Abstract
Purpose
The aim of this study is to determine and to compare the oncological outcomes of bilateral reduction mammoplasty to standard breast-conserving surgery for breast cancer.
Methods
One hundred sixty-two patients who received a quadrantectomy because of breast cancer (group 1) and 106 breast cancer patients with macromastia who underwent breast-conserving surgery via bilateral reduction mammoplasty (group 2) between 2003 and 2010 were enrolled in this study.
Results
The mean follow-up time was 37 months for group 1 and 33 months for group 2. Surgical margins were wider than 2 mm in 82.7% and 10 mm in 76.5% of the patients in group 1. Eleven percent of patients had positive surgical margins in this group. When compared to group 2, the rates were 89%, 84%, and 8.4%, respectively. Three patients (1.8%) in group 1 and one patient (0.9%) in group 2 had local recurrence of the disease and received a mastectomy. No statistical significances were noted for either local recurrence or overall survival between the two groups.
Conclusion
Bilateral reduction mammoplasty has some advantages as compared to the standard conventional breast-conserving surgery techniques without having any unfavorable effects on surgical margin confidence, local recurrence, and survival rates.
Keywords: Breast neoplasms, Mammoplasty, Oncoplastic surgery, Segmental mastectomy
INTRODUCTION
Excluding the patients who must receive a mastectomy for breast cancer, every other patient is a candidate for breast-conserving surgery (BCS) according to willingness. Randomly controlled studies show that BCS has similar survival rates with mastectomy [1,2]. Quadrantectomy and lumpectomy have been the conventional standard surgical techniques used to achieve BCS for many years. Oncoplastic surgery is now becoming the preferred technique at an accelerated pace. Oncoplastic surgery describes surgical techniques for wide excision of breast tissues containing tumor and the healing of the residual breast tissues with the best cosmetic results. The type of oncoplastic surgery is established according to the tumor location in the breast, tumor size, and the rate of tumor/breast volume. These are also the factors affecting cosmetic and oncological results [3].
Macromastia has been considered a relative contra-indication to BCS because of difficulties with postoperative radiation therapy and cosmesis. Breast volumes of these patients cannot be reduced sufficiently with techniques such as quadrantectomy or segmental mastectomy. Bilateral reduction mammoplasty (BRM), which has long been used for the treatment of macromastia, has recently become a preferred technique in the surgical treatment of breast cancer patients with macromastia. BRM is known to provide symmetry in the contralateral breasts, more effective radiotherapy planning, and the removal of the neoplasm with wider surgical margins [4-6].
As the oncoplastic surgery technique used varies according to tumor and patient's characteristics, the oncological outcomes of every technique must be reported separately. In the present study, the need of re-excision, local recurrence, and survival results of breast cancer patients managed with conventional standard BCS and BRM techniques were stated. Our aim in this study is to determine and compare the oncological outcomes of BRM to standard BCS in a single center. We examined the tumor characteristics, re-excision rates, local recurrence rates, as well as recurrences of free survival and overall disease-free survival rates.
METHODS
One hundred and sixty-two patients who received a quadrantectomy because of breast cancer (group 1) and 106 breast cancer patients with macromastia who underwent BCS via BRM (group 2) between 2003 and 2010 at Ankara Oncology Hospital were enrolled in this prospective study. Quadrantectomy and BRM managed patients were consecutive in the study. Although there were 286 patients at the beginning of the study, 18 of them did not attain their follow-up and were excluded.
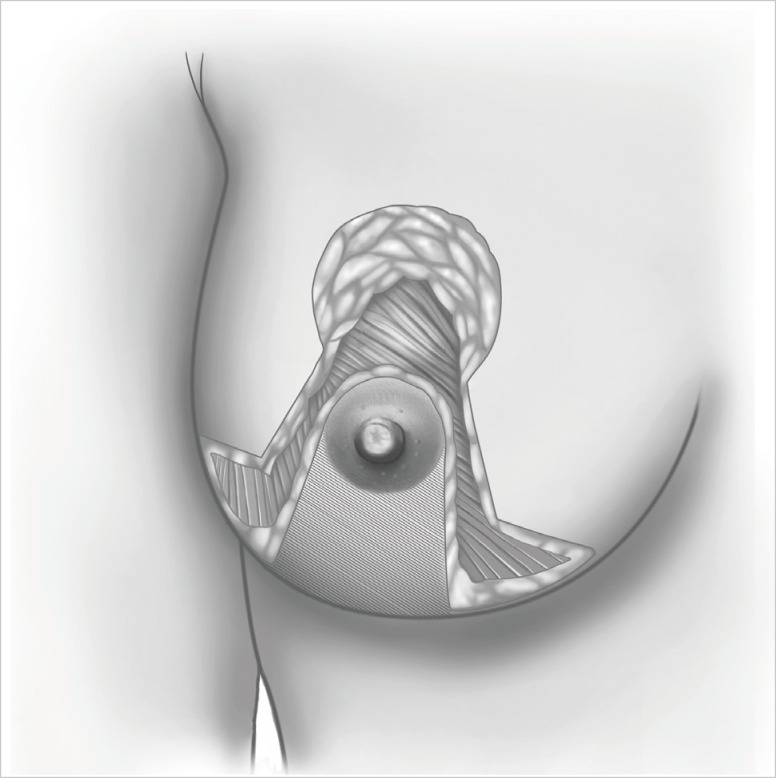
The study was approved by the local ethics committee. All the patients were informed with written explanations on both techniques. All the drawings and operations were carried out by the breast surgeons from our clinic. Quadrantectomy is achieved by removing both the tumor and surrounding nominal tissue as well as fascia pectoralis with radical incisions on the tumor. In order to observe the similarity of tumor localization between the groups, only the patients with upper inner- and upper outer-quadrant lesions were included in the study. In the patients with inadequate breast volumes for reduction mammoplasty or whom have the tumor localized outside the border of reduction incisions, the quadrantectomy was preferred, and there was no further attempts to close the dead space after quadrantectomy. Patients with breast hypertrophy causing breast overweight, overvolume and/or breast malposition were regarded as macromasty. Any numerical definition was not used for pathological hypertrophy. Mammography and breast ultrasonography were routinely applied on the patients in order to display the tumor. Magnetic resonance imaging (MRI) was used when necessary. Liver ultrasonography was routinely used for disease staging. The patients who have had invasive breast cancer were enrolled in the study, where the patients who had in situ carcinoma and multicentric or multifocal diseases were excluded. The inferior pedicle flap technique was used for upper quadrant lesions in tumors involving breasts and for symmetrizing in contralateral breasts in the patients receiving BRM (Figure 1). Nipple-areola complex is brought up to 19 to 21 cm of the line drawn between midclavicula and nipple. Level I-II axillary dissection was carried out for patients with metastatic sentinel lymph nodes proven at a frozen section, and for patients with unidentified sentinel lymph nodes and clinically axillary positive lymph nodes. Postoperative pictures of two groups can be noted in Figure 2. Intraoperative frozen section was not applied to surgical margins. Supplementary radiotherapy was used for each group of patients with a 50-Gy dose to the entire breast and an additional 10-Gy boost dose to the tumor bed.
Figure 1.
The illustration of bilateral reduction mammoplasty technique.
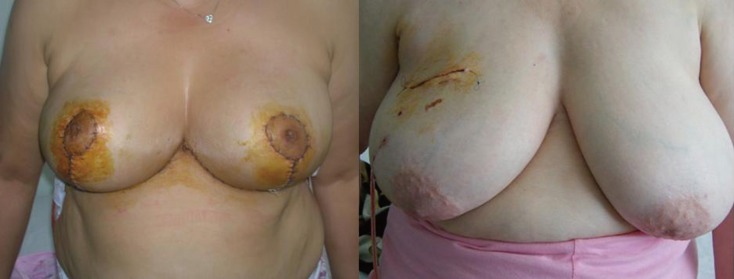
Figure 2.
Postoperative pictures of bilateral reduction mammoplasty and quadrantectomy groups.
In the first 2 years of the postoperative period, the patients came in every 3 months for controls. During the third to the fifth year of the postoperative period, patients attended the controls every 6 months. Thereafter, they came for controls only once per year. A physical examination of liver functionality and tumor markers (CA 15-3 and carcinoembryonic antigen) were carried out during the controls. Mammography and breast ultrasonography were applied once a year. MRI was also used when necessary. Further radiographic scanning was performed on the patients who had suspicious conditions for recurrence of the disease. Age, histopathological type, tumor size, re-excision rate, local recurrence, distant metastasis, and weight of the specimens were analyzed. Basic postoperative information and examinations of the patients in every follow-up were recorded accordingly in the datebase. Patients who did not attend the follow-up in the last 3 months were invited for control in order to examine the local recurrence and distant metastasis. The tumors that were determined in the same quadrant with the same pathology were regarded as local recurrences. Specimens, before being fixed, were directly put into a graduated cylinder and the volumes were conformed to the water displacement method.
Statistical analysis was achieved by SPSS version 17 (SPSS Inc., IBM, Chicago, USA). The Mann-Whitney U test was used to compare abnormal continuous data while the chi-squared test was used to compare categorical variables. Survival curves were compared using the Kaplan-Meier test.
RESULTS
The groups were homogenous when age and tumor size were considered. The median follow-up time was 37 months (range, 20-124 months) for group 1 and 33 months (range, 16-88 months) for group 2.
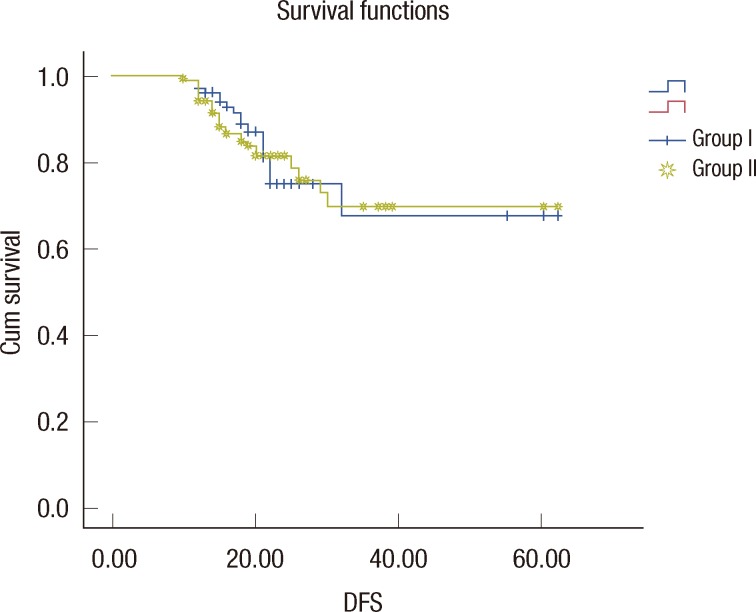
Sixty-four patients were in stage I, 160 patients were in stage II and 44 patients were in stage IIIA. Table 1 summarizes the characteristics of the patients in our sample population. Mean weight of the reduction mammoplasty specimen for the cancerous side was 960±58 g. Mean specimen weight of the quadrantectomy group was 157±32 g (p=0.001). In group 1, 82.7% of the patients had a surgical margin wider than 2 mm, and 76.5% of the patients in the same group had a surgical margin wider than 10 mm, where 11% patients had positive surgical margin. When compared to group 2, the rates were 89%, 84%, and 8.4%, respectively. Re-excision was applied on 18 positive surgical margin patients together with additional 6 close margin patients (total of 24 patients, 15%) in group 1. In this group, re-excision was as mastectomy in 8 patients. Re-excision was applied on 11 patients (10.3%) in group 2 where there were 9 positive surgical margin patients and 2 close margin patients. Mastectomy was not applied on any of these patients. Re-excision rate in the BRM-managed patients is found to be much lower (p=0.04). Three patients (1.8%) in group 1 and one patient (0.9%) in group 2 had local recurrences of the disease and received a mastectomy. Adjuvant chemotherapy and hormonotherapy rates were 57% and 81% in group 1 and 61% and 79% in group 2, respectively. No statistical significances were noted for either local recurrence or overall survival between the two groups. Five-year disease-free survival and overall survival rates according to disease stages are shown in Table 2 and Figure 3.
Table 1.
Characteristics of the patients
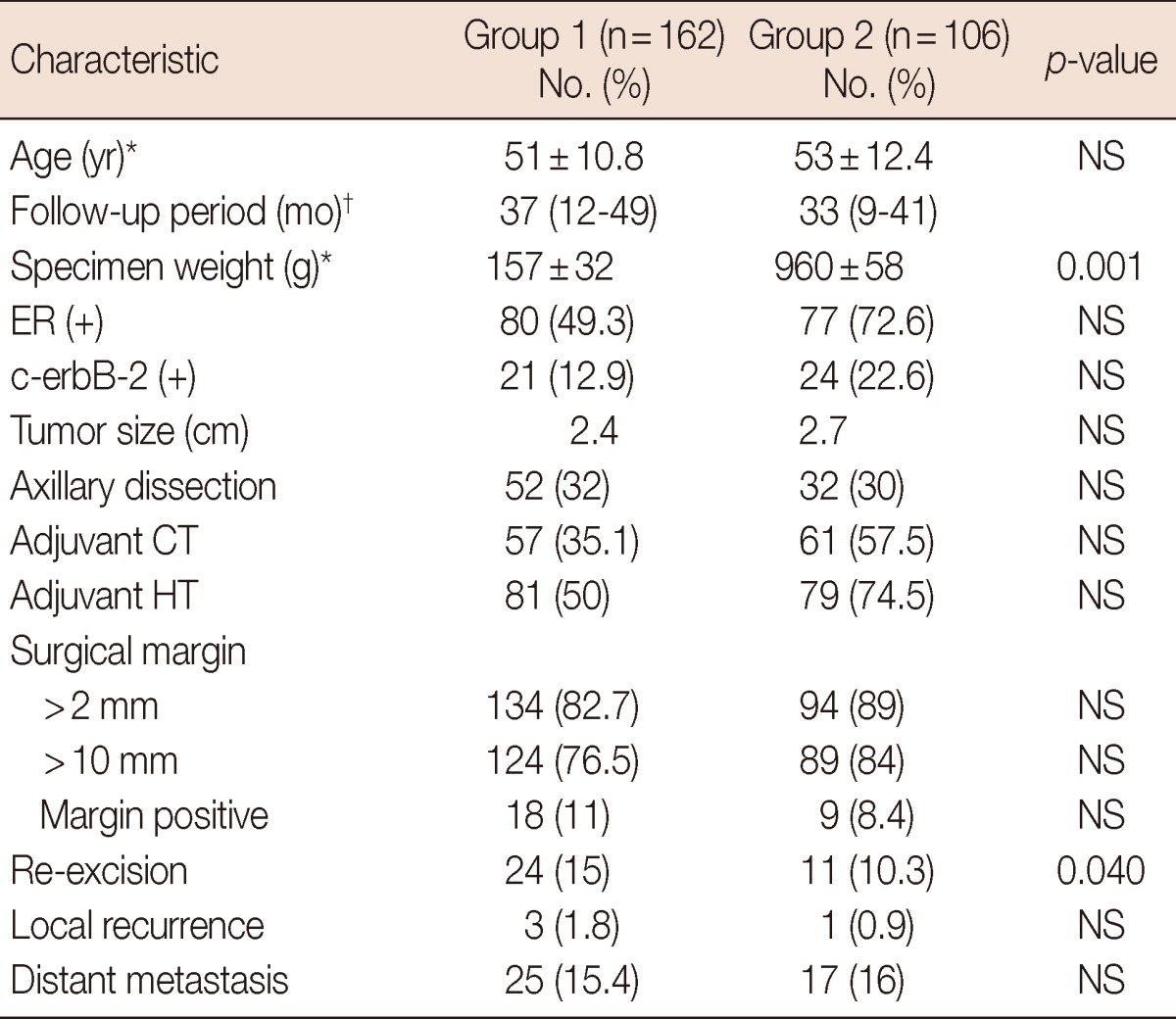
NS=not significant; ER=estrogen receptor; CT=chemotherapy; HT=hormonal therapy.
*Mean±SD; †Median (range).
Table 2.
Five-year disease-free survival and overall survival rates
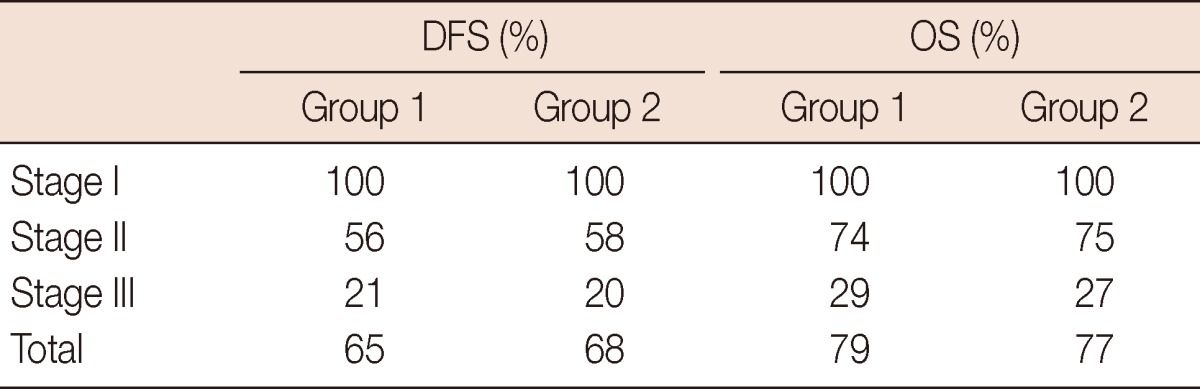
DFS=disease-free survival; OS=overall survival.
Figure 3.
Five-year disease-free survival (DFS).
DISCUSSION
The aim of BCS is to help the patient live with optimal and satisfactory cosmetic result after surgery, as well as to perform equal local recurrences and long period survival rates compared to mastectomy. Among the standard BCS techniques, quadrantectomy, which removes the tumor with breast skin down to the pectoral fascia, is shown to be oncologically superior to wide local excisions, such as lumpectomy [7]. Besides the worst cosmetic results in quadrantectomy performed on breast cancer patients with macromasty, it is difficult to plan radiotherapy in a quadrantectomy performed on pendulous and large breasts as well. Such patients receive a much higher dose of radiotherapy [8]. BRM has been used to manage macromasty for many years. Surgeons hesitate to use this technique in breast cancer patients with macromasty because of the uncertain oncological results. There are some recent studies that show this technique as to minimize the radiotherapy dosage in patients. Furthermore, in these studies it is also shown that by using this technique there are much fewer skin reaction issues and mastitis compared to conventional operation techniques [9,10]. Since 2003, BRM has been managed in our clinic by the inferior flap technique for the tumors that are localized in upper quadrants, as well as infero-lateral and infero-medial quadrants.
Most oncoplastic surgeries and BRM-related series in the abstract mention cosmetic results and complications; there are few studies on survival rates and local controls of the disease [11]. However, as the tumor and patient characteristics differ, oncoplastic surgery techniques should be compared to other BCS techniques in term of survivals and recurrence rates. In studies where oncoplastic surgery is compared to quadrantectomy, it is claimed that oncoplastic surgery techniques are superior to quadrantectomy in obtaining a wider surgical margin [12,13]. In the study of Giacalone et al. [14], positive surgical margin and re-excision rates are found to be lower in the oncoplastic surgery group, with 42 oncoplastic surgeries and 57 BCS applied patients. In the series related to oncoplastic surgery results, close surgical margin rates are below 10% [15,16]. Clough et al. [6] found surgical margin involvements in 11 (10.9%) of their 101 breast cancer patients whom were treated with oncoplastic surgery. McCulley and Macmillan [17] reported a series of 50 breast cancer patients treated with therapeutic mammaplasty, in which 4 patients (8%) required other operations due to surgical margin involvements. In these series, three different oncoplastic techniques were used. Caruso et al. [18] reported a series of 61 breast cancer patients treated with BRM. The margins were positive in 5 patients (8.2%). However, it is mentioned that surgical margin confidence is achieved with re-excision in all of the patients.
Fitoussi et al. [19] found a local recurrence rate of 6.8% with 540 patients in their oncoplastic surgery related study. The positive marginal rate was 18.9%, which led to mastectomy in 9.4% of the patients. Rietjens et al. [20] found a 5-year local recurrence rate of 3% and a distant metastasis rate of 13% in their study concerning the 148 patients. Chakravorty et al. [21] reported the long term results of oncoplastic surgery and BCS. There were 440 BCS and 150 oncoplastic surgery techniques (wise pattern therapeutic mammoplasty, Grisotti and Benelli procedures) applied to patients within their study. The need for new surgical applications was much higher in the BCS group (14.5%) than in the oncoplastic surgery group (6.6%) (p=0.01). During an average 28 month follow-up period, local recurrence occurred in 2.7% of the oncoplastic surgery group and 2.2% of the BCS group; however, this is not statistically significant. The groups were similar based on local recurrence and overall survival results. Oncoplastic techniques draw attention due to their success in reliable surgical margins and re-excision rates when compared to wide local excisions [22].
Our study is one of the larger series evaluating oncoplastic conservation and is unique in that it compares BRM and BCS from the same center. Our local recurrence rate of 0.9% in BRM with a 33-month follow-up is compatible with published series.
In our study, we caught the similarity in surgical marginal confidence, local recurrence, and long term survival rates among the two groups. More tissue is removed with BRM, however the increased re-excision rates in quadrantectomy is because of the patients who had close surgical margin. Re-excision was applied on 6 of the patients in quadrantectomy group and 2 of the patients in BRM group for the reason of closed surgical margins. Here, the tip is that BRM achieves better cosmetic results and makes the radiotherapy planning easier, which means lower radiotherapy doses. Therefore, it can be concluded that BRM has some advantages when compared to standard conventional BCS techniques without having any unfavorable effects on surgical margin confidence, local recurrence, and survival rates.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 3.Morrow M. Minimally invasive surgery for breast cancer. BMJ. 2009;338:b557. doi: 10.1136/bmj.b557. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Marubini E, Mariani L, Galimberti V, Luini A, Veronesi P, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Pelletiere CV, Wolfe AJ, Tsangaris TN, Pennanen MF. Experience with reduction mammaplasty combined with breast conservation therapy in the treatment of breast cancer. Plast Reconstr Surg. 2003;111:1102–1109. doi: 10.1097/01.PRS.0000046491.87997.40. [DOI] [PubMed] [Google Scholar]
- 6.Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003;237:26–34. doi: 10.1097/00000658-200301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 9.Fernando IN, Ford HT, Powles TJ, Ashley S, Glees JP, Torr M, et al. Factors affecting acute skin toxicity in patients having breast irradiation after conservative surgery: a prospective study of treatment practice at the Royal Marsden Hospital. Clin Oncol (R Coll Radiol) 1996;8:226–233. doi: 10.1016/s0936-6555(05)80657-0. [DOI] [PubMed] [Google Scholar]
- 10.Brierley JD, Paterson IC, Lallemand RC, Rostom AY. The influence of breast size on late radiation reaction following excision and radiotherapy for early breast cancer. Clin Oncol (R Coll Radiol) 1991;3:6–9. doi: 10.1016/s0936-6555(05)81031-3. [DOI] [PubMed] [Google Scholar]
- 11.Asgeirsson KS, Rasheed T, McCulley SJ, Macmillan RD. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2005;31:817–823. doi: 10.1016/j.ejso.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Kaur N, Petit JY, Rietjens M, Maffini F, Luini A, Gatti G, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2005;12:539–545. doi: 10.1245/ASO.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Giacalone PL, Roger P, Dubon O, El Gareh N, Rihaoui S, Taourel P, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2007;14:605–614. doi: 10.1245/s10434-006-9098-5. [DOI] [PubMed] [Google Scholar]
- 14.Giacalone PL, Roger P, Dubon O, El Gareh N, Daurés JP, Laffargue F. Lumpectomy vs oncoplastic surgery for breast-conserving therapy of cancer. A prospective study about 99 patients. Ann Chir. 2006;131:256–261. doi: 10.1016/j.anchir.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg. 1999;104:409–420. doi: 10.1097/00006534-199908000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Nos C, Fitoussi A, Bourgeois D, Fourquet A, Salmon RJ, Clough KB. Conservative treatment of lower pole breast cancers by bilateral mammoplasty and radiotherapy. Eur J Surg Oncol. 1998;24:508–514. doi: 10.1016/s0748-7983(98)93356-x. [DOI] [PubMed] [Google Scholar]
- 17.McCulley SJ, Macmillan RD. Therapeutic mammaplasty-analysis of 50 consecutive cases. Br J Plast Surg. 2005;58:902–907. doi: 10.1016/j.bjps.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Caruso F, Catanuto G, De Meo L, Ferrara M, Gallodoro A, Petrolito E, et al. Outcomes of bilateral mammoplasty for early stage breast cancer. Eur J Surg Oncol. 2008;34:1143–1147. doi: 10.1016/j.ejso.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Fitoussi AD, Berry MG, Famà F, Falcou MC, Curnier A, Couturaud B, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases [outcomes article] Plast Reconstr Surg. 2010;125:454–462. doi: 10.1097/PRS.0b013e3181c82d3e. [DOI] [PubMed] [Google Scholar]
- 20.Rietjens M, Urban CA, Rey PC, Mazzarol G, Maisonneuve P, Garusi C, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast. 2007;16:387–395. doi: 10.1016/j.breast.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Chakravorty A, Shrestha AK, Sanmugalingam N, Rapisarda F, Roche N, Querci Della, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38:395–398. doi: 10.1016/j.ejso.2012.02.186. [DOI] [PubMed] [Google Scholar]
- 22.Down SK, Jha PK, Burger A, Hussien MI. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast J. 2013;19:56–63. doi: 10.1111/tbj.12047. [DOI] [PubMed] [Google Scholar]