Abstract
During the past 20 years, the 5-HT6 receptor has received increasing attention and become a promising target for improving cognition. Several studies with structurally different compounds have shown that not only antagonists but also 5-HT6 receptor agonists improve learning and memory in animal models. A large number of publications describing the development of ligands for this receptor have come to light, and it is now quite evident that 5-HT6 receptors have great pharmaceutical potential in terms of related patents. However, 5-HT6 receptor functionality is much more complex than initially defined. According to the existing data, different cellular pathways may be activated, depending on the drug being used. This article reviews preclinical and clinical evidence of the effects that 5-HT6 receptor compounds have on cognition. In addition, the biochemical and neurochemical mechanisms of action through which 5-HT6 receptor compounds can influence cognition will be described. Overall, several 5-HT6-targeted compounds can reasonably be regarded as powerful drug candidates for the treatment of Alzheimer's disease.
Introduction
Alzheimer's disease (AD), the most common cause of dementia among older people, is characterized by behavioral disorders and a progressive decline in memory function. Senile plaques, neurofibrillary tangles, and cholinergic dysfunction are major hallmarks of the disease. Clinical and preclinical studies point to neuronal and synaptic loss and synaptic impairment and associated neurochemical alterations of several transmitter systems as the main factors underlying both cognitive and neuropsychiatric symptoms. The use of acetylcholinesterase inhibitors for treating cognitive decline in AD, based on early findings of a cholinergic deficit, has been clinically applied for more than a decade but provides only modest benefits in most patients. Therefore, there is still an ongoing search for new treatments that will demonstrate greater efficacy against cognitive dysfunction.
Increasing evidence supports the role of the serotonergic system in learning and memory processes. Extensive serotonergic denervation has been described in AD, although it is not yet fully understood whether these changes are a cause or a consequence of the neuro-degeneration in the illness [1]. The identification of seven serotonin (5-HT) receptor families (5-HT1 to 5-HT7), the 5-HT transporter (SERT) in mammalian species, and the drugs that are selective for these sites has helped clarify their specific roles in learning and memory.
The 5-HT6 receptor is the most recently identified member of the 5-HT receptor superfamily. The 5-HT6 receptor is involved in affective disorders, anxiety and depression, epilepsy, and obesity. Initially, interest in the 5-HT6 receptors was triggered by evidence showing that certain anti-psychotics are able to bind to these receptors. Now, however, interest in these receptors lies in the role that they play as well as the therapeutic potential of 5-HT6 receptor compounds in learning and memory processes. Currently, some 5-HT6 receptor ligands are being subjected to clinical development processes for future use as potential anti-dementia, anti-psychotic, and anti-obese drugs, although the mechanisms associated with the 5-HT6 receptor activation/blockade are not completely understood. In any case, information regarding the pharmacology of 5-HT6 receptors is still quite limited.
This article will focus on preclinical and clinical studies that describe the effects of 5-HT6 receptor compounds on cognition and the purported mechanism of action by which 5-HT6 receptor compounds may affect learning and memory in AD. Several up-to-date reviews on this receptor can be found in the literature [2-4]. This paper gives a comprehensive review on the state of art of the 5-HT6 receptors, focusing on articles published in recent years (Figure 1).
Figure 1.
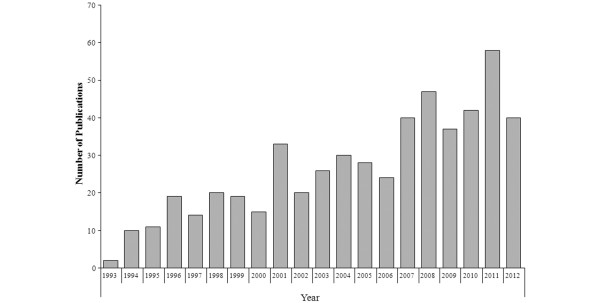
Medline search for '5-HT6 receptors'. Since the initial studies describing the cloning of the receptor (1993), 5-HT6 receptors have attracted wide interest. In the past 20 years, 540 published studies have directly or indirectly focused on these receptors, studying them from a pharmacological, physiological, behavioral, or biochemical point of view.
Structure and localization of 5-HT6 receptors
Initially cloned from striatal tissue [5], the rat 5-HT6 receptor gene encodes a protein of 438 amino acids and shares 89% homology with the human form [6,7]. The 5-HT6 receptor belongs to the G-protein-coupled receptor (GPCR) family, displaying seven transmembrane domains. They are quite different from all other 5-HT receptors: they are characterized by a short, third cytoplasmatic loop and a long C-terminal tail and contain one intron located in the middle of the third cytoplasmatic loop. The 5-HT6 receptor has no known functional isoforms. A non-functional truncated splice variant of the 5-HT6 receptor has been identified but appears not to have any physiological significance. Kohen and colleagues [6] identified a silent polymorphism at base pair 267 (C267T). Although there is evidence linking this polymorphism to several syndromes that affect cognition, including dementia, AD, and schizophrenia, these findings have not always been replicated and their significance has not yet been determined.
5-HT6 receptor expression is restricted mainly within the central nervous system (CNS). In situ hybridization and northern blot studies revealed an exclusive distribution of 5-HT6 mRNA in the rat CNS, and the highest density was found in the olfactory tubercle, followed by the frontal and entorhinal cortices, dorsal hippocampus (that is, dentate gyrus and CA1, CA2, and CA3 regions), nucleus accumbens, and striatum. Lower levels were observed in the hypothalamus, amygdala, substantia nigra, and several diencephalic nuclei. These findings have been corroborated by immunolocalization and radioligand-binding studies, which showed a similar distribution of 5-HT6 receptor protein in the rat CNS [8,9]. Therefore, 5-HT6 receptors appear to be localized in brain areas involved in learning and memory processes.
5-HT6 receptor signaling
Interestingly, it has been suggested that both 5-HT6 receptor agonists and antagonists may have pro-cognitive activities, implying that activation and inhibition of this receptor could evoke similar responses. The selective 5-HT6 receptor agonist LY-586713 caused a bell-shaped dose-response curve on hippocampal brain-derived neurotrophic factor (BDNF) mRNA expression. It also increased the Arc mRNA levels, and this effect was blocked by the 5-HT6 receptor antagonist SB-271046. However, in some brain regions, the antagonist was not able to block the agonist effect and, in fact, induced an increase in Arc expression [10], consistent with a potential differential mechanism. An excellent review [11] regarding the effects of 5-HT6 receptor agonists and antagonists on cognition in normal adult rats and in rodent models of psychiatric disorders, as well as data obtained from some clinical studies, suggested that agonists and antagonists are able to act on receptors located on distinct neuronal populations.
The mechanism for paradoxically similar effects of agonist/antagonists on cognition could be related to the existence of alternative biochemical pathways activated by 5-HT6 receptors. The 5-HT6 receptor is a GPCR that positively stimulates adenylate cyclase activity, meaning that, upon agonist activation, cAMP formation is increased. In fact, activity on adenylate cyclase confers the classic definition as agonist/antagonist on 5-HT6 receptors. 5-HT6 receptor coupling to Gαs has been widely described, but coupling of 5-HT6 receptors to other Gα protein subunits (Gαi/o or Gαq), using a scintillation proximity assay/antibody-immunocapture technique, has also been recently reported [12]. In addition, the coupling of 5-HT6 receptors to Ca2+ signaling by using a chimeric G-protein has been reported [13]. It has been reported that, with a yeast two-hybrid assay, the carboxyl-terminal region of the 5-HT6 receptor interacts with the Fyn-tyrosine kinase, a member of the Src family of non-receptor protein-tyrosine kinases [14]. This same study showed that the activation of 5-HT6 receptor activated the extracellular signal-regulated kinase1/2 via a Fyn-dependent pathway. These findings suggest that Fyn plays an important role in 5-HT6 receptor-mediated signaling pathways in the CNS. In addition, improvement in learning, associated with the administration of the 5-HT6 receptor antagonist SB-271046 in the Morris water maze learning task, is associated with increased phosphor-extracellular signal-regulated kinase1/2 (pERK1/2) levels [15]. All of these data suggest that 5-HT6 receptors activate the ERK1/2 via a Fyn-dependent pathway (Figure 2). At this point, it is worth mentioning a purported relationship between Fyn and Tau. Tau is a microtubule-associated protein and, in a hyperphosphorylated state, a main component of neurofibrillary tangles, one of the pathologic hallmarks of AD. Most of the Tau phosphorylation sites that have been routinely characterized are serine and threonine residues, but recent reports state that Tau can be phosphorylated at tyrosine residues by kinases, including Fyn. In addition, pERK1 is one of the kinases involved in Tau phosphorylation. Therefore, it is possible to suggest that modulation of 5HT6 receptors might lead to increased tau phosphorylation. In other words, it is even possible to speculate that 5HT6 receptor modulation might, in the short term, improve cognitive function (as described in the following sections) but, over a longer term, enhance the neurodegenerative processes in AD. A physical inter action between 5-HT6 receptor and the Jun activation domain-binding protein-1 (Jab-1), using different experimental approaches, has also been described, suggesting another signal transduction pathway for these receptors [16].
Figure 2.
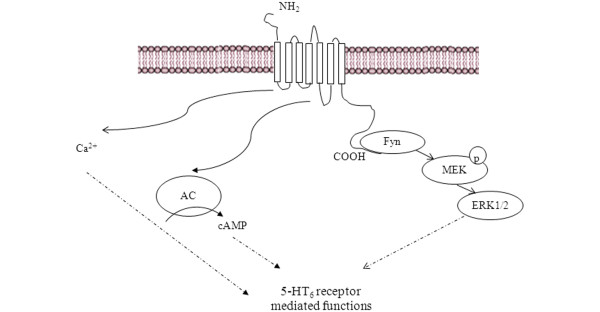
Biochemical mechanisms mediating 5-HT6 receptor functions. In addition to activating cAMP signaling pathways, 5-HT6 receptors activate Ca2+ signaling and the extracellular signal-regulated kinase 1/2 (ERK1/2) via Fyn-dependent pathway.
However, it should be noticed that drugs that are considered to be a reference agonist/antagonist upon 6-HT6 receptors might be regulating 5-HT6 receptor-independent events. In an investigation of the effects of EMD386088, a 5-HT6 receptor agonist, on cell viability, it was found that EMD386088 potentiated cell death in different cultured neuronal cell lines and that these cytotoxic effects, regardless of the presence of 5-HT6 receptors, were mediated by the downregulation of ERK1/2 activities. Furthermore, the specific 5-HT6 receptor antagonist SB258585 potentiated cell death and induced an increase in the concentration of intracellular Ca2+, whereas EMD386088, or 5-HT, did not affect calcium concentration [17]. Therefore, these compounds that have been intensively used as 5-HT6 receptor ligands could display 5-HT6 receptor-independent effects.
Neurochemical mechanisms mediating 5-HT6 receptor functions
A postsynaptic location of 5-HT6 receptors is expected because quantitative reverse transcription-polymerase chain reaction distribution of serotonin 5-HT6 receptor mRNA in the CNS of rats subjected to a selective serotonergic lesion using 5,7-dihydroxytryptamine has shown that 5-HT6 receptors are present within 5-HT projection fields and not in serotonergic raphe neurons [18]. Therefore, 5-HT6 receptors appear to be located in neurons that are not serotonergic.
It has been consistently described that the influence of 5-HT6 receptors on memory is mediated, at least partially, by increased cholinergic neurotransmission. Behavioral studies have shown that 5-HT6 receptor blockade leads to an increase in behaviors such as the number of yawns or stretches in rats. These behaviors are largely dependent on the cholinergic system because they are reversed by muscarinic antagonists. Further supporting this cholinergic mediation, 5-HT6 receptor antagonists increase acetylcholine release both in vitro [19] and in vivo [20].
However, the purported localization of 5-HT6 receptors on cholinergic neurons was discarded because a selective cholinergic lesion, induced by injection of the selective immunotoxin 192-IgG-Saporin, failed to alter the density of 5-HT6 receptor mRNA or protein expression in the deafferentated frontal cortex [19]. Therefore, the effects of 5-HT6 receptor ligands on cholinergic neurons could be mediated by other neurotransmitter systems, such as the glutamatergic system [21]. Treatment with a 5-HT6 receptor antagonist or atypical anti-psychotics with high affinities for 5-HT6 receptors, such as clozapine, enhanced glutamate levels in the frontal cortex and hippo-campus. On the other hand, 5-HT6 receptor agonism attenuated stimulated glutamate levels elicited by high KCl treatment [22]. A recent work aimed to study the effect of 5-HT6 receptor activation on glutamatergic transmission by means of whole-cell patch-clamp electrophysiological recordings from medium spiny neurons of the striatum and layer V pyramidal neurons of the prefrontal cortex. 5-HT6 receptor activation by the novel agonist ST1936 reduced the frequency of spontaneous excitatory postsynaptic currents. 5-HT6 receptor activation also reduced the amplitude of spontaneous excitatory postsynaptic currents recorded from medium spiny neurons, suggesting a mechanism of action involving postsynaptic 5-HT6 receptors. The inhibitory effect of ST1936 on glutamatergic transmission was prevented by the selective 5-HT6 receptor antagonist SB258585 [23].
It has also been shown that 5-HT6 receptors may be expressed on GABAergic spiny neurons of the striatum. The co-localization of glutamic acid decarboxylase and 5-HT6 receptors in rat cerebral cortex and hippocampus has also been demonstrated, and almost 20% of 5-HT6- like immunoreactive neurons have been shown to be GABAergic [24]. It can be suggested, on the basis of all these data regarding localization of 5-HT6 receptors and on the basis of data from releasing experiments [22,25], that 5-HT6 receptor agonists/antagonists modulate cholinergic or glutamatergic systems (or both) via disinhibition of GABAergic neurons.
5-HT6 receptor ligands
Since the initial discovery of the first ligands in the late1990s by using high-throughput screening technologies on compound libraries, a growing number of scientific publications and patent applications have developed [26]. The synthesis of 5-HT6 receptor ligands has been very successful, and a number of highly potent ligands have been reported [27].
At the preclinical level, 5-HT6 receptor medicinal chemistry is benefiting from knowledge that has been acquired since the discovery of the receptor using tools such as pharmacophore modeling, three-dimensional molecular docking or structure similarity algorithms. As a result, an increasing number and diversity of novel, highly selective 5-HT6 receptor ligands of all functional types have been reported, although the principal efforts have been focused on antagonism. Some of these compounds have been used extensively as pharmacological tools (that is, Ro-04-6790 or SB-271046). The search for new 5-HT6 receptor ligands continues. A new 5-HT6 receptor agonist, ST1936, was recently reported. ST1936 bound to human 5-HT6 receptors with good affinity (Ki = 28.8 nM) and behaved as a full 5-HT6 agonist on cloned cells; it was able to increase Ca2+ concentration and phosphorylation of Fyn kinase and regulate the activation of ERK1/2 (downstream target of Fyn kinase). These effects were completely antagonized by 5-HT6 receptor blockade with selective antagonists [28]. Epiminocyclohepta[b]indole analogs [29], tetracyclic tryptamines with the rigidized N-arylsulphonyl, N-arylcarbonyl, and N-benzyl substituents [30], or conformationally restricted N(1)-arylsulfonyl-3-aminoalkoxy indoles [31] have been shown to have acceptable ADME (absorption, distribution, metabolism, and excretion) properties, adequate brain penetration, and favorable pharmacokinetic profile. Using a newly devised chemocentric informatics methodology for drug discovery integration showed that selective estrogen receptor modulators are putative ligands of 5-HT6 receptors [32]. Positive results in animal models of cognition have been reported for both lead compounds (that is, L-483518, Ro-4368144, BGC20-761, or E-6801) and newly synthesized ligands, further confirming the involvement of this receptor in cognitive processes and its therapeutic potential. However, despite encouraging results at a preclinical level, very few 5-HT6 receptor selective ligands (and all of them acting as antagonists) have reached the clinical phases of development for the treatment of cognitive disorders.
The development of a positron emission tomography(PET) radioligand for imaging 5-HT6 receptors in the brain would, for the first time, enable in vivo imaging of this target along with assessment of its involvement in disease pathophysiology. Based on the aforementioned, the development of N-[3,5-dichloro-2-(methoxy)phenyl]-4(methoxy)-3-(1-piperazinyl)benzenesulfonamide(SB399885), a selective and high-affinity (pKi= 9.11) 5-HT6 antagonist radiolabeled with carbon-11 by O-methylation of the corresponding desmethyl analog with [11C]MeOT, has been described. PET studies with [11C]SB399885 in baboons showed fast uptake followed by rapid clearance in the brain. Poor brain entry and inconsistent brain uptake of [11C]SB399885 compared with known 5-HT6 receptor distribution limit its usefulness [33]. Recently, the development of GSK215083 (Glaxo-SmithKline, Uxbridge, Middlesex, UK) has been reported. This compound was radiolabeled with 11C via methylation. The in vivo properties of 11C-GSK215083 have been evaluated in pigs, non-human primates, and human subjects. 11C-GSK215083 readily entered the brain in all three species, leading to a heterogeneous distribution (striatum > cortex > cerebellum) that is consistent with reported 5-HT6 receptor densities and distribution determined by tissue-section autoradiography in preclinical species and humans [34].
Experimental approaches to the role of 5-HT6 receptors in cognition
Following the discovery of 5-HT6 receptor ligands with good brain penetration, a growing body of preclinical evidence has supported the use of 5-HT6 receptor antagonism for treating cognitive dysfunction. In two excellent reviews, Meneses and colleagues [4] (2011) and Fone [11] (2008) described the effects of 5-HT6 receptor agonists and antagonists on cognition. The first indirect evidence of 5-HT6 receptor involvement in memory was obtained by using antisense oligonucleotides. A few years later, pharmacological blockade of 5-HT6 receptor was shown to produce promnesic or antiamnesic effects (or both) in a number of memory tasks, including water maze, passive avoidance, autoshaping, fear conditioning, novel object recognition, or social memory [35]. Further support came from studies based on how learning paradigms decrease 5-HT6 receptor expression [15,36], whereas 5-HT6 receptor overexpression of 5-HT6 receptors in the striatum, achieved by targeted gene delivery, led to cognition impairments in a reward-based instrumental learning task, a striatum-dependent learning model [37]. Different 5-HT6 receptor antagonists have been reported to be active in the novel object discrimination test in rats and to improve water maze retention, even in aged rats [38], although failing to alter acquisition of spatial learning. In senescent mice, the effects of 5-HT6 receptor blockade with SB-271046 were assessed in the novel object recognition test for evaluating recognition memory (a component of episodic-like memory) and in spontaneous alternation task in the T-maze for evaluating working memory. It was found that deficits in consolidation of both non-spatial recognition memory and working memory performances were reversed by 5-HT6 receptor blockade [39].
One of the more consistent findings regarding the involvement of 5-HT6 receptors in memory is the ability of the 5-HT6 receptor antagonist to reverse a scopolamine-induced cognitive deficit in the Morris water maze or novel object recognition test [40]. This finding would be in line with the hypothesis that 5-HT6 receptor functions are mediated, at least partially, by a modulation of the cholinergic neurotransmission. In an extensive study regarding the effects of the 5-HT6 receptor antagonist SB-271046 in mice presenting a scopolamine-induced cholinergic disruption of memory, it was found that SB-271046 was able to reverse the scopolamine-induced deficits in working memory and to reverse the deficits of acquisition and retrieval of aversive learning, whereas scopolamine-induced deficits in episodic-like memory (acquisition and retrieval) were partially counteracted by 5-HT6 receptor blockade. However, SB-271046 alone failed to affect working memory, recognition memory, and aversive learning performances [39], but it appears that 5-HT6 receptor blockade is more consistently effective in alleviating memory deficits than increasing memory in normally functioning animals [41]. Interestingly, a combined treatment of SB-271046 with an acetylcholinesterase inhibitor produced an additive increase in passive avoidance and significantly reversed scopolamine-induced amnesic effects [41]. Similarly, this combined administration of subthreshold doses of two novel selective 5-HT6 antagonists, compounds CMP X and CMP Y, with the acetylcholinesterase inhibitor donepezil (Aricept®; Eisai, Tokyo, Japan) (approved for symptomatic treatment of AD) enhanced memory performance in young Wistar rats with cognitive deficits induced by scopolamine [40]. This suggests that the administration of 5-HT6 receptor antagonists with acetylcholinesterase inhibitors has potentially additive-enhancing effects on cognition.
Lu AE58054, a 5-HT6 receptor antagonist, reversed cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats [42]. Ro 04-6790 also reversed impairment in learning consolidation produced by the NMDA receptor antagonist MK-801, and the antagonist PRX-07034 restored the impairment of novel object recognition in the social isolation rearing model, both of which showed behavioral changes that resemble the core defects observed in schizophrenia [11]. SB271046 has also been shown to reverse memory disturbances in experimental models of stress-related psychiatric disorders that have been associated with an impairment of the hypothalamic-pituitary-adrenal axis reactivity [43].
In contrast to the works cited above, those by Russell and Dias [44] and Lindner and colleagues [45] failed to detect any effects of Ro 04-6790 or SB-271046 upon acquisition of an autoshaping task, scopolamine-induced deficits in contextual fear conditioning, or retention of a water maze task. In the same way, two selective 5-HT6 receptor antagonists, Ro-4368554 and SB-258585, showed differential effects on cognition, depending on the paradigm that was used [46]. Both compounds showed cognition-enhancing effects in object recognition, whereas only SB-258585 was able to prevent the scopolamine-induced deficit in the Morris water maze test. Neither Ro-4368554 nor SB-258585 prevented scopolamine-induced impairment in contextual fear conditioning. Similarly, both compounds were ineffective on MK801-induced deficits in contextual fear conditioning and spatial working memory. In addition, Fone [11], Kendall and colleagues [47], and Meneses and colleagues [4] reported that selective 5-HT6 receptor agonists appear to restore memory impairments in the novel object discrimination paradigm. More intriguing were the results obtained when combining non-active doses of the 5-HT6 receptor agonist E-6801 and the 5-HT6 receptor antagonist SB-271046, which produced an improvement in novel object discrimination. In addition, E-6801, alone and at a non-active dose, was able to synergistically improve the activity of non-active doses of donezepil (an acetylcholinesterase inhibitor) and memantine (an NMDAreceptor antagonist) [47]. Thus, both 5-HT6 receptor agonist and antagonist compounds show pro-cognitive activity in preclinical studies, although the explanation for their paradoxically analogous effect is still not clear.
5-HT6 receptors and Alzheimer's disease
Significant reductions in 5-HT6 receptor density in cortical areas of patients with AD have been found, although the reductions in 5-HT6 receptor density were unrelated to cognitive status before death [48]. Since 5-HT6 receptor blockade induces acetylcholine release, reductions in 5-HT6 receptors may represent an effort to restore acetylcholine levels in a deteriorated cholinergic system. In addition, it has been reported that a dysregulation of 5-HT6 receptor activation by 5-HT in the temporal cortex may be related to behavioral symptoms in AD [49]. In this sense, preclinical data suggest a possible role for 5-HT6 receptors in depression and anxiety. Two selective 5-HT6 antagonists (SB-399885 and SB-271046) and donepezil (an acetylcholinesterase inhibitor) were evaluated in the rat forced swimming test because this test is known to identify drugs with antidepressant activity. Systemic administration of the 5-HT6 receptor antagonist produced a significant reduction in the immobility time in the rat forced swimming test, with a similar profile in terms of 5-HT6 receptor occupancy, measured by binding assay. These data suggest that 5HT6 antagonists, at doses corresponding to those that occupy central 5-HT6 receptors, could have an antidepressive effect in humans. This may differentiate 5-HT6 antagonists from acetylcholinesterase inhibitors with respect to mood control in the symptomatic treatment of AD [50]. However, once again, the results of pharmacological studies are equivocal since both blockade and stimulation of 5-HT6 receptors may evoke antidepressant-and anxiolytic-like effects.
A number of 5-HT6 receptor antagonists have successfully undergone phase I clinical studies (healthy volunteers) and some have been evaluated in clinical phase II studies (patients) for the treatment of AD [51]. Two of these compounds appear to be showing positive results. Two phase II trials using SB-742457 (GlaxoSmithKline) have recently been completed in subjects with mild-to-moderate AD. The first was a dose-ranging trial comparing SB-742457 with placebo, and the second was an exploratory study with SB-742457 and donepezil arms. Overall, these studies demonstrated that SB-742457 is well tolerated in patients with AD. SB-742457 produced an improvement in both cognitive and global function in AD as assessed by ADAS-cog (Alzheimer's Disease Assessment Scale-cognitive subscale) and CIBIC+ (Clinician's Interview-Based Impression of Change-plus Caregiver Input), respectively [52]. Other clinical phase II studies are being performed, either alone or as add-on therapy with the acetylcholine esterase inhibitor, donepezil. This is the case for Lu-AE-58054 (SGS-518; Lundbeck, Copenhagen, Denmark) or PF-05212365 (SAM-531; Pfizer Inc, New York, NY, USA). Other compounds that are in different phases of clinical trials are SUVN-502 (Suven Life Sciences Ltd., Hyderabad, India) or AVN-322 (Avineuro Pharmaceuticals, San Diego, CA, USA) or PRX-07034 (Epix Pharmaceuticals, Lexington, MA, USA). In any case, treatment with 5-HT6 receptor antagonists provides symptomatic treatment that might improve cognition, perhaps via modulating neurotransmitter-related mechanisms.
Besides these selective compounds, dimebon (latrepirdine, also known as dimebolin), originally developed as an antihistamine drug, is worth mentioning. This compound shows good affinity for 5-HT6 receptors (ki = 34 nM). Dimebon received widespread publicity as a potential therapy for AD following a very positive phase 2 study [53]. However, a more recent multinational phase 3 study showed no improvements [54].
Concluding remarks
Since the discovery of 5-HT6 receptor in 1993 and subsequent development of selective antagonists, a growing number of studies support the use of serotonin 5-HT6 receptor antagonism as a promising mechanism for treating cognitive dysfunction. Over the past 20 years, several studies with structurally different compounds have shown that not only antagonists but also 5-HT6 receptor agonists improve learning and memory in animal models. In addition, the potential therapeutic use of 5-HT6 receptor ligands in mood disorders associated with AD, such as anxiety, depression, or schizophrenia, has been reported. Therefore, ligands acting on 5-HT6 receptors are attracting attention as potential candidates for the treatment of AD. However, the full characterization of the functional profile of the 5-HT6 receptor is still pending.
Currently, 5-HT6 receptors have obvious pharmaceutical potential in terms of related patents. Several 5-HT6-targeted compounds, mainly antagonists, are regarded as powerful drug candidates for the treatment of a range of neuropathological disorders, including AD [26]. However, the failure of compounds such as dimebolin points to the hypothesis that the crucial point regarding compounds acting on 5-HT6 receptors is the intracellular pathways activated after the interaction of the compound with the receptor. Therefore, perhaps it is a question not only of developing an agonist or antagonist with good affinity but also of developing compounds able to activate the necessary mechanisms for the pro-cognitive effects. It is expected that, in the near future, the drug discovery process will benefit from the complexity of functional responses associated with 5-HT6 receptors and that new molecules will enter in the scenario of treating AD.
Note
This article is part of a series on Cognitive enhancers for ageing and Alzheimer's disease, edited by Howard Fillit. Other articles in this series can be found at http://alzres.com/series/cogenhancers
Abbreviations
5-HT: serotonin; AD: Alzheimer's disease; CNS: central nervous system; ERK1/2: extracellular signal-regulated kinase 1/2; GPCR: G-protein-coupled receptor; pERK1/2: phosphor-extracellular signal-regulated kinase 1/2; PET: positron emission tomography.
Competing interests
The author declares that she has no competing interests.
Acknowledgements
The author thanks Eva Martisova for her help in preparing the manuscript and Laura Stokes for carefully editing the manuscript.
References
- Francis PT, Ramírez MJ, Lai MK. Neurochemical basis for symptomatic treatment of Alzheimer's disease. Neuropharmacol. 2010;59:221–229. doi: 10.1016/j.neuropharm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Codony X, Vela JM, Ramírez MJ. 5-HT(6) receptor and cognition. Curr Opin Pharmacol. 2011;11:94–100. doi: 10.1016/j.coph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Catena Dell'Osso M, Bordi F, Borsini F. Serotonin receptors of type 6 (5-HT6): what can we expect from them? Curr Med Chem. 2011;18:2783–2790. doi: 10.2174/092986711796011283. [DOI] [PubMed] [Google Scholar]
- Meneses A, Pérez-García G, Ponce-Lopez T, Castillo C. 5-HT6 receptor memory and amnesia: behavioral pharmacology--learning and memory processes. Int Rev Neurobiol. 2011;96:27–24. doi: 10.1016/B978-0-12-385902-0.00002-4. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem. 1996;66:47–56. doi: 10.1046/j.1471-4159.1996.66010047.x. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- Gérard C, Martres MP, Lefevre K, Miquel MC, Verge D, Lanfumey L, Doucet E, Hamon M, El Mestikawy S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–219. doi: 10.1016/S0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Verge D. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacol. 1999;21:68S–76S. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- de Foubert G, O'Neill MJ, Zetterström TS. Acute onset by 5-HT(6)-receptor activation on rat brain brain-derived neurotrophic factor and activity-regulated cytoskeletal-associated protein mRNA expression. Neuroscience. 2007;147:778–785. doi: 10.1016/j.neuroscience.2007.04.045. [DOI] [PubMed] [Google Scholar]
- Fone KC. An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology. 2008;55:1015–1022. doi: 10.1016/j.neuropharm.2008.06.061. [DOI] [PubMed] [Google Scholar]
- Dupuis DS, Mannoury la Cour C, Chaput C, Verrièle L, Lavielle G, Millan MJ. Actions of novel agonists, antagonists and antipsychotic agents at recombinant rat 5-HT6 receptors: a comparative study of coupling to G alphas. Eur J Pharmacol. 2008;588:170–177. doi: 10.1016/j.ejphar.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Nawoschik S, Kowal D, Smith D, Spangler T, Ochalski R, Schechter L, Dunlop J. Characterization of the 5-HT6 receptor coupled to Ca2+ signalling using an enabling chimeric G-protein. Eur J Pharmacol. 2003;472:33–38. doi: 10.1016/S0014-2999(03)01855-7. [DOI] [PubMed] [Google Scholar]
- Yun HM, Kim S, Kim H, Kostenis E, Kim JI, Seong JY, Baik JH, Rhim H. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem. 2007;282:5496–5505. doi: 10.1074/jbc.M606215200. [DOI] [PubMed] [Google Scholar]
- Marcos B, Cabero M, Solas M, Aisa B, Ramirez MJ. Signalling pathways associated with 5-HT6 receptors: relevance for cognitive effects. Int J Neuropsychopharmacol. 2010;13:775–784. doi: 10.1017/S146114570999054X. [DOI] [PubMed] [Google Scholar]
- Yun HM, Baik JH, Kang I, Jin C, Rhim H. Physical interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J Biol Chem. 2010;285:10016–10029. doi: 10.1074/jbc.M109.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HM, Rhim H. 5-HT6 receptor ligands, EMD386088 and SB258585, differentially regulate 5-HT6 receptor-independent events. Toxicol In Vitro. 2011;25:2035–2040. doi: 10.1016/j.tiv.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Gérard C, el Mestikawy S, Lebrand C, Adrien J, Ruat M, Traiffort E, Hamon M, Martres MP. Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse. 1996;23:164–173. doi: 10.1002/(SICI)1098-2396(199607)23:3<164::AID-SYN5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Marcos B, Gil-Bea FJ, Hirst WD, García-Alloza M, Ramírez MJ. Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release. Eur J Neurosci. 2006;24:1299–1306. doi: 10.1111/j.1460-9568.2006.05003.x. [DOI] [PubMed] [Google Scholar]
- Riemer C, Borroni E, Levet-Trafit B, Martin JR, Poli S, Porter RH, Bös M. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-bromo-6-pyrrolidin-1-ylpyridine4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem. 2003;46:1273–1276. doi: 10.1021/jm021085c. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Li P. The 5-HT6 receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology. 2001;25:662–668. doi: 10.1016/S0893-133X(01)00265-2. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, Brandt MR, Dawson LA, Cole D, Bernotas R, Robichaud A, Rosenzweig-Lipson S, Beyer CE. Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology. 2008;33:1323–1335. doi: 10.1038/sj.npp.1301503. [DOI] [PubMed] [Google Scholar]
- Tassone A, Madeo G, Schirinzi T, Vita D, Puglisi F, Ponterio G, Borsini F, Pisani A, Bonsi P. Activation of 5-HT6 receptors inhibits corticostriatal glutamatergic transmission. Neuropharmacology. 2011;61:632–637. doi: 10.1016/j.neuropharm.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- West PJ, Marcy VR, Marino MJ, Schaffhauser H. Activation of the 5-HT(6) receptor attenuates long-term potentiation and facilitates GABAergic neurotransmission in rat hippocampus. Neuroscience. 2009;164:692–701. doi: 10.1016/j.neuroscience.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Ivachtchenko AV, Ivanenkov YA, Tkachenko SE. 5-hydroxytryptamine subtype 6 receptor modulators: a patent survey. Expert Opin Ther. 2010;20:1171–1196. doi: 10.1517/13543776.2010.494661. [DOI] [PubMed] [Google Scholar]
- Liu KG, Robichaud AJ. 5-HT6 medicinal chemistry. Int Rev Neurobiol. 2010;94:1–33. doi: 10.1016/B978-0-12-384976-2.00001-0. [DOI] [PubMed] [Google Scholar]
- Riccioni T, Bordi F, Minetti P, Spadoni G, Yun HM, Im BH, Tarzia G, Rhim H, Borsini F. ST1936 stimulates cAMP, Ca2+, ERK1/2 and Fyn kinase through a full activation of cloned human 5-HT6 receptors. Eur J Pharmacol. 2011;661:8–14. doi: 10.1016/j.ejphar.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Henderson AJ, Guzzo PR, Ghosh A, Kaur J, Koo JM, Nacro K, Panduga S, Pathak R, Shimpukade B, Tan V, Xiang K, Wierschke JD, Isherwood ML. Epiminocyclohepta[b]indole analogs as 5-HT6 antagonists. Bioorg Med Chem Lett. 2012;22:1494–1498. doi: 10.1016/j.bmcl.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Nirogi RV, Kambhampati R, Kothmirkar P, Konda J, Bandyala TR, Gudla P, Arepalli S, Gangadasari NP, Shinde AK, Deshpande AD, Dwarampudi A, Chindhe AK, Dubey PK. Synthesis and structure-activity relationship of novel conformationally restricted analogues of serotonin as 5-HT6 receptor ligands. J Enzyme Inhib Med Chem. 2012;27:443–450. doi: 10.3109/14756366.2011.595713. [DOI] [PubMed] [Google Scholar]
- Nirogi RV, Kambhampati R, Daulatabad AV, Gudla P, Shaikh M, Achanta PK, Shinde AK, Dubey PK. Design, synthesis and pharmacological evaluation of conformationally restricted N-arylsulfonyl-3-aminoalkoxy indoles as a potential 5-HT6 receptor ligands. J Enzyme Inhib Med Chem. 2011;26:341–349. doi: 10.3109/14756366.2010.510471. [DOI] [PubMed] [Google Scholar]
- Hajjo R, Setola V, Roth BL, Tropsha A. Chemocentric informatics approach to drug discovery: identification and experimental validation of selective estrogen receptor modulators as ligands of 5-hydroxytryptamine-6 receptors and as potential cognition enhancers. J Med Chem. 2012;55:5704–5719. doi: 10.1021/jm2011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Majo VJ, Prabhakaran J, Milak MS, John Mann J, Parsey RV, Kumar JS. Synthesis and in vivo evaluation of [O-methyl-11C] N-[3,5-dichloro-2(methoxy)phenyl]-4-(methoxy)-3-(1-piperazinyl)benzenesulfonamide as an imaging probe for 5-HT6 receptors. Bioorg Med Chem. 2011;19:5255–5259. doi: 10.1016/j.bmc.2011.06.090. [DOI] [PubMed] [Google Scholar]
- Parker CA, Gunn RN, Rabiner EA, Slifstein M, Comley R, Salinas C, Johnson CN, Jakobsen S, Houle S, Laruelle M, Cunningham VJ, Martarello L. Radiosynthesis and characterization of 11C-GSK215083 as a PET radioligand for the 5-HT6 receptor. J Nucl Med. 2012;53:295–303. doi: 10.2967/jnumed.111.093419. [DOI] [PubMed] [Google Scholar]
- Meneses A. Effects of the 5-HT6 receptor antagonist Ro 04-6790 on learning consolidation. Behav Brain Res. 2001;118:107–110. doi: 10.1016/S0166-4328(00)00316-8. [DOI] [PubMed] [Google Scholar]
- Huerta-Rivas A, Pérez-García G, González-Espinosa C, Meneses A. Time-course of 5-HT6(6) receptor mRNA expression during memory consolidation and amnesia. Neurobiol Learn Mem. 2010;93:99–110. doi: 10.1016/j.nlm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–1530. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, Heidbreder CA, Atkins AR, Lacroix LP, Dawson LA, Foley AG, Regan CM, Upton N. SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol. 2006;553:109–119. doi: 10.1016/j.ejphar.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Da Silva Costa-Aze V, Dauphin F, Boulouard M. 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology. 2012;2221:99–115. doi: 10.1007/s00213-011-2627-3. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Prickaerts J, van Loevezijn A, Venhorst J, de Groote L, Houba P, Reneerkens O, Akkerman S, Kruse CG. Two novel 5-HT6 receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats. Neurobiol Learn Mem. 2011;96:392–402. doi: 10.1016/j.nlm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Marcos B, Chuang TT, Gil-Bea FJ, Ramírez MJ. Effects of 5-HT6 receptor antagonism and cholinesterase inhibition in models of cognitive impairment in the rat. Br J Pharmacol. 2008;155:434–440. doi: 10.1038/bjp.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Bang-Andersen B, Grayson B, Bymaster FP, Cohen MP, DeLapp NW, Giethlen B, Kreilgaard M, McKinzie DL, Neill JC, Nelson DL, Nielsen SM, Poulsen MN, Schaus JM, Witten LM. Lu AE58054, a 5-HT(6) antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int J Neuropsychopharmacol. 2010;13:1021–1033. doi: 10.1017/S1461145710000659. [DOI] [PubMed] [Google Scholar]
- Marcos B, Aisa B, Ramírez MJ. Functional interaction between 5-HT(6) receptors and hypothalamic-pituitary-adrenal axis: cognitive implications. Neuropharmacology. 2010;54:708–714. doi: 10.1016/j.neuropharm.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Russell MG, Dias R. Memories are made of this (perhaps): a review of serotonin 5-HT(6) receptor ligands and their biological functions. Curr Top Med Chem. 2002;2:643–654. doi: 10.2174/1568026023393877. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Hodges DB Jr, Hogan JB, Orie AF, Corsa JA, Barten DM, Polson C, Robertson BJ, Guss VL, Gillman KW, Rose GM, Jones KM, Gribkoff VK. An assessment of the effects of serotonin 6 (5-HT6) receptor antagonists in rodent models of learning. J Pharmacol Exp Ther. 2003;307:682–691. doi: 10.1124/jpet.103.056002. [DOI] [PubMed] [Google Scholar]
- Gravius A, Laszy J, Pietraszek M, Sághy K, Nagel J, Chambon C, Wegener N, Valastro B, Danysz W, Gyertyán I. Effects of 5-HT6 antagonists, Ro-4368554 and SB-258585, in tests used for the detection of cognitive enhancement and antipsychotic-like activity. Behav Pharmacol. 2011;22:122–135. doi: 10.1097/FBP.0b013e328343d804. [DOI] [PubMed] [Google Scholar]
- Kendall I, Slotten HA, Codony X, Burgueño J, Pauwels PJ, Vela JM, Fone KC. E-6801, a 5-HT6 receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology. 2011;213:413–430. doi: 10.1007/s00213-010-1854-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Hirst WD, Chen CP, Lasheras B, Francis PT, Ramírez MJ. Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer's disease. Neuropsychopharmacology. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]
- Marcos B, García-Alloza M, Gil-Bea FJ, Chuang TT, Francis PT, Chen CP, Tsang SW, Lai MK, Ramirez MJ. Involvement of an altered 5-HT6-{6} receptor function in behavioral symptoms of Alzheimer's disease. J Alz Dis. 2008;14:43–50. doi: 10.3233/jad-2008-14104. [DOI] [PubMed] [Google Scholar]
- Hirano K, Piers TM, Searle KL, Miller ND, Rutter AR, Chapman PF. Procognitive 5-HT6 antagonists in the rat forced swimming test: potential therapeutic utility in mood disorders associated with Alzheimer's disease. Life Sci. 2009;84:558–562. doi: 10.1016/j.lfs.2009.01.019. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov homepage. http://www.ClinicalTrials.gov
- Upton N, Chuang TT, Hunter AJ, Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease. Neurotherapeutics. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. dimebon investigators. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Jones RW. Dimebon disappointment. Alzheimers Res Ther. 2010;2:25. doi: 10.1186/alzrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]


