Abstract
Streptococcus pyogenes, bearing M-protein on its surface, resists opsonization by normal human serum and subsequent phagocytosis by human polymorphonuclear leukocytes. Previous studies have shown that M-protein positive organisms are poorly opsonized by the alternate pathway of complement. In an attempt to define further the role of the surface components of S. pyogenes in this process, we examined the ability of clindamycin, an antibiotic that inhibits protein biosynthesis, to alter bacterial opsonization.
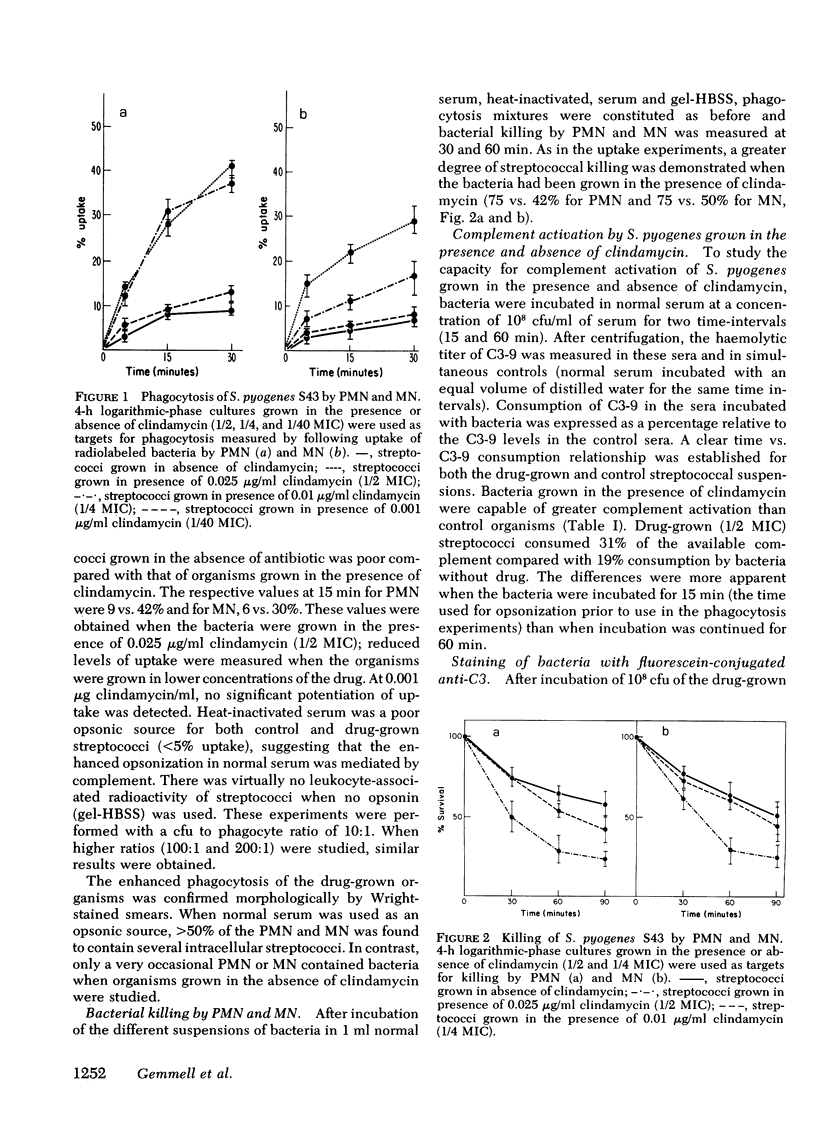
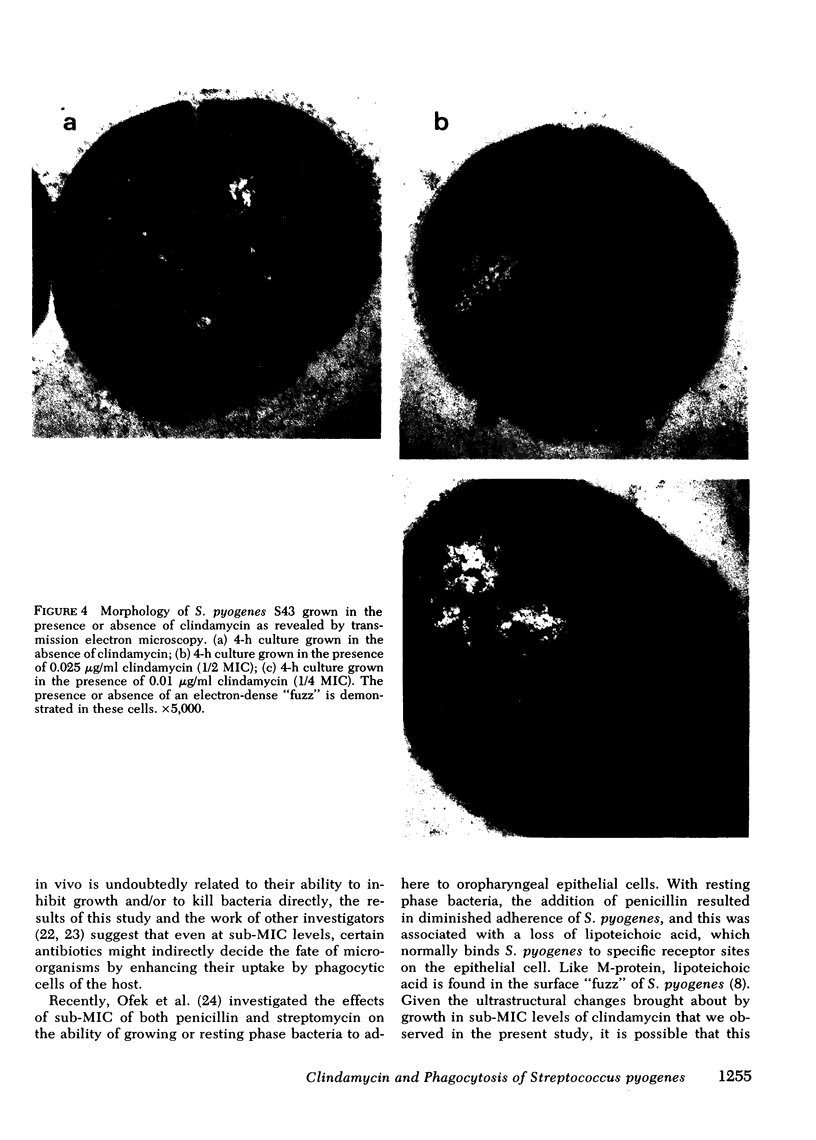
An M-protein positive strain of S. pyogenes was grown in varying concentrations of clindamycin at levels lower than those which inhibited growth, i.e., at levels less than the minimal inhibitory concentration. These bacteria were incubated with purified human polymorphonuclear leukocytes and peripheral blood monocytes. Significant enhancement of bacterial opsonization, phagocytosis, and killing resulted. Measurement of complement consumption and binding of the third component of complement (C3) onto the bacterial surface demonstrated that organisms grown in the presence of clindamycin activated complement more readily and fixed more C3 on their surface. Electron microscopy revealed the probable basis for these findings. Streptococci exposed to clindamycin during growth were largely denuded of surface “fuzz,” the hairlike structures bearing M-protein.
We conclude that the incorporation of clindamycin at concentrations that fail to inhibit growth of S. pyogenes nevertheless causes significant changes in the capacity of these bacteria to resist opsonization by serum complement. These findings support the hypothesis that M-protein inhibits bacterial opsonization by interfering with effective complement activation on the bacterial surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Becker C. G. Enhancing effect of type specific antistreptococcal antibodies on emergence of streptococci rich in M-protein. Proc Soc Exp Biol Med. 1967 Jan;124(1):331–335. doi: 10.3181/00379727-124-31736. [DOI] [PubMed] [Google Scholar]
- Bisno A. L. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun. 1979 Dec;26(3):1172–1176. doi: 10.1128/iai.26.3.1172-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Interference with the mannose binding and epithelial cell adherence of Escherichia coli by sublethal concentrations of streptomycin. J Clin Invest. 1979 Jun;63(6):1219–1228. doi: 10.1172/JCI109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H., Warren G. H. Enhanced susceptibility of penicillin-resistant staphylococci to phagocytosis after in vitro incubation with low doses of nafcillin. Proc Soc Exp Biol Med. 1974 Jul;146(3):707–711. doi: 10.3181/00379727-146-38177. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Page A. R., Pickering R. J., Good R. A. Complement activity and inflammatory neutrophil exudation in man. Studies in patients with glomerulonephritis, essential hypocomplementemia and agammaglobulinemia. Int Arch Allergy Appl Immunol. 1967;32(1):64–90. doi: 10.1159/000229917. [DOI] [PubMed] [Google Scholar]
- Lorian V., Atkinson B. A. Effect of serum and blood on Enterobacteriaceae grown in the presence of subminimal inhibitory concentrations of ampicillin and mecillinam. Rev Infect Dis. 1979 Sep-Oct;1(5):797–806. doi: 10.1093/clinids/1.5.797. [DOI] [PubMed] [Google Scholar]
- McLean R. H., Geiger H., Burke B., Simmons R., Najarian J., Vernier R. L., Michael A. F. Recurrence of membranoproliferative glomerulonephritis following kidney transplantation. Serum complement component studies. Am J Med. 1976 Jan;60(1):60–72. doi: 10.1016/0002-9343(76)90534-9. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eisenstein B. I., Alkan M. L., Sharon N. Suppression of bacterial adherence by subminimal inhibitory concentrations of beta-lactam and aminoglycoside antibiotics. Rev Infect Dis. 1979 Sep-Oct;1(5):832–837. doi: 10.1093/clinids/1.5.832. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Schmeling D., Cleary P. P., Wilkinson B. J., Kim Y., Quie P. G. Inhibition of alternative complement pathway opsonization by group A streptococcal M protein. J Infect Dis. 1979 May;139(5):575–585. doi: 10.1093/infdis/139.5.575. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Poste G., Greenham L. W., Mallucci L., Reeve P., Alexander D. J. The study of cellular "microexudates" by ellipsometry and their relationship to the cell coat. Exp Cell Res. 1973 Apr;78(2):303–313. doi: 10.1016/0014-4827(73)90073-6. [DOI] [PubMed] [Google Scholar]
- Sandberg T., Stenqvist K., Svanborg-Edén C. Effects of subminimal inhibitory concentrations of ampicillin, chloramphenicol, and nitrofurantoin on the attachment of Escherichia coli to human uroepithelial cells in vitro. Rev Infect Dis. 1979 Sep-Oct;1(5):838–844. doi: 10.1093/clinids/1.5.838. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]







