell function, and previous results demonstrate that, in the presence of oxidative stress, reduced MafA expression in the pancreatic β-cells accompanies β-cell dysfunction (6, 7, 27). Interestingly, transgenic expression of antioxidants, glutathione peroxidase or thioredoxin, in β-cells of db/db mice substantially reversed the development of hyperglycemia and the reduction in MafA expression (7, 27). These studies support the potential link between oxidative stress, reduced MafA expression/function, and the development of β-cell dysfunction. In this study, using cell culture system we have defined the molecular mechanism underlying the oxidative stress/p38 MAPK-mediated reduction in MafA expression and provide evidence that preventing this reduction minimizes the loss of β-cell function under oxidative stress.
We show that, under nonoxidative conditions independent of the action of GSK3 and its priming kinase, p38 MAPK-triggers degradation of MafA via T57 (Figure 1). Additionally, we demonstrate that p38 MAPK physically interacts with MafA under both oxidative and nonoxidative conditions (Figure 3). Furthermore, both WT-MafA and MafA with mutations in p38 MAPK-target sites bound p38 MAPK, demonstrating that the interaction between MafA and p38 MAPK does not require the presence of phosphorylatable p38 MAPK-target sites (Figure 3). This observation is consistent with the presence of distinct sites in p38 MAPK substrates for the docking of the kinase and the phosphoacceptor site (28). Interestingly, it is likely that the binding between MafA and p38 MAPK (Figure 3) might be enhanced under oxidative stress, a condition that activates p38 MAPK (6, 29–31). The binding of p38 MAPKα to its downstream kinase target MK2 is dissociated by the activation of p38 MAPK, which allows p38 MAPK to then bind its downstream substrate transcription factor MEF2 (32). It is likely that enhanced activation of p38 MAPK in the presence of tBHP (6) dissociated p38 MAPK from its other partners in β-cells, resulting in a higher proportion of total p38 MAPK available to interact with MafA.
Our results also demonstrate that phosphorylation of MafA by p38 MAPK, both in the absence and presence of oxidative stress (Figures 2 and 5), mediates its degradation via UPP. p38 MAPK influences the stability of other proteins via UPP; phosphorylation by p38 MAPK increases degradation of proteins like cyclin D3 and Bcl-2 (33, 34), but stabilizes Atrogin-1 (35) and Twist1 (36) by reducing their ubiquitination.
A key finding of our study is that the MafA phosphorylated by p38 MAPK is degraded by different mechanisms under nonoxidative and oxidative conditions. We previously showed that the phosphorylation at either T57 or T134 by p38 MAPK was sufficient to trigger MafA degradation under nonoxidative conditions (6). Here we showed that under nonoxidative conditions, T57 is phosphorylated by p38 MAPK independent of GSK3 activity (Figure 1). Yet, in the presence of tBHP, p38 MAPK triggers MafA degradation by phosphorylation of only T134 and not T57 (Figure 6A). The migration of WT-MafA as a hyperphosphorylated form, and that of A57 as a hypophosphorylated MafA (Figure 3B) in the presence of tBHP demonstrates that S61, T57, T53, and S49 are phosphorylated by GSK3 and p38 MAPK under this condition. In contrast, the A134 MafA, which contains a phosphorylatable T57, was resistant to p38 MAPK-dependent degradation (Figure 6A). It is likely that the mechanism(s) that recognizes and triggers MafA degradation in response to T57 phosphorylation under nonoxidative conditions is inhibited by the oxidative stress. Consistent with this assumption, we observed that the proteasomal activator PA28γ, which binds both the proteasome and MafA phosphorylated by GSK3 and enhances the proteasomal degradation of MafA (21), was reduced in the presence of tBHP (Figure 6B). The activity of PA28 is regulated by oxidative stress: under low level of oxidative stress hepatitis C virus core protein stimulated interaction between PA28 and the proteasome and enhanced proteasome activity, but under high oxidative stress this activity was suppressed (37). It is possible that, in addition to reduction in its expression, the inhibition in PA28 activity under high oxidative stress in β-cells prevents the degradation of MafA phosphorylated at T57. Another possibility could be that, under oxidative stress, the F-box protein that regulates ubiquitination and degradation of MafA does not recognize the phosphorylated-T57 degradation signal (phosphodegron). Several F-box proteins recognize GSK3 phosphorylated targets, including β-transducin repeat-containing (β-TRCP) (38–40). Oxidative stress modifies the cysteine residues of β-TRCP and reduces both its binding to and degradation of phosphorylated IκBα (41). It is possible that a similar oxidative stress-sensitive F-box regulates degradation of T57-phosphorylated MafA. Identification of F-box protein involved in degradation of MafA phosphorylated by GSK3 is essential to determining whether the reduction in PA28γ, F-box protein, or both, prevent degradation of MafA phosphorylated by GSK3 under oxidative stress.
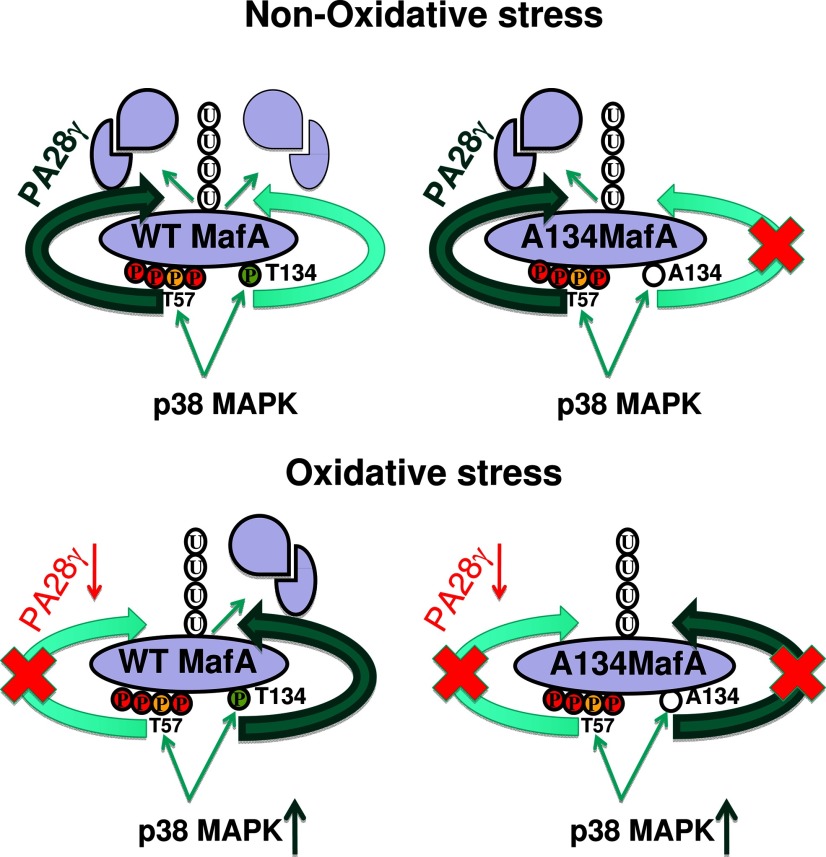
To explain p38 MAPK-dependent MafA degradation under oxidative and nonoxidative conditions, we suggest a model (Figure 8) in which: 1) T57 phosphorylated by either p38 MAPK or GSK3 is recognized by the same downstream recognition system (eg, PA28γ; Figure 6B) that mediates its ubiquitination and proteasomal degradation, 2) T134 phosphorylated by p38 MAPK is recognized by a distinct recognition system for its ubiquitination and degradation. Under nonoxidative conditions both pathways would be functional and, therefore, phosphorylation at either T57 or T134 could trigger p38 MAPK-dependent degradation of MafA. However, under oxidative stress, the pathway involved in T57-mediated degradation (eg, PA28γ), but not its phosphorylation, might be inhibited, whereas the pathway regulating degradation of phosphorylated T134 is active or even enhanced (Figure 8). The presence of such multiple pathways regulating degradation of a single protein provides a means to regulate its expression in response to different physiologic/pathologic conditions and is not unique to MafA and has been shown to regulate degradation of β-catenin, replication licensing factor Cdt1, and a tumor suppressor RASSF1C protein (40, 42–46). Although, both GSK3 and p38 MAPK regulate MafA degradation in pancreatic islets and MafA expression is reduced in β-cells in animal models of oxidative stress and diabetes (6, 7, 27), we suggest that determining the in vivo role of p38 MAPK and T134 MafA phosphorylation in β-cell function will require replacing endogenous MafA gene with A134MafA.
Figure 8.
A schematic model for p38 MAPK-mediated MafA degradation under oxidative and nonoxidative conditions. Red circles represent GSK3 phosphorylation sites, yellow circles represent common T57 phosphorylation site for p38 MAPK and GSK3, green circles represent T134 phosphorylation site, and P denotes phosphorylation. The absence of phosphorylation at the A134 site is shown by an empty circle. Potential pathways for degradation of WT-MafA and A134-MafA under oxidative and nonoxidative conditions are shown. Circles with U indicate ubiquitination; intact and degraded MafA are shown by single ovals and 2 structures, respectively. Reduced expression of PA28γ under oxidative stress is shown by downward arrow and red font; higher expression levels are indicted by bold green font for PA28γ, and dark green arrow shows increased p38 MAPK expression under oxidative stress. Darker green arrow indicates dominant MafA degradation pathway under oxidative stress.
Oxidative stress enhances both expression and activity of p38 MAPK, thereby triggering MafA degradation (Refs. 6 and 47 and this study). Insulin-producing cells express all 4 p38 MAPK isoforms (48). Our results with pharmacologic p38 MAPK α and -β selective inhibitor (SB20) support the role of these isoforms in regulating the stability of MafA. Mice lacking p38 MAPKδ, which is expressed in limited cell types including β-cells, showed improved glucose tolerance and enhanced insulin secretion by activation of PKD (22). Because all p38 MAPK isoforms recognize the same target site (47, 49), it is likely that loss of p38 MAPKδ would enhance MafA expression, with a resultant increase in expression of genes involved in insulin synthesis and secretion (5, 11, 12). A134-MafA would be resistant to all p38 MAPK isoforms and hence should be able to counter any detrimental effect of activation of p38 MAPKδ in response to oxidative stress. Our finding that A134-MafA in the presence of tBHP did not enhance the levels of active PKD (Figure 7A), but successfully reversed the tBHP-mediated inhibition of GSIS (Figure 7B), suggests that MafA-mediated improvement in GSIS under this condition occurs by a mechanism distinct from PKD-mediated induction of insulin secretion.
In summary, we provide evidence for novel mechanisms of p38 MAPK-mediated degradation of MafA under nonoxidative and oxidative conditions and show that limiting MafA degradation under oxidative stress could prevent development of β-cell dysfunction. Because in vivo reduction in oxidative stress enhances MafA expression and improves β-cell function, we suggest that the strategies based on the molecular mechanisms regulating degradation of MafA under oxidative and nonoxidative conditions will provide novel ways in which to enhance β-cell function and ameliorate diabetes.
Materials and Methods
Plasmid preparation
The HSV-tagged human MafA (MafA) plasmid and its derivatives with mutations in phosphoacceptor sites A57, A134, and A57A134 were described previously (6), and A65, A57A65, A65A134, or A57A65A134 were generated using the QuikChange Site-Directed Mutagenesis kit according to manufacturer's instructions (Stratagene, La Jolla, California). Ubiquitin-HA (8X) was a gift from Dr. Dirk Bohman University of Rochester (Rochester, Minnesota) (50), whereas pEGFP-N1 (CLONTECH Laboratories, Inc., Mountain View, California), pcDNA3.1/Zeo (Invitrogen, Carlsbad, California), and p38 MAPKα (Addgene, Cambridge, Massachusetts) expression plasmids were purchased.
Cell culture and reagents
MIN6 cells (passage 15–35) (from Dr. J-I Miyazaki, Osaka, Japan) were cultured in DMEM plus 10% fetal bovine serum or in glucose-free DMEM (Invitrogen) plus 10% dialyzed fetal bovine serum (Invitrogen) and the indicated glucose concentrations (Sigma, St. Louis, Missouri) supplemented with 5μL/liter of β-mercaptoethanol and Penicillin/Streptomycin (Sigma). p38 MAPK inhibitor SB203580 (SB20) and proteasome inhibitor MG132 (Calbiochem, San Diego, California), GSK3 inhibitor SB216763 (SB21) (Tocris, Ellisville, Missouri), and tert-Butyl hydroperoxide (tBHP) N-ethylmaleimide, cycloheximide (CHX), and DMSO were purchased from Sigma.
Transient transfection and adenoviral transduction of cells
MIN6 cells seeded at 6 × 106 cells/mL in 100-mm plate were cotransfected with 4 μg of indicated plasmid and 0.5 μg of pEGFP-N1 plasmid as described elsewhere (51), resulting in approximately 50% transfection efficiency. To detect protein-protein interactions by coimmunoprecipitation, bait (MafA:HSV and its derivatives) and prey (Ub:HA or p38 MAPK) expression plasmids were cotransfected at 3 μg each. After 24 hours, cells were trypsinized and seeded in 6-well plates; the next day DMSO or indicated inhibitors (20 μM SB203580, 20 μM SB216763, 10 μM MG132, 50 μM cycloheximide) were added, and cells were cultured in medium containing 0.8 mM glucose for the indicated times in the presence or absence of 100 μM of tBHP before harvesting. For adenoviral infection experiments, MIN6 cells were infected with adenoviruses expressing dominant negative p38 MAPKα (DNp38 MAPKα) (6, 20) or GFP (control) and 48 hours later, cells were treated in the presence or absence of 100 μM tBHP for 5 hours before harvesting.
Coimmunoprecipitation and immunoblot analysis
Total cell lysates were prepared as described elsewhere (2). For coimmunoprecipitation studies N-ethylmaleimide, an inhibitor of deubiquitinating enzymes, was included in lysis buffer, and immunoprecipitation was performed using ExactaCruz antigen detection system (Santa Cruz Biotechnology, Inc., Santa Cruz, California). Equal concentrations of proteins were boiled in β-mercaptoethenol containing Laemmli buffer for 5 minutes and resolved on 7.5 or 10% SDS-polyacrylamide gel by electrophoresis, and Western blots were performed as described elsewhere (2). The following primary antibodies were used for immunoblot analysis: rabbit α-HSV (Abcam, Cambridge, Massachusetts), goat α-actin (Santa Cruz Biotechnology), mouse α-HA (Sigma), rabbit α-MafA (2), mouse αGFP (CLONTECH Laboratories), rabbit α-p38 MAPK (Bethyl Laboratories, Montgomery, Texas), α-total-PKD and α-phospho-PKD (Cell Signaling Technology, Danvers, Massachusetts), and α-PA28γ (Cell Signaling Technology). Secondary antibodies were α-rabbit IgG (Bio-Rad Laboratories, Hercules, California) and α-mouse IgG (Bio-Rad Laboratories) and α-goat IgG (Santa Cruz Biotechnology).
Isolation of rat islets and culture
Islets from adult Sprague Dawley rats were isolated using collagenase digestion and cultured in RPMI-1640 medium (Mediatech, Inc, Manassas, Virginia) as described elsewhere(52). After overnight culture, islets of similar sizes were handpicked and cultured in 60-mm dishes for 5 hours in 2.2 mM glucose in the absence or presence of p38 MAPK inhibitor 20 μM SB203580 and 100 μM tBHP.
Glucose-stimulated insulin secretion
MIN6 cells were cotransfected with pEGFP-N1 and WT-, T57A-, or T134A-HSV-tagged MafA expression plasmids at 10 μg /100-mm plate. To maintain comparable transfection efficiency for different experimental groups in a given experiment, 24 hours after transfection cells from large plates were seeded in 6-well plates as above. After another 24 hours these cells were treated, or not, with 75 μM tBHP for the last 6 hours. Immediately after treatment, cells were washed twice with PBS and preincubated twice for 1 hour in 0.8 mM glucose KRB buffer (12) containing 0.1% (wt/vol) BSA in a 37°C humidified air incubator. The cells were then incubated for an additional hour in KRB buffer containing 0.8 mM (basal) or 16.7 mM (stimulatory) glucose. At the end of incubation, media insulin concentrations were measured using Rat Insulin ELISA kit and Mouse Insulin Standards, both from Crystal Chem (Downers Grove, Illinois). Secretion data were normalized to total cellular protein levels determined by Bradford assay (Bio-Rad).
Statistical analysis
Data are presented as mean ± SEM. Differences between groups were compared by 2-tailed Student's test and considered significant when P < .05.
Acknowledgments
We thank Dr. Susan Bonner-Weir for reviewing the manuscript. We also thank Yulan Ai and Pia Auk-Emblem for technical assistance, and Jennifer Hollister-Lock for islet isolation. All work at the Joslin Diabetes Center.
This study was supported by research grants from National Institutes of Health (NIH) Grant RO1 DK060127 and ADA, the Herbert Graetz Fund, Shirley and William Fleisher Family Foundation, and the Alexander and Margaret Stewart Trust. I.E.K. was supported by a postdoctoral fellowship from NIH Grant T32 DK07260. We acknowledge support from NIH DK-36836 Diabetes Endocrinology Research Center grant for Media and Assay cores.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A
- alanine
- CHX
- cycloheximide
- DMSO
- dimethylsulfoxide
- DN
- dominant-negative
- GFP
- green fluorescent protein
- GSIS
- glucose-stimulated insulin secretion
- GSK3
- glycogen synthase kinase 3
- HA
- hemagglutinin
- PKD
- protein kinase D
- S
- serine
- T
- threonine
- tBHP
- tert-butyl hydroperoxide
- β-TRCP
- β-transducin repeat-containing
- Ub:HA
- HA-tagged ubiquitin
- UPP
- ubiquitin proteasomal pathway
- WT
- wild-type.
References
- 1. Olbrot M, Rud J, Moss LG, Sharma A. Identification of β-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci USA. 2002;99:6737–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura W, Kondo T, Salameh T, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev Biol. 2006;293:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Artner I, Le Lay J, Hang Y, et al. MafB: an activator of the glucagon gene expressed in developing islet α- and β-cells. Diabetes. 2006;55:297–304 [DOI] [PubMed] [Google Scholar]
- 4. Zhang C, Moriguchi T, Kajihara M, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Artner I, Hang Y, Mazur M, et al. MafA and MafB regulate genes critical to β-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kondo T, El Khattabi I, Nishimura W, et al. p38 MAPK is a major regulator of MafA protein stability under oxidative stress. Mol Endocrinol. 2009;23:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harmon JS, Bogdani M, Parazzoli SD, et al. β-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler AE, Robertson RP, Hernandez R, Matveyenko AV, Gurlo T, Butler PC. β-Cell nuclear musculoaponeurotic fibrosarcoma oncogene family A (MafA) is deficient in type 2 diabetes. Diabetologia. 2012;55:2985–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 10. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 11. Wang H, Brun T, Kataoka K, Sharma AJ, Wollheim CB. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia. 2007;50:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguayo-Mazzucato C, Koh A, El Khattabi I, et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat β cells. Diabetologia. 2011;54:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bliss CR, Sharp GW. Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am J Physiol. 1992;263:E890–E896 [DOI] [PubMed] [Google Scholar]
- 14. Han SI, Aramata S, Yasuda K, Kataoka K. MafA stability in pancreatic β cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol Cell Biol. 2007;27:6593–6605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rocques N, Abou Zeid N, Sii-Felice K, et al. GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol Cell. 2007;28:584–597 [DOI] [PubMed] [Google Scholar]
- 16. Benkhelifa S, Provot S, Nabais E, Eychène A, Calothy G, Felder-Schmittbuhl MP. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol Cell Biol. 2001;21:4441–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ochi H, Ogino H, Kageyama Y, Yasuda K. The stability of the lens-specific Maf protein is regulated by fibroblast growth factor (FGF)/ERK signaling in lens fiber differentiation. J Biol Chem. 2003;278:537–544 [DOI] [PubMed] [Google Scholar]
- 18. Sii-Felice K, Pouponnot C, Gillet S, et al. MafA transcription factor is phosphorylated by p38 MAP kinase. FEBS Lett. 2005;579:3547–3554 [DOI] [PubMed] [Google Scholar]
- 19. Guo S, Burnette R, Zhao L, et al. The stability and transactivation potential of the mammalian MafA transcription factor are regulated by serine 65 phosphorylation. J Biol Chem. 2009;284:759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Huang S, Sah VP, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168 [DOI] [PubMed] [Google Scholar]
- 21. Kanai K, Aramata S, Katakami S, Yasuda K, Kataoka K. Proteasome activator PA28γ stimulates degradation of GSK3-phosphorylated insulin transcription activator MAFA. J Mol Endocrinol. 2011;47:119–127 [PubMed] [Google Scholar]
- 22. Sumara G, Formentini I, Collins S, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;1–11 doi:10.1155/2012/703538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pitocco D, Zaccardi F, Di Stasio E, et al. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nourooz-Zadeh J, Tajaddini-Sarmadi J, McCarthy S, Betteridge DJ, Wolff SP. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes. 1995;44:1054–1058 [DOI] [PubMed] [Google Scholar]
- 26. Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA. 1999;96:10857–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamamoto M, Yamato E, Toyoda S, et al. Transgenic expression of antioxidant protein thioredoxin in pancreatic β cells prevents progression of type 2 diabetes mellitus. Antioxid Redox Signal. 2008;10:43–49 [DOI] [PubMed] [Google Scholar]
- 28. Yang SH, Galanis A, Sharrocks AD. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19:4028–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lander HM, Jacovina AT, Davis RJ, Tauras JM. Differential activation of mitogen-activated protein kinases by nitric oxide-related species. J Biol Chem. 1996;271:19705–19709 [DOI] [PubMed] [Google Scholar]
- 30. Klotz LO, Pellieux C, Briviba K, Pierlot C, Aubry JM, Sies H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur J Biochem. 1999;260:917–922 [DOI] [PubMed] [Google Scholar]
- 31. Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142 [DOI] [PubMed] [Google Scholar]
- 32. ter Haar E, Prabhakar P, Prabakhar P, Liu X, Lepre C. Crystal structure of the p38 α-MAPKAP kinase 2 heterodimer. J Biol Chem. 2007;282:9733–9739 [DOI] [PubMed] [Google Scholar]
- 33. Casanovas O, Jaumot M, Paules AB, Agell N, Bachs O. P38SAPK2 phosphorylates cyclin D3 at Thr-283 and targets it for proteasomal degradation. Oncogene. 2004;23:7537–7544 [DOI] [PubMed] [Google Scholar]
- 34. Markou T, Dowling AA, Kelly T, Lazou A. Regulation of Bcl-2 phosphorylation in response to oxidative stress in cardiac myocytes. Free Radic Res. 2009;43:809–816 [DOI] [PubMed] [Google Scholar]
- 35. Li JJ, Zhang TP, Meng Y, Du J, Li HH. Stability of F-box protein atrogin-1 is regulated by p38 mitogen-activated protein kinase pathway in cardiac H9c2 cells. Cell Physiol Biochem. 2011;27:463–470 [DOI] [PubMed] [Google Scholar]
- 36. Hong J, Zhou J, Fu J, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71:3980–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Osna NA, White RL, Krutik VM, Wang T, Weinman SA, Donohue TM., Jr Proteasome activation by hepatitis C core protein is reversed by ethanol-induced oxidative stress. Gastroenterology. 2008;134:2144–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kitagawa K, Hiramatsu Y, Uchida C, et al. Fbw7 promotes ubiquitin-dependent degradation of c-Myb: involvement of GSK3-mediated phosphorylation of Thr-572 in mouse c-Myb. Oncogene. 2009;28:2393–2405 [DOI] [PubMed] [Google Scholar]
- 39. Busino L, Millman SE, Scotto L, et al. Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat Cell Biol. 2012;14:375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou X, Li TT, Feng X, et al. Targeted polyubiquitylation of RASSF1C by the Mule and SCFβ-TrCP ligases in response to DNA damage. Biochem J. 2012;441:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banerjee S, Zmijewski JW, Lorne E, Liu G, Sha Y, Abraham E. Modulation of SCF β-TrCP-dependent I κB α ubiquitination by hydrogen peroxide. J Biol Chem. 2010;285:2665–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle. 2009;8:4032–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926 [DOI] [PubMed] [Google Scholar]
- 45. Xiao JH, Ghosn C, Hinchman C, et al. Adenomatous polyposis coli (APC)-independent regulation of β-catenin degradation via a retinoid X receptor-mediated pathway. J Biol Chem. 2003;278:29954–29962 [DOI] [PubMed] [Google Scholar]
- 46. Nishitani H, Sugimoto N, Roukos V, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417 [DOI] [PubMed] [Google Scholar]
- 48. Makeeva N, Myers JW, Welsh N. Role of MKK3 and p38 MAPK in cytokine-induced death of insulin-producing cells. Biochem J. 2006;393:129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Z, Gibson TB, Robinson F, et al. MAP kinases. Chem Rev. 2001;101:2449–2476 [DOI] [PubMed] [Google Scholar]
- 50. Treier M, Staszewski LM, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the δ domain. Cell. 1994;78:787–798 [DOI] [PubMed] [Google Scholar]
- 51. Harrington RH, Sharma A. Transcription factors recognizing overlapping C1–A2 binding sites positively regulate insulin gene expression. J Biol Chem. 2001;276:104–113 [DOI] [PubMed] [Google Scholar]
- 52. King A, Lock J, Xu G, Bonner-Weir S, Weir GC. Islet transplantation outcomes in mice are better with fresh islets and exendin-4 treatment. Diabetologia. 2005;48:2074–2079 [DOI] [PubMed] [Google Scholar]