Abstract
Purpose
The aim of this study was to determine whether plasma lecithin:cholesterol acyltransferase (pLCAT) and erythrocyte membrane Na+-K+-ATPase ase (emNaKATPs) activity have a correlation in breast cancer. This study compared these parameters at time points before and after treatment with radiotherapy.
Methods
The levels of pLCAT and emNaKATPs were assessed in 30 patients with breast carcinoma and 20 control subjects. While emNaKATPs was measured with spectrophotometric method, pLCAT levels was measured using a specific enzyme-linked immunosorbent assay.
Results
pLCAT levels, both before and after radiotherapy, were found to be decreased in breast cancer patients than in the controls groups (p<0.001 and p<0.001, respectively). Also, pLCAT levels after radiotherapy were found to be decreased in breast cancer patients than the pLCAT levels before radiotherapy (p<0.001). The emNaKATPs activity were higher in the control group than in the breast cancer patients before/after radiotherapy (RT) (p<0.001 and p<0.001, respectively). At the same time, emNaKATPs activity before RT was higher in the breast cancer patients than emNaKATPs activity after RT (p<0.001). There was a significant correlation between pLCAT and emNaKATPs activity in breast cancer patients receiving radiotherapy (r=0.63, p<0.001), but no correlation between in breast cancer patients before RT and control group (r=0.023, p>0.05).
Conclusion
The results of the present study demonstrated that decreased pLCAT and emNaKATPs activity levels in breast cancer patients after/before RT than control group. In addition, decreased emNaKATPs activity in breast cancer patients receiving radiotherapy may be due to decreased pLCAT concentrations and RT beam. In our opinion, altered activities of pLCAT and emNaKATPs are linked to the treatment effect of radiotherapy. These data may clarify the development of cell membrane dysfunction and lipid metabolism in breast cancer patients receiving radiotherapy.
Keywords: Acytransferases, Breast neoplasms, Lecithins, Na+-K+-ATPase, Radiotherapy
INTRODUCTION
Breast cancer is one of the most common cancers in the world. A combinatorial mode of surgery, chemo, radiation, and hormonal therapy is used for treatment of breast cancer. When surgical removal of the cancer mass is not possible or surgery might weaken the patient radiotherapy is an important modality for cancer treatment [1]. Radiotherapy (RT) has a key role in the treatment of different cancer types. Approximately 60% of all cancer patients receive RT alone or in conjunction with surgery or chemotherapy [2,3]. The nausea, hair loss, skin irritation, anemia, infertility, cardiovascular disease, cognitive impairment and the development of secondary cancers are the most common side effects of RT. The aim of RT is to destroy cancer cells in the area of the body, but at the same time it influence some of the normal cells membrane.
Although plasma lecithin: cholesterol acyltransferase (pLCAT) is also expressed in small amounts in the testes and in astrocytes in the brain it is synthesized primarily by the liver [4,5]. In addition pLCAT transesterifies and hydrolyzes platelet activating factor and oxidizes phospholipids with long chains in the stereochemical numbering-2 position [6-8]. Hence, pLCAT is expected to contribute to the antioxidant properties of high density lipoprotein (HDL) [9]. All things considered that the results of pLCAT deficiency for the complete HDL proteome are unknown, it is not clear whether the influences of pLCAT deficiency on the antioxidant properties of HDL are a direct effect of the damaged pLCAT function or due to other anomalies of the HDL proteome. Nevertheless, since oxidation of plasma lipoproteins is an important action in the formation of atherogenic particles, damaged pLCAT function is predicted to activate the pathogenesis of atherosclerosis. In addition to its role in reverse cholesterol transport, pLCAT directly or indirectly interferes with several other physiological mechanisms that might affect the development of membrane dysfunctions [6-9]. Erythrocyte membrane Na+-K+-ATPase (emNaKATPs) is a heterodimeric transmembrane protein that regulates ion homeostasis, neuronal signaling, substrate transportation and muscle contraction. Furthermore, it acts as a signal transducer for the regulation of many cellular mechanisms, including those interested in tumor cell growth. emNaKATPase is a ubiquitous cation transporter of the cell, and establishes the cell's working potential across the plasma membrane. emNaKATPase is an enzyme of critical importance to cellular metabolism. Therefore, any biological defect in its function can predictably influence a variety of cellular mechanisms [10]. pLCAT and emNaKATPs activity may suggest a direct or indirect relationship to cancer or RT.
However, pLCAT and emNaKATPs activity in patients with breast cancer accepted RT-remains to be determined. Thus, the aim of the present study was to determine the effect of their activity in breast cancer.
METHODS
We studied 30 patients with breast cancer and 20 healthy women. Breast cancer staging was determined by the American Joint Committee on Cancer Guidelines. None of the subjects in this study were taking any lipid-lowering medication. They did not have diabetes or any other systemic diseases. All of patients had undergone a mastectomy or lumpectomy. After having undergone a mastectomy or a lumpectomy, all patients underwent chemotherapy treatment. Per protocol, RT had to start within 6 weeks after completion of the adjuvant chemotherapy. In the mastectomy group, a dose of 45-Gy was delivered in 25 fractions over 5 weeks to the chest wall using tangential photon fields, and in cases of pN1 status, to the supraclavicular, infraclavicular, and axillary nodes using an anterior field matched to the tangential fields. Breast-conserved patients received, an addition, a sequential boost of 5.4-Gy delivered in 3 fractions to the initial tumor bed using a direct electron field. The study was restricted to women with invasive breast cancer; women with ductal carcinoma in situ were not included. The study was being performed with the approval of the Ethics Committee of the Ataturk University medical faculty(approval no: B.30.2.ATA.0.01.00/91) and all participants have provided written informed consent. At baseline, immediately after diagnosis, blood samples were collected from patients for data measurement. From each patient, 10 mL of blood was drawn by venipucture into heparinized glass tubes. Blood samples were centrifuged at 4,000 rpm for 10 minutes and the plasma samples obtained were stored at -80℃ until assayed and packed cells washed in triplicate, using physiological saline. After treatment, blood samples were obtained in the same manner from breast cancer patients that were undergoing radiotherapy. pLCAT level was measured using a specific enzyme-linked immunosorbent assay (Cat no: CSB-E13469h; Cusabio, Wuhan, China). All lipid profile tests-cholesterol, triglyceride, HDL-were performed as routine tests in all patients and in the control group. But routine tests did not show in paper. All chemicals were obtained from Sigma-Aldrich (St. Louis, USA).
The data are presented as mean±standard deviation. The statistical analysis was performed using SPSS version 19.0 (IBM-SPSS, Chicago, USA). Comparisons between groups were done using Mann-Whitney U tests for continuous data, as much of the data was skewed. The relations between data were investigated by linear regression analysis.
Membrane preparation
The erythrocyte membranes were adapted according to Wood and Beutler [11]. Red cells were separated from plasma by centrifugation at 2,000×g for 10 minutes. Red blood cells (RBCs) were washed once with 0.9% NaCl, and 1.5-mL of packed red cells was added to a 50-mL polyethylene centrifuge tube. Then 50 mL of hemolysing solution (1×10-4 Na2EDTA and 7×10-3 M Tris, pH=7.4) were added to packed erythrocytes. Then this suspension was centrifuged at 20,000×g for 10 minutes. The supernatant was decanted very slowly and carefully for not losing the obtained ghosts (packed erythrocyte membrane). These ghosts were mixed for 1 minute with a magnet and magnetic stirrer. Next, hemolysing solution was added again and the procedure was repeated 3 times. The final pellet of membranes was resuspended in 1-mL of Tris-EDTA buffer, and stored at -70℃ until analysis. Protein concentration was determined by the lowry method with albumin as a standard (125-190 µg/100 µL) [12].
Na+-K+-ATPase assay
The reaction medium for total ATPase was adapted as described by Muriel and Mourelle [13]. Standard reaction mixture contained 5-mmol/L KCL, 150-mmol/L NaCl, 2.5-mmol/L MgCl2, and 0.1-mmol/L EDTA in 600-µL of 20-mmol/L imidazole buffer. The ghost suspension was added in a volume of 0.5-mL and the mixture was placed in a water bath at 37℃ for 10 minutes. The reaction was started with the addition of 2.5-mmol/L of disodium ATP in a volume of 0.1-mL. At the end of 1 hour, the tubes were placed in an ice bath. To stop the reaction, we added 1-mL of 15% trichloroacetic acid. Pi hydrolysed from the reaction was measured by the method of Muñoz et al. [14]. ATPase activity assayed in the presence of 1-mmol/L ouabain was subtracted from the total Mg+2-dependent ATPase activity to calculate the activity of the ouabain-sensitive Na+-K+-ATPase. The results have been expressed as µmol of Pi per milligrams of membrane protein per hour.
RESULTS
Table 1 shows the characteristics of the study group. Values of pLCAT and emNaKATPs activity in the study subjects are presented in Table 2. pLCAT levels both before and after radiotherapy were found to be lower in breast cancer patients than in the controls groups (p<0.001 and p<0.001, respectively). Also, pLCAT level after radiotherapy was found to be decreased in breast cancer patients than in the before radiotherapy patients (p<0.001). The emNaKATPs activities were higher in the control group than in the breast cancer patients before and after RT (p<0.001, p<0.001, respectively). At the same time, emNaKATPs activities before RT were higher in the breast cancer patients than they were after RT (p<0.001). There were statistically significant correlation between pLCAT and emNaKATPs activities in breast cancer patients receiving radiotherapy (r=0.63, p<0.001), but no correlation between in breast cancer patients before RT and control groups (r=0.023, p>0.05). In addition, no correlation was detected between pLCAT levels and other parameters in breast cancer patients. Similarly, there was no correlation between emNaKATPase activities and other parameters in patients. There was no correlation between pLCAT and emNaKATPase with the stage of cancer in the participating breast cancer patients in this study. In a like manner, there was no any correlation between lipid profiles and these enzymes activities in breast cancer patients (not data see). The p-value lower than 0.05 was accepted as statistically significant.
Table 1.
Clinical and demographic characteristics of study subjects
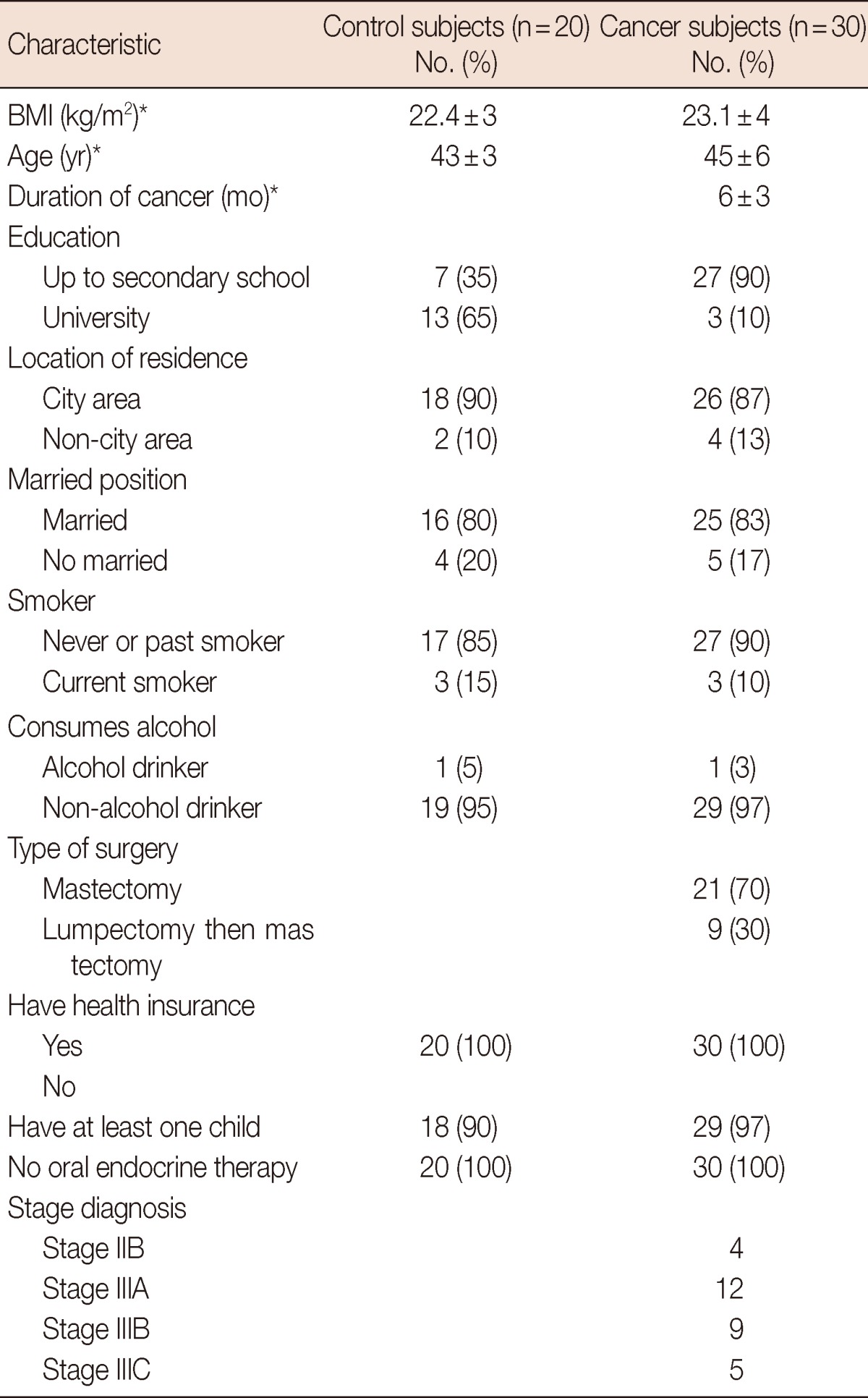
BMI=body mass index.
*Mean±SD.
Table 2.
Levels of erythrocyte membrane Na+-K+-ATPase activity and pLCAT activity in control and patients groups
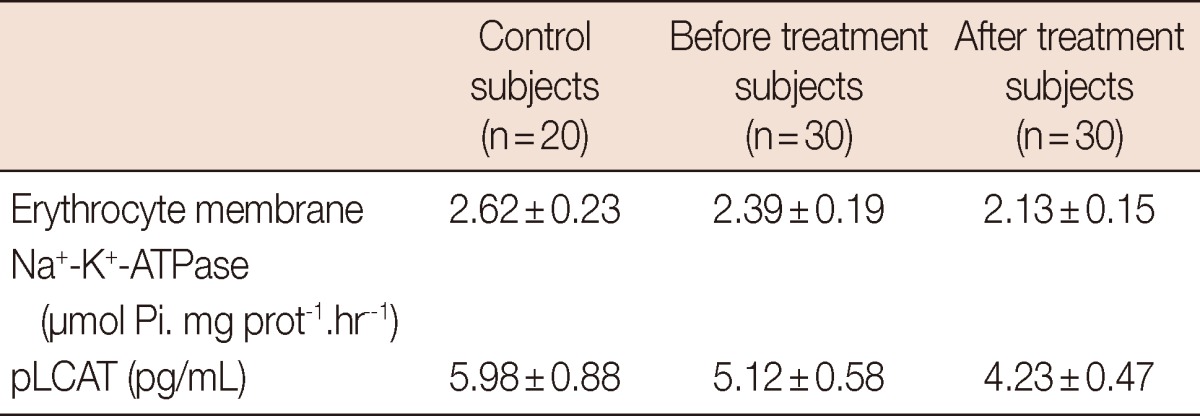
Data are presented as mean±SD. Comparisons are made between control and breast cancer subjects. p<0.001 vs. control group and before treatment. p<0.001 vs. control group and after treatment. p<0.001 vs. after treatment and before treatment.
pLCAT=plasma lecithin:cholesterol acyltransferase.
DISCUSSION
In the present study a significant decrease in pLCAT and emNaKATPase levels were observed in patients with breast cancer before and after RT. LCAT enzyme reversibly binds to lipoproteins and is primarily found on HDL, which probably anticipates its rapid clearance from the circulation. ApoA-I is the most dominant activator of pLCAT, which enables it to convert free cholesterol into cholesteryl esters (CE) on HDL by a transesterification reaction involving the transfer of a fatty acid at the sn-2 position of phosphatidylcholine, or lecithin, to the free hydroxyl group of cholesterol [4,15]. Significantly, upon esterification of cholesterol in HDL, LCAT keeps the gradient of free cholesterol between the cellular membrane and the surface of the HDL particle, which is concepted generate a continuous flow of cholesterol from the cell to lipoproteins and block the transfer of cholesterol back to the cell [16-18]. According to our study, pLCAT deficiency may arise as a consequence of either a defect in the enzyme, or defects in its synthesis and/or secretion in breast cancer before or after radiotherapy. An early study did show that both fractional and molar LCAT rates were positively correlated with obesity in women, but not in men [19]. However, a different and more recent study found increased pLCAT in obese individuals of both sexes [20]. As LCAT was considered the main driving force in the reverse cholesterol transport pathway, it was soon thought that the enzymatic activity of LCAT could be involved in the protection against atherosclerotic lesion formation and membrane dysfunction. Moreover, LCAT activity was raised with increased severity of coronary atherosclerosis.
A change in lipid mechanisms in malignant conditions modifies the activity of certain lipid metabolizing enzymes such as LCAT, which leads to striking changes in the pattern of integrated membrane metabolism and ultimately the reason for the observed change in lipid levels after radiotherapy. Erythrocyte membrane Na+-K+-ATPase and other ATPase molecules regulates intracellular ion homeostasis. The activities of these membrane ATPases are greatly influented by free radicals, lipid peroxides, and molecules like CE and ATP [21].
The observed significant decreases of the emATPase in the breast cancer patients might be due to the decreased plasma CE and increased ionizing radiation under RT condition. According to our study, plasma cholesterol ester concentration would be changed by decreased pLCAT activity; CE is an important member of erythrocyte membrane, the activity of emNaKATPase might be decreased in patients with breast cancer after or before RT. An important observation of this study is that while activity of pLCAT and emNaKATPase were slightly reduced in breast cancer patients before RT, the activity of pLCAT and emNaKATPase are reduced significantly in breast cancer patients receiving radiotherapy. The first reason for abnormally decreased emNaKATPase activity may be affected the result of altered erythrocyte membrane CE concentration, which regulates membrane viscosity. From the present data, it may be speculated that there might be an association in the decreased plasma LCAT and decreased emNaKATPase activities. Lysophosphatidyl cholesterol (LPC), being an amphiphilic molecule and as a product of LCAT activity, is rapidly incorporated into cell membranes that in turn increases membrane fluidity. Decreased pLCAT activity leads to decreased levels of LPC in cell membrane that may change physicochemical properties of the membrane leading to conformational changes in erythrocyte membrane, which result in decreased emNaKATPase activity. In conditions with decreased pLCAT activity, cholesterol being a substrate of LCAT, may accumulate and its rigid sterol ring may react with acyl-side chains of membrane phospholipids and thereby result in the restriction of membrane motility and fluidity. LCAT contributes to maintaining a concentration gradient of free cholesterol between the cell membrane and plasma, and defective LCAT activity may result in a increase in the plasma cholesterol concentration. Both before and after radiotherapy, the decrease in LCAT reactivity could effect the reverse cholesterol transport of HDL and thereby, contribute to the atherosclerotic process in breast cancer patients after RT. Our results are confirmed by the results of previous studies performed in this area [22,23]. In both groups, the reduction of emNaKATPase activity may be related to qualitative alterations in the phospholipid components of the membrane and modifications in phosphoinositide metabolism linked to the polyol biosynthetic pathway. The second reason may be ionizing radiation. Some studies showed that ionizing radiation is one of the important exogenous sources of DNA damage, cell membrane dysfunction, and oxidative stres [24,25].
As a result, we found decreased emNaKATPase and pLCAT activities in breast cancer before and after RT. According to our study, altered activities of pLCAT and emNaKATPs are linked to the treatment effects of radiotherapy. Genetic studies are required to define the relationship between emNaKATPase and pLCAT activities and radiotherapy in breast cancer.
ACKNOWLEDGEMENTS
We gratefully acknowledge Ass. Prof. Dr. Orhan Sezen who helped with the study design and with the physical evaluation of the first study patients. We are grateful to all patients who participated in this clinical trial.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Hall EJ. Radiation, the two-edged sword: cancer risks at high and low doses. Cancer J. 2000;6:343–350. [PubMed] [Google Scholar]
- 2.Hogle WP. The state of the art in radiation therapy. Semin Oncol Nurs. 2006;22:212–220. doi: 10.1016/j.soncn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 4.Jonas A. Lecithin cholesterol acyltransferase. Biochim Biophys Acta. 2000;1529:245–256. doi: 10.1016/s1388-1981(00)00153-0. [DOI] [PubMed] [Google Scholar]
- 5.Yang CY, Manoogian D, Pao Q, Lee FS, Knapp RD, Gotto AM, Jr, et al. Lecithin:cholesterol acyltransferase. Functional regions and a structural model of the enzyme. J Biol Chem. 1987;262:3086–3091. [PubMed] [Google Scholar]
- 6.Liu M, Subbaiah PV. Hydrolysis and transesterification of platelet-activating factor by lecithin-cholesterol acyltransferase. Proc Natl Acad Sci U S A. 1994;91:6035–6039. doi: 10.1073/pnas.91.13.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal J, Wang K, Liu M, Subbaiah PV. Novel function of lecithin-cholesterol acyltransferase. Hydrolysis of oxidized polar phospholipids generated during lipoprotein oxidation. J Biol Chem. 1997;272:16231–16239. doi: 10.1074/jbc.272.26.16231. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian VS, Goyal J, Miwa M, Sugatami J, Akiyama M, Liu M, et al. Role of lecithin-cholesterol acyltransferase in the metabolism of oxidized phospholipids in plasma: studies with platelet-activating factor-acetyl hydrolase-deficient plasma. Biochim Biophys Acta. 1999;1439:95–109. doi: 10.1016/s1388-1981(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- 10.Clausen T. Na+-K+- pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- 11.Wood L, Beutler E. Temperature dependence of sodium-potassium activated erythrocyte adenosine triphosphatase. J Lab Clin Med. 1967;70:287–294. [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Muriel P, Mourelle M. The role of membrane composition in ATPase activities of cirrhotic rat liver: effect of silymarin. J Appl Toxicol. 1990;10:281–284. doi: 10.1002/jat.2550100409. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz MA, Balón M, Fernandez C. Direct determination of inorganic phosphorus in serum with a single reagent. Clin Chem. 1983;29:372–374. [PubMed] [Google Scholar]
- 15.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 16.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 17.Murphy JR. Erythrocyte metabolism. IV. Equilibration of cholesterol-4-C-14 between erythrocytes and variously treated sera. J Lab Clin Med. 1962;60:571–578. [PubMed] [Google Scholar]
- 18.Czarnecka H, Yokoyama S. Regulation of cellular cholesterol efflux by lecithin:cholesterol acyltransferase reaction through nonspecific lipid exchange. J Biol Chem. 1996;271:2023–2028. doi: 10.1074/jbc.271.4.2023. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland WH, Temple WA, Nye ER, Herbison PG. Lecithin: cholesterol acyltransferase activity, plasma and lipoprotein lipids and obesity in men and women. Atherosclerosis. 1979;34:319–327. doi: 10.1016/s0021-9150(79)80009-x. [DOI] [PubMed] [Google Scholar]
- 20.Magkos F, Mohammed BS, Mittendorfer B. Plasma lipid transfer enzymes in non-diabetic lean and obese men and women. Lipids. 2009;44:459–464. doi: 10.1007/s11745-009-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perumal SS, Shanthi P, Sachdanandam P. Energy-modulating vitamins: a new combinatorial therapy prevents cancer cachexia in rat mammary carcinoma. Br J Nutr. 2005;93:901–909. doi: 10.1079/bjn20051439. [DOI] [PubMed] [Google Scholar]
- 22.Finotti P, Palatini P. Reduction of erythrocyte (Na+-K+-)ATPase activity in type 1 (insulin-dependent) diabetic subjects and its activation by homologous plasma. Diabetologia. 1986;29:623–628. doi: 10.1007/BF00869260. [DOI] [PubMed] [Google Scholar]
- 23.Testa I, Rabini RA, Fumelli P, Bertoli E, Mazzanti L. Abnormal membrane fluidity and acetylcholinesterase activity in erythrocytes from insulin-dependent diabetic patients. J Clin Endocrinol Metab. 1988;67:1129–1133. doi: 10.1210/jcem-67-6-1129. [DOI] [PubMed] [Google Scholar]
- 24.Pang D, Winters TA, Jung M, Purkayastha S, Cavalli LR, Chasovkikh S, et al. Radiation-generated short DNA fragments may perturb non-homologous end-joining and induce genomic instability. J Radiat Res. 2011;52:309–319. doi: 10.1269/jrr.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonanno M, de Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat Res. 2011;175:405–415. doi: 10.1667/RR2461.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


