Abstract
The molecular mechanisms that regulate the pivotal transformation processes observed in the follicular wall following the preovulatory LH surge, are still not established, particularly for cells of the thecal layer. To elucidate thecal cell (TC) and granulosa cell (GC) type-specific biologic functions and signaling pathways, large dominant bovine follicles were collected before and 21 hours after an exogenous GnRH-induced LH surge. Antral GCs (aGCs; aspirated by follicular puncture) and membrane-associated GCs (mGCs; scraped from the follicular wall) were compared with TC expression profiles determined by mRNA microarrays. Of the approximately 11 000 total genes expressed in the periovulatory follicle, only 2% of thecal vs 25% of the granulosa genes changed in response to the LH surge. The majority of the 203 LH-regulated thecal genes were also LH regulated in GCs, leaving a total of 57 genes as LH-regulated TC-specific genes. Of the 57 thecal-specific LH-regulated genes, 74% were down-regulated including CYP17A1 and NR5A1, whereas most other genes are being identified for the first time within theca. Many of the newly identified up-regulated thecal genes (eg, PTX3, RND3, PPP4R4) were also up-regulated in granulosa. Minimal expression differences were observed between aGCs and mGCs; however, transcripts encoding extracellular proteins (NID2) and matrix modulators (ADAMTS1, SASH1) dominated these differences. We also identified large numbers of unknown LH-regulated GC genes and discuss their putative roles in ovarian function. This Research Resource provides an easy-to-access global evaluation of LH regulation in TCs and GCs that implicates numerous molecular pathways heretofore unknown within the follicle.
In cattle the periovulatory transformation of the 17β-estradiol (E2)-producing dominant follicle during the follicular phase is accompanied by a profound morphologic reorganization of somatic cell layers (ie, granulosa and theca) to produce the ephemeral progesterone (P4)-producing corpus luteum (1, 2). This conversion or luteinization process of the dominant follicle has been well studied with respect to changes in steroid hormone production and the compartmentalization of gonadotropin receptors and steroidogenic enzymes (3, 4) and forms the basis of the “two-cell-two gonadotropin hypothesis of estrogen biosynthesis” (5). Although it is understood that paracrine interactions occur between the theca and granulosa tissue layers and that they are essential for folliculogenesis and likely luteal function, much yet remains to be discovered with respect to these interactions (6). This is particularly true for the role of the LH-responsive thecal cells (TCs) of which very little is known.
Immunocytochemical and in situ hybridization studies in rat have demonstrated that granulosa cells (GCs) can have markedly different levels of LH receptor (LHCGR) expression dependent upon their position within the follicular wall. Higher LHCGR levels are found in mural GCs located close to the basement membrane vs those close to the antrum (7, 8). Indeed, oocyte-derived factors present within the follicular fluid are known to affect the mural GCs, with those residing near the antrum being under a greater influence. An even greater difference in gene expression between GCs intimately associated with the oocyte (ie, corona radiata and cumulus) and the mural granulosa has already been established (8–10).
Expression profiling studies performed over the last couple of years in cattle and other species have shed light on changes that occur during the early and intermediate processes of follicular differentiation such as recruitment, selection, and dominance (11–14). Furthermore, studies aimed at understanding the changes in gene expression following the ovulatory LH surge have been completed in rodent, domestic animals, and primate species. In mice and rats, early events (ie, 1 h after a preovulatory LH/human chorionic gonadotropin [hCG] surge) through 12 hours after the LH surge (ovulation occurs at ∼13–15 h) have been evaluated in GCs (15–17). In primates, a recent analysis of whole periovulatory follicles collected at 0, 12, 24, and 36 hours post-LH in primates was completed (18). In cows and buffalo, suppression-subtractive hybridization (19) and gene-specific microarrays (20, 21) have been used to interrogate granulosa-specific gene expression changes occurring after the preovulatory LH surge. In the paper by Ndiaye et al (19), bovine GCs were evaluated before and 23 hours after hCG administration, and these authors reported only the down-regulated genes (32 in total). Another bovine study, conducted by Gilbert et al. (20), compared GC pre-LH surge gene expression to granulosa collected 6 and 22 hours post-GnRH (using custom arrays). Lastly a recent study, focusing on genes involved in steroid biosynthesis and using RNAseq-technology, was used to comparatively survey expression profiles in cells of the follicular wall of heifers and lactating cows (22). However, to date, no study has provided a comprehensive examination of the effect of the LH surge on all layers of the bovine follicular wall, granulosa, and theca interna, particularly evaluating the differential effects of location of GCs within the follicular wall (ie, antral vs membrane associated).
The present study examines large dominant bovine follicles collected before and 21 hours after a GnRH-induced preovulatory LH surge; follicles were dissected and antral (aspirated) GC (aGC)-, membrane-associated GC (mGC)-, and TC-specific expression profiles were analyzed by mRNA microarray analysis. The bovine model is useful because ultrasonography allows accurate tracking of the developing follicle and because a mono-ovulatory species enhances its relevance to human correlates as well as its general importance to animal husbandry of this economically important species. The results of this study provide the first comprehensive expression profiling analysis of both LH receptor-positive cell types within the periovulatory follicle and cell type-specific regulatory pathways, and functions affected by the preovulatory LH surge are identified.
Materials and Methods
Animals and tissue samples
Follicles were collected from normally cycling cows (n = 6) of the Holstein-Friesian breed after slaughter and immediately processed as described elsewhere (3, 23). Briefly, for collecting large dominant follicles before the preovulatory LH surge, numbers, sizes, and relative locations of follicles (≥5 mm) of a growing cohort were monitored by ultrasonography in normally cycling cows after ovulation and at 3 days and 21 hours before slaughtering using a transrectal 5 MHz linear transducer (Aloka SSD-500, Aloka GmbH, Meerbusch, Germany). In this way, the growth of individual follicles could be tracked. These cows were slaughtered at days 7 (n = 1) and 8 (n = 2) of the estrous cycle (day 0 = day of estrus) during the first follicular wave. In these animals the largest growing follicle (as determined by ultrasound monitoring) was collected, whereas stagnating or regressing follicles were easily identified and avoided. To collect the late preovulatory follicles after the LH surge, cows at day 8 (n = 3) of the cycle (first follicular wave) were ultrasonographically examined and treated with 500 μg PGF2α (PGF forte, Veyx Pharma GmbH, Schwarzenborn, Germany) to induce luteolysis of the functional corpus luteum. Forty-eight hours later, all animals were scanned and injected with 100 μg of a GnRH analog (Gonavet Veyx, Depherelin, Veyx Pharma GmbH) to induce the LH surge. The animals were slaughtered 23 hours after the GnRH injection, which elicits a preovulatory LH surge about 2 hours after administration, and the largest growing follicle of each animal was dissected away from the remaining ovarian tissue (3, 23).
All procedures involving living animals (injections, ultrasonographic examinations) were according to the German law for animal protection (TierSchG) and in compliance with the European legislation on the care and use of animals.
Isolation of different follicular cell types
Two separate fractions of GCs were successively isolated from each follicle, aGC and membrane-associated granulosa (mGC) previously described as mural granulosa in Refs. (3 and 23). Briefly, the aGC fraction, together with the antral fluid, were collected by puncturing the antral follicle with a 18G needle, and the antral fluid, together with the cumulus cells and free floating or only slightly adherent cells of the GC layer, were aspirated. It should be noted that the cumulus cells and oocyte were not separated from the other aGCs aspirated from the follicles, but they likely make up a very small percentage of the actual transcripts within the aGC preparation, because it is well established that large numbers of mural GCs are removed by aspiration. The fluid was centrifuged (2 min, 400 relative centrifugal force, room temperature), the cell-free supernatant was frozen and stored at −20°C for determination of steroid concentrations, and the sedimented cells were frozen in liquid nitrogen and stored at −80°C for RNA preparation. The follicle was then cut open with scissors, and the follicular wall was peeled away from the remaining ovarian stroma and submersed in Ca2+ and Mg2+-free PBS buffer (pH 7.4). Membrane-associated GCs (mGCs) were then gently scraped off with a small scalpel blade. Detached cells and surrounding buffer were collected with a pipette, centrifuged (2 min, 400 relative centrifugal force), after which the supernatant was discarded and the sedimented cells were frozen in liquid nitrogen and stored at −80°C until RNA preparation. To isolate the theca cells (TCs), the inner (antral) side of the follicular wall was again carefully scraped with a scalpel blade and washed several times in PBS in order to further eliminate residual GCs attached to the basement membrane. The remaining basement membrane and the TCs attached to its outer side were then transferred to RNAlater solution (Qiagen, Hilden, Germany), stored at 4°C overnight, and then transferred to −20°C for long-term storage until RNA preparation.
Determination of follicular P4 and E2 content
Concentrations of P4 were determined using a competitive single-antibody [3H]RIA (24). Briefly, follicular fluid of each sample (10 μL each) was diluted with assay buffer (90 μL). Ten microliters of the diluted samples were used to measure directly, ie, without extraction, P4 and E2 concentrations. The tracer, [1,2,6,7-3H] progesterone, was purchased from GE Healthcare (Freiburg, Germany). The antibody raised in rabbits was further purified by affinity chromatography on protein A superose (GE Healthcare). The intra- and interassay coefficients of variation (CVs) were 7.4% and 9.8%, respectively. Radioactivity was measured with a β-counter with integrated RIA calculation (TriCarb2900; PerkinElmer, Rodgau, Germany).
Concentrations of E2 were determined with an ultrasensitive [125I]RIA (DSL, Sinsheim, Germany). The standard curve was established between 5 and 750 pg/mL. Radioactivity was measured with an automatic γ-counter with integrated RIA calculation (Wizard; PerkinElmer). The detection limit of the method was 3 pg/mL. The intra- and interassay CVs were 8.4% and 10.2%, respectively.
The intrafollicular ratio of E2:P4 was calculated to characterize the endocrinologic status of isolated follicles. Before the preovulatory LH surge only E2-active (E2:P4 > 1) large dominant follicles were processed for cell type-specific transcriptome analysis because it has been demonstrated by others that E2-inactive follicles (E2:P4 < 1) isolated before the LH surge had reduced capacities to specifically bind gonadotropins and display clear histologic signs of atresia (25).
RNA preparation for mRNA microarray analysis
Total RNA was isolated from granulosa and theca with Trizol (Ambion, Forest City, California), per the manufacturer's instruction. RNA quality was assessed with Agilent Bioanalyzer Nano Chips (Agilent Technologies, Santa Clara, California), and all samples had sharp 18S and 28S peaks as well as RNA integrity values over 8.0, indicating high-quality RNA. The RNA was subjected to Gene Chip Eukaryotic Target Labeling Assay (Affymetrix). Biotin-labeled cRNA was fragmented according to Affymetrix protocols. The fragmented cRNA from each sample was hybridized to individual Affymetrix bovine gene array chips using the GeneChip Fluidics Station 400 protocol (Affymetrix), and the hybridized chips were scanned using the Agilent GeneArray Scanner (Affymetrix). Before analysis, all probes that did not show significant probe level hybridization (measured by Affymetrix MAS5.0 algorithm) in any of the 18 samples were filtered out. The raw signal was background corrected, normalized, and gene-level summarized using the Robust Multichip Average (RMA) procedure (26) using default RMA settings. To allow for statistical analysis, 3 preovulatory dominant follicles isolated from 3 different cows (ie, experimental unit) and 3 periovulatory (eg, ∼21-post LH-surge) follicles isolated from 3 different cows were dissected and the aGC, mGC, and TC preparations were collected, such that a total of 18 individual bovine Affymetrix GeneChips were used. The array results have been uploaded in the GEO database (GSE46996).
cDNA synthesis and quantitative RT-PCR
Gene-specific primers for cDNA synthesis and amplification of different transcripts were designed according to published mRNA sequences (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). For cDNA synthesis, 0.25 μg total RNA was reverse transcribed in a 25-μL reaction volume using M-MLV reverse transcriptase, RNase H Minus, Point Mutant (Promega, Mannheim, Germany) with gene-specific, or random and oligo-dT primers. The freshly synthesized cDNA samples were cleaned with the High Pure PCR Product Purification Kit (Roche, Mannheim, Germany) and eluted in 50 μL elution buffer. The identity of products generated with different primer pairs was determined by sequencing.
For qPCR, 0.5 and 0.25 μL of each purified cDNA sample were amplified with the LC 480 SYBR Green I Master Kit (Roche) in 12 μL total reaction volume. Measures from both reactions were averaged, considering the different amounts of cDNA. Amplification and quantification of products were performed in a LightCycler 480 instrument (Roche) under the following cycling conditions: preincubation at 95°C for 5 minutes, followed by 40 cycles denaturation at 95°C for 20 seconds, annealing at 60°C for 15 seconds, extension at 72°C for 15 seconds, and single-point fluorescence acquisition at 83°C for 10 seconds. The melting peaks of all samples were routinely determined by melting curve analysis in order to ascertain that only the expected products had been generated. Additionally, the length of all PCR products was monitored by agarose gel electrophoresis analysis (3% agarose, ethidium bromide stained). Cloned PCR products of the respective transcripts were used to generate external standard curves. Routinely, dilutions of standards covering 5 orders of magnitude (5 × 10−16 to 5 × 10−12 g DNA per reaction) were freshly diluted from stocks of 10−9 g DNA/μL and coamplified during each run. Abundance of target transcript was calculated relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. In a previous study it has been shown that GAPDH transcript abundance is similar in the aGC, mGC, and TC fractions of large dominant bovine follicles before and after the LH surge (3).
Statistics and bioinformatics
Correlations between mRNA microarray data and levels of transcript abundance (relative to GAPDH transcripts), as determined by quantitative RT-PCR (qRT-PCR), were calculated by Pearson Product Moment analysis; pair-wise comparisons by t test analysis using the SigmaPlot 12.0 software (Jandel Scientific, San Rafael, California).
Gene set enrichment analysis was performed with the Ingenuity Pathway Analysis tool (Ingenuity IPA 8.0, Ingenuity Systems Inc., Redwood City, California) to map differentially expressed transcripts to specific biologic functions and canonical pathways. If the calculated P value was below .05, mapping of the respective transcript to a specific biologic function or canonical pathway was considered significant. For clarity, significant canonical pathways were combined to super ordinate categories (see Results).
Results
Morphologic and physiologic characterization of follicles before and after the LH surge
Ultrasonography of each developing follicle was completed to ensure that only the single largest growing follicles (ie, destined preovulatory follicles) were collected for subsequent analyses. Physiologic (antral E2 and P4 concentrations), morphologic (size, vascularization, color etc.), and molecular markers were used to confirm that the individual follicles collected from the 6 different cows were characteristic of either preovulatory (n = 3) and post-LH (n = 3) follicles before gene expression analysis. Before the LH surge, the mean growing follicle diameter was 13.8 ± 1.2 mm, and the ratio of antral fluid E2:P4 was 4.9 ± 1.5, which was significantly different from follicles of the post-LH group, which were 20.5 ± 0.9 mm and 0.6 ± 0.2, respectively (Supplemental Table 2). Analysis of PTGS2 transcript levels within the GCs of the LH-treated periovulatory follicles were markedly (∼280-fold) elevated in comparison to the preovulatory follicles (Table 1). Thus, the preovulatory follicles were E2 active, which is an important criterion for healthy (nonatretic) large dominant follicles (25), whereas those after LH treatment had a significantly reduced E2:P4 ratio and increased PTGS2 expression, which are both reliable biomarkers for impending ovulation (3, 23, 27–29).
Table 1.
Hybridization Values, Fold Changes, and Correlation Coefficients of Microarray and qRT-PCR Comparison for LH-Responsive TC- and GC-Specific Genes
| Gene Symbol | Gene Name | Fold Change qRT-PCRa |
Microarray HSI − Mean RMA Hybridization |
Correlation of qRT-PCR and HSI r Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aGC |
mGC |
TC |
|||||||||
| aGC | mGC | TC | bLH | aLH | bLH | aLH | bLH | aLH | |||
| CYP17A1 | Cytochrome P450; family XVII, 17α-hydroxylase | −77 | 47 | 36 | 266 | 26 | 3452 | 45 | 1.00 | ||
| FSHR | FSH receptor | −4.1 | 285 | 70 | 294 | 75 | 29 | 19 | 0.91 | ||
| CYP19A1 | Cytochrome P450; family 19; aromatase | −398 | −539 | −80 | 6762 | 17 | 13603 | 25 | 1255 | 16 | 0.97 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | 274 | 290 | 32 | 16 | 4287 | 13 | 3876 | 20 | 643 | 0.94 |
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 motif; 1 | 29 | 8.5 | 45 | 1272 | 184 | 1569 | 1138 | 2552 | 0.89 | |
| CCND2 | Cyclin D2 | −82 | −27 | 5297 | 64 | 3993 | 146 | 796 | 296 | 0.92 | |
| CYP11A1 | Cytochrome P450; family 11; subfamily A; polypeptide 1 | 8180 | 549 | 9733 | 974 | 7146 | 2852 | 0,83 | |||
| HSD3B1 | hydr.-δ-5-ster. dehydr.; 3β- and steroid δ-isom. 1 | 7002 | 5677 | 9315 | 4268 | 3571 | 2716 | 0.89 | |||
| LHCGR | LH/CG receptor | −7.9 | −17 | −7.4 | 280 | 35 | 790 | 46 | 380 | 52 | 0.95 |
| NR5A1 | Nuclear receptor subfamily 5; group A; member 1 | −2.8 | 529 | 352 | 405 | 228 | 642 | 229 | 0.73 | ||
| NR5A2 | Nuclear receptor subfamily 5; group A; member 2 | 8205 | 5549 | 9327 | 5559 | 1093 | 1164 | 0.89 | |||
| PCNA | Proliferating cell nuclear antigen | −4.2 | −2.7 | 2300 | 553 | 2263 | 841 | 2443 | 1942 | 0.81 | |
| PTX3 | Pentraxin 3; long | 644 | 711 | 108 | 14 | 9232 | 15 | 10921 | 85 | 9158 | 0.93 |
| STAR | Steroidogenic acute regulatory protein | 57 | 287 | 823 | 963 | 9578 | 4471 | 0.96 | |||
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 85 | 38 | 10 | 72 | 6151 | 241 | 9231 | 1439 | 14888 | 0.85 |
Boldface symbols are the cell-specific and LH-induced (PTGS2) validation genes we evaluated.
Only significant LH responses by array are depicted; bLH, before LH surge; aLH, after LH surge; correlation coefficients (r) calculated by Pearson Product Moment analysis including all 18 samples; all P values were <.001.
Microarray analysis, cell-specific markers, and qRT-PCR validation
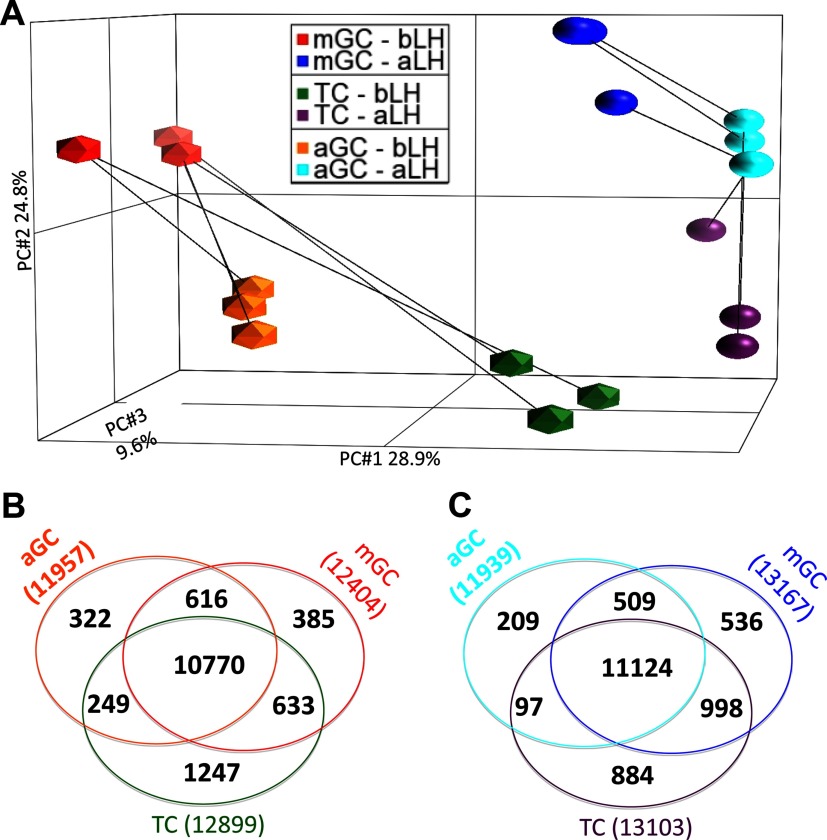
Principal component analysis of the data revealed that the global gene expression patterns observed in aGCs, mGCs, and TCs from follicles before (n = 3) and after LH surge (n = 3) were tightly clustered within cell types and across treatment groups (before and after LH; Figure 1A). Analysis of the microarray data revealed that of the 24 016 probe sets which represent 11 548 independent annotated bovine genes and 7091 unannotated expressed sequence tags, approximately half were determined as significantly present, based on the significance of the probe level hybridization of their transcripts to the microarray in all 3 biologic replicates (calculated using Affymetrix MAS 5.0 algorithm) in all aGC, mGC, or TC samples either before and after LH, respectively (Figure 1, B and C). The majority of the probe sets, 10 770 and 11 124 were present in all 3 cell types before and after the LH surge, respectively. No striking differences in actual numbers of present genes within a cell type before and after the LH surge were found for the aGC, mGC, or TC samples (comparison of gene numbers across cell types in Figure 1, B and C). In order to estimate the degree of potential contamination of the GC preparations with thecal RNA and vice versa, the microarray hybridization signals of several well-established genes that are commonly associated with one cell type or the other (CYP19A1 and FSHR in GC; and CYP17A1 in TC) were examined (Table 1). Hybridization signal intensity (HSI: RMA normalized fluorescence intensity values surrogate to the transcript hybridization signal) for CYP19A1 in aGCs and mGCs before the LH surge were robust (6762- and 13606-HSI, respectively); however, TCs collected before LH surge also had detectable levels (1255-HSI) of CYP19A1, possibly indicating a small degree of contamination of the TC preparation with this highly expressed GC mRNA. Statistical analysis indicates that the aGC and mGC CYP19A1 levels were significantly (false discovery rate [fdr] < 0.01) greater (5.4- and 10.8-fold, respectively) than those in TCs before the LH surge. After the LH surge, the CYP19A1 HSI dropped to low levels (<25) in all cell types. Another established GC-specific transcript, FSHR, that has a lower initial HSI in aGCs and mGCs (285- and 294-HSI) before the LH surge, was 9.8- and 10.1-fold greater (fdr < 0.02) than the HSI level (<29) observed in TCs, respectively. After the LH surge FSHR expression dropped (70- and 75-HSI) as expected in aGCs and mGCs, respectively, whereas TC values remained low and nearly unchanged (29-HSI vs 19-HSI; Table 1). Examination of CYP17A1 in the 3 cellular preparations indicated that TC levels (3452-HSI) were 73.5- and 13.0-fold greater than those in aGCs (47-HSI) and mGCs (266-HSI). After the LH surge, CYP17A1 levels were low (HSI < 45) and not different across cell types.
Figure 1.
mRNA Microarray Analysis of Present Transcripts within Cells of the aGCs, mGCs, and TC before and after the Preovulatory LH Surge. A, Principal component analysis shown for the 3 cell types, before (bLH) and after (aLH) the LH surge. Each symbol represents an individual replicate and the line connects those from the same cow. B and C, Venn diagrams showing members of probe sets significantly called present, based on the significance of the probe level hybridization to the microarray in all 3 biologic replicates (panel B represents before the LH surge and panel C represents after the LH surge).
Using a panel of 15 genes expressed at varying levels in GCs and TCs we compared the hybridization signals and relative qRT-PCR results for these genes (Table 1). The transcript abundance levels of these 15 selected transcripts, 7 of which were significantly up- or down-regulated by the LH surge, were correlated to qRT-PCR results for all 18 samples. The Pearson product moment correlation test indicated that all 15 transcripts showed a highly significant (P < .001) correlation between qRT-PCR and microarray hybridization values with correlation coefficients between 0.73 and 1.00 (Table 1). Because of this high correlation for genes expressed at divergent levels (low and high expression genes) combined with the tight clustering of biologic replicates observed in the principal component analysis analysis, we postulate that the microarray results are accurate and that they reflect actual relative changes before and after the LH surge for the remaining genes detected in the microarrays.
Cell-specific gene expression markers in GCs and TCs
Comparison of aGCs to mGCs before or after LH yielded few statistically significant differences. Only 1 gene, PLXNB2 (encoding plexin B2) was 2.67-fold greater (fdr < 0.05) in mGCs vs aGCs before LH, whereas 2 genes, HS6ST1 (heparan sulfate 6-O-sulfotransferase 1; 1.52-fold increase) and SSR1 (signal sequence receptor; α −1.88-fold decrease) were different (fdr < 0.05) between mGCs and aGCs after the LH surge. Even increasing the fdr to < 0.1 resulted in no further annotated genes being detected with a fold difference of ≥1.5-fold.
Comparison of TC expression to mGCs or aGCs before LH indicated that 3564 or 3650 genes, respectively, had a ≥1.5-fold (fdr < 0.05) difference in expression across these cell types; of these the majority (2696 genes) were consistently differentially regulated in both GC populations compared with TCs (Supplemental Table 3). After the LH surge 596 or 3833 genes were different (fdr < 0.05) at the ≥1.5-fold level between TCs and mGCs or aGCs, respectively; of these 541 were consistently differentially regulated in both cell populations compared with TCs (Supplemental Table 4). In summary, these data are therefore consistent with the observation that aGCs and mGCs are very similar and quite divergent from TCs.
Using a different approach to identify cell-specific genes (comparison of the significance of the transcript HSI calls, ie, absent vs present calls), we identified 34 genes as specific to the TC preparation: (ie, present in all 6 TC samples and absent [below background hybridization] in all 12 GC samples; with a significance of P < .05; Supplemental Table 5). A large number of these TC-specific transcripts are implicated in endothelial cell function (ie, PECAM1, TMEM100, LMO2, CD300LG, PEAR1). This is likely due to the unavoidable contamination of the TC with the capillary plexus surrounding the highly vascular dominant follicle. The complete lack of these endothelial markers in the avascular GC preparations of all 12 samples is reassuring. Analysis of the GCs identified 15 genes as present in either mGCs or aGCs and absent from all TC samples; 6 of the 15 genes were shown to be LH regulated in the GCs (Supplemental Table 6).
Cell-specific expression of gene transcripts influenced by the LH surge
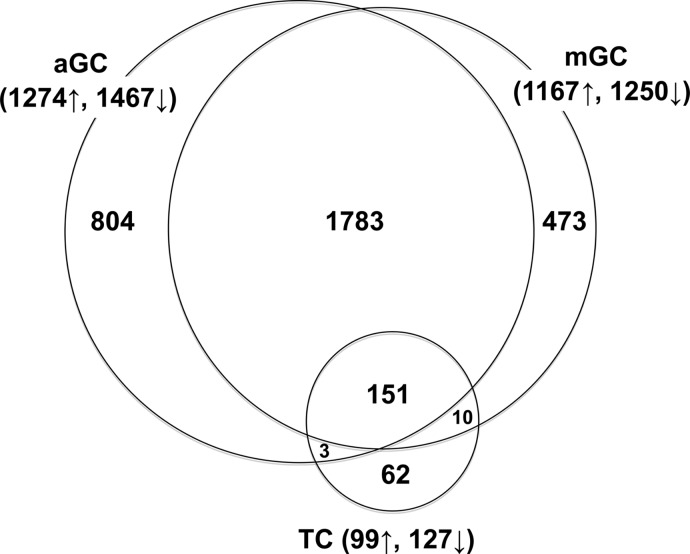
To determine those genes that are regulated by the LH surge, we included only those probe sets that showed significant probe level hybridization (P ≤ .05, Affymetrix MAS 5.0 algorithm) and that exhibited an absolute fold change that was equal to or exceeded 1.5-fold and had a stringent Benjamini-Hochberg adjusted significance P value (ie, fdr) of 0.01 or less. This resulted in the selection of 2741 and 2417 differently expressed probe sets in aGCs and mGCs, and 226 in TC samples before and after LH, respectively (Figure 2). The relative number of gene transcripts which were down-regulated (aGCs 54%, mGCs 52%, and TCs 56%) was slightly more than numbers of gene transcripts which were up-regulated (Figure 2). Consistent with their similar tissue of origin, aGCs and mGCs shared 1783 transcripts that were similarly and significantly up- or down-regulated (Figure 2). A large number of LH-regulated probe sets (804 and 473) were differentially expressed in only aGCs or mGCs, respectively (Figure 2). However, in most cases these probe sets that were significant for either aGCs or mGCs had a calculated similar fold change (ie, within 2-fold) for the other cell type but failed to reach significance in the other cell type under the highly stringent conditions we established. Comparison of the fold changes for all 3060 probe sets that were LH regulated in GCs (see Supplemental Table 7), showed that only 29 genes exhibited more than a 3-fold difference (P < .05) in response to LH between aGCs and mGCs (Table 2).
Figure 2.
Venn Diagram Showing Numbers of Significantly LH Affected Probe Set Hybridization Signals in GC and TC Preparations. Total numbers of up- (↑) or down-regulated (↓) probe sets are shown in brackets.
Table 2.
LH Regulated Genes That Are Differentially Expressed Between aGCs and mGCs
| Gene Name or Chromosomal Location | Gene Symbola | Fold Change Between LH Response of aGCs vs mGCs | P Value | LH Regulated in aGCs fdr < 0.01 | LH Regulated in mGCs fdr < 0.01 | HSI − Mean RMA Hybridization Value Before LH |
HSI − Mean RMA Hybridization Value After LH |
||
|---|---|---|---|---|---|---|---|---|---|
| aGC | mGC | aGC | mGC | ||||||
| Nidogen 2 | NID2 | 16.52 | .002 | Yes | Yes | 23 | 558 | 3616 | 5224 |
| Similar to Collagen α-1(XIV) chain precursor | LOC781493 | 8.91 | .001 | Yes | No | 14 | 138 | 371 | 417 |
| Caveolin 1 | CAV1 | 7.54 | .001 | Yes | Yes | 16 | 75 | 6548 | 6235 |
| Fibronectin 1 | FN1 | 5.10 | .002 | Yes | No | 89 | 744 | 1200 | 1978 |
| Claudin 11 | CLDN11 | 4.91 | .011 | Yes | Yes | 12 | 57 | 3518 | 3409 |
| Dickkopf homolog 3 | DKK3 | 4.77 | .001 | Yes | Yes | 15 | 92 | 2273 | 2952 |
| Collagen, type V, α 2 | COL5A2 | 3.82 | .029 | Yes | No | 15 | 256 | 215 | 956 |
| CART prepropeptide | CARTPT | 3.68 | .001 | No | Yes | 45 | 155 | 46 | 43 |
| Chr2:110382-336-110382643(−) | (IGFBP5) | 3.67 | .033 | Yes | No | 71 | 540 | 573 | 1181 |
| Dihydropyrimidinase-like 3 | DPYSL3 | 3.56 | .001 | Yes | YES | 21 | 81 | 252 | 277 |
| ρ family GTPase 3 | RND3 | 3.55 | .001 | Yes | Yes | 29 | 93 | 4046 | 3710 |
| ADAM metallopeptidase with thrombospondin type 1 motif; 1 | ADAMTS1 | 3.34 | .001 | Yes | Yes | 45 | 184 | 1272 | 1569 |
| Chromosome 8 open reading frame 4 | C27H8orf4 | 3.32 | .025 | Yes | Yes | 11 | 37 | 654 | 662 |
| Cyclin D2 | CCND2 | −3.01 | .002 | Yes | Yes | 5297 | 3993 | 64 | 146 |
| SPARC-related modular calcium binding 2 | SMOC2 | −3.02 | .015 | Yes | No | 942 | 717 | 192 | 442 |
| Hydroxysteroid (17-β) dehydrogenase 1 | HSD17B1 | −3.05 | .010 | Yes | Yes | 2452 | 1039 | 49 | 62 |
| Heme oxygenase 1 | HMOX1 | −3.07 | .033 | Yes | No | 367 | 218 | 90 | 164 |
| NADH dehydrogenase (ubiquinone) 1 α subcomplex; 4-like 2 | NDUFA4L2 | −3.10 | .005 | Yes | No | 62 | 21 | 20 | 22 |
| 6-Phosphofructo-2-kinase/fructose-2;6-biphosphatase 3 | PFKFB3 | −3.28 | .011 | No | Yes | 283 | 95 | 504 | 556 |
| Chromosome 12 open reading frame 32 | C5H12orf32 | −3.35 | .002 | Yes | No | 226 | 236 | 32 | 113 |
| CD14 molecule | CD14 | −3.39 | .048 | No | Yes | 77 | 20 | 146 | 130 |
| Neural precursor cell expressed; developmentally down-regulated 9 | NEDD9 | −3.41 | .032 | Yes | No | 130 | 118 | 21 | 65 |
| KIAA0101 protein (PCNA-assoc factor) | KIAA0101 | −3.45 | .018 | Yes | Yes | 1782 | 1439 | 45 | 126 |
| SAM and SH3 domain containing 1 | SASH1 | −3.51 | .028 | Yes | No | 105 | 65 | 16 | 35 |
| chr2:88862297–88862784 (−) | (Ankrd44) | −4.04 | .009 | Yes | No | 405 | 163 | 70 | 114 |
| chr11:16969669-16970066 (+) | — | −4.26 | .006 | Yes | Yes | 701 | 267 | 27 | 43 |
| Complement component 1; s subcomponent | C1S | −5.63 | .005 | No | Yes | 20 | 41 | 18 | 210 |
| Guanylate cyclase activator 1A (retina) | GUCA1A | −6.07 | .008 | Yes | No | 485 | 97 | 21 | 25 |
| chr1:73033634-73034079 (+) | — | −8.03 | .003 | No | Yes | 19 | 200 | 102 | 1737 |
Gene symbols enclosed in parentheses are probe sets that recognize transcripts with high homology to a named gene in another species.
Chromosomal locations are based on the Cow October 2011 assembly. Dashes reflect negative or down regulated gene.
Of the 226 probe sets shown to be LH regulated in TCs, only 62 probe sets (representing 57 independent genes) were significantly regulated by LH in TC only. Most (74%) of these TC-specific LH-regulated transcripts (Table 3) were significantly down-regulated after LH-surge, with CYP17A1 encoding steroid 17α-hydroxylase (P450c17), the key enzyme of androgen biosynthesis showing the strongest response. Of the remaining 164 probe sets that were LH regulated in both TCs and GCs all but 2 of these showed the same (ie, either up or down) regulation across the different cell types. The 2 genes CACNA1H (calcium channel; voltage-dependent; T type; α 1H subunit) and SIPA1 (signal-induced proliferation-associated 1) were down-regulated (−2.13-fold, fdr < 0.01; −1.92-fold, fdr < 0.01) in TCs after LH. In contrast, CACNA1H and SIPA1 were up-regulated in aGCs (2.74-fold, fdr < 0.01; 1.46-fold, fdr > 0.5) or mGCs (1.05-fold, fdr > 0.5; 1.69-fold, fdr < 0.01), respectively. The remaining 162 differentially expressed probe sets (representing 136 independent genes), were divided into high, intermediate, and low expression (based on HSI) for depiction in (Table 4). Fold-changes varied widely with the greatest change being the up-regulation of PTX3 (108-, 711-, 643-fold) and the down-regulation of CYP19A1 (−80-, −539-, −397-fold), in TCs, mGCs, and aGCs, respectively. Comparison of these LH-regulated genes to previous studies indicated that a large number of the identified genes have not been previously shown to be LH-regulated in bovine GCs (see Table 4).
Table 3.
LH-Regulated Genes Specific to TCs
| Gene Name or Chromosomal Location | Gene Symbola | Fold Change | HSI − Mean RMA Hybridization Value |
|
|---|---|---|---|---|
| Before LH | After LH | |||
| Cytochrome P450c17, 17α-hydroxylase/17;20 lyase | CYP17A1 | −77.1 | 3452 | 45 |
| Ankyrin repeat and sterile α motif domain containing 4B | ANKS4B | −19.1 | 234 | 12 |
| Solute carrier family 1; member 3 | SLC1A3 | −10.5 | 195 | 19 |
| chr16:69743779-69744290 (−) | (TRAF5) | −9.5 | 107 | 11 |
| Chemokine (C-X-C motif) ligand 14 | CXCL14 | −9.2 | 142 | 15 |
| Tetraspanin 33 | TSPAN33 | −8.8 | 270 | 31 |
| Crystallin; μ | CRYM | −7.8 | 328 | 42 |
| Hydroxyprostaglandin dehydrogenase 15-(NAD) | HPGD | −7.1 | 875 | 124 |
| Asporin | ASPN | −6.5 | 709 | 109 |
| dCTP pyrophosphatase 1 | DCTPP1 | −5.5 | 2181 | 400 |
| Oncoprotein induced transcript 3 | OIT3 | −5.0 | 222 | 45 |
| ChrUn_JH123563:7386-7628 - AffyID Bt.16424.1.S1_at | — | −4.9 | 609 | 124 |
| Transmembrane 7 superfamily member 2 | TM7SF2 | −4.7 | 624 | 132 |
| Ryanodine receptor 3 | RYR3 | −4.5 | 85 | 19 |
| Pyridine nucleotide-disulfide oxidoreductase domain 2 | PYROXD2 | −4.4 | 216 | 50 |
| Angiopoietin 4 | ANGPT4 | −4.0 | 192 | 48 |
| BCL2-related protein A1 | BCL2A1 | −3.9 | 193 | 49 |
| Protein phosphatase 2; regulatory subunit B; β | PPP2R2B | −3.9 | 45 | 12 |
| Perilipin 5 | PLIN5 | −3.7 | 44 | 12 |
| chr5:17947935-17948135 (−) | — | −3.7 | 28 | 8 |
| Guanylate binding protein 4 | GBP4 | −3.7 | 106 | 29 |
| Transient receptor potential cation channel, subfamily M, member 4 | TRPM4 | −3.3 | 102 | 30 |
| Chemokine (C-C motif) ligand 26 | CCL26 | −3.3 | 135 | 41 |
| Small integral membrane protein 10 | SMIM10 | −3.1 | 147 | 48 |
| Cytochrome P450; family 4; subfamily F; polypeptide 3 | CYP4F3 | −3.0 | 64 | 21 |
| Potassium large conductance calcium-activated channel; subfamily M; β member 1 | KCNMB1 | −3.0 | 52 | 18 |
| Nuclear receptor subfamily 5; group A; member 1 | NR5A1 | −2.8 | 642 | 229 |
| Similar to DKFZP586J0619 protein | INTS1 | −2.6 | 347 | 131 |
| ADP-ribosyltransferase 3 | ART3 | −2.6 | 94 | 36 |
| NLR family; CARD domain containing 5 | NLRC5 | −2.6 | 59 | 23 |
| Gap junction protein; β 5; 31.1 kDa | GJB5 | −2.5 | 243 | 98 |
| Diacylglycerol O-acyltransferase homolog 2 (mouse) | DGAT2 | −2.2 | 41 | 18 |
| Carbohydrate (keratan sulfate Gal-6) sulfotransferase 1 | CHST1 | −2.2 | 228 | 102 |
| Mevalonate kinase | MVK | −2.0 | 571 | 288 |
| Fibroblast growth factor 1 (acidic) | FGF1 | −1.9 | 30 | 16 |
| RAN guanine nucleotide release factor | RANGRF | −1.9 | 115 | 60 |
| Mitochondrial ribosome recycling factor | MRRF | −1.9 | 445 | 233 |
| chr29:48560683-48560989 (+) | (CCND1) | −1.9 | 23 | 12 |
| ATP synthase subunit g, mitochondrial-like | ATP5 liter | −1.7 | 2599 | 1561 |
| Solute carrier family 25; member 34 | SLC25A34 | −1.7 | 33 | 20 |
| Solute carrier family 25: member 23 | SCL25A23 | −1.6 | 67 | 41 |
| Fragile X mental retardation; autosomal homolog 1 | FXR1 | −1.6 | 1542 | 970 |
| Kruppel-like factor 10 | KLF10 | 2.3 | 581 | 1311 |
| ras Homolog gene family; member B | RHOB | 2.4 | 1375 | 3341 |
| chr19:32921507-32921922 (+) | (Hs3st3b1) | 2.6 | 10 | 25 |
| chr11:86149219-86149711 (−) | (FAM84A) | 2.7 | 23 | 60 |
| Chromosome 21 open reading frame 7 ortholog | C1H21ORF7 | 2.9 | 18 | 52 |
| Adenomatosis polyposis coli down-regulated 1 | APCDD1 | 3.0 | 71 | 213 |
| Carbonic anhydrase IV | CA4 | 3.8 | 45 | 169 |
| chr8:52926349-52926740 (+) | (Rorb) | 3.9 | 11 | 42 |
| Coagulation factor V (proaccelerin; labile factor) | F5 | 4.0 | 13 | 53 |
| chr8:26339534-26340004 (−) | (Acer2) | 4.3 | 78 | 340 |
| Nuclear protein; transcriptional regulator; 1 | NUPR1 | 4.9 | 63 | 306 |
| ATP-binding cassette; subfamily G (WHITE); member 1 | ABCG1 | 6.9 | 14 | 93 |
| Solute carrier family 7; member 5 | SLC7A5 | 7.7 | 54 | 419 |
| Ceruloplasmin (ferroxidase) | CP | 8.2 | 20 | 166 |
| Lysyl oxidase-like 4 | LOXL4 | 18.8 | 98 | 1847 |
Gene symbols enclosed in parentheses are probes sets that recognize transcripts with high homology to a named gene in another species.
Chromosomal locations are based on the Cow October 2011 assembly. Dashes reflect a negative or down regulated gene.
Table 4.
LH-Regulated Genes in GCs and mGCs and TCs
| Gene Name or Chromosomal Location | Gene Symbola | aGC Fold Change | mGC Fold Change | TC Fold Change | HSI − Mean RMA Hybridization Value Before LH |
HSI − Mean RMA Hybridization Value After LH |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| aGC | mGC | TC | aGC | mGC | TC | |||||
| High Expression Genes | ||||||||||
| Cytochrome P450; family 19; subfamily A; polypeptide 1 | CYP19A1 | −397.6 | −539.3 | −80.1 | 6762 | 13603 | 1255 | 17 | 25 | 16 |
| Carbohydrate sulfotransferase 8 | CHST8 | −103.8 | −89.7 | −15.6 | 2313 | 2044 | 270 | 22 | 23 | 17 |
| chr4:81639977-81640459 (+) | — | −63.5 | −44.1 | −56.9 | 22293 | 23339 | 5402 | 351 | 529 | 95 |
| Hydroxysteroid (17-β) dehydrogenase 1 | HSD17B1 | −50.5 | −16.5 | −5.7 | 2452 | 1039 | 197 | 49 | 63 | 34 |
| Brain-expressed X-linked 2 | BEX2 | −47.6 | −52.9 | −37.3 | 15996 | 16449 | 7717 | 336 | 311 | 207 |
| Troponin I type 3 (cardiac) | TNNI3 | −41.0 | −27.9 | −10.5 | 10015 | 7713 | 5333 | 244 | 276 | 507 |
| Inhibin; β A | INHBA | −29.5 | −29.4 | −44.6 | 24933 | 27321 | 9370 | 846 | 930 | 210 |
| chr23:7266183-7266577 (+) | (GCLC) | −24.9 | −18.8 | −7.9 | 8260 | 8319 | 2630 | 331 | 442 | 335 |
| Solute carrier family 35, member G1 | SLC35G1 | −20.4 | −22.5 | −10.8 | 3422 | 3945 | 740 | 168 | 175 | 69 |
| Glutamic-pyruvate transaminase | GPT | −19.2 | −26.5 | −4.6 | 1748 | 1990 | 276 | 91 | 75 | 60 |
| Janus kinase 3 | JAK3 | −18.0 | −17.7 | −12.5 | 2269 | 2816 | 2496 | 126 | 159 | 200 |
| ST3 β-galactoside α-2;3-sialyltransferase 4 | ST3GAL4 | −15.8 | −13.4 | −3.0 | 8672 | 11352 | 2212 | 549 | 849 | 726 |
| Arsenic (+3 oxidation state) methyltransferase | AS3MT | −9.0 | −9.5 | −18.6 | 347 | 358 | 2497 | 38 | 38 | 134 |
| Serpin peptidase inhibitor; clade A; member 3 | SERPINA3 | −8.7 | −7.6 | −3.9 | 3539 | 2669 | 3235 | 408 | 351 | 832 |
| Stearoyl-CoA desaturase 5 | SCD5 | −6.3 | −6.6 | −2.7 | 7996 | 11245 | 5720 | 1265 | 1701 | 2133 |
| ADP-ribosylation factor GTPase activating protein 3 | ARFGAP3 | −6.1 | −5.1 | −2.7 | 6010 | 7954 | 5284 | 988 | 1571 | 1978 |
| chr27:17010375-17010926 (−) | (SORBS2) | −6.1 | −4.9 | −3.2 | 3427 | 2903 | 4173 | 565 | 592 | 1319 |
| Guanine nucleotide binding protein (G protein); γ 10 | GNG10 | −5.4 | −4.7 | −1.8 | 10398 | 9768 | 4266 | 1911 | 2068 | 2332 |
| chr25:38495947-38496497(−) | — | −5.3 | −5.9 | −2.3 | 9275 | 7888 | 2375 | 1758 | 1339 | 1031 |
| Biliverdin reductase A | BLVRA | −5.0 | −4.1 | −2.2 | 4220 | 3271 | 1814 | 848 | 803 | 838 |
| chr27:36195193-36195591 (+) | (PLEKHA2) | −4.8 | −4.2 | −1.9 | 4214 | 3869 | 1608 | 872 | 926 | 835 |
| G protein-coupled receptor 125 | GPR125 | −4.6 | −5.1 | −2.4 | 2078 | 2619 | 1215 | 455 | 515 | 499 |
| Enoyl-CoA δ isomerase 1 | ECI1 | −3.6 | −2.1 | −2.2 | 1164 | 744 | 684 | 319 | 357 | 304 |
| Transcription elongation factor A (SII)-like 1 | TCEAL1 | −3.3 | −2.5 | −2.5 | 923 | 858 | 1418 | 277 | 344 | 562 |
| MAP/microtubule affinity-regulating kinase 1 | MARK1 | −3.2 | −3.6 | −3.0 | 2561 | 2565 | 1775 | 811 | 703 | 593 |
| Membrane-spanning 4-domains; subfamily A; member 8B | MS4A8B | −3.1 | −6.0 | −7.0 | 1775 | 3444 | 4092 | 564 | 575 | 585 |
| Ligand of numb-protein × 2 | LNX2 | −3.1 | −3.0 | −3.3 | 1899 | 1623 | 1337 | 611 | 544 | 411 |
| Ubiquitin specific peptidase 48 | USP48 | −2.5 | −2.3 | −1.8 | 1048 | 1140 | 640 | 418 | 493 | 347 |
| Transcriptional adaptor 3 | TADA3 | −2.0 | −1.8 | −1.9 | 1489 | 1180 | 827 | 749 | 648 | 425 |
| chr21:13524038-13524353 (−) | LOC100848010 | −2.0 | −1.7 | −2.0 | 2416 | 2418 | 2002 | 1237 | 1456 | 1019 |
| chr8:101776156-101776718 (+) | (ZNF462) | −1.8 | −2.1 | −2.0 | 845 | 896 | 535 | 461 | 429 | 270 |
| ORM1-like 2 (S. cerevisiae) | ORMDL2 | −1.6 | −1.7 | −1.6 | 965 | 1010 | 1103 | 593 | 608 | 696 |
| Progesterone receptor membrane component 1 | PGRMC1 | −1.3 | −1.6 | −1.6 | 1299 | 2355 | 2342 | 1023 | 1459 | 1480 |
| Myeloid cell leukemia sequence 1 | MCL1 | 2.3 | 2.4 | 1.8 | 645 | 574 | 914 | 1495 | 1378 | 1622 |
| SWI/SNF related; matrix associated; actin dependent regulator of chromatin; subfamily a; member 1 | SMARCA1 | 3.4 | 3.0 | 3.1 | 3258 | 3635 | 2445 | 11008 | 10934 | 7674 |
| WD repeat domain 26 | WDR26 | 3.5 | 2.5 | 1.8 | 1323 | 1515 | 926 | 4579 | 3796 | 1701 |
| SLAIN motif family; member 1 | SLAIN1 | 3.5 | 3.9 | 4.4 | 631 | 526 | 122 | 2236 | 2065 | 534 |
| chr3:8282216-8282683 (−) | (ATF6) | 4.2 | 3.6 | 2.1 | 904 | 1001 | 681 | 3828 | 3587 | 1428 |
| UDP-GlcNAc:βGal β-1;3-N-acetylglucosaminyltransferase 2 | B3GNT2 | 4.4 | 4.4 | 3.5 | 1779 | 1735 | 847 | 7905 | 7630 | 2982 |
| chr1:50742017-50742481 (−) | (CBLB) | 4.7 | 4.6 | 3.4 | 237 | 267 | 151 | 1118 | 1218 | 513 |
| CDC42 small effector 2 | CDC42SE2 | 5.5 | 5.5 | 2.6 | 696 | 705 | 510 | 3814 | 3859 | 1331 |
| TCDD-inducible poly(ADP-ribose) polymerase | TIPARP | 5.7 | 5.5 | 3.5 | 265 | 272 | 224 | 1513 | 1485 | 774 |
| Solute carrier family 25; member 17 | SLC25A17 | 6.4 | 4.6 | 2.2 | 551 | 831 | 646 | 3551 | 3829 | 1428 |
| N-Acylsphingosine amidohydrolase (acid ceramidase) 1 | ASAH1 | 6.5 | 6.0 | 3.9 | 324 | 342 | 567 | 2090 | 2051 | 2190 |
| Monoamine oxidase A | MAOA | 7.6 | 6.2 | 4.2 | 209 | 206 | 174 | 1583 | 1270 | 739 |
| Plasminogen activator; tissue | PLAT | 9.3 | 3.6 | 4.2 | 601 | 1897 | 801 | 5588 | 6754 | 3336 |
| Translocator protein (18 kDa) | TSPO | 9.3 | 8.3 | 3.5 | 156 | 170 | 279 | 1457 | 1413 | 977 |
| Retinol binding protein 1; cellular | RBP1 | 10.2 | 16.5 | 7.6 | 1039 | 758 | 1013 | 10634 | 12524 | 7657 |
| chr28:30192805-30192992 (+) | (SAMD8) | 10.6 | 8.6 | 4.7 | 251 | 354 | 188 | 2660 | 3054 | 890 |
| Metallothionein 2A | MT2A | 12.9 | 14.8 | 22.6 | 890 | 629 | 306 | 11508 | 9286 | 6917 |
| chr12:67614705-67615018(+) | (GPC6) | 13.4 | 11.9 | 6.8 | 244 | 280 | 198 | 3267 | 3341 | 1341 |
| Spermidine/spermine N1-acetyltransferase 1 | SAT1 | 15.0 | 12.7 | 2.7 | 1185 | 1213 | 4102 | 17742 | 15349 | 11043 |
| Regenerating islet-derived family; member 4 | REG4 | 17.6 | 10.5 | 5.2 | 88 | 134 | 360 | 1540 | 1413 | 1873 |
| chr23:35873098-35873230 (−) | (SOX4) | 18.9 | 11.0 | 3.8 | 391 | 558 | 670 | 7400 | 6143 | 2564 |
| chr26:11594846-11595263 (−) | (SLC16A12) | 19.0 | 12.6 | 26.4 | 57 | 106 | 14 | 1079 | 1337 | 360 |
| Thrombospondin 2 | THBS2 | 19.6 | 10.0 | 5.4 | 34 | 92 | 535 | 660 | 911 | 2863 |
| Nudix (nucleoside diphosphate linked moiety X)-type motif 10 | NUDT10 | 22.0 | 22.4 | 12.1 | 118 | 130 | 54 | 2587 | 2910 | 650 |
| Regulator of calcineurin 1 | RCAN1 | 23.1 | 20.2 | 3.9 | 167 | 181 | 288 | 3863 | 3647 | 1120 |
| SEMA5A sema domain, 7 thrombospondin repeats, transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 5A) | SEMA5A | 32.9 | 25.7 | 4.8 | 33 | 49 | 240 | 1101 | 1265 | 1144 |
| Nudix (nucleoside diphosphate linked moiety X)-type motif 11 | NUDT11 | 34.9 | 29.7 | 13.9 | 176 | 258 | 187 | 6152 | 7668 | 2596 |
| FBJ murine osteosarcoma viral oncogene homolog | FOS | 40.3 | 40.2 | 7.2 | 122 | 134 | 431 | 4898 | 5396 | 3088 |
| Procollagen C-endopeptidase enhancer 2 | PCOLCE2 | 43.1 | 21.6 | 7.6 | 25 | 55 | 189 | 1079 | 1181 | 1447 |
| TNF receptor superfamily; member 6b; decoy | TNFRSF6B | 51.6 | 81.1 | 10.0 | 28 | 25 | 36 | 1429 | 2005 | 361 |
| Neurotensin | NTS | 92.8 | 84.8 | 9.4 | 20 | 21 | 50 | 1887 | 1759 | 467 |
| TIMP metallopeptidase inhibitor 1 | TIMP1 | 106.8 | 41.3 | 9.6 | 52 | 251 | 1563 | 5600 | 10386 | 15001 |
| Chromosome 5 open reading frame, human C12orf75 | C5H12ORF75 | 108.6 | 76.2 | 18.9 | 26 | 37 | 156 | 2791 | 2803 | 2941 |
| Vanin 2 | VNN2 | 116.0 | 140.9 | 39.6 | 29 | 22 | 30 | 3364 | 3056 | 1170 |
| chr5:5940972-5941399 (−) | (PHLDA1) | 116.0 | 104.7 | 16.3 | 23 | 25 | 39 | 2665 | 2589 | 641 |
| chr6:22210314-22210785 (+) | (CXXC4) | 141.4 | 83.9 | 18.6 | 17 | 24 | 19 | 2432 | 2032 | 351 |
| ρ Family GTPase 3 | RND3 | 141.7 | 39.9 | 3.4 | 29 | 93 | 644 | 4046 | 3710 | 2217 |
| Prostaglandin-endoperoxide synthase 2 | PTGS2 | 274.0 | 289.6 | 32.4 | 16 | 13 | 20 | 4287 | 3876 | 643 |
| Periostin; osteoblast specific factor | POSTN | 325.0 | 194.8 | 13.3 | 10 | 21 | 114 | 3121 | 4067 | 1514 |
| chr23:19331670-19332170 (+) | (RUNX2) | 602.3 | 276.5 | 25.3 | 9 | 20 | 61 | 5282 | 5645 | 1536 |
| Pentraxin 3; long | PTX3 | 643.9 | 711.4 | 108.0 | 14 | 15 | 85 | 9232 | 10921 | 9158 |
| Intermediate Expression Gene | ||||||||||
| chr3:9463181-94632151 (+) | (Dab1) | −19.5 | −15.6 | −11.0 | 372 | 254 | 229 | 19 | 16 | 21 |
| Solute carrier family 5 (sodium/glucose cotransporter); member 11 | SLC5A11 | −19.1 | −28.5 | −13.1 | 227 | 369 | 329 | 12 | 13 | 25 |
| Ethanolamine kinase 2 | ETNK2 | −11.2 | −8.6 | −4.1 | 822 | 745 | 451 | 74 | 87 | 110 |
| Transmembrane protein 156 | TMEM156 | −9.1 | −7.3 | −3.2 | 728 | 468 | 169 | 80 | 65 | 53 |
| Troponin T type 1 (skeletal; slow) | TNNT1 | −8.3 | −4.6 | −15.4 | 148 | 105 | 898 | 18 | 23 | 58 |
| LH/CG receptor | LHCGR | −7.9 | −17.2 | −7.4 | 280 | 790 | 380 | 35 | 46 | 52 |
| Breast cancer antiestrogen resistance 3 | BCAR3 | −7.7 | −6.6 | −2.6 | 907 | 1074 | 328 | 118 | 162 | 125 |
| chr3:24537369-24537825 (−) | (PDE4DIP) | −4.5 | −3.2 | −2.7 | 397 | 446 | 865 | 88 | 140 | 320 |
| Aldolase C; fructose-bisphosphate | ALDOC | −4.4 | −2.4 | −2.5 | 939 | 563 | 582 | 212 | 236 | 232 |
| Protein phosphatase 1; regulatory (inhibitor) subunit 1A | PPP1R1A | −2.9 | −3.9 | −8.8 | 124 | 155 | 527 | 43 | 40 | 60 |
| unc-119 homolog (C. elegans) | UNC119 | −2.8 | −2.8 | −4.2 | 238 | 213 | 495 | 84 | 77 | 118 |
| Cox2 chaperone homolog | COX20 | −2.6 | −2.0 | −1.7 | 259 | 211 | 191 | 101 | 108 | 115 |
| Phosphofructokinase; muscle | PFKM | −2.5 | −2.4 | −2.6 | 327 | 442 | 616 | 132 | 186 | 236 |
| Estrogen receptor 1 | ESR1 | −2.4 | −2.7 | −1.9 | 391 | 426 | 174 | 162 | 160 | 91 |
| Propionyl CoA carboxylase; β polypeptide | PCCB | −2.2 | −2.2 | −2.2 | 362 | 674 | 977 | 165 | 303 | 446 |
| Chromosome 19 open reading frame, human C17orf101 | C19H17orf101 | −1.8 | −1.7 | −1.8 | 203 | 240 | 243 | 114 | 145 | 136 |
| chr10:70504545-70504958 (+) | (TMEM260) | −1.6 | −2.0 | −1.8 | 202 | 300 | 258 | 124 | 153 | 140 |
| chr18:53779152-53779618 (+) | (ARHGAP35) | −1.5 | −1.7 | −1.6 | 330 | 357 | 284 | 223 | 205 | 182 |
| Polyhomeotic homolog 2 | PHC2 | 2.7 | 2.6 | 2.0 | 216 | 196 | 201 | 589 | 507 | 401 |
| Filamin A interacting protein 1 | FILIP1 | 4.1 | 3.7 | 2.3 | 103 | 106 | 66 | 419 | 390 | 154 |
| Zinc finger; DHHC-type containing 23 | ZDHHC23 | 4.2 | 2.6 | 2.4 | 118 | 165 | 56 | 499 | 427 | 133 |
| Retinol binding protein 4; plasma | RBP4 | 4.7 | 4.2 | 3.9 | 50 | 83 | 50 | 237 | 349 | 195 |
| Similar to TPR repeat-containing protein 39 | TTC39C | 5.7 | 5.0 | 5.3 | 121 | 127 | 44 | 682 | 633 | 231 |
| chr8:99285533-99286070 (−) | (ABCA1) | 5.8 | 8.8 | 5.1 | 36 | 32 | 84 | 212 | 280 | 432 |
| Cylindromatosis | CYLD | 5.9 | 4.4 | 1.7 | 156 | 193 | 177 | 914 | 845 | 303 |
| A kinase (PRKA) anchor protein 11 | AKAP11 | 6.5 | 4.9 | 3.2 | 135 | 149 | 75 | 875 | 729 | 238 |
| Inositol 1;4;5-trisphosphate 3-kinase A | ITPKA | 6.7 | 6.3 | 2.1 | 95 | 96 | 175 | 634 | 606 | 373 |
| Fibulin 1 | FBLN1 | 6.7 | 4.4 | 4.0 | 97 | 226 | 130 | 652 | 994 | 523 |
| Solute carrier family 25; member 12 | SLC25A12 | 9.5 | 9.7 | 3.3 | 95 | 94 | 141 | 905 | 913 | 468 |
| chr9:42277341-42277566 (+) | — | 15.6 | 14.0 | 4.0 | 31 | 37 | 31 | 490 | 523 | 123 |
| TNF receptor superfamily; member 12A | TNFRSF12A | 16.5 | 20.3 | 6.0 | 17 | 30 | 81 | 286 | 617 | 486 |
| Poly (ADP-ribose) polymerase family; member 8 | PARP8 | 21.6 | 19.3 | 6.6 | 47 | 44 | 48 | 1027 | 858 | 316 |
| Regulator of G-protein signaling 2 | RGS2 | 22.1 | 14.7 | 4.4 | 22 | 26 | 26 | 490 | 376 | 113 |
| chr20:4115985-4116308 (−) | (Sh3pxd2b) | 23.8 | 18.3 | 4.6 | 27 | 36 | 119 | 649 | 662 | 550 |
| Protein phosphatase 4; regulatory subunit 4 | PPP4R4 | 23.9 | 19.6 | 8.0 | 39 | 57 | 69 | 930 | 1113 | 547 |
| KIAA0226-like | KIAA0226 liter | 45.9 | 35.0 | 7.7 | 12 | 11 | 13 | 557 | 390 | 97 |
| Glutamate receptor; ionotrophic; AMPA 3 | GRIA3 | 80.5 | 71.3 | 8.7 | 9 | 10 | 9 | 749 | 680 | 74 |
| Low Expression Genes | ||||||||||
| Rh family; B glycoprotein (gene/pseudogene) | RHBG | −11.1 | −10.8 | −7.0 | 99 | 98 | 89 | 9 | 9 | 13 |
| chr23:30536840-30537310 (−) | (Hist1h4 h) | −8.1 | −9.0 | −2.9 | 149 | 156 | 91 | 18 | 17 | 31 |
| Collectin subfamily member 11 | COLEC11 | −5.1 | −7.1 | −5.1 | 143 | 284 | 173 | 28 | 40 | 34 |
| Cytochrome P450; family 2; subfamily C; polypeptide 87 | CYP2C87 | −4.5 | −4.5 | −3.2 | 108 | 92 | 82 | 24 | 21 | 26 |
| Cytochrome P450; family 4; subfamily F; polypeptide 3 | CYP4F3 | −4.2 | −6.4 | −16.0 | 58 | 64 | 241 | 14 | 10 | 15 |
| Usher syndrome 1C binding protein 1 | USHBP1 | −4.1 | −4.3 | −3.2 | 140 | 127 | 159 | 34 | 30 | 50 |
| chr8:67614674-67615116 (−) | — | −3.1 | −3.4 | −2.5 | 184 | 203 | 96 | 60 | 60 | 39 |
| Tetraspanin 33 | TSPAN33 | −2.6 | −3.8 | −8.8 | 31 | 48 | 186 | 12 | 12 | 21 |
| Chemokine (C-C motif) ligand 25 | CCL25 | −2.4 | −2.5 | −2.7 | 199 | 167 | 220 | 83 | 66 | 82 |
| Shisa homolog 3 (Xenopus laevis) | SHISA3 | −2.1 | −2.9 | −3.3 | 19 | 29 | 34 | 9 | 10 | 10 |
| SH3-domain GRB2-like 2 | SH3GL2 | −1.9 | −3.1 | −3.6 | 190 | 225 | 114 | 99 | 74 | 32 |
| Sushi domain containing 3 | SUSD3 | −1.4 | −2.0 | −3.7 | 45 | 58 | 116 | 31 | 29 | 31 |
| tRNA splicing endonuclease 54 homolog (S. cerevisiae) | TSEN54 | −1.3 | −1.5 | −1.8 | 95 | 126 | 167 | 71 | 83 | 92 |
| Teashirt zinc finger homeobox 2 | TSHZ2 | −1.3 | −1.6 | −2.2 | 55 | 74 | 195 | 41 | 46 | 89 |
| LY6/PLAUR domain containing 1 | LYPD1 | 2.8 | 3.6 | 3.2 | 21 | 17 | 14 | 59 | 63 | 45 |
| chr10:27864596-27865146 (+) | (KATNBL1) | 3.0 | 2.4 | 1.9 | 101 | 100 | 49 | 301 | 246 | 90 |
| chr16:68337216-68337707 (−) | (VASH2) | 3.2 | 3.7 | 2.7 | 25 | 33 | 39 | 80 | 122 | 107 |
| chr2:53798017-53798235 (+) | (ZEB2) | 3.8 | 3.6 | 6.9 | 49 | 44 | 23 | 185 | 157 | 160 |
| Potassium large conductance calcium-activated channel; subfamily M; β member 4 | KCNMB4 | 5.6 | 7.6 | 2.2 | 25 | 23 | 58 | 139 | 176 | 127 |
| Retinoic acid induced 14 | RAI14 | 8.7 | 8.2 | 2.7 | 35 | 22 | 67 | 306 | 183 | 181 |
| Carbonic anhydrase XIII | CA13 | 8.7 | 11.8 | 7.1 | 17 | 16 | 14 | 148 | 185 | 100 |
| RAS protein activator like 1 (GAP1 like) | RASAL1 | 9.4 | 6.9 | 3.2 | 34 | 37 | 22 | 318 | 257 | 71 |
| Transmembrane protein 22 | TMEM22 | 11.3 | 9.6 | 2.2 | 11 | 11 | 11 | 126 | 108 | 23 |
| chr11:22800828-22801173 (−) | — | 13.7 | 14.0 | 3.9 | 14 | 13 | 14 | 197 | 188 | 53 |
| Meis homeobox 1 | MEIS1 | 32.3 | 22.8 | 2.9 | 8 | 11 | 60 | 258 | 246 | 178 |
Gene symbols enclosed in parentheses are probes sets that recognize transcripts with high homology to a named gene in another species.
Chromosomal locations are based on the Cow October 2011 Assembly. Dashes reflect a negative or down regulated gene.
Gene set enrichment analysis
To uncover biologic functions that are significantly affected by the LH surge, differentially expressed transcripts were analyzed with the Ingenuity Pathway Analysis (IPA) tool. Altogether 1614, 1373, and 146 of the differentially expressed transcripts, which had been identified in aGCs, mGCs, and TCs, respectively, could be significantly assigned to specific IPA biofunctions. Both GC fractions were affected by LH and yielded very similar biofunctional categories, which included “cell cycle, cellular assembly and organization,” and “DNA replication, recombination, and repair,” and “cellular movement.” The “functions annotations” within these broad categories had increased “predicted activation states” that were broadly associated with cellular migration (eg, “organization of cytoplasm/cytoskeleton,” “formation of plasma membrane projections,” “microtubule dynamics;” Supplemental Table 8). In addition, these cells had significantly decreased “predicted activation states” that were associated the “functions annotations” (“segregation of chromosomes,” “proliferation of cells”: Supplemental Table 8).
IPA analysis of the 146 LH-regulated theca genes indicated that the following categories were highlighted: “lipid metabolism,” “small molecule biochemistry,” “endocrine/reproductive system development and function” and “cell death and survival” were highlighted.
The “functions annotations” within these categories all had significant decreased “predicted activation states” associated with “modification of androstenedione” or “apoptosis of epidermal cells” (Supplemental Table 9).
Discussion
This study describes, for the first time, the comparative gene expression profile of TCs cells from preovulatory follicles (before LH surge) to periovulatory follicles (collected ∼21 h after a GnRH-induced LH surge) of any species. Comparison of LH-regulated TC genes with the other LH-responsive cell (ie, granulosa) within the same follicle, also provides additional novel insights into ovarian physiology as well as helps with interpretation of previous “granulosa cell” only studies. LH responsiveness of the mural GCs has long been known to vary with respect to location of GCs with respect to distance from the basement membrane that separates it from the theca. Our results indicate that these differences are mostly quantitative because the actual genes that were expressed in the antral (aspirated) and membrane-associated (scraped) granulosa are not that different, with only a few genes exhibiting any major quantitative differences with respect to position within the follicular wall. Lastly, this study provides a robust and comprehensive accounting of gene expression changes that occur following the LH surge in bovine follicular cells and the identification of a number of novel LH-regulated genes in the bovine follicle, both in GCs and TCs.
Validation of experimental model and cellular preparations
The morphologic, physiologic, and molecular characterization (principal component analysis) of these dominant follicles collected before and after the LH surge, clearly indicate that very little variation was observed across cows, again indicating a very consistent experimental procedure. The increased mean size and the dramatic decrease in the E2:P4 ratio indicates that the conversion from an E2-active to E2-inactive follicles in the wake of the preovulatory LH surge is consistent with previous studies using this same paradigm to collect preovulatory follicles (before LH surge) and periovulatory follicles (collected ∼21 h after a GnRH-induced LH surge) as described earlier (3) as was the induction of PTGS2. The consistency of the array data was further validated by the very high correlations (R = 0.73 to 1.0) for 15 genes when compared with qRT-PCR levels. Because of these highly uniform correlation coefficients, we can be very confident in the relative fold-differences predicted by the arrays.
The purity of GC and TC sample preparations was verified by analysis of CYP17A1 (17α-hydroxylase) or CYP19A1 (aromatase) and FSHR, which have been reported to be restricted to the theca and granulosa layers, respectively (4) by in situ hybridization. Our results are consistent with earlier studies that show both steroidogenic genes and the FSHR gene are largely down-regulated by the preovulatory LH surge (3, 30, 31). The presence of CYP17A1 and CYP19A1 in mGC and TC, respectively, at very low levels, might be explained by slight contamination with the respective other cell type. Following the scraping of the basement membrane, this is probably not avoidable, nor is the incidental sloughing of some TC into the mGC preparation following scraping. Interestingly, 2 of the top LH down-regulated genes as reported by Gilbert et al. (20) in mural bovine GCs, CXCL14 (chemokine [C-X-C motif] ligand 14) and ASPN (asporin), were found in our experiments to be primarily expressed in TCs (142- and 709-HSI) compared with aGCs and mGCs (HSI < 20), respectively. Furthermore, we show that these 2 reportedly GC LH-regulated genes (20) were only LH-regulated in TCs (Table 3), decreasing significantly after the LH surge. This and other previous studies examining GC gene expression in periovulatory follicles did not examine purity of their cellular preparations and based their GC derivation solely on the method of isolation (scraping), which is identical to our procedure for mGCs. Consistent with their distance from the TCs, the aGCs had significantly less CYP17A1 than mGC, yet was still detectable (by hybridization) even though the experimental procedure (needle aspiration) was highly unlikely to allow a significant amount of cross-contamination. This leaves open several alternatives, 1) GCs/TCs may actually express very low levels of “contaminating” mRNA that are sufficient to be detected via the sensitive methods we have used or 2) alternatively, extracellular RNA in the form of exosome encapsulated/associated RNA released from GCs, TCs, or follicular fluid could be associating with the basement membrane or other cell preparations effectively contaminating them. The lack of detection of oocyte-derived transcripts (eg, BMP15 and GDF9 and ZP2, ZP3, and ZP4) in the aGC preparation illustrates that the levels of detectable contaminating RNAs (eg, CYP17A1, CYP19A1, etc.) is of a greater magnitude than that can be detected from a single oocyte; however, it is no reliable indication for the actual absence of oocyte, which might be present in these preparations. The high induction of PTGS2 expression in the GCs following the GnRH-induced LH surge is a conserved mechanism that occurs about 18 hours post-hCG treatment in bovine follicles in vivo and is considered a reliable marker for approaching ovulation (29, 32). Overall these results indicate a very small degree of contamination that may only be evident for those genes with extremely high expression such as CYP17A1 and CYP19A1, because low/intermediate expressing genes (FSHR), although still being detected, were far more cell selective. On the other hand, the uneven distribution of LHCGR transcripts particularly between the aGC and mGC samples indicates that both granulosa preparations, in fact, represent different fractions. As expected from data of others (7), LHCGR transcripts predominated in the GCs close to the basement membrane (mGC [HSI-790] compared with the aspirated cells [aGCs, HSI-280] or TCs [HSI-380] before LH, respectively).
Theca-specific transcriptome is considerably less affected by the preovulatory LH surge than the granulosa-specific transcriptome
Principal component analysis revealed that the LH surge exerted a significant influence on the expression profiles of theca and granulosa samples, leading to the arrangement of the samples into clearly defined clusters, before and after LH. Also theca and granulosa samples could be clearly separated due to their cell type-specific expression profiles. Whereas 25% and 21% of all expressed genes showed a significant reaction to the GnRH-induced LH surge in aGCs and mGCs respectively, only 2% of the genes present in TCs were significantly up- or down-regulated. The vast majority of genes that are LH regulated in granulosa and theca showed the same direction of change (up or down) in both cell types. The strongly varying extent of LH response in theca and granulosa, however, cannot be simply explained by a lower number of LH binding sites (LH receptors) on cells of the theca compared with those of the granulosa. Previous studies, as well as the present study, indicate that both cell types, granulosa and theca, expressed the corresponding LHCGR transcripts at relatively high levels before the onset of the LH surge (see Table 1 and Refs. 3, 4, and 33). One might speculate that the signal transduction pathway downstream of the LH receptor might be different in GCs and TCs during the periovulatory period. This is the first demonstration that the TC transcriptome shows considerably less alteration in the wake of the preovulatory LH surge compared with that of the GCs. This result might be expected if one considers the reduced morphologic and biochemical changes that occur in TCs (loss of androgen biosynthesis, minimal cell hypertrophy) compared with GCs (loss of cell proliferation, loss of aromatase activity, induction of numerous steroidogenic genes, extensive cell hypertrophy).
Novel genes identified in bovine TCs that are regulated by the LH surge
Although critical roles in periovulatory follicular function and subsequent luteal function have been defined for TCs, previous studies examining TC cell gene expression have been limited to individual genes (3, 34, 35) or cultured TC experiments (36). This study is therefore the first to globally identify thecal genes that exhibit changes in mRNA expression in vivo following the LH surge. In total 203 genes (combined results of Tables 3 and 4) exhibited differential expression in the TCs following the LH surge. However, only 57 of these 199 were specifically LH regulated in TCs (Table 3). Of the remaining 141 LH-regulated genes found common to both granulosa and theca, only about 10% or 19 of these LH-regulated genes could be absolutely confirmed to be LH regulated in theca because they had higher (HSI) expression levels than in corresponding GC preparations. A great number of the other 141 genes (Table 4) showed similar levels across the cell types, but without further analysis (ie, in situ hybridization or laser capture dissection followed by qRT-PCR confirmation) cannot be assured as truly LH-regulated genes in TC, and not cross-contamination by granulosa. The 19 additional thecal LH-regulated genes included AS3MT, SORBS2, TCEAL1, MS4A8B, ORMDL2, MCL1, ASAH1, SAT1, THBS2, FOS, PCOLCE2, TIMP1, RND3, TNNT1, PDE4DIP, PPP1R1A, PFKM, PCCB, SIPA1 (encoding arsenic methyltransferase, transcription elongation factor A-like 1, sorbin and SH3 domain containing 2, membrane spanning 4-domains; subfamily A; member 8B, ORM1-like 2, myeloid cell leukemia sequence 1, N-acylsphingosine amidohydrolase 1 [acid ceramidase], spermidine/speriine N1-acetyltransferase 1, thromospondin 2, FOS, procollagen C-endopeptidase enhancer 2, TIMP1, Rho family GTPase 3, troponin T type 1, phosphodiesterase 4D interacting protein [PDE4DIP], transcript variant 8, phosphofructokinase, propionyl CoA carboxylase; β polypeptide, protein phosphatase 1; regulatory subunit 1A, signal-induced proliferation-associated 1, respectively).
Not surprisingly, CYP17A1 mRNA, which encodes the critical TC enzyme involved in androgen biosynthesis had the greatest decrease (1/77 of pre-LH values) following the LH surge. Transcriptional regulation of the CYP17A1 gene has been well studied (31, 37) and steroidogenic factor-1 (SF-1 encoded by NR5A1) one of its primary transcriptional regulators was also decreased to approximately one-third of its pre-GnRH level following the LH surge in TC (Table 3). As an established mediator of steroidogenic activity and known transcriptional activator of CYP17A1, this loss of SF-1 in TCs and lack of LH effect on GCs is consistent with previous observations, which indicate that NR5A2 (also known as LRH-1) is the primary steroidogenic transcription factor in GCs (38). We additionally identified acid ceramidase (ASAH1) as one of 19 genes induced (∼4- to 6-fold) by LH that was common to both TCs and GCs. Recently, ASAH1, which catalyzes the conversion of ceramide to sphingoside (generating sphingoside 1 phosphate) was demonstrated to be induced in adrenocortical cells by ACTH/cAMP, and this was associated with changes in adrenal steroidogenesis, by decreasing the expression of a number of steroidogenic enzymes including CYP17A1 (39, 40). This is the first demonstration that LH may have a similar effect in TCs. Another unique aspect of sphinoside-1 phosphate is its antagonist actions on NR5A1 transcriptional activity (41). Thus, in addition to a decrease in SF-1 expression, acid ceremide-induced sphingosine may further block SF-1 action, ultimately leading to the sharp reduction in CYP17A1 expression we observed. With respect to the remaining LH-regulated thecal genes, a large number are associated with lipid metabolism and regulation of apoptosis (see Supplemental Table 9), both of which would be considered as important in luteinization and formation of the corpus luteum.
To our surprise we found PTX3 (pentraxin 3) as a highly abundant and LH-regulated gene also in TCs. It is well established that this protein plays a role as a modulator of the inflammatory response (42). In the context of the ovarian follicle, however, its expression has only been reported in the context of GCs of periovulatory follicles, where it mediates cumulus cell-oocyte interactions (43). In a recent paper it was also found that it interferes with fibroblast growth factor 2 actions in luteal endothelial cells, thus inhibiting angiogenesis (44). Suggestively, pentraxin 3 might be involved in the regulation of angiogenesis also in the intensely vascularized theca layer. However, the function of its heavy LH-induced up-regulation during the periovulatory period still seems obscure, because this protein is an inhibitor of angiogenesis, whereas the luteinization process is accompanied by intense vascular growth.
The thecal-specific gene with the greatest LH induction we identified was LOXL4 (encoding lysyl oxidase-like 4). This gene's expression increased 18.8-fold (HSI-98 to HSI-1847) following the LH surge, with no concomitant LH induction in aGCs or mGCs (HSI was <148 at all time points in granulosa). Lysyl oxidases are an extracellular copper enzyme that cross-link lysine side chains in mature fibrillar collagen or elastin, regulating the tensile strength and structural integrity of connective tissues (45). LOXL4 shares it amine oxidase motif and the scavenger receptor cysteine-rich protein domain with 4 other family members, lysyl oxidase (LOX) and lysyl oxidase like 1–4 (45). Our study detected expression of LOX, LOXL1, and LOXL2 in TCs and GCs, with TCs having higher levels of LOX (HSI-140) and LOXL1 (HSI-450) than GCs (HSI < 30 for both), whereas equal levels of LOXL2 (all HSI < 62) were found in TCs and GCs. However, no LH regulation was observed for these other lysyl oxidase family members within TC or GC compartments. Conversely, although whole-tissue Northern blot analyses have demonstrated presence of LOXL4 in human ovary as well as numerous other tissues (46), this is the first indication of LH regulation of this gene in any ovarian tissue. Previous studies examining LOX expression and activity have shown it to be down-regulated during early folliculogenesis by FSH (47, 48). In other cells, LOXL4 has been shown to block cell migration by suppressing the synthesis of laminins and α3 integrin and the activity of matrix metallopeptidase 2 (49), and recent studies have shown the full-length protein to be a tumor suppressor gene (50). Thus, the high induction of LOXL4 seen in TCs might be a critical regulatory protein within the extracellular matrix in the highly dynamic periovulatory follicle, and this gene awaits further study. Lastly, most of the newly identified TC genes have not been studied within the context of the periovulatory follicle; therefore this study provides a critical first step in our understanding this largely understudied follicular cell.
Variation in mural GC transcriptome based on location within the periovulatory follicle
Immunohistochemical analyses and in situ hybridization studies have shown that LHCGR levels can vary with distance from the basement membrane (7). The relative expression of LHCGR in our aGC and mGC preparations before the LH surge (Table 1) supports this observation although it did not reach significance. Recent studies examining the gene profile in GC types, mural vs cumulus granulosa, have observed large differences (51–53). Our study did not aim to investigate this difference but instead wished to determine whether differences in the mural granulosa (ie, those near the antrum vs those near the membrane) could be elucidated. Indeed although our aGC samples would likely be contaminated with cumulus cells and the oocyte, we failed to detect oocyte-specific genes or the differences observed in these other papers (51–53). Our study further indicates only minor differences in the 2 different mural GC preparations: mGCs (ie, membrane-associated) and aGCs (aspirated). This inability to detect changes could easily be attributed to a pronounced contamination of the aGC population with mGCs, a result that cannot be prevented using the current procedures. To further evaluate whether a quantitative difference might exist we compared the fold changes in response to LH across the cells (See Supplemental Table 7 for complete list) and identified a small number of genes (29) that were different (Table 2). We observed a preferential loss (one-fourth of its pre-LH levels) of CARTPT transcript in mGCs that was unaffected in aGCs. Decreased follicular fluid levels and expression of CARTPT have been previously tied to bovine follicle selection and increased health status of the dominant follicle in bovine (54). Many of the other genes that were quantitatively differentially expressed (>3-fold difference) between aGCs and mGCs were either extracellular matrix proteins or proteins that interact with or metabolize these proteins. Interestingly, extracellular matrix genes were also enriched in cumulus cells in comparison with mural granulosa in human preovulatory follicles (52). Extracellular matrix proteins separate the granulosa and theca (ie, basement membrane) or are of a specialized type (also known as focimatrix proteins) between GCs (55). Most of these proteins (ie, collagen α-1, claudin 11, a disintegrin-like and metallopeptidase with thrombospondin type 1 motif [ADAMTS1]), had slightly higher expression in the mGCs vs aGCs before the LH surge and fairly equivalent levels after the LH surge. Nidogen 2 and fibronectin 1, both extracellular matrix proteins, had clearly greater expression in the mGC (558- and 744-HSI) compared with almost background levels (23- and 89-HSI) in aGCs before LH, and almost equivalent levels after the LH surge (see Table 2). Two additional genes which modulate extracellular signaling, neural precursor cell expressed; developmentally down-regulated 9 (NEDD9) and SAM and SH3 domain containing 1 (SASH1) were LH-regulated in aGCs, exclusively. These two proteins have been linked to cancer cell migration and invasion (56, 57). Whether these genes may play a role in the ovulation by oocyte migration model as proposed by Akison et al. (58) is unknown. Lastly, several genes involved in cell cycle control, CCND2 (cyclin D2) and KIAA0101 (also known as proliferating cell nuclear antigen-associated factor) were both found to undergo a pronounced decrease in expression after the LH surge, with the decline being of slightly greater magnitude in the aGCs than in the mGCs (see Table 2).
Novel genes identified in bovine GCs that are regulated by the LH surge
Our study identified a large number of heretofore unknown LH-regulated genes in bovine granulosa, even though this is not the first study to characterize the periovulatory GCs following the LH surge. Indeed, a recent study examining late post-LH changes of GC-specific expression profiles in bovine follicles (20) having remarkably different data was reported. Surprisingly, only 121 of LH-regulated genes are common between both studies. In addition, when comparing the transcript abundance data of individual genes, remarkable differences are evident. From the 15 cell marker transcripts shown in Table 1, 14 are represented on both microarray systems (ADAMTS1, CCND2, CYP11A1, CYP19A1, FSHR, LHCGR, NR5A1, NR5A2, PCNA, PTGS2, PTX3, STAR, TIMP1, HSD3B1). From these 14, only CCND2, STAR, and TIMP1 were differentially expressed in the Gilbert et al. (20) data set (see Supplemental Data 1). The fact that PTGS2 was not up-regulated in the study of Gilbert et al. (20), at 22 hours post-LH, is surprising. PTGS2 expression is a conserved mechanism that occurs about 18 hours post-hCG treatment in bovine follicles in vivo (29, 32) and can be considered as a reliable marker for approaching ovulation. A thorough comparison of the 2 studies is presented in Supplemental Data 1. Consistent with our study, of 23 granulosa-derived transcripts that were found down-regulated 23 hours after hCG application using substractive hybridization (19), 13 (ANAPC5, ARFGAP3, CYP19A1, FNDC3B, FSHR, GJA1, IDH3A, INHBA, LRP8, PRC1, RPA2, SCD, TRIB2) were confirmed. In this regard, however, the deviating animal models might be responsible for some of the differences observed between studies. In the study of Gilbert et al. (20) follicles were harvested following an ovarian superovulation regime. In contrast, the monitoring (ultrasonography during estrous cycle, signs of heat, morphologic parameters, antral E2 and P4 concentrations, appearance of PTGS2 transcripts) and hormone treatment regime used during the present study was developed to obtain only a single, well-characterized large dominant preovulatory follicle per animal, thus mimicking the natural cycling situation as close as possible.
We identified 2 members of the Ig-like domain-containing superfamily, IGSF1 and IGSF11, encoding Ig superfamily; members 1 and 11, as LH-regulated genes in GC. These genes have not been identified in GC before and, interestingly, both are regulated by LH in the opposite way: whereas the x-chromosomal IGSF1 is clearly up-regulated in aGCs and mGCs (10.1- and 10.9-fold in aGCs and mGCs, respectively), IGSF11 is significantly down-regulated in both GC populations (−4.9 and −6.7 fold in aGCs and mGCs, respectively). Generally, the Ig domain has evolved to serve diverse biologic functions including growth and development, signaling, adhesion, and protein-carbohydrate interactions (59). Specifically, IGSF11 has been clearly connected to carcinogenesis (60, 61). The role of these specific Igs in luteinizing GC and, in particular, the complementary regulatory characteristic is not yet clear. However considering their function in other tissues, both might play a role during the intense tissue reorganization of the granulosa layer during the formation of the corpus luteum. This might also be applicable to another interesting gene, AMIGO2 (adhesion molecule with Ig-like domain 2), which is up-regulated 6.0- and 3.0-fold in aGCs and mGCs, respectively, by LH. AMIGO2 is a member of the AMIGO/Alvin family, which are transmembrane proteins characterized by 6 leucine-rich repeats and a single Ig-like extracellular domain. AMIGO proteins are mainly found in the nervous system, where they function as novel cell adhesion molecules that facilitate neuronal growth (62). Correspondingly, during the formation of the corpus luteum AMIGO2 that is up-regulated to relatively high levels in aGCs and mGCs by the preovulatory LH surge (1020-HSI and 1117-HSI, respectively; Supplemental Table 7) might also be involved in mediating cell adhesion and regulating migration of GC.
In conclusion, the data of the present study demonstrate that 1) theca cell-specific transcriptome is considerably less affected by the preovulatory LH surge than that of GCs, consistent with the greater changes induced in GCs as they undergo luteinization, 2) aGCs and mGCs show very similar gene expression profiles, thus suggesting a widely equivalent molecular equipment of both GC preparations and 3) numerous novel LH-regulated genes in GCs and TCs were identified. This is particularly true for the TCs where, with the exception of a few known TC marker genes, almost all of the genes were identified as LH regulated for the first time. This research resource comparing the transcriptomic profiles of both thecal and granulosa follicular cells from preovulatory (pre-LH) and post-LH (ie, periovulatory) follicles provides an easily accessible and searchable resource that should greatly benefit the scientific community.
Supplementary Material
Acknowledgments
We thank Maren Anders of the Reproductive Biology Unit at the Leibniz Institute for Farm Animal Biology for excellent technical assistance.
This work was supported by Grant VA135/5-2 from the Deutsche Forschungsgemeinschaft (DFG) and by Grant HD061580 from the National Institute of Health (to L.K.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aGC
- antral (aspirated) granulosa cell
- E2
- 17β-estradiol
- fdr
- false discovery rate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GC
- granulosa cell
- hCG
- human chorionic gonadotropin
- HSI
- hybridization signal intensity
- IPA
- Ingenuity Pathway Analysis
- mGC
- membrane-associated granulosa cell
- P4
- progesterone
- qRT-PCR
- quantitative RT-PCR
- RMA
- Robust Multichip Average
- SF-1
- steroidogenic factor-1
- TC
- thecal cell.
References
- 1. Smith MF, McIntush EW, Smith GW. Mechanisms associated with corpus luteum development. J Anim Sci. 1994;72:1857–1872 [DOI] [PubMed] [Google Scholar]
- 2. Murphy BD. 2004 Luteinization. In: Leung PC, Adashi EY, eds. The Ovary. 2nd ed Burlington, MA: Academic Press; 2004;185–199 [Google Scholar]
- 3. Nimz M, Spitschak M, Schneider F, Fürbass R, Vanselow J. Down-regulation of genes encoding steroidogenic enzymes and hormone receptors in late preovulatory follicles of the cow coincides with an accumulation of intrafollicular steroids. Domest Anim Endocrinol. 2009;37:45–54 [DOI] [PubMed] [Google Scholar]
- 4. Bao B, Garverick HA. Expression of steroidogenic enzyme and gonadotropin receptor genes in bovine follicles during ovarian follicular waves: a review. J Anim Sci. 1998;76:1903–1921 [DOI] [PubMed] [Google Scholar]
- 5. Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin model' revisited. Mol Cell Endocrinol. 1994;100:51–54 [DOI] [PubMed] [Google Scholar]
- 6. Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207 [DOI] [PubMed] [Google Scholar]
- 8. Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–446:431–446 [DOI] [PubMed] [Google Scholar]
- 9. Markholt S, Grøndahl ML, Ernst EH, Andersen CY, Ernst E, Lykke-Hartmann K. Global gene analysis of oocytes from early stages in human folliculogenesis shows high expression of novel genes in reproduction. Mol Hum Reprod. 2012;18:96–110 [DOI] [PubMed] [Google Scholar]
- 10. Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–984 [DOI] [PubMed] [Google Scholar]
- 11. Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Z, Youngquist RS, Garverick HA, Antoniou E. Molecular mechanisms regulating bovine ovarian follicular selection. Mol Reprod Dev. 2009;76:351–366 [DOI] [PubMed] [Google Scholar]
- 13. Zielak AE, Canty MJ, Forde N, et al. Differential expression of genes for transcription factors in theca and granulosa cells following selection of a dominant follicle in cattle. Mol Reprod Dev. 2008;75:904–914 [DOI] [PubMed] [Google Scholar]
- 14. Hayashi KG, Ushizawa K, Hosoe M, Takahashi T. Differential genome-wide gene expression profiling of bovine largest and second-largest follicles: identification of genes associated with growth of dominant follicles. Reprod Biol Endocrinol. 2010;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jo M, Gieske MC, Payne CE, et al. Development and application of a rat ovarian gene expression database. Endocrinology. 2004;145:5384–5396 [DOI] [PubMed] [Google Scholar]
- 16. Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–1670 [DOI] [PubMed] [Google Scholar]
- 17. Carletti MZ, Christenson LK. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction. 2009;137:843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu F, Stouffer RL, Müller J, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17:152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ndiaye K, Fayad T, Silversides DW, Sirois J, Lussier JG. Identification of downregulated messenger RNAs in bovine granulosa cells of dominant follicles following stimulation with human chorionic gonadotropin. Biol Reprod. 2005;73:324–333 [DOI] [PubMed] [Google Scholar]
- 20. Gilbert I, Robert C, Dieleman S, Blondin P, Sirard MA. Transcriptional effect of the LH surge in bovine granulosa cells during the peri-ovulation period. Reproduction. 2011;141:193–205 [DOI] [PubMed] [Google Scholar]
- 21. Rao JU, Shah KB, Puttaiah J, Rudraiah M. Gene expression profiling of preovulatory follicle in the buffalo cow: effects of increased IGF-I concentration on periovulatory events. PLoS One. 2011;6:e20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walsh SW, Mehta JP, McGettigan PA, et al. Effect of the metabolic environment at key stages of follicle development in cattle: focus on steroid biosynthesis. Physiol Genomics. 2012;44:504–517 [DOI] [PubMed] [Google Scholar]
- 23. Vanselow J, Spitschak M, Nimz M, Fürbass R. DNA methylation is not involved in preovulatory down-regulation of CYP11A1, HSD3B1, and CYP19A1 in bovine follicles but may have a role in permanent silencing of CYP19A1 in large granulosa lutein cells. Biol Reprod. 2010;82:289–298 [DOI] [PubMed] [Google Scholar]
- 24. Schneider F, Brüssow KP. Effects of a preovulatory administered depot gonadotrophin-releasing hormone agonist on reproductive hormone levels and pregnancy outcome in gilts. Reprod Fertil Dev. 2006;18:857–866 [DOI] [PubMed] [Google Scholar]
- 25. Ireland JJ, Roche JF. Development of antral follicles in cattle after prostaglandin-induced luteolysis: changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors. Endocrinology. 1982;111:2077–2086 [DOI] [PubMed] [Google Scholar]
- 26. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264 [DOI] [PubMed] [Google Scholar]
- 27. Nimz M, Spitschak M, Fürbass R, Vanselow J. The pre-ovulatory luteinizing hormone surge is followed by down-regulation of CYP19A1, HSD3B1, and CYP17A1 and chromatin condensation of the corresponding promoters in bovine follicles. Mol Reprod Dev. 2010;77:1040–1048 [DOI] [PubMed] [Google Scholar]
- 28. Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Doré M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Human Reproduction Update. 2004;10:373–385 [DOI] [PubMed] [Google Scholar]
- 29. Sirois J. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology. 1994;135:841–848 [DOI] [PubMed] [Google Scholar]
- 30. Voss AK, Fortune JE. Levels of messenger ribonucleic acid for cytochrome P450 17α-hydroxylase and P450 aromatase in preovulatory bovine follicles decrease after the luteinizing hormone surge. Endocrinology. 1993;132:2239–2245 [DOI] [PubMed] [Google Scholar]
- 31. Conley AJ, Kaminski MA, Dubowsky SA, Jablonka-Shariff A, Redmer DA, Reynolds LP. Immunohistochemical localization of 3β-hydroxysteroid dehydrogenase and P450 17α-hydroxylase during follicular and luteal development in pigs, sheep, and cows. Biol Reprod. 1995;52:1081–1094 [DOI] [PubMed] [Google Scholar]
- 32. Liu J, Carrière PD, Doré M, Sirois J. Prostaglandin G/H synthase-2 is expressed in bovine preovulatory follicles after the endogenous surge of luteinizing hormone. Biol Reprod. 1997;57:1524–1531 [DOI] [PubMed] [Google Scholar]
- 33. Bao B, Garverick HA, Smith GW, Smith MF, Salfen BE, Youngquist RS. Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome P450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles. Biol Reprod. 1997;56:1158–1168 [DOI] [PubMed] [Google Scholar]
- 34. Li Q, Jimenez-Krassel F, Ireland JJ, Smith GW. Gene expression profiling of bovine preovulatory follicles: gonadotropin surge and prostanoid-dependent up-regulation of genes potentially linked to the ovulatory process. Reproduction. 2009;137:297–307 [DOI] [PubMed] [Google Scholar]
- 35. Mamluk R, Wolfenson D, Meidan R. LH receptor mRNA and cytochrome P450 side-chain cleavage expression in bovine theca and granulosa cells luteinized by LH or forskolin. Domest Anim Endocrinol. 1998;15:103–114 [DOI] [PubMed] [Google Scholar]
- 36. Wood JR, Nelson VL, Ho C, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278:26380–26390 [DOI] [PubMed] [Google Scholar]
- 37. Komar CM, Berndtson AK, Evans AC, Fortune JE. Decline in circulating estradiol during the periovulatory period is correlated with decreases in estradiol and androgen, and in messenger RNA for p450 aromatase and p450 17α-hydroxylase, in bovine preovulatory follicles. Biol Reprod. 2001;64:1797–1805 [DOI] [PubMed] [Google Scholar]
- 38. Zhao H, Li Z, Cooney AJ, Lan ZJ. Orphan nuclear receptor function in the ovary. Front Biosci. 2007;12:3398–3405 [DOI] [PubMed] [Google Scholar]
- 39. Lucki N, Sewer MB. The cAMP-responsive element binding protein (CREB) regulates the expression of acid ceramidase (ASAH1) in H295R human adrenocortical cells. Biochim Biophys Acta. 2009;1791:706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lucki NC, Bandyopadhyay S, Wang E, Merrill AH, Sewer MB. Acid ceramidase (ASAH1) is a global regulator of steroidogenic capacity and adrenocortical gene expression. Mol Endocrinol. 2012;26:228–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Urs AN, Dammer E, Sewer MB. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–5258 [DOI] [PubMed] [Google Scholar]
- 42. Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012;2012:920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varani S, Elvin JA, Yan C, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167 [DOI] [PubMed] [Google Scholar]
- 44. Zalman Y, Klipper E, Farberov S, et al. Regulation of angiogenesis-related prostaglandin f2α-induced genes in the bovine corpus luteum. Biol Reprod. 2012;86:92. [DOI] [PubMed] [Google Scholar]
- 45. Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398 [DOI] [PubMed] [Google Scholar]
- 46. Mäki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20:493–496 [DOI] [PubMed] [Google Scholar]
- 47. Harlow CR, Rae M, Davidson L, Trackman PC, Hillier SG. Lysyl oxidase gene expression and enzyme activity in the rat ovary: regulation by follicle-stimulating hormone, androgen, and transforming growth factor-β superfamily members in vitro. Endocrinology. 2003;144:154–162 [DOI] [PubMed] [Google Scholar]
- 48. Slee RB, Hillier SG, Largue P, Harlow CR, Miele G, Clinton M. Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology. 2001;142:1082–1089 [DOI] [PubMed] [Google Scholar]
- 49. Kim DJ, Lee DC, Yang SJ, et al. Lysyl oxidase like 4, a novel target gene of TGF-β1 signaling, can negatively regulate TGF-β1-induced cell motility in PLC/PRF/5 hepatoma cells. Biochem Biophys Res Commun. 2008;373:521–527 [DOI] [PubMed] [Google Scholar]
- 50. Sebban S, Golan-Gerstl R, Karni R, Vaksman O, Davidson B, Reich R. Alternatively spliced lysyl oxidase-like 4 isoforms have a pro-metastatic role in cancer. Clin Exp Metastasis. 2013;30:103–117 [DOI] [PubMed] [Google Scholar]
- 51. Gilchrist RB, Ritter LJ, Myllymaa S, et al. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119:3811–3821 [DOI] [PubMed] [Google Scholar]
- 52. Grøndahl ML, Andersen CY, Bogstad J, Borgbo T, Boujida VH, Borup R. Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol Hum Reprod. 2012;18:572–584 [DOI] [PubMed] [Google Scholar]
- 53. Kõks S, Velthut A, Sarapik A, et al. The differential transcriptome and ontology profiles of floating and cumulus granulosa cells in stimulated human antral follicles. Mol Hum Reprod. 2010;16:229–240 [DOI] [PubMed] [Google Scholar]
- 54. Smith GW, Sen A, Folger JK, Ireland JJ. Putative role of cocaine- and amphetamine-regulated transcript (CARTPT) in dominant follicle selection in cattle. Soc Reprod Fertil Suppl. 2010;67:105–117 [PubMed] [Google Scholar]
- 55. Irving-Rodgers HF, Catanzariti KD, Aspden WJ, D'Occhio MJ, Rodgers RJ. Remodeling of extracellular matrix at ovulation of the bovine ovarian follicle. Mol Reprod Dev. 2006;73:1292–1302 [DOI] [PubMed] [Google Scholar]
- 56. Chang JX, Gao F, Zhao GQ, Zhang GJ. Role of NEDD9 in invasion and metastasis of lung adenocarcinoma. Exp Ther Med. 2012;4:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meng Q, Zheng M, Liu H, et al. SASH1 regulates proliferation, apoptosis, and invasion of osteosarcoma cell. Mol Cell Biochem. 2013;373:201–210 [DOI] [PubMed] [Google Scholar]
- 58. Akison LK, Alvino ER, Dunning KR, Robker RL, Russell DL. Transient invasive migration in mouse cumulus oocyte complexes induced at ovulation by luteinizing hormone. Biol Reprod. 2012;86:125. [DOI] [PubMed] [Google Scholar]
- 59. Srinivasan M, Roeske RW. Immunomodulatory peptides from IgSF proteins: a review. Curr Protein Pept Sci. 2005;6:185–196 [DOI] [PubMed] [Google Scholar]
- 60. Watanabe T, Suda T, Tsunoda T, et al. Identification of immunoglobulin superfamily 11 (IGSF11) as a novel target for cancer immunotherapy of gastrointestinal and hepatocellular carcinomas. Cancer Sci. 2005;96:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ailan H, Xiangwen X, Daolong R, et al. Identification of target genes of transcription factor activator protein 2 gamma in breast cancer cells. BMC Cancer. 2009;9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen Y, Aulia S, Li L, Tang BL. AMIGO and friends: an emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Res Rev. 2006;51:265–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.