Abstract
The oncogene nuclear receptor coactivator amplified in breast cancer 1 (AIB1) is a transcriptional coactivator that is overexpressed in various types of human cancers. However, the molecular mechanisms controlling AIB1 expression in the majority of cancers remain unclear. In this study, we identified a novel interacting protein of AIB1, forkhead-box protein G1 (FoxG1), which is an evolutionarily conserved forkhead-box transcriptional corepressor. We show that FoxG1 expression is low in breast cancer cell lines and that low levels of FoxG1 are correlated with a worse prognosis in breast cancer. We also demonstrate that transient overexpression of FoxG1 can suppress endogenous levels of AIB1 mRNA and protein in MCF-7 breast cancer cells. Exogenously expressed FoxG1 in MCF-7 cells also leads to apoptosis that can be rescued in part by AIB1 overexpression. Using chromatin immunoprecipitation, we determined that FoxG1 is recruited to a region of the AIB1 gene promoter previously characterized to be responsible for AIB1-induced, positive autoregulation of transcription through the recruitment of an activating, multiprotein complex, involving AIB1, E2F transcription factor 1, and specificity protein 1. Increased FoxG1 expression significantly reduces the recruitment of AIB1, E2F transcription factor 1 and E1A-binding protein p300 to this region of the endogenous AIB1 gene promoter. Our data imply that FoxG1 can function as a pro-apoptotic factor in part through suppression of AIB1 coactivator transcription complex formation, thereby reducing the expression of the AIB1 oncogene.
Amplified in breast cancer 1 (AIB1, ACTR, RAC3, SRC3, NCOA3, and p/CIP) belongs to the p160 family of steroid receptor coactivators and is found to be frequently amplified in multiple human cancers (1). Similar to the other p160 coactivators, AIB1 can associate with hormone-bound nuclear receptors and potentiate transcriptional activation by enhancing transcriptional complex assembly and through local chromatin remodeling (2–4). AIB1 is an oncogene and has been strongly implicated in the development of hormone-responsive and nonresponsive cancers (5, 6) by coactivating not only nuclear receptors but also nonreceptor transcription factors such as E2F transcription factor 1 (E2F1), nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and PEA3 (7–10). In mouse models, AIB1 overexpression results in the development of mammary hyperplasia and tumorigenesis (11). The overexpression of AIB1 has been observed in 30%–60% of human breast tumors, and a strong correlation exists between high levels of AIB1 and high epidermal growth factor receptor 2 levels, larger tumor size, higher tumor grade, increased cancer reoccurrence, and worse prognosis (12).
AIB1 expression can be controlled at multiple levels. AIB1 protein levels are regulated by a number of proteasomal degradation pathways (13–15). In terms of AIB1 mRNA, we have previously reported that all-trans retinoic acid, antiestrogens and tamoxifen, and TGF-β can up-regulate AIB1 transcripts, whereas estrogen can suppress AIB1 gene expression (16). In addition, a recent study demonstrates that transcription of the AIB1 gene is controlled by regulatory sequences within the −250 to +350 bp region of its promoter that enable AIB1 to autoregulate and enhance the expression of its own gene (7, 17). In these studies, an specificity protein 1 (Sp1)-binding site downstream of exon 1 was described within the −250/+350 region that also recruited E2F1. This enables AIB1 to complex with E2F1, and this Sp1-associated transcription complex significantly increases the coactivation of the AIB1 gene (17).
AIB1 is also known to bind directly to other coactivators such as histone acetyltransferase E1A-binding protein p300 (300)/CREB-binding protein (CBP), p300/CBP-associated cofactor p/CAF, and coactivator-associated arginine methyltransferase 1, and enhances transcriptional activation by bringing these potent cofactors capable of modifying chromatin organization to the target gene promoter (3, 18, 19). The ability to interact with a wide range of transcriptional cofactors allows AIB1 to act as a potent coactivator (12). In contrast, only a few transcriptional corepressors that interact with the steroid receptor coactivator family proteins are known (20, 21). Therefore, we conducted broad screens of AIB1-interacting proteins using mass spectrometry (MS) to detect low-abundance AIB1 binding partners that may potentially suppress AIB1 function and negatively regulate the AIB1 gene expression (reviewed in Ref. 22). We focused on AIB1-interacting proteins that segregated under the category of “transcriptional repressors,” and here we demonstrate that the winged-helix, DNA-binding transcriptional corepressor forkhead-box protein G1 (FoxG1; also known as brain factor 1, BF1) which we identified as an AIB1-interacting protein, can down-regulate AIB1 promoter activity and suppress both AIB1 transcript and protein expression in MCF-7 cells. FoxG1 belongs to the forkhead-box family of transcriptional regulators and is a protein mainly expressed in the brain and testis in human (23, 24). FoxG1 controls the development of the telencephalon and cerebral corticogenesis (23) and is shown to interact with global transcriptional corepressors and histone deacetylases to potentiate transcriptional repression (25). FoxG1 can also directly interact with androgen receptor (AR) and suppress AR-mediated transactivation (24). Whereas FoxG1 knockout mice develop cerebral hypoplasia and die at birth, humans with FoxG1 haploinsufficiency show severe mental retardation and microcephaly (23, 26, 27). The prominent developmental phenotype associated with FoxG1 pathology has focused most investigations of FoxG1 function on the brain and neurogenesis; therefore not much is known about the role of FoxG1 in cancer and the molecular mechanism underlying FoxG1 function.
Our data indicate that FoxG1 is directly recruited to the AIB1 promoter. We report a mechanism by which FoxG1 overexpression compromises the integrity of an Sp-1-associated activating transcriptional complex. This complex is required for the up-regulation of AIB1 gene expression, and FoxG1 reduces its recruitment by disassembly and detachment of the activating complex from the AIB1 promoter. We also show that FoxG1 down-regulates AIB1 expression, which leads to apoptosis in human breast cancer cells.
Results
AIB1 interacts with the transcriptional corepressor FoxG1
To identify proteins that interact with AIB1, we performed AIB1-specific immunoprecipitations (IPs) of lysates from human embryonic kidney (HEK)293T cells transfected with a FLAG-tagged AIB1 construct. After IP with a FLAG antibody, we isolated FLAG-associated immunocomplexes by denaturing gel electrophoresis, followed by Coomassie blue staining. Twelve visible bands were excised, and extracted proteins were subjected to MS analysis (described in Ref. 22). FoxG1 was identified as a candidate AIB1-interacting protein through our MS evaluation. It was of interest for further investigation because it is known to function in certain contexts as a transcriptional repressor (25) and could potentially regulate AIB1 function and gene expression. To verify AIB1 interaction with FoxG1, we performed coimmunoprecipitation experiments. FLAG-AIB1 was expressed by transient transfection together with hemagglutinin (HA)-FoxG1 in HEK293T cells, and HA-FoxG1 was detected in the immunoprecipitates of FLAG-AIB1 from whole-cell lysates (Figure 1A). We also confirmed the interaction of endogenous AIB1 and FoxG1 in MCF-7 breast cancer cells that harbor the 20q AIB1 gene amplicon and express high levels of AIB1 protein and a detectable amount of FoxG1 (Figure 1B). These IP results confirm the MS data and demonstrate that FoxG1 is present in complexes that coimmunoprecipitate with AIB1.
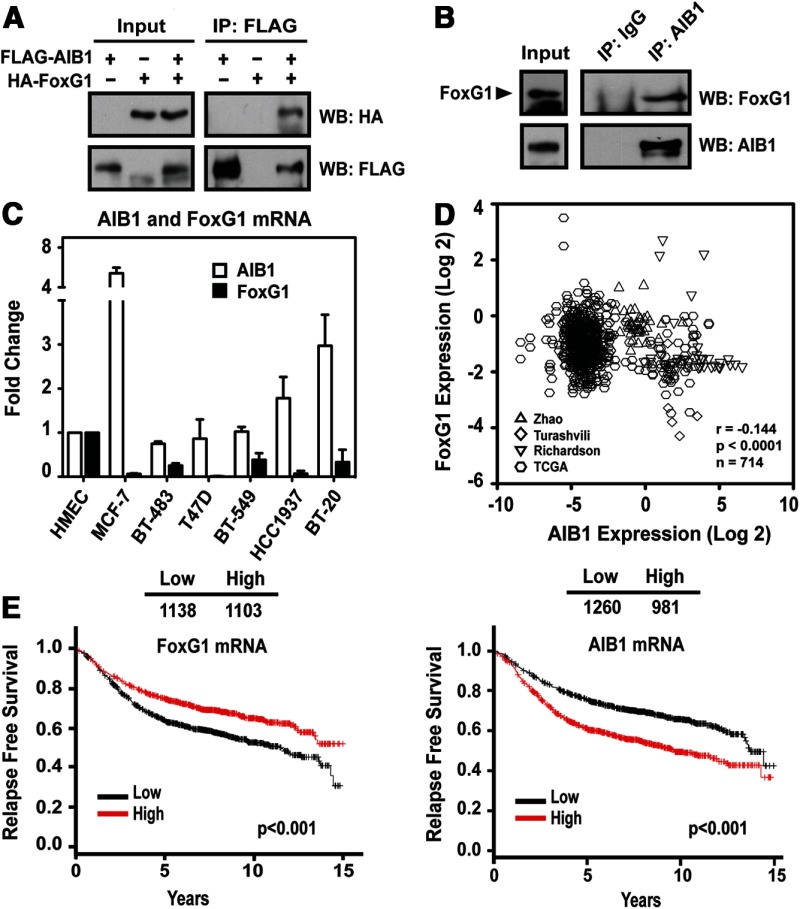
Figure 1.
AIB1 and FoxG1 Interact in Mammalian Cells. A, AIB1 interacts with FoxG1 in HEK293T cells. FLAG-AIB1 was cotransfected with HA-FoxG1 constructs. Whole-cell lysates were collected 48 hours after transient transfection, and used for IP and WB analysis with anti-FLAG and anti-HA antibodies as indicated. B, Interaction of endogenous AIB1 with FoxG1 in MCF-7 breast cancer cells. Nuclear lysates were prepared from MCF-7 cells and immunoprecipitated with an AIB1 antibody or control IgG. FoxG1 protein associated with AIB1 in the IP was detected by WB as indicated. C, AIB1 and FoxG1 mRNA expression levels in breast cancer cell lines. Total RNA was harvested from breast cancer cell lines to determine the relative gene expression for AIB1 and FoxG1. D, FoxG1 and AIB1 mRNA expression are inversely correlated. Data from Zhao et al (28), Turashvili et al (29), Richardson et al (30), and TCGA (The Cancer Genome Atlas-Invasive Breast Carcinoma Gene Expression Data) were analyzed using Oncomine (www.oncomine.org). Higher expression of FoxG1 coincides with lower expression of AIB1 and vice versa. E, Analysis of the levels of AIB1 and FoxG1 mRNA on a gene expression microarray of breast cancer samples from patients with known RFS times provided by KM Plotter (http://www.kmplot.com) (KM analysis parameters are described in Material and Methods) (31)..
FoxG1 is predominantly expressed in the brain and its non-neuronal expression in normal tissues is low (23, 24). The expression of FoxG1 in human cancer has not been widely reported. In a comparison of normal breast cells and breast cancer cell lines, we found that the mRNA expression level of FoxG1 was significantly lower in breast cancer cell lines, irrespective of their estrogen receptor (ER) status, as compared with FoxG1 expression in the normal human mammary epithelial cells (HMECs) (Figure 1C). These data suggest a loss of FoxG1 expression from normal to cancerous transition (Figure 1C) and indicate that reduced FoxG1 expression might have prognostic significance in human breast cancer. Our reanalysis of published microarray data (www.oncomine.org) from human breast cancer clinical samples of 4 independent studies (Refs. 28, 29, and 30, and The Cancer Genome Atlas-Invasive Breast Carcinoma Gene Expression Data/TCGA) further supported this hypothesis and showed a significant (P < .0001) negative correlation between AIB1 and FoxG1 mRNA expression over 714 samples, in which higher expression of FoxG1 coincided with lower expression of AIB1 and vice versa (Figure 1D).
In addition, we used unbiased gene expression data compiled by Kaplan-Meier (KM) Plotter (http://www.kmplot.com) (31) from a series of suitable studies that allow for an analysis of clinical outcomes correlated with a single gene expression. We saw that higher FoxG1 mRNA levels, in 2241 breast cancer samples, correlated with increased relapse-free survival (RFS) rate (Figure 1E) (KM analysis parameters are described in Materials and Methods). In contrast, in the same data set, elevated AIB1 transcript levels correlated with reduced RFS (Figure 1E), which is consistent with previous reports on AIB1 prognostic significance in human breast cancer (reviewed in Ref. 12).
FoxG1 induces apoptosis in MCF-7 cells
The expression pattern for AIB1 and FoxG1 in MCF-7 cells was of interest because these cells significantly overexpress AIB1 and show low levels of FoxG1 mRNA expression compared with HMECs (Figure 1C). We therefore chose to study the phenotypic effect of FoxG1 overexpression on MCF-7 cells. Twenty-four hours after expression vector transfection, overexpressing FoxG1 led to cell detachment from culture dishes, and the induction of apoptosis as determined by annexin V staining (Figure 2A, upper panel). The early and total apoptosis average indices for duplicate samples of 14.5 and 28.64% in FoxG1-expressing cells were significantly elevated compared with MCF-7 cells transfected with the control empty vector (EV) with average apoptosis indices of 1.68 and 9.4%, respectively (Figure 2A, lower panel). Increased expression of FoxG1 in MCF-7 cells was correlated with the apoptotic response.
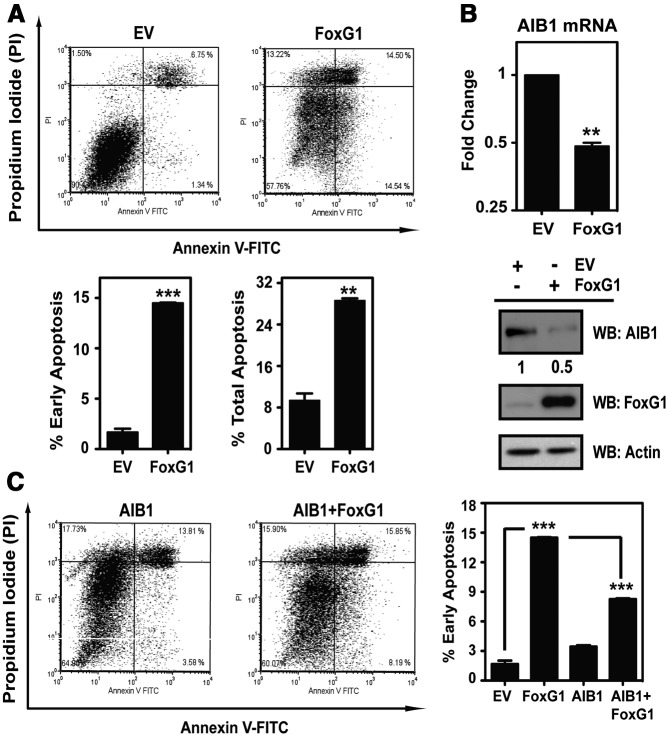
Figure 2.
FoxG1 Induces Apoptosis and Down-Regulates AIB1 Expression in MCF-7 Cells. A, MCF-7 cells were transfected with either an EV control or FoxG1 constructs. Cells were subjected 24 hours after transfection to Annexin V apoptosis analysis. The percentages of cells in early and late apoptosis are represented by bottom right and top right quadrants of the FACS analysis, respectively. Percent total apoptosis was the total percentage of cells in both early and late apoptosis. The mean ± SEM values were obtained from duplicate samples from each transfection condition. ***, P < .001; **, P < .01 relative to EV. Statistical analysis was done by Student's t test. B, Analysis of endogenous AIB1 expression in MCF-7 cells overexpressing FoxG1. Cells were transfected with EV or FoxG1 as in panel A. Total RNA and whole-cell lysates were collected to determine the relative levels of mRNA and protein for AIB1. Cells transfected with EV were arbitrarily set at 1, and cells expressing FoxG1 were analyzed in reference to it. Student's t test. **, P < .01 relative to EV. Relative protein levels were determined by WB with antibodies as indicated. C, AIB1 rescues MCF-7 cells from FoxG1-induced apoptosis. MCF-7 cells were transfected separately with expression vectors for either EV control, FoxG1, AIB1, or AIB1 and FoxG1 together. Cells were assessed for apoptosis as in panel A. ***, P < .001, EV vs FoxG1; or FoxG1 vs AIB1+FoxG1. One-way ANOVA with Bonferroni posttest. FITC, fluorescein isothiocyanate.
Previous studies reported increased incidence of apoptosis in MCF-7 cells when AIB1 expression is down-regulated by small interfering RNA-directed gene silencing (32). Thus, because AIB1 and FoxG1 form a complex (Figure 1, A and B), we conjectured that a portion of the FoxG1-induced apoptotic effect might be mediated through changes in AIB1 expression. Consistent with this notion, FoxG1 overexpression caused a 2-fold decrease in both AIB1 mRNA and protein expression levels (Figure 2B). We also observed a similar effect of FoxG1 on AIB1 expression in the metastatic MDA-MB-231 cells. In these cells, FoxG1 overexpression caused a 3% to 6% increase in early apoptosis and a near 60% reduction in the levels of AIB1 protein (Supplemental Figure 1, A and B, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). This indicates that FoxG1 can have a negative regulatory effect on AIB1 expression also in other different subtypes of breast cancer cells that are ER negative.
We next asked whether the FoxG1 induction of apoptosis was mediated directly by the loss of AIB1 by determining whether exogenously expressed AIB1 was sufficient to rescue these cells from FoxG1-induced apoptosis. Our analysis revealed that AIB1 coexpression with FoxG1 allowed an approximately 50% rescue from apoptosis compared with cells transfected with only FoxG1 (Figure 2C, right panel; compare second bar with the fourth bar). Thus, our phenotypic studies argue that a significant portion of the FoxG1 induction of apoptosis in MCF-7 cells is mediated through down-regulation of AIB1 expression.
FoxG1 represses AIB1 promoter activity
Our data demonstrate that FoxG1 overexpression represses the levels of AIB1 transcript (Figure 2B); thus, we hypothesized that FoxG1 could directly regulate the AIB1 promoter. Previous studies have identified a region in the AIB1 gene promoter responsible for the positive autoregulation of transcription through the recruitment of an activating transcriptional protein complex involving AIB1, E2F1, and Sp1 (17). This critical positive regulatory sequence covers a −250 to +350 span up- and downstream of the transcription start site of the AIB1 gene. An intronic Sp1-binding site, marked by a GC box at +150/+160 bp is required for the AIB1 promoter activation by E2F1 and AIB1. Moreover, the Sp1-binding sequence serves as a docking site for the recruitment of the AIB1-E2F1 complex, through which AIB1 can act as a coactivator on its own promoter, establishing a positive autoregulatory loop of AIB1 gene expression (Figure 3A) (17).
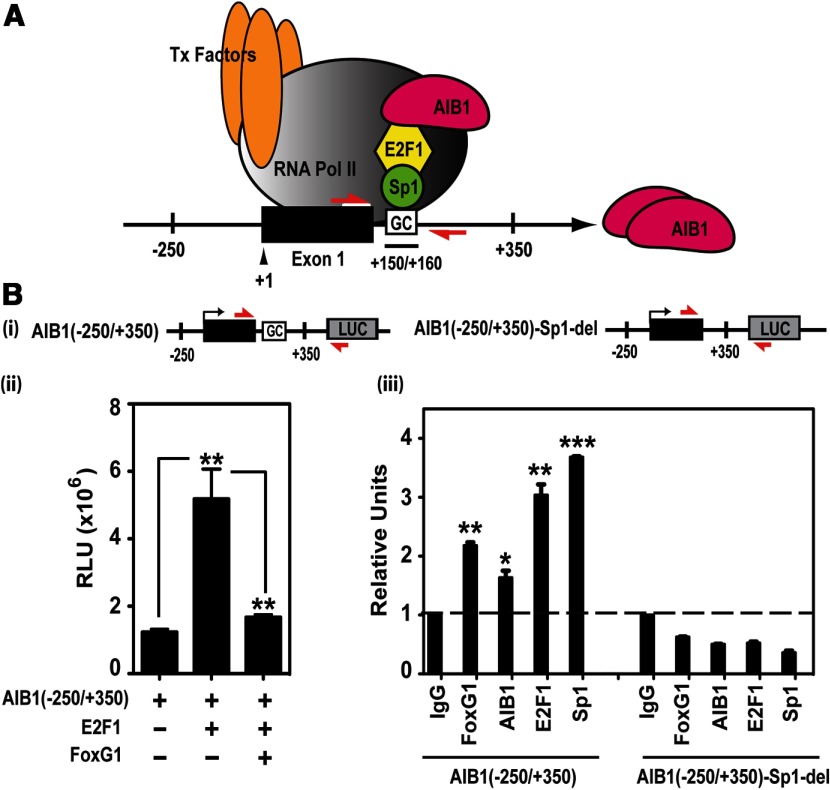
Figure 3.
FoxG1 Represses AIB1 Gene Promoter Activity. A, Model of the AIB1 gene promoter. Model showing an activating transcriptional complex consisting of AIB1, E2F1, and Sp1, anchored to DNA through a Sp1-binding site, the GC box, which is downstream of exon 1 (black box) in the −250 to +350 bp region of the AIB1 promoter. The red arrows represent the locations and orientations of the AIB1 promoter-specific primers. B (panel i), AIB1 WT and mutant Sp1 site-deleted promoter luciferase reporters. The red arrows are primers that specifically detect these reporters. B (panel ii), FoxG1 represses the activity of the AIB1 promoter reporter. MCF-7 cells were transfected with WT AIB1 (−250/+350) reporter alone, or together with E2F1 in the presence or absence of FoxG1. A representative graph is shown from 2 independent experiments, and data were analyzed by 1-way ANOVA with Bonferroni posttest. **, P < .01 when E2F1 is compared with promoter alone; or FoxG1 and E2F1 together relative to E2F1. B (panel iii), Protein association to the Sp1-binding site in the transfected AIB1 gene promoter. HEK293T cells were transfected with either the WT AIB1 reporter or the mutant reporter where the Sp1-binding sequence is deleted. Cells were processed for ChIP 6 hours after transfection. Recruitment of FoxG1, AIB1, E2F1, and Sp1 to both the WT and mutant reporters was assessed with a pair of primers that specifically detect the transfected reporter DNA (panel i, red arrows). The IgG-ChIP was arbitrarily set as 1, and all the samples were analyzed and plotted in reference to IgG. Data represent 2 independent experiments and were analyzed by Student's t test. ***, P < .001; **, P < .01; *, P < .05 compared with IgG control.
To determine whether FoxG1 impacted AIB1 promoter activity, we cotransfected MCF-7 cells with an E2F1 expression vector in the presence or absence of FoxG1 and a wild-type (WT) AIB1 promoter-luciferase reporter containing the intact positive regulatory sequence of the AIB1 gene promoter (Figure 3B, panel i). E2F1 significantly enhanced the AIB1 promoter reporter activity, as shown previously (Figure 3B, panel ii) (17), whereas the addition of FoxG1 suppressed E2F1-induced AIB1 promoter activation back to the basal level, indicating an inhibitory role for FoxG1 in AIB1 gene expression (Figure 3B, panel ii).
Because the intronic GC-rich, Sp1 binding sequence is essential for the recruitment of Sp1, AIB1, and E2F1 (17), we next tested whether FoxG1 is also recruited to the AIB1 gene promoter through this element. We performed chromatin immunoprecipitation (ChIP) assays using HEK293T cells transfected with either the WT luciferase reporter AIB1(−250/+350) or the same reporter with a deleted Sp1 site: AIB1(−250/+350)-Sp1-del (Figure 3B, panel i). PCR primers with a forward primer positioned on exon 1 of the AIB1 gene and a reverse primer in the luciferase sequence were used to specifically detect and distinguish the transfected luciferase vectors from the endogenous AIB1 promoter (Figure 3B, panel i, red arrows). Our ChIP analysis showed that FoxG1 is associated with the WT AIB1(−250/+350) reporter but not the mutant reporter AIB1(−250/+350)-Sp1-del (Figure 3B, panel iii). Consistent with published literature (17), our data also demonstrated that AIB1, E2F1, and Sp1 bind to the WT AIB1(−250/+350) reporter, whereas the Sp1 site deletion abolished recruitment of these proteins (Figure 3B, panel iii). These results indicate that the Sp1-binding site is not only required for Sp1, E2F1, and AIB1 binding to the AIB1 promoter, but it is also critical for the recruitment of FoxG1.
FoxG1 forms a complex with AIB1 and E2F1 on the endogenous AIB1 gene promoter
We next performed ChIP assays to investigate whether FoxG1 is directly recruited to the −250/+350 region of the endogenous AIB1 gene promoter in MCF-7 cells. Using a pair of AIB1 promoter-specific primers in which the forward primer is positioned on exon 1 of the AIB1 gene and the reverse primer is situated downstream of the Sp1-binding sequence (Figure 3A, red arrows), we found that an antibody specific to FoxG1, but not the IgG control, successfully immunoprecipitated endogenous FoxG1 on the AIB1 promoter (Figure 4A). As a negative control we demonstrated no specific FoxG1 binding relative to the IgG control to a region in the coding sequence of exon 4 of the AIB1 gene (Figure 4A). We also show that FoxG1 is not recruited specifically to the nontarget albumin promoter (Figure 4A). Together, these ChIP data support FoxG1 specific recruitment to the AIB1 promoter.
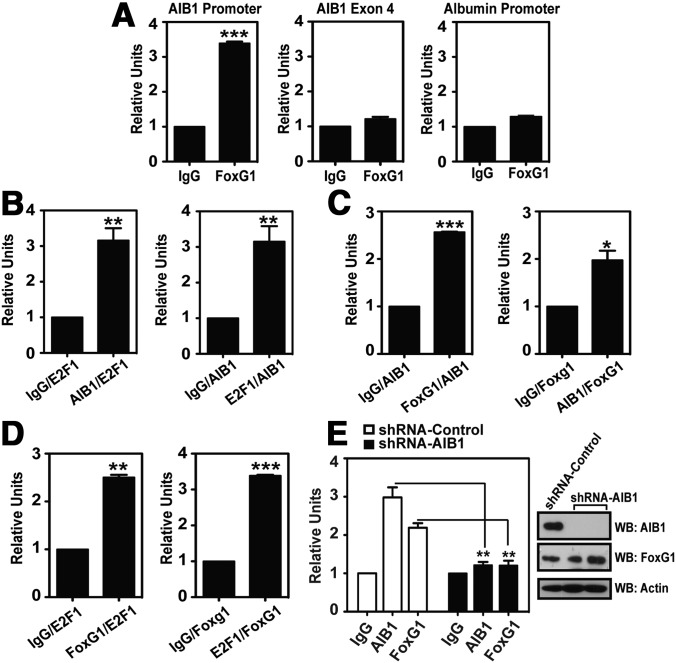
Figure 4.
FoxG1 Forms a Complex with AIB1 and E2F1 on the AIB1 Gene Promoter. A, FoxG1 is recruited to the endogenous AIB1 promoter. ChIP assays were performed in MCF-7 cells, where endogenous FoxG1-DNA complex was immunoprecipitated with anti-FoxG1 antibody or isotype IgG. Protein-enriched DNA was analyzed by RT-PCR using AIB1 promoter-specific primers (Figure 3A, red arrows) or primers that will either amplify a region in exon 4 of the AIB1 gene or the albumin promoter. ChIP results were analyzed by Student's t test, where ***, P < .001 relative to IgG. B–D, Two-step reChIP assays were performed in MCF-7 cells, and all DNA samples were subjected to 2 rounds of ChIPs. Sonicated chromatin was immunoprecipitated first with AIB1 or E2F1- (panel B), FoxG1 or AIB1- (panel C), or FoxG1 or E2F1 antibodies (panel D), followed by reChIP with antibodies specific to E2F1 or AIB1 (panel B), AIB1 or FoxG1 (panel C), or E2F1 or FoxG1 (panel D). As a negative control, isotype IgG was used for the first-round ChIPs followed by reChIP of the respective second-round antibodies. The endogenous AIB1 promoter bound to each immunocomplex as indicated in the figure was analyzed by qPCR using the AIB1 promoter-specific primers. Student's t test. ***, P < .001;4 **, P < .01; *, P < .05 when compared with each respective IgG-reChIP control. E, FoxG1 recruitment to the endogenous AIB1 promoter is dependent on AIB1. Endogenous AIB1 was depleted by infection of MCF-7 cells with lentiviral vectors expressing shRNAs targeting a distinct sequence in AIB1 (shRNA-AIB1) or control scrambled shRNA (shRNA-Control). Ninety-six hours after lentiviral infection, cells were subjected to ChIP analyses in which protein-DNA complexes were immunoprecipitated with antibodies against either AIB1 or FoxG1, or an isotype IgG. Student's t test. **, P < .01; shRNA-Control vs shRNA-AIB1. The amount of AIB1 protein knocked down 96 hours after infection was assessed by WB with antibodies as indicated.
AIB1 has been shown to directly interact with E2F1, and the AIB1-E2F1 complex is essential for E2F1-regulated gene transcription (7). Individual binding of AIB1 and E2F1 to the −250/+350 region of the endogenous AIB1 gene promoter is well established (17), and our goal was to determine whether AIB1 and E2F1 are recruited to this region as a complex. We performed reciprocal ChIP-reChIP (reChIP) assays to address this question. Cross-linked and sonicated chromatin was prepared from MCF-7 cells, incubated and immunoprecipitated first with either AIB1 or E2F1 antibodies. The AIB1- or E2F1-ChIP, followed by “release” of the protein-enriched chromatin, was subjected to a subsequent ChIP with antibodies specific to either E2F1 or AIB1, respectively. As a control, isotype IgG was used for the first round of ChIP followed by E2F1- or AIB1-reChIP. The 2-step reciprocal reChIP successfully precipitated the endogenous AIB1 promoter, indicating that AIB1 and E2F1 form a protein complex at the −250/+350 region of the AIB1 promoter (Figure 4B).
We next assayed for endogenous FoxG1 participation in the AIB1-E2F1 complex by reciprocal reChIP assays. We found that the endogenous AIB1 promoter was precipitated from FoxG1/AIB1 and FoxG1/E2F1 reChIP immunoprecipitates, as well as AIB1/FoxG1 and E2F1/FoxG1 reChIP immunoprecipitates (Figure 4, C and D). These data suggest simultaneous chromatin co-occupancy of the 3 proteins and indicate that there is basal level recruitment of endogenous AIB1, E2F1, and FoxG1 to the −250/+350 regulatory sequence of the AIB1 promoter.
We have confirmed an interaction between AIB1 and FoxG1 (Figure 1, A and B) and shown that the two proteins are recruited to the endogenous AIB1 gene promoter as a complex (Figure 4C). To examine whether FoxG1 occupancy at the endogenous AIB1 promoter is dependent on the corecruitment of AIB1, we infected MCF-7 cells with lentiviral vectors expressing short hairpin RNAss (shRNAs) targeting AIB1 (shRNA-AIB1) or control scrambled shRNAs (shRNA-Control). Depletion of AIB1 protein by shRNA silencing led to a 3-fold decrease in AIB1 occupancy at the endogenous AIB1 gene promoter as well as a 2-fold reduction in the recruitment of FoxG1 to the promoter (Figure 4E). This decrease in FoxG1 occupancy at the AIB1 promoter was not due to a reduction in FoxG1 expression because its protein levels remained unchanged whereas the levels of AIB1 protein were significantly reduced (Figure 4E, Western blot). Together our data indicate that the presence of AIB1 is required for the corecruitment of FoxG1 to the AIB1 gene promoter.
FoxG1 compromises the integrity of the activating complex on the AIB1 gene promoter
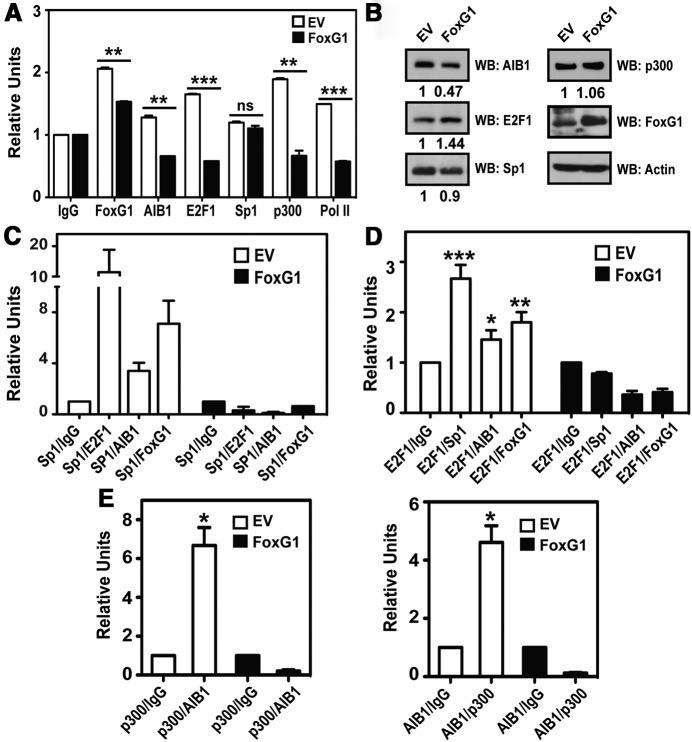
We next performed ChIP assays to investigate the effect of overexpressing FoxG1 (at levels that suppress AIB1 mRNA expression and cause apoptosis as shown in Figure 2, A and B) on the transcription complex present on the endogenous AIB1 promoter at +150/+160 bp (Figure 3A). ChIP assays were performed from MCF-7 cells that had been transfected with either an EV control or a FoxG1-expression vector. We demonstrate that increased expression of FoxG1 resulted in greater than 50% decline in the promoter-binding activities of AIB1, E2F1, p300, and RNA polymerase II (Pol II), although there was no significant change in Sp1 recruitment at the promoter level (Figure 5A). The loss of promoter binding was not due to FoxG1-induced changes in gene expression because protein levels of these factors were either unchanged or increased (eg, E2F1) when FoxG1 was overexpressed (Figure 5B). Interestingly, we did not observe increased chromatin occupancy of FoxG1 when it was overexpressed (Figure 5A), suggesting that once cellular expression of FoxG1 is above a threshold level, it can promote rapid disassembly of the Sp1-associated complex without affecting the direct binding of Sp1 to the +150/+160 AIB1 promoter-binding element. To test this, we performed reciprocal reChIP experiments to assess the integrity of the transcriptional protein complex with increased expression of FoxG1. We show that FoxG1 overexpression in MCF-7 cells caused significant reduction in the recruitment of protein complexes comprising Sp1 and E2F1, AIB1, and FoxG1 respectively, or E2F1 and Sp1, AIB1, and FoxG1 respectively (Figure 5, C and D). Most interestingly, we observed that overexpressing FoxG1 led to a near 3- to 12-fold decrease (depending on the orientation of the reChIP) in the corecruitment of E2F1 complexed with Sp1 to the AIB1 promoter (Figure 5, C and D; compare “Sp1/E2F1” and “E2F1/Sp1” between EV, white bar, and FoxG1, black bar).
Figure 5.
FoxG1 Destabilizes the Sp1-Associated Transcription Complex on the AIB1 Gene Promoter. A, FoxG1 overexpression leads to decreased recruitment of the members of the transcriptional complex to the endogenous AIB1 promoter. ChIP assays were performed in MCF-7 cells transfected with EV or FoxG1 vectors, by enriching protein-bound endogenous AIB1 promoter with antibodies as indicated. Student's t test, in which ***, P < .001; **, P < .01 were FoxG1-expressing cells (black bars) relative to EV (white bars). B, Relative protein levels after FoxG1 transfection in MCF-7 cells are shown by WB and probed with antibodies as indicated. C and D, Overexpressing FoxG1 compromises the integrity of the transcription complex. The immunocomplexes associated with the AIB1 promoter were assessed by reChIP experiments, where chromatin was immunoprecipitated sequentially first with an anti-Sp1 antibody, followed by reChIP with antibodies specific to either E2F1, AIB1, or FoxG1; or first with an anti-E2F1 antibody, followed by reChIP with antibodies specific to either Sp1, AIB1, or FoxG1. The Sp1-ChIP and E2F1-ChIP were also followed by a reChIP of IgG as a negative control. ***, P < .001; **, P < .01; *, P < .05 relative to E2F1/IgG. Student's t test. E, Overexpressing FoxG1 causes reduction in p300-AIB1 co-occupancy at the AIB1 promoter. MCF-7 cells were transfected with EV or FoxG1 as in panel A and harvested for reChIP experiments by performing reciprocal and sequential ChIPs using antibodies specific to p300, followed by AIB1, or to AIB1, followed by p300. The AIB1 promoter-specific primers were used to assess the relative occupancy of the AIB1–p300 complex at the endogenous AIB1 promoter. *, P < .05 relative to p300/IgG or AIB1/IgG. Student's t test.
The recruitment of p300 to the +150/+160 Sp1-associated complex has not been described previously, and overexpression of FoxG1 also reduces its association with the complex at the AIB1 promoter (Figure 5A), without a reduction in p300 protein expression (Figure 5B). The binding of p300 to AIB1 is known to promote and stabilize transcriptional complex formation and exert a positive effect on gene transcription (3, 33, 34). Therefore, we wanted to determine whether overexpressing FoxG1 had any effect on the recruitment of the AIB1-p300 complex. We assessed co-occupancy of AIB1 and p300 at the AIB1 promoter by reciprocal reChIP and discovered a 5- to 8-fold reduction in the recruitment of AIB1-p300 complex to the AIB1 promoter in MCF-7 cells transfected with FoxG1 as compared with control (Figure 5E).
FoxG1 disrupts AIB1's coactivator function
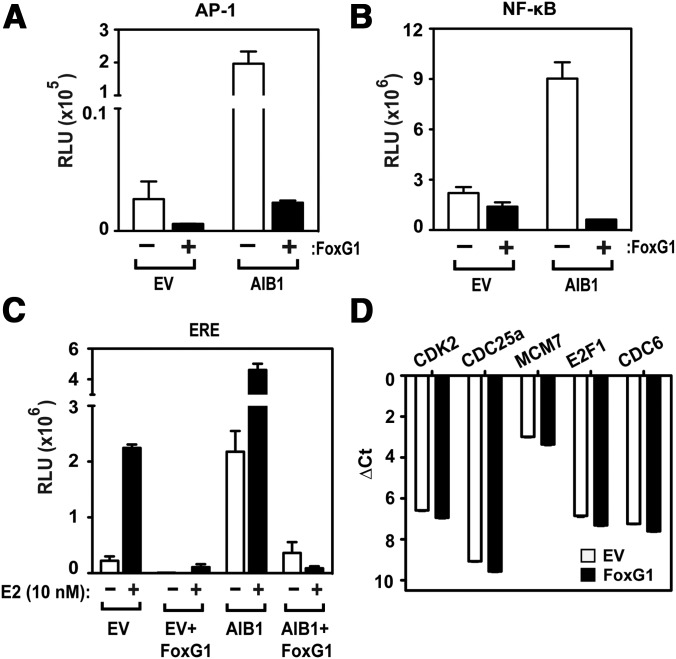
We next tested whether the effect of FoxG1 on AIB1-containing transcription complexes was limited to the Sp1-binding site in the AIB1 gene promoter or whether other promoter elements known to involve AIB1 were also affected. AIB1 has been shown previously to coactivate NF-κB and AP-1 (8, 10). Therefore we examined the impact of FoxG1 on promoters containing these transcription factor-binding sites. As reported previously (35), AIB1 overexpression induced transcription from all these reporters (Figure 6, A–C) but concomitant FoxG1 overexpression caused a significant reduction in the AIB1-induced transcription of the AP-1 and NF-κB promoters (Figure 6, A and B). We also observed a near-complete reversal of AIB1 coactivation on the estrogen-responsive promoter (ERE) reporter in the presence of FoxG1 and estrogen (Figure 6C). Interestingly, FoxG1 expression caused a slight decrease in AIB1 coactivation on the promoter-reporters in the EV-transfected cells (Figure 6, A–C). HEK293T cells express endogenous AIB1 protein, which may coactivate the reporters and affect transcription from the EV control. Thus, FoxG1 overexpression should dampen basal AIB1-coactivating activity. To assess the impact of FoxG1 expression on endogenous genes in MCF-7 cells, we used qPCR to generate an mRNA expression profile of 168 genes that are known to participate in or respond to ER or NF-κB signaling. In addition, 5 housekeeping genes were included as a loading control (ACTB, B2M, GAPDH, HPRT1, RPLP0). Using a cutoff of gene expression changes of more than 1.5-fold and P < .01, we found that in the NF-κB gene set, 30 genes were up-regulated and 14 were down-regulated (40 were unchanged); in the ER-responsive gene set, 8 genes were up-regulated and 42 genes were down-regulated (34 were unchanged) after overexpression of FoxG1. In Supplemental Table 1 we detailed some of the notable genes involved in breast cancer pathogenesis that were down-regulated by FoxG1 from the NF-κB and ER gene array sets. However, the repressive effect of FoxG1 on AIB-coactivated gene promoters was not universal because we found in parallel experiments that FoxG1 overexpression had no impact on endogenous E2F1-regulated genes such as CDK2, CDC25a, MCM7, E2F1, and CDC6 in MCF-7 cells (Figure 6D). These data indicate that FoxG1 is important not only for the control of AIB1 promoter, but also for some AIB1-regulated steroid-dependent and -independent transcription, although the impact of FoxG1 is dependent on the promoter context.
Figure 6.
FoxG1 Disrupts AIB1's Coactivator Function. A and B, FoxG1's effect on steroid-independent promoters. HEK293T cells were transfected with AIB1 expression constructs as indicated with either a multimerized AP-1 reporter (panel A) or a multimerized NF-κB reporter (panel B), in the presence or absence of FoxG1. c-fos and c-jun expression vectors were also cotransfected with the AP-1 reporter. Cells were lysed 24 hours after transfection to measure luciferase activity. C, FoxG1's effect on estrogen-stimulated transcription. AIB1 was cotransfected with ERα and ERE constructs into hormone-stripped HEK293T cells, with or without cotransfection of FoxG1. Cells were treated with ethanol (−) or 10 nM estradiol (E2) (+) for 24 hours and analyzed for reporter activity. Results are expressed as changes in the level of activation compared with EV-transfected cells. D, FoxG1 has no effect on E2F1-regulated gene expression. MCF-7 cells were transfected with EV or FoxG1, and total RNA was harvested from cells to determine the relative gene expression for CDK2, CDC25A, MCM7, E2F1, and CDC6. The Ct values were normalized to actin expression as control.
Discussion
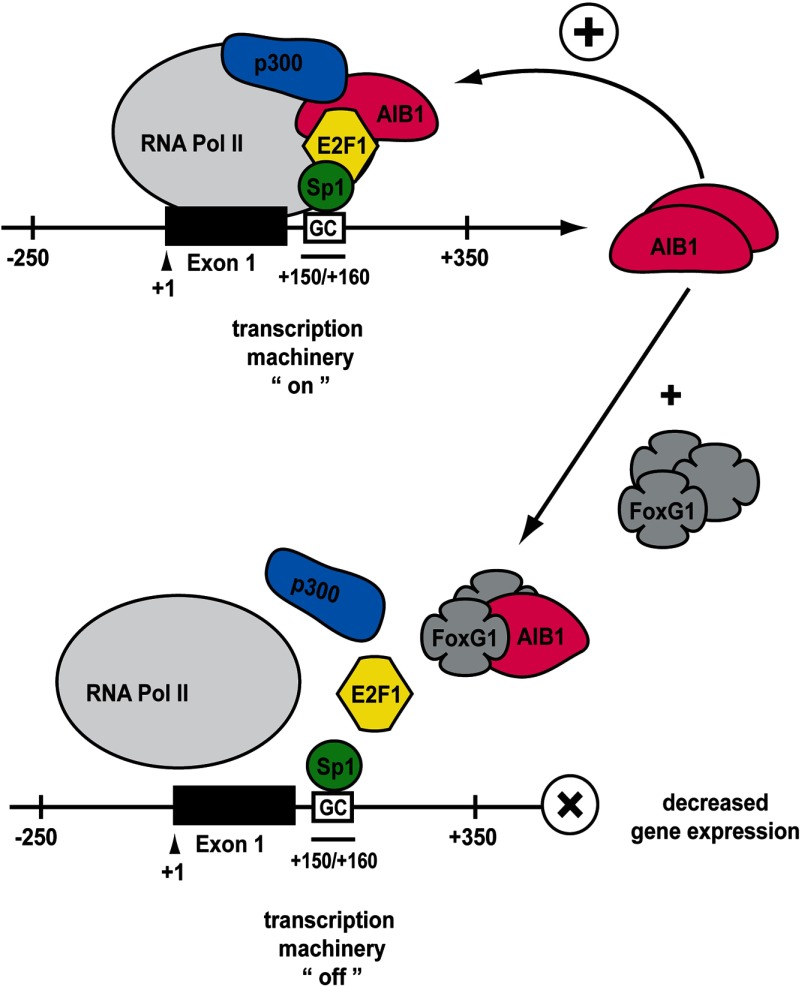
The control of the overall levels and activity of the nuclear receptor coactivator AIB1 in a cell occurs at multiple levels including control of AIB1 levels of gene transcription (17, 36), control of AIB1 mRNA stability and degradation (with eg, miRNA-17–5p (37)), control of protein modification including phosphorylation (35, 38–40), acetylation (3), and sumoylation (41) and control of proteasomal degradation of AIB1 protein (13, 14, 42). In the current study we have determined that FoxG1 can control levels of AIB1 mRNA by directly influencing the transcription of the AIB1 gene. Based on our data we propose a model (Figure 7) whereby the Sp1 site at +150/+160 bp of the AIB1 gene promoter is directly repressed by increasing levels of FoxG1. AIB1 can complex with E2F1 and together regulate the activity of its own promoter (17, 36). E2F1 can regulate AIB1 promoter activity by interacting with Sp1 bound at +150/+160 bp which, via direct binding to DNA, appears to anchor the E2F1-AIB1 coactivating complex to the AIB1 gene promoter (Figure 7) (17). This allows AIB1 to coactivate and enhance the transcriptional activity of its own promoter. Our data show a reduction in the recruitment of the “anchorage complex”, E2F1-Sp1, as well as the essential “coactivating complex”, E2F1-AIB1, to the AIB1 promoter when FoxG1 is overexpressed (Figure 5, C and D). Our data also indicate that p300 is recruited as part of the activating complex necessary for high levels of AIB1 gene transcription (Figures 5A and 7). CBP/p300 can bind AIB1 directly, promote stable formation of the transcription complex, and has strong histone acetylase activity necessary for altering local chromatin structure and activating transcription (3, 34). We show that overexpressing FoxG1 led to a dramatic reduction in the recruitment of p300-AIB1 complex to the AIB1 promoter (Figure 5E). Our data indicate that as FoxG1 levels rise in the cell, the Sp1-associated transcription complex is disrupted, causing E2F1, AIB1, and p300 to dissociate from Sp1, thus reducing AIB1 gene transcription. Interestingly, Hsia et al (43) have shown that E2F family proteins together with AAA+ nuclear coregulator cancer-associated proteins are recruited to the AIB1 gene promoter by binding to multiple noncanonical E2F binding sequences within the AIB1 first exon and intron regions and are able to directly control AIB1 expression in breast cancer cells. However, we believe that the inhibition of AIB1 gene transcription by FoxG1 requires no other elements of the AIB1 gene promoter because deletion of the Sp1 binding sequence alone effectively prevents recruitment of FoxG1, AIB1, E2F1, and Sp1 to the AIB1 promoter reporter (Figure 3B, panel iii).
Figure 7.
A Proposed Model for the role of FoxG1 in Regulating AIB1 Gene Expression. FoxG1 binds to, and reduces, AIB1 binding to the components of the activating transcription complex that is required for the up-regulation of AIB1 gene expression. In the presence of increased FoxG1 levels, the activating complex disassembles and disassociates from the AIB1 promoter, leading to reduced AIB1 gene transcription.
Previous studies have shown that FoxG1 can cause transcriptional repression by binding DNA directly (44) and nucleating a repressosome by recruiting histone deacetylase 1 and TLE (transcriptional corepressor of the groucho/transducin-like enhancer of split) family proteins (25). However, a search (http://www.ncbi.nlm.nih.gov/nuccore) of the region flanking the Sp1 site in the AIB1 gene promoter using AIB1 genomic DNA (GenBank accession no. AL353777) shows no match for the consensus FoxG1-binding sequence, AATGTAAACA, which is evolutionarily conserved and commonly shared by avian, rat, and human FoxG1 gene (45). Furthermore, the interaction of AIB1 with FoxG1 in cell lysates occurs in the absence of tethering DNA. This suggests that in the context of the Sp1-binding site in the AIB1 promoter, FoxG1 inhibits transcription by disrupting an activating transcription complex bearing histone acetylase activity, rather than by forming a de novo repressosome after direct DNA binding to the AIB1 gene promoter. Also consistent with this paradigm of FoxG1-induced repression mechanism is that, in our ChIP assays in cells overexpressing FoxG1, we did not observe increased FoxG1 binding to the AIB1 promoter near the Sp1-binding site. In fact, as exogenous FoxG1 levels increase in the cell, the amount of FoxG1 present in the Sp1-associated transcription complex decreases along with the loss of AIB1 and E2F1. This suggests that at higher concentrations of FoxG1, there is an increase in its access or affinity for AIB1 binding, possibly though dimerization, and this in turn would accelerate degeneration and disassembly of the activating transcription complex. Similar models of repression have been seen with Foxp1, a transcriptional repressor of the forkhead protein family, which has been shown to be tumor suppressive in several types of cancers (46). Foxp1 can homo- and heterodimerize with Foxp2 and Foxp4, and dimerization is required for interacting with other transcription cofactors and for executing transcriptional repression (47).
Our data also suggest that FoxG1 may fall under the category of “short-range repressors,” which generally act within 100 bp of, or bind adjacently to, a transcriptional activator, causing inhibition through “quenching” (48–50). Short-range repressors may also directly interact with an activating cofactor and interfere with its activity or block its access to the basal transcriptional machinery (51, 52). It is been demonstrated that several members of the short-range repressors mediate transcriptional repression in a repressor concentration-dependent manner, in which higher repressor protein levels (as compared with low levels) are sufficient to switch a gene from an active to an inactive state (52–54). Therefore, it is possible that activation of the AIB1 gene promoter occurs when FoxG1 protein levels are lowered or lost in a cell. Because high AIB1 expression can lead to uncontrolled cell proliferation and tumorigenesis (11), it is possible that cells employ FoxG1 to control and dampen AIB1 transcription. FoxG1 thus may serve as a short-range repressor for the AIB1 promoter, because we have shown that FoxG1 binding to AIB1 leads to the detachment of the critical activating protein complexes from the AIB1 gene promoter, which subsequently causes the disintegration of the Sp1-associated positive regulatory transcription complex (Figure 5, C and D). In this sense, FoxG1 acts like a tumor suppresser, and this would be consistent with the loss of FoxG1 expression as cells evolve from normal to cancerous (Figure 1C).
A question that arises from the proposed model in Figure 7 is: How does FoxG1 cause destabilization of the Sp1-associated transcription complex? One possibility is that through binding, FoxG1 induces a conformational change in AIB1 that leads to reduced affinity between AIB1 and the other members of the Sp1 transcription complex. Future studies will be directed to examine the domains of AIB1 and FoxG1 responsible for complex formation and repression of gene transcription. Another intriguing observation from our study is that, despite the disruptive effect FoxG1 exerts on AIB1-mediated coactivation, we found that this is not limited to, or exclusive to, the Sp1-regulatory sequence. In fact, we observed dramatic reductions in AIB1 coactivation at NF-κB and AP-1 regulatory elements (Figure 6, A and B). Although not all AIB1-associated promoter elements are influenced by FoxG1, because the transcription of a number of E2F1-driven genes was unaffected by increasing amounts of FoxG1 in the cell. This implies that FoxG1 repression of a gene promoter activity is context specific for AIB1 in a transcription complex. A recent genome-wide location analysis of AIB1 chromatin affinity sites in 17β-estradiol-treated MCF-7 cells demonstrated a significant overlap of AIB1 with FoxA1 binding sites in the breast cancer cell DNA (55). FoxA1 is another member of the forkhead family and a determining factor for ER function and endocrine response (56). It would be interesting to investigate the portion of AIB1 genomic binding sites that are also engaged by FoxG1, and to determine whether such a population represents a subset of FoxG1-regulated genes.
Reintroducing AIB1 into FoxG1-induced apoptotic MCF-7 cells was able to only partially restore viability in these cells (Figure 2C). This could indicate that FoxG1 induction of apoptosis also involves genes that are not directly regulated by AIB1. However, complete replenishment of endogenous AIB1 levels is difficult to achieve after knockdown, and also the temporal response of different AIB1 regulated genes can be variable. Global ChIP assays have revealed that AIB1 is widely distributed in the genome (55), and our data have shown that FoxG1 down-regulates AIB1 coactivation of AP-1 and NF-κB transcription (Figure 6, A and B). AP-1 is known to promote the expression of genes involved in cell cycle progression (57), and NF-κB-dependent gene transcription is crucial for proproliferation and antiapoptosis signals (58). Thus reduction in AIB1 levels by FoxG1 repression likely has effects on multiple AIB1-regulated pathways that result in apoptosis. Of note is that FoxG1 is also known to antagonize TGF-β signaling by binding to, and blocking, the action of Sma and Mad related protein-3/4 proteins (SMAD3/4), both of which are major signal transducers of TGF-β (59). Interestingly, AIB1 is one of a number of TGF-β-responsive genes in A549 human lung carcinoma cells (60), and TGF-β can significantly up-regulate AIB1 gene transcription in MCF-7 cells (16). Thus FoxG1 inhibition of TGF-β signaling might also be involved in the FoxG1-mediated apoptosis in MCF-7 cells.
The overexpression of the oncogene AIB1 is associated with worse disease outcome in multiple types of tumors (61). However, loss of AIB1 can also be pro-oncogenic in certain contexts, such as in B cell lymphoma (62). Similarly, although FoxG1 can interact with AR directly in vitro and acts as a corepressor to both AR- and PR-mediated transactivation (24), FoxG1 is also shown to be up-regulated in ovarian cancer (63), and its gene amplification is associated with the development of bladder cancer and medulloblastoma (64, 65). This suggests that FoxG1 can be either pro- or antioncogenic, depending on the cellular environment. Our analysis of the microarray data generated from breast cancer populations indicates that lower levels of FoxG1 segregate with worse clinical outcome (Figure 1E). Because estrogen is reported to suppress cellular AIB1 expression in MCF-7 cells (16), it is possible that ER status may contribute to the better prognosis associated with higher FoxG1 levels in patients. However, the risk of relapse with low FoxG1 was not significantly different in the ER-positive and -negative patient populations analyzed in Figure 1E (data not shown). Overall our observations in the present study suggest that FoxG1 can act like a tumor suppressor in breast cancer, and down-regulation of FoxG1 function could represent an important mechanism to drive AIB1-dependent survival and growth. Mimicking FoxG1 binding to AIB1 with small molecule inhibitors is therefore a possible therapeutic approach in AIB1-overexpressing cancers.
Materials and Methods
Plasmids
HA-p300, E2F1, and internal ribosome entry site-FoxG1 constructs were kindly provided by Dr. Maria L. Avantaggiati (Georgetown University, Washington D.C.), Dr. Hongwu Chen (University of California, Davis, California), and from Dr. Joan Massague (Sloan-Kettering Institute, New York, New York), respectively. FLAG-AIB1 plasmid was previously described (35, 66). HA-FoxG1 was generated first by restriction enzyme digestion of the internal ribosome entry site-FoxG1 construct with EcoRI and BamHI, followed by insertion of the excised FoxG1-coding region into phCMV2.
Cell lines and transient transfection
HEK293T, MDA-MB-231, and MCF-7 were obtained from the Tissue Culture Shared Resource at Georgetown University. HEK293T and MDA-MB-231 were grown in DMEM (Invitrogen, Carlsbad, California) and MCF-7 was cultured in phenol red-free Iscove's modified Eagle's medium (IMEM, Invitrogen). All the mediums were supplemented with 10% FBS. The HMECs were purchased and cultured in commercially supplied medium (BulletKit; Lonza, Walkersville, Maryland). Transient transfection was performed in HEK293T and MCF-7 cells with FuGENE 6 (Roche, Indianapolis, Indiana) and FuGENE HD (Promega Corp, Madison, Wisconsin), respectively.
Western blot (WB), nuclear extraction, IP
For interaction of AIB1 with FoxG1 in HEK293T, cells were transfected with 8 μg of either FLAG-AIB1, HA-FoxG1, or FLAG-AIB1 and HA-FoxG1 together. Cells were washed 48 hours after transfection with cold 1× PBS and lysed in 1% Nonidet P-40 lysis buffer containing 1 mM NaO3VO4 and 1× Complete protease inhibitor tablet (Roche). Whole-cell lysates were subjected to IP with anti-FLAG M2 affinity gel (Sigma, St Louis, Missouri) as described previously (67), and samples were subjected to SDS-PAGE. WB was probed with antibodies against FLAG (M2, Sigma) or HA (Y-11, Santa Cruz Biotechnology, Inc, Santa Cruz, California).
For the endogenous interaction of AIB1 with FoxG1, MCF-7 cells were plated in 15-cm dishes, and nuclear lysates were prepared from cells as per the protocol recommended by the CelLytic NuCLEAR Extraction kit (NXTRACT, Sigma). Nuclear lysates (2 mg) were used to immunoprecipitate AIB1 with anti-AIB1 antibody (611105, BD Biosciences, San Jose, California). The amount of FoxG1 associated with AIB1 was detected with a FoxG1 antibody (ab3394, Abcam, Cambridge, Massachusetts), and the precipitated AIB1 was probed using FLAG M2 antibody (Sigma).
For protein expression levels in MCF-7 cells overexpressing FoxG1, cells were transfected with an EV control or FoxG1 constructs for 24 hours. Whole-cell lysates were prepared as indicated in step 1 above, and relative protein levels were assessed with the following antibodies: AIB1 (5E11, Cell Signaling Technology Inc, Danvers, Massachusetts); E2F1 (KH95, Santa Cruz Biotechnology); Sp1 (PEP2, Santa Cruz Biotechnology); p300 (C-20, Santa Cruz Biotechnology); FoxG1 (ab3394, Abcam), and human actin (C4, Millipore Corp., Billerica, Massachusetts).
Kaplan-Meier (KM) analysis
KM survival curves are generated from Kaplan-Meier Plotter (http://www.kmplot.com) (31). Analysis parameters used to generate FoxG1 and AIB1 mRNA KM plots are the following: Affymetrix identifications of “206018_at” and “209062_x_at” were used for FoxG1 and RAC3/AIB1, respectively; data were plotted for RFS at 15-year follow-up threshold; patient data were split and analyzed by median using all probe sets per gene; database “n = 2361” was used to generate all the KM plots in this study.
Annexin V apoptosis assay and flow cytometry
MCF-7 cells were grown in 10-cm dishes with IMEM containing 10% FBS and transfected with an EV control or FoxG1-expressing constructs. After 24 hours, cells were trypsinized and stained with fluorescein isothiocyanate-conjugated Annexin V and propidium iodide as per the protocol recommended by the Trevigen Apoptotic Cell System Annexin V kit (Trevigen, Inc., Gaithersburg, Maryland). The percentage of cells in early and late apoptosis was determined using fluorescence-activated cell sorting (FACS) on a Facstar-Plus Dual Laser flow cytometer (Becton Dickinson, Franklin Lakes, New Jersey), provided by the Flow Cytometry Shared Resource at Georgetown University. Duplicate samples from each transfection condition were subjected to FACS analysis for apoptotic index.
ChIP and reChIP
For ChIP assays using transfected HEK293T, cells in 15-cm dishes were transfected with 12 μg of either WT AIB1(−250/+350) or mutant AIB1(−250/+350)-Sp1-del promoter reporter constructs. Cells were fixed 6 hours after transfection with 1% formaldehyde (3.7% formaldehyde, 100 mM NaCl, 50 mM Tris/HCl, [pH 8.0], 1 mM EDTA, 0.5 mM EGTA) for 10 minutes at 37°C, and the reaction was stopped by 0.125 M of glycine solution for 5 minutes at room temperature. ChIP procedures were carried out essentially as described previously (68). Total protein (1 mg) was immunoprecipitated overnight with 5 μg of antibodies against either FoxG1 (ab18259, Abcam), AIB1/NCoA3 (C-20, Santa Cruz Biotechnology), E2F1 (1:1 mixture of C-20 and KH95; Santa Cruz Biotechnology), Sp1 (PEP2x, Santa Cruz Biotechnology) or IgG (as negative control). After reversal of cross-links and protein digestion, DNA was purified using GENECLEAN Turbo kit (Obiogene, Inc, Carlsbad, Califirnia). Real time PCR (qPCR) (iCycler, Bio-Rad Laboratories, Inc., Hercules, California) was performed in triplicate using IQ SYBR Green Supermix (Bio-Rad Laboratories) and 2 μL purified ChIP DNA to examine protein recruitment to the WT and mutant reporter constructs with the following primers: 5′-GCGAGTTTCCGATTTAAAGC (complementary to the 5′-AIB1 promoter sequence) and 5′-CTTTATGTTTTTGGCGTCTTCCA (complimentary to the 5′-reporter sequence) (17).
For the association of FoxG1 with the endogenous AIB1 promoter, MCF-7 cells were plated in 15-cm dishes and grown until 70%–80% confluence. Cells were cross linked and fragmented for ChIP assays as described above. The protein-DNA complexes were immunoprecipitated with 5 μg of negative control rabbit IgG (Santa Cruz Biotechnology) or anti-FoxG1 antibody. Purified DNA was analyzed by qPCR with a pair of AIB1 promoter-specific primers: 5′-GCGAGTTTCCGATTTAAAGC and 5′-GCCTTGGCAGATCTGAAG (17). To further verify the specificity of FoxG1 binding to the AIB1 promoter, ChIP DNA samples were also analyzed by qPCR with primers amplifying an region in exon 4 of the AIB1 gene (5′-AGACGGGAGCAGGAAAGTAA and 5′-CGCACATTTATCTGGTTTGACATTG) or primers that detect the albumin promoter (5′-TGGGGTTGACAGAAGAGAAAAGC and 5′-TACATTGACAAGGTCTTGTGGAG) (17). To investigate protein recruitment to the AIB1 promoter in MCF-7 cells overexpressing FoxG1, cells were transfected with either an EV control or FoxG1 constructs. Cells were collected 24 hours after transfection and sonicated, and crude chromatin solution was diluted and incubated overnight at 4°C with specific antibodies against FoxG1, AIB1, E2F1, Sp1, p300 (C-20x, Santa Cruz Biotechnology), Pol II (C-21x, Santa Cruz Biotechnology), and IgG as negative control. Purified DNA was analyzed by qPCR using the AIB1 promoter-specific primers.
The 2-step reChIP experiments were performed in MCF-7 cells. Chromatin was precleared with 20 μL Magna ChIP Protein A+G Magnetic Beads (16663, Millipore Corp.), and 2 mg chromatin DNA was immunoprecipitated with 40 μL beads and 5 μg of either AIB1, E2F1, or FoxG1 antibodies in the first round of ChIPs. The ChIP precipitates were gently washed as in the usual ChIP assay, and the chromatin-protein complexes were eluted from the beads in 75 μL TE buffer (10 mM Tris, pH 8.0; 1 mM EDTA, pH 8.0) containing 1× Complete protease inhibitor (Roche) and 10 mM dithiothreitol for 30 minutes at 37°C. After centrifugation, the supernatant from each sample was diluted with 1.5 mL ChIP dilution buffer (20 mM Tris, pH 8.0; 2 mM EDTA, pH 8.0; 150 mM NaCl; 1% Triton X-100) containing 1× Complete protease inhibitor and subjected to the second round of ChIP with 40 μL beads and 5 μg antibodies specifically against either E2F1, AIB1, or FoxG1. All first-round ChIPs were also followed by an IgG-ChIP as a negative control. Cross-links were reversed in the precipitated complexes with 200 mM NaCl for 16 hours at 65°C and proteins were digested with 1 μg of proteinase K for 1 hour at 45°C. For reChIP assays performed in MCF-7 cells with exogenously expressed FoxG1, sonicated chromatin was prepared from cells transfected with either an EV control or FoxG1 vectors and subjected to reChIP procedures as described above with the exception of first-round IPs using anti-E2F1 antibody, followed by second-round ChIPs with either IgG (negative control), Sp1, AIB1, or FoxG1 antibodies. Recovered ChIP DNA was purified and analyzed by qPCR with the AIB1 promoter-specific primers.
Data (Ct values obtained from qPCR) collected from all ChIP and reChIP experiments in this study were first calculated as percentage of their respective inputs. The IgG-ChIPs and reChIPs were then arbitrarily set as 1, and all the samples were analyzed and plotted in reference to IgG.
shRNA constructs and lentivirus infection
shRNA-AIB1 (5-TGGTGAATCGAGACGGAAACA-3) was subcloned into the EcoRI and AgeI restriction sites in PLKO.1 puro (Addgene, Cambridge, Massachusetts) (35). Control scrambled shRNA (shRNA-Control) was purchased from Addgene. Lentivirus production was performed as described previously using the recommended protocols for production of lentiviral particles with packaging plasmid (pCMV-dR8.2 dvpr) and envelope plasmid (pCMV-VSVG) (Addgene) (69).
Luciferase reporter assay
Cells were plated in triplicate for all the luciferase reporter assays. MCF-7 cells (50 000 cells per well in a 24-well dish) were transfected in serum-free IMEM with 0.5 μg WT AIB1(−250/+350) promoter reporter construct alone, or together with 0.25 μg E2F1, with or without cotransfection of 0.25 μg FoxG1. Cells were lysed 24 hours after transfection, in 100 μL 1× passive lysis buffer (Promega) and incubated at room temperature for 30 minutes on a rocker. Luciferase values were measured using the luciferase reporter assay kit (Promega). Protein concentration for each sample was determined using the BCA protein assay (Pierce Chemical Co., Rockford, Illinois), and luciferase values were normalized with their protein concentrations. Reporter assays using HEK293T cells were performed as described above. Cells were transfected in DMEM without serum with AIB1 expression plasmids and either 0.2 μg multimerized AP-1 or NF-κB reporter constructs (Stratagene, Santa Clara, California), in the presence or absence of 0.5 μg FoxG1. c-fos and c-jun expression vectors (25 ng) were also cotransfected with the AP-1 reporter. Hormone-stripped HEK293T cells used for ERE reporter assays were transfected with AIB1- (0.5 μg), ERα (20 ng) constructs, and ERE luciferase vector (0.2 μg), with or without 0.5 μg FoxG1 for 24 hours. Cells were then treated with hormone for 24 hours before assessing for luciferase activity.
RNA extraction and real time PCR
Total RNA was harvested using RNeasy mini kit (Qiagen, Valencia, California) and reverse transcribed with iScript can synthesis kit (Bio-Rad) using 1 μg of total RNA. cDNA fragments were amplified in triplicate by RT-qPCR (iCycler, Bio-Rad) of 45 cycles with primers listed in Table 1 (7, 36). For AIB1 and FoxG1 gene expression in the 7 tested cell lines, frozen cell pellets of BT-483, T47D, BT-549, HCC-1937, and BT-20 cell lines were obtained from the Tissue Culture Shared Resource at Georgetown University. HMEC and MCF-7 cells were plated in 10-cm dishes and grown in culture until 70%–80% confluence. The fold change in AIB1 and FoxG1 gene expression was normalized first to the human actin gene, and then calculated by the comparative Ct method, with relative transcript levels determined as y = 2^-ΔCT. AIB1 and FoxG1 mRNA expression of the 6 cancer cell lines was further normalized to the normal HMEC (set as 1), which expresses the lowest levels of AIB1 in the group. For E2F1-regulated gene expression in MCF-7 cells overexpressing FoxG1, cells were transfected with an EV control or FoxG1 constructs. After 24 hours of transfection, the ΔCt values of E2F1-regulated genes were obtained by normalizing to actin. Gene expression data for NF-κB and ER signaling and target genes were collected using NF-κB Signaling Targets RT (2) Profiler PCR Array and ER Signaling Pathway Activity RT (2) Profiler PCR Array (Qiagen). Expression of each gene was first normalized to the housekeeping genes and then to EV.
Table 1.
qPCR Primers Used in This Study
| Primers | Forward Sequence | Reverse Sequence |
|---|---|---|
| AIB1 | AGACGGGAGCAGGAAAGTAA | CGCACATTTATCTGGTTTGACATTG |
| FoxG1 | AGAAGAACGGCAAGTACGAGA | TGTTGAGGGACAGATTGTGGC |
| CDK2 | TTTGCTGAGATGGTGACTCGC | CACTGGAGGAGGGGTGAGATTAG |
| CDC25a | TGAAGAATGAGGAGGAGACCCC | CTGATGTTTCCCAGCAACTGTATG |
| MCM7 | AAGCCAGGAGTGCCAAACCAAC | GCAGCAGTGCCTTCTTCACATC |
| E2F1 | CGCATCTATGACATCACCAACG | GAAAGTTCTCCGAAGAGTCCACG |
| CDC6 | AAAGAGAATGGTCCCCCTCACTC | AGTTTTTCCAGTTCCAGGAGCAC |
| Actin | CCTGGCACCCAGCACAAT | GCCGATCCACACGGAGTACT |
Statistical analysis
All experiments were performed independently at least 2 times, and results are presented as means ± SEM. Data were analyzed by unpaired Student's t test for comparison of 2 groups or 1-way ANOVA with Bonferroni posttest for comparison of more than 2 groups. Statistical significance is indicated in each figure by asterisks: ***, P < .001; **, P < .01; *, P < .05.
Supplementary Material
Acknowledgments
This work was supported in whole or in part, by National Institutes of Health Grant R01 CA113477 from National Cancer Institute (to A.T.R.). This work was also supported by Center of Excellence Grant W81XWH-06–10590 (to V.C.J., A.W., and A.T.R.). Flow cytometry was performed by Lombardi Comprehensive Cancer Center core facilities, supported in part by cancer center support grant CA051008.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIB1
- amplified in breast cancer 1
- AP-1
- activator protein-1
- AR
- androgen receptor
- CBP
- CREB-binding protein
- ChIP
- chromatin immunoprecipitation
- E2F1
- E2F transcription factor 1
- ER
- estrogen receptor
- ERE
- estrogen-responsive promoter
- EV
- empty vector
- FACS
- fluorescence-activated cell sorting
- FoxG1
- forkhead-box protein G1
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- HMEC
- human mammary epithelial cell
- IMEM
- Iscove's modified Eagle's medium
- IP
- immunoprecipitation
- KM
- Kaplan-Meier
- MS
- mass spectrometry
- NF-κB
- nuclear factor-κB
- p300
- E1A-binding protein p300
- Pol II
- polymerase II
- reChIP
- ChIP-reChIP
- RFS
- relapse-free survival
- shRNA
- short hairpin RNA
- Sp1
- specificity protein 1
- WB
- Western blot
- WT
- wild-type.
References
- 1. Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anzick SL, Kononen J, Walker RL, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968 [DOI] [PubMed] [Google Scholar]
- 3. Chen H, Lin RJ, Schiltz RL, et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580 [DOI] [PubMed] [Google Scholar]
- 4. Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem. 1998;273:27645–27653 [DOI] [PubMed] [Google Scholar]
- 5. Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692 [DOI] [PubMed] [Google Scholar]
- 6. Hsia EY, Zou JX, Chen H-W. The roles and action mechanisms of p160/SRC coactivators and the ANCCA coregulator in cancer. Prog Mol Biol Transl Sci. 2009;87:261–298 [DOI] [PubMed] [Google Scholar]
- 7. Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol. 2004;24:5157–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ. Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res. 2006;66:11039–11046 [DOI] [PubMed] [Google Scholar]
- 9. Qin L, Liao L, Redmond A, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Werbajh S, Nojek I, Lanz R, Costas MA. RAC-3 is a NF-kappa B coactivator. FEBS Lett. 2000;485:195–199 [DOI] [PubMed] [Google Scholar]
- 11. Torres-Arzayus MI, Font de Mora J, Yuan J, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274 [DOI] [PubMed] [Google Scholar]
- 12. Lahusen T, Henke RT, Kagan BL, Wellstein A, Riegel AT. The role and regulation of the nuclear receptor co-activator AIB1 in breast cancer. Breast Cancer Res Treat. 2009;116:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mani A, Oh AS, Bowden ET, et al. E6AP mediates regulated proteasomal degradation of the nuclear receptor coactivator amplified in breast cancer 1 in immortalized cells. Cancer Res. 2006;66:8680–8686 [DOI] [PubMed] [Google Scholar]
- 14. Wu RC, Feng Q, Lonard DM, O'Malley BW. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell. 2007;129:1125–1140 [DOI] [PubMed] [Google Scholar]
- 15. Pavet V, Portal MM, Moulin JC, Herbrecht R, Gronemeyer H. Towards novel paradigms for cancer therapy. Oncogene. 2011;30:1–20 [DOI] [PubMed] [Google Scholar]
- 16. Lauritsen KJ, List HJ, Reiter R, Wellstein A, Riegel AT. A role for TGF-beta in estrogen and retinoid mediated regulation of the nuclear receptor coactivator AIB1 in MCF-7 breast cancer cells. Oncogene. 2002;21:7147–7155 [DOI] [PubMed] [Google Scholar]
- 17. Mussi P, Yu C, O'Malley BW, Xu J. Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol. 2006;20:3105–3119 [DOI] [PubMed] [Google Scholar]
- 18. Teyssier C, Chen D, Stallcup MR. Requirement for multiple domains of the protein arginine methyltransferase CARM1 in its transcriptional coactivator function. J Biol Chem. 2002;277:46066–46072 [DOI] [PubMed] [Google Scholar]
- 19. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852 [DOI] [PubMed] [Google Scholar]
- 20. Li X, Kimbrel EA, Kenan DJ, McDonnell DP. Direct interactions between corepressors and coactivators permit the integration of nuclear receptor-mediated repression and activation. Mol Endocrinol. 2002;16:1482–1491 [DOI] [PubMed] [Google Scholar]
- 21. Karmakar S, Gao T, Pace MC, Oesterreich S, Smith CL. Cooperative activation of cyclin D1 and progesterone receptor gene expression by the SRC-3 coactivator and SMRT corepressor. Mol Endocrinol. 2010;24:1187–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu ZZ, Kagan BL, Ariazi EA, et al. A Proteomic analysis of pathways involved in estrogen-induced growth and apoptosis of breast cancer cells. PLoS One. 2011;6:e20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152 [DOI] [PubMed] [Google Scholar]
- 24. Obendorf M, Meyer R, Henning K, et al. FoxG1, a member of the forkhead family, is a corepressor of the androgen receptor. J Steroid Biochem Mol Biol. 2007;104:195–207 [DOI] [PubMed] [Google Scholar]
- 25. Yao J, Lai E, Stifani S. The winged-helix protein brain factor 1 interacts with groucho and hes proteins to repress transcription. Mol Cell Biol. 2001;21:1962–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59 [DOI] [PubMed] [Google Scholar]
- 27. Shoichet SA, Kunde SA, Viertel P, et al. Haploinsufficiency of novel FOXG1B variants in a patient with severe mental retardation, brain malformations and microcephaly. Hum Genet. 2005;117:536–544 [DOI] [PubMed] [Google Scholar]
- 28. Zhao H, Langerød A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turashvili G, Bouchal J, Baumforth K, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132 [DOI] [PubMed] [Google Scholar]
- 31. Györffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731 [DOI] [PubMed] [Google Scholar]
- 32. Oh A, List HJ, Reiter R, et al. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res. 2004;64:8299–8308 [DOI] [PubMed] [Google Scholar]
- 33. Korzus E, Torchia J, Rose DW, et al. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707 [DOI] [PubMed] [Google Scholar]
- 34. Torchia J, Rose DW, Inostroza J, et al. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684 [DOI] [PubMed] [Google Scholar]
- 35. Oh AS, Lahusen JT, Chien CD, et al. Tyrosine phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced by Abl kinase and is required for its activity in cancer cells. Mol Cell Biol. 2008;28:6580–6593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Louie MC, Revenko AS, Zou JX, Yao J, Chen HW. Direct control of cell cycle gene expression by proto-oncogene product ACTR, and its autoregulation underlies its transforming activity. Mol Cell Biol. 2006;26:3810–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hossain A, Kuo MT, Saunders GF. Mir-17–5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu RC, Qin J, Hashimoto Y, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by IkB kinase. Mol Cell Biol. 2002;22:3549–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu RC, Qin J, Yi P, et al. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signaling pathways. Mol Cell. 2004;15:937–949 [DOI] [PubMed] [Google Scholar]
- 40. Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu H, Sun L, Zhang Y, et al. Coordinated regulation of AIB1 transcriptional activity by sumoylation and phosphorylation. J Biol Chem. 2006;281:21848–21856 [DOI] [PubMed] [Google Scholar]
- 42. Naeem H, Cheng D, Zhao Q, et al. The activity and stability of the transcriptional coactivator p/CIP/SRC-3 are regulated by CARM1-dependent methylation. Mol Cell Biol. 2007;27:120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsia EY, Kalashnikova EV, Revenko AS, Zou JX, Borowsky AD, Chen HW. Deregulated E2F and the AAA+ coregulator ANCCA drive proto-oncogene ACTR/AIB1 overexpression in breast cancer. Mol Cancer Res. 2010;8:183–193 [DOI] [PubMed] [Google Scholar]
- 44. Li J, Thurm H, Chang HW, Iacovoni JS, Vogt PK. Oncogenic transformation induced by the Qin protein is correlated with transcriptional repression. Proc. Natl Acad Sci USA. 1997;94:10885–10888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li J, Chang HW, Lai E, Parker EJ, Vogt PK. The oncogene qin codes for a transcriptional repressor. Cancer Res. 1995;55:5540–5544 [PubMed] [Google Scholar]
- 46. Banham AH, Beasley N, Campo E, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820–8829 [PubMed] [Google Scholar]
- 47. Li S, Weidenfeld J, Morrisey EE. Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol Cell Biol. 2004;24:809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gray S, Levine M. Short-range transcriptional repressors mediate both quenching and direct repression within complex loci in Drosophila. Genes Dev. 1996;10:700–710 [DOI] [PubMed] [Google Scholar]
- 49. Arnosti DN, Gray S, Barolo S, Zhou J, Levine M. The gap protein knirps mediates both quenching and direct repression in the Drosophila embryo. EMBO J. 1996;15:3659–3666 [PMC free article] [PubMed] [Google Scholar]
- 50. Barolo S, Levine M. Hairy mediates dominant repression in the Drosophila embryo. EMBO J. 1997;16:2883–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li C, Manley JL. Even-skipped represses transcription by binding TATA binding protein and blocking the TFIID-TATA box interaction. Mol Cell Biol. 1998;18:3771–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sauer F, Jäckle H. Concentration-dependent transcriptional activation or repression by Krüppel from a single binding site. Nature. 1991;353:563–566 [DOI] [PubMed] [Google Scholar]
- 53. Kosman D, Small S. Concentration-dependent patterning by an ectopic expression domain of the Drosophila gap gene knirps. Development. 1997;124:1343–1354 [DOI] [PubMed] [Google Scholar]
- 54. Hewitt GF, Strunk BS, Margulies C, et al. Transcriptional repression by the Drosophila giant protein: cis element positioning provides an alternative means of interpreting an effector gradient. Development. 1999;126:1201–1210 [DOI] [PubMed] [Google Scholar]
- 55. Lanz RB, Bulynko Y, Malovannaya A, et al. Global characterization of transcriptional impact of the SRC-3 coregulator. Mol Endocrinol. 2010;24:859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Y, Lu C, Shen Q, Munoz-Medellin D, Kim H, Brown PH. AP-1 blockade in breast cancer cells causes cell cycle arrest by suppressing G1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene. 2004;23:8238–8246 [DOI] [PubMed] [Google Scholar]
- 58. Biswas DK, Shi Q, Baily S, et al. NF-κ B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA 2004;101:10137–10142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223 [DOI] [PubMed] [Google Scholar]
- 60. Akiyama N, Matsuo Y, Sai H, Noda M, Kizaka-Kondoh S. Identification of a series of transforming growth factor β-responsive genes by retrovirus-mediated gene trap screening. Mol Cell Biol. 2000;20:3266–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ma G, Ren Y, Wang K, He J. SRC-3 has a role in cancer other than as a nuclear receptor coactivator. Int J Biol Sci. 2011;7:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coste A, Antal MC, Chan S, et al. Absence of the steroid receptor coactivator-3 induces B-cell lymphoma. EMBO J. 2006;25:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chan DW, Liu VW, To RM, et al. Overexpression of FOXG1 contributes to TGF-β resistance through inhibition of p21WAF1/CIP1 expression in ovarian cancer. Br J Cancer. 2009;101:1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim TH, Jo SW, Lee YS, et al. Forkhead box O-class 1 and Forkhead box G1 as prognostic markers for bladder cancer. J Korean Med Sci. 2009;24:468–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adesina AM, Nguyen Y, Mehta V, et al. FOXG1 dysregulation is a frequent event in medulloblastoma. J Neurooncol. 2007;85:111–122 [DOI] [PubMed] [Google Scholar]
- 66. Reiter R, Wellstein A, Riegel AT. An isoform of the coactivator AIB1 that increases hormone and growth factor sensitivity is overexpressed in breast cancer. J Biol Chem. 2001;276:39736–39741 [DOI] [PubMed] [Google Scholar]
- 67. Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res. 2007;67:7256–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chien CD, Kirilyuk A, Li JV, et al. Role of the nuclear receptor coactivator AIB1-Δ 4 splice variant in the control of gene transcription. J Biol Chem. 2011;286:26813–26827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Al-Otaiby M, Tassi E, Schmidt MO, et al. Role of the nuclear receptor coactivator AIB1/SRC-3 in angiogenesis and wound healing. Am J Pathol. 2012;180:1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.