Abstract
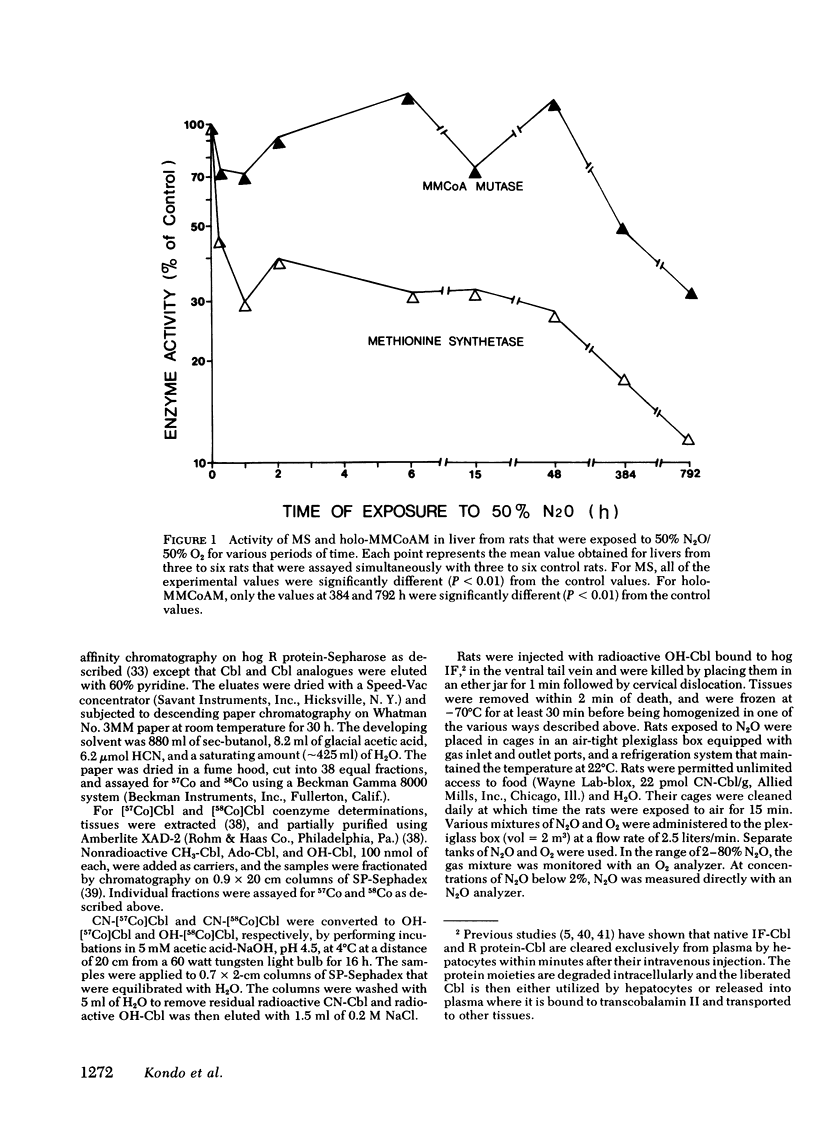
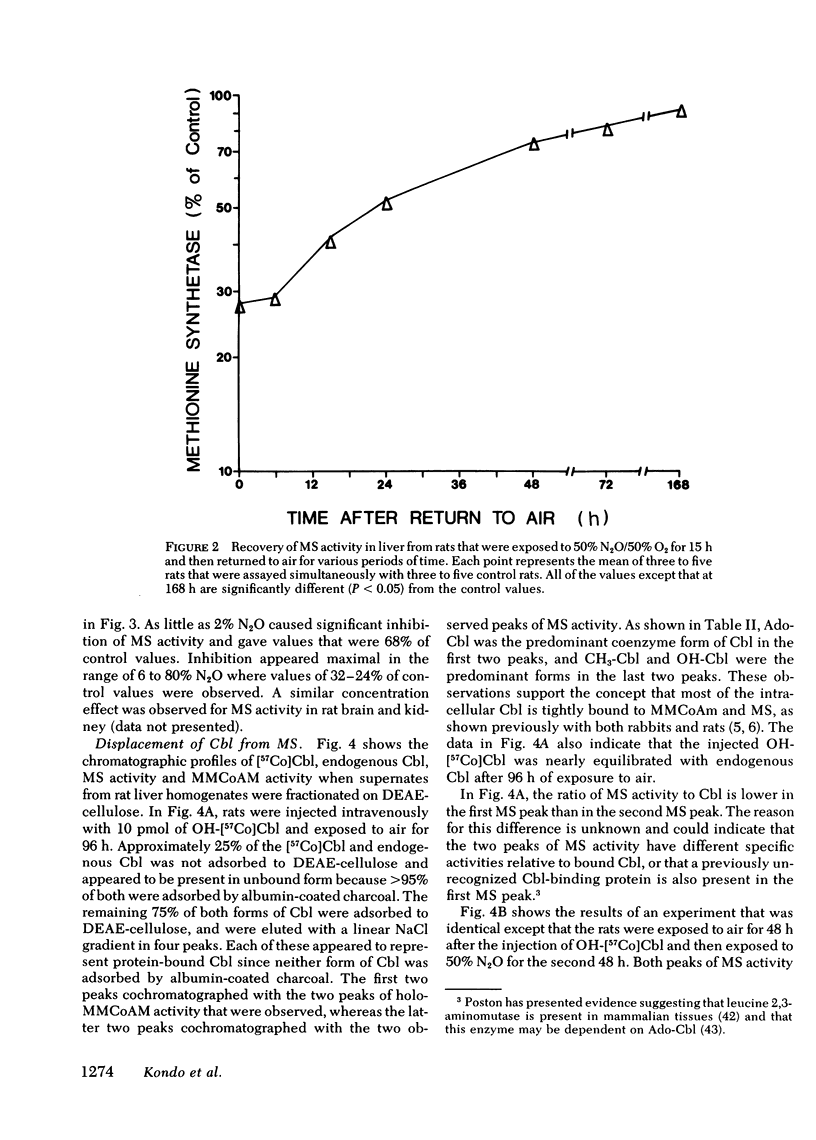
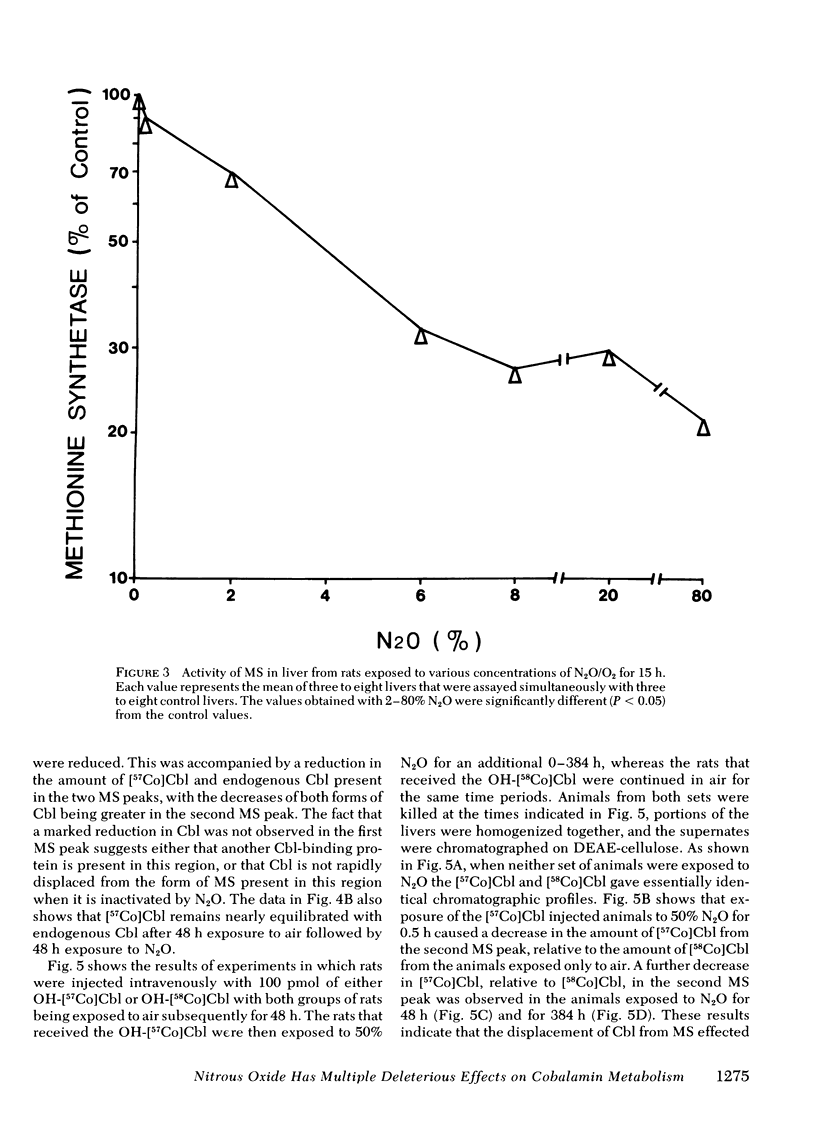
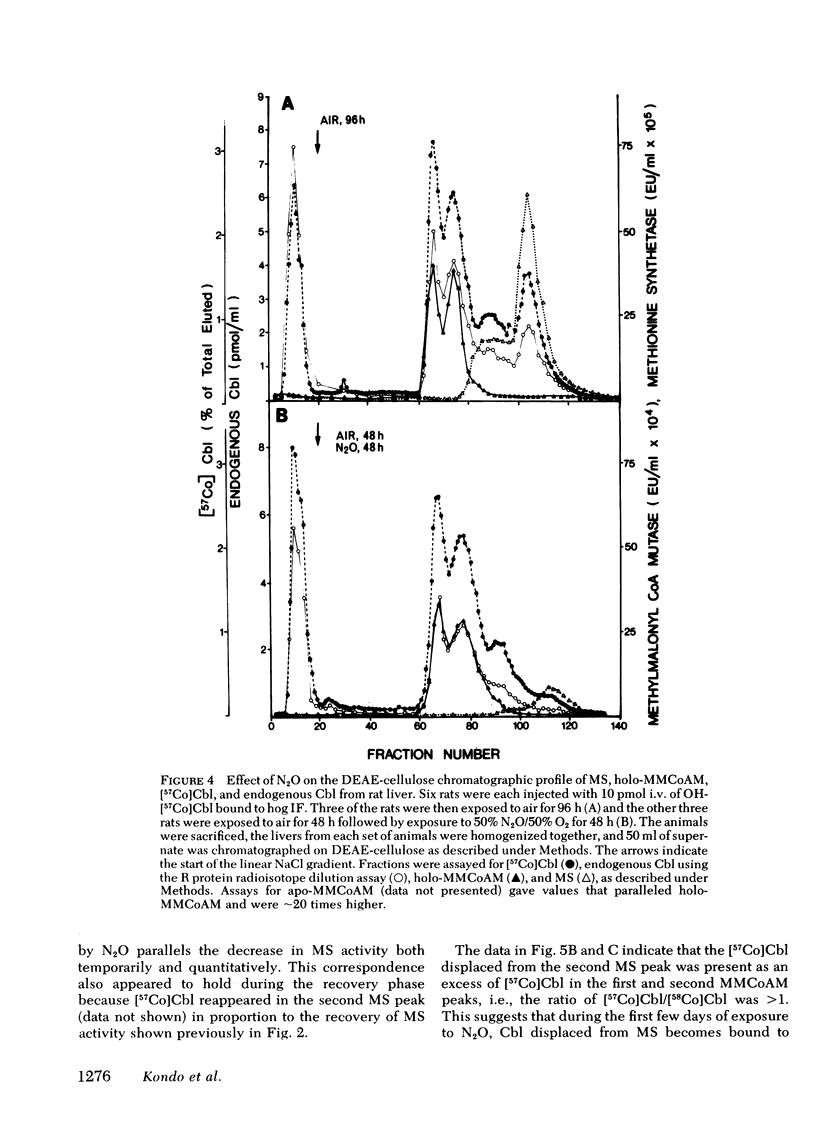
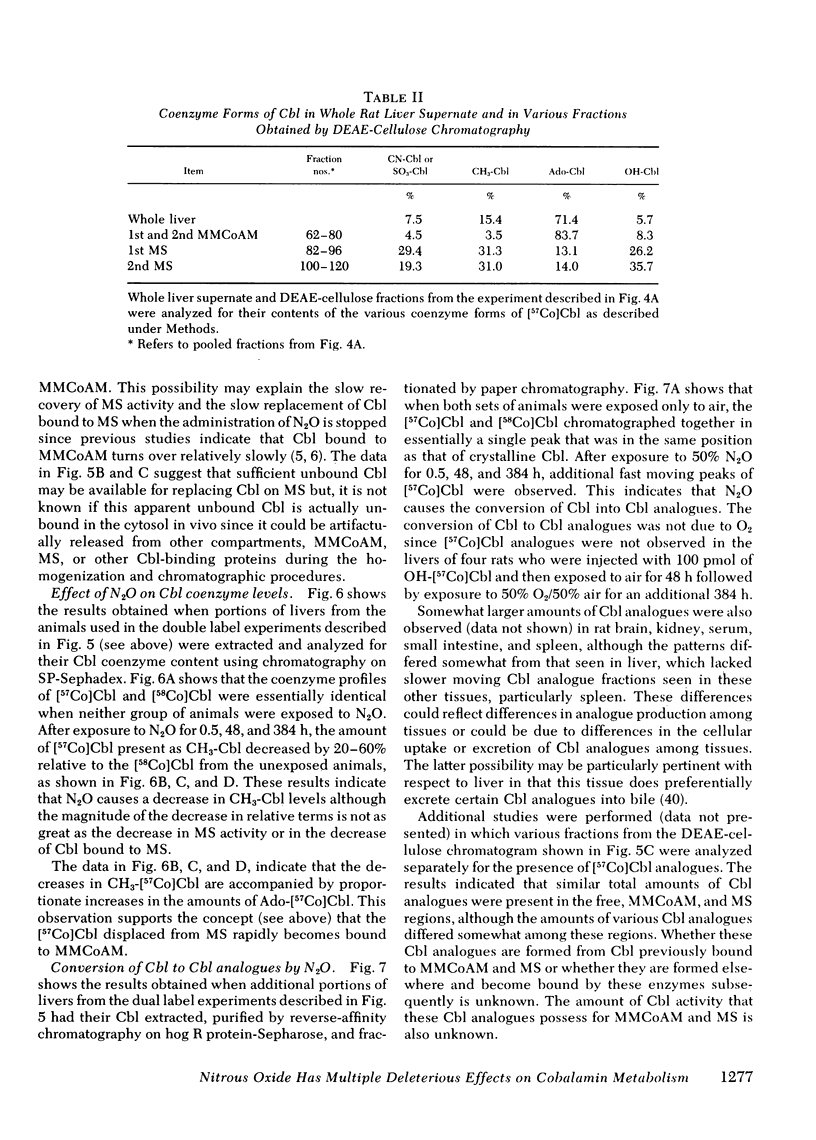
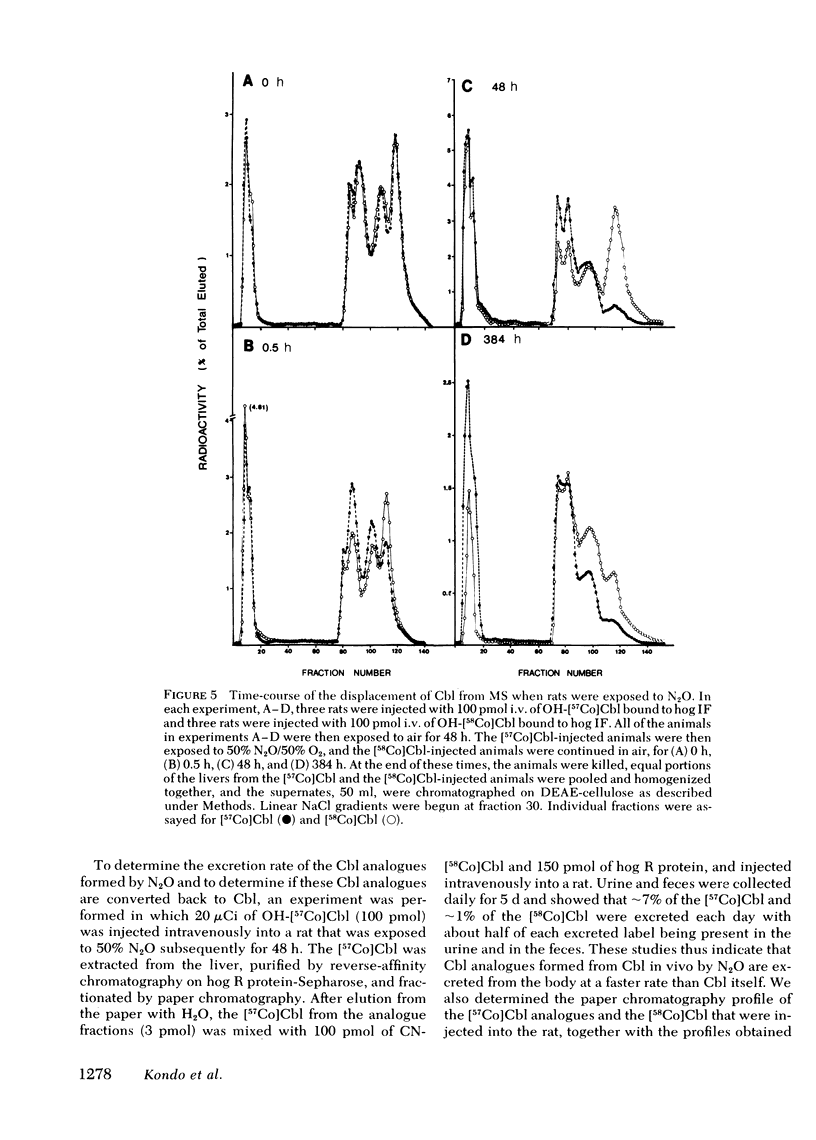
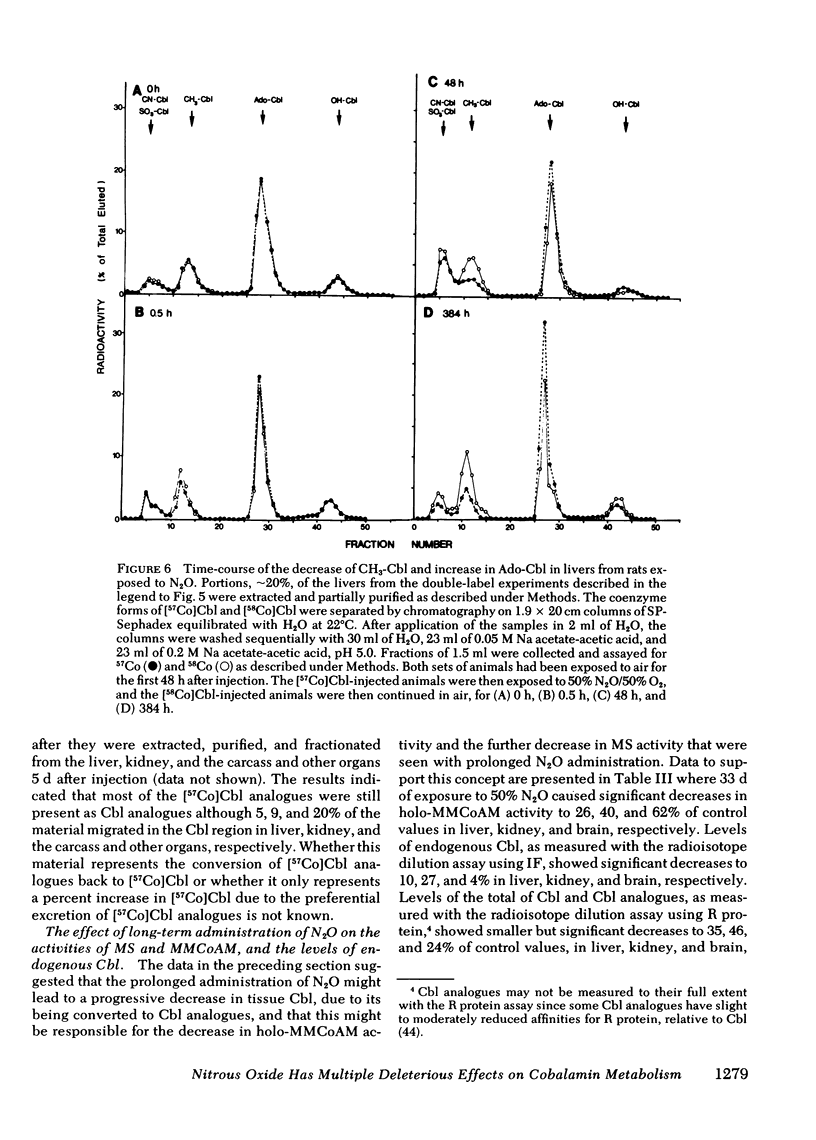
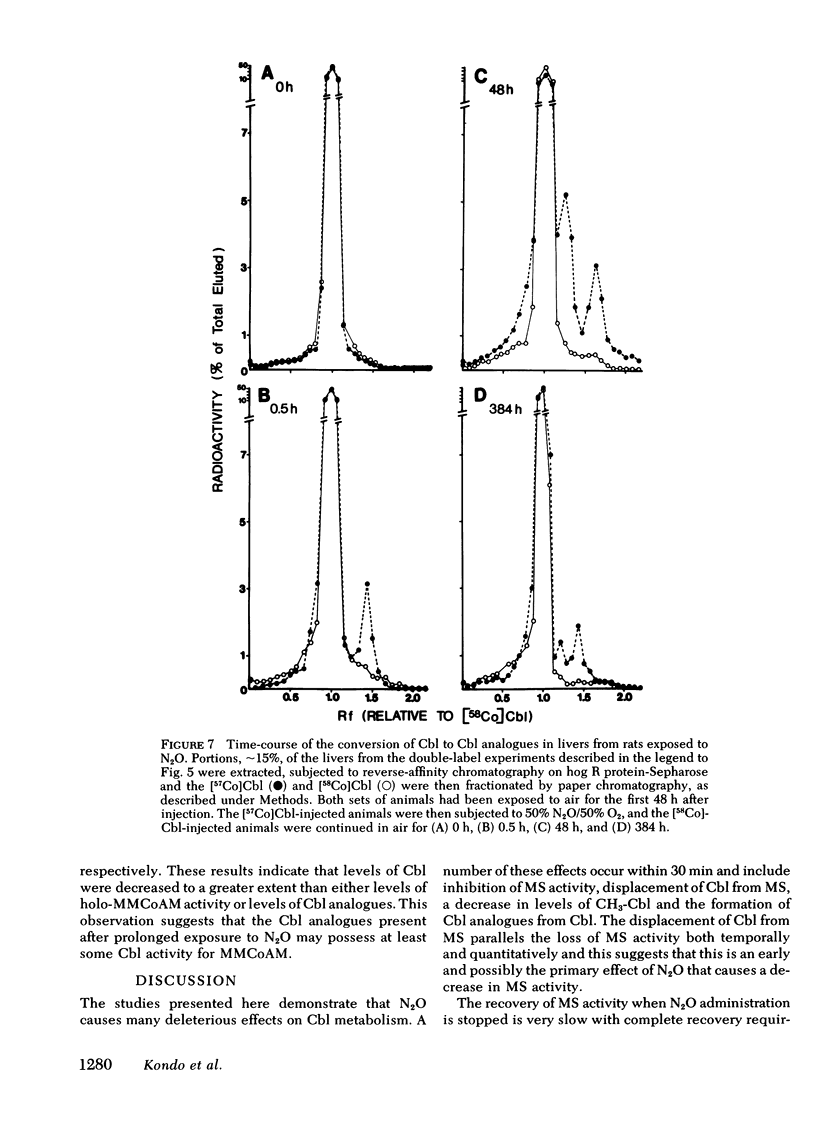
In man, use of the general anesthetic nitrous oxide, N2O, is associated with hematologic and neurologic abnormalities that mimic those seen in cobalamin (Cbl, vitamin B12) deficiency. We have measured a number of aspects of Cbl metabolism in rts exposed to various concentrations of N2O for various periods of time. As little as 2% N2O given for 15 h resulted in 30% inhibition of methionine synthetase (MS) in rat liver. With 50% N2O, inhibition of 70% occurred with 1 h and did not change during the next 48 h. Under these conditions, no inhibition of methylmalonyl-CoA mutase (MMCoAM) was observed. The recovery of MS activity was slow and was only 80% of control values 72 h after N2O was stopped. Studies employing rats previously injected with [57Co]Cbl showed that N2O displaced [57Co]Cbl from MS in a manner that temporally and quantitatively paralleled the loss of MS activity. Recovery of MS activity paralleled the reappearance of [57Co]Cbl on MS. N2O also caused the hepatic content of CH3-[57Co]Cbl to decrease by 20-60%. When [57Co]-Cbl was extracted from liver and analyzed by paper chromatography, [57Co]Cbl analogues were present (10-40% of total [57Co]Cbl) in rats exposed to N2O, but not in control animals. When rats were exposed to 50% N2O for 33 d, the total of endogenous Cbl and Cbl analogues in liver decreased to 35% of control values and endogenous Cbl decreased to 10% of control values. At this time, MS activity was 15% of control values and MMCoAM was only 26% of control values. We conclude that N2O causes multiple defects in Cbl metabolism that include the following: (a) rapid inhibition of MS activity with a slow recovery when N2O is stopped; (b) displacement of Cbl from MS; (c) decreased CH3-Cbl; (d) conversion of Cbl to Cbl analogues; (e) the gradual development of Cbl deficiency and (f) an eventual decrease in MMCoAM activity with a further decrease in MS activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agamanolis D. P., Chester E. M., Victor M., Kark J. A., Hines J. D., Harris J. W. Neuropathology of experimental vitamin B12 deficiency in monkeys. Neurology. 1976 Oct;26(10):905–914. doi: 10.1212/wnl.26.10.905. [DOI] [PubMed] [Google Scholar]
- Allen R. H., Mehlman C. S. Isolation of gastric vitamin B 12 -binding proteins using affinity chromatography. II. Purification and properties of hog intrinsic factor and hog nonintrinsic factor. J Biol Chem. 1973 May 25;248(10):3670–3680. [PubMed] [Google Scholar]
- Amess J. A., Burman J. F., Rees G. M., Nancekievill D. G., Mollin D. L. Megaloblastic haemopoiesis in patients receiving nitrous oxide. Lancet. 1978 Aug 12;2(8085):339–342. doi: 10.1016/s0140-6736(78)92941-0. [DOI] [PubMed] [Google Scholar]
- Brot N., Weissbach H. The role of cobamides in methionine synthesis. Enzymatic formation of holoenzyme. J Biol Chem. 1966 May 10;241(9):2024–2028. [PubMed] [Google Scholar]
- Burger R. L., Schneider R. J., Mehlman C. S., Allen R. H. Human plasma R-type vitamin B12-binding proteins. II. The role of transcobalamin I, transcobalamin III, and the normal granulocyte vitamin B12-binding protein in the plasma transport of vitamin B12. J Biol Chem. 1975 Oct 10;250(19):7707–7713. [PubMed] [Google Scholar]
- CANNATA J. J., FOCESI A., Jr, MAZUMDER R., WARNER R. C., OCHOA S. METABOLISM OF PROPIONIC ACID IN ANIMAL TISSUES. XII. PROPERTIES OF MAMMALIAN METHYLMALONYL COENZYME A MUTASE. J Biol Chem. 1965 Aug;240:3249–3257. [PubMed] [Google Scholar]
- Cullen M. H., Rees G. M., Nancekievill D. G., Amess J. A. The effect of nitrous oxide on the cell cycle in human bone marrow. Br J Haematol. 1979 Aug;42(4):527–534. doi: 10.1111/j.1365-2141.1979.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Deacon R., Lumb M., Perry J., Chanarin I., Minty B., Halsey M. J., Nunn J. F. Selective inactivation of vitamin B12 in rats by nitrous oxide. Lancet. 1978 Nov 11;2(8098):1023–1024. doi: 10.1016/s0140-6736(78)92341-3. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Rosenberg L. E. Improved techniques for the extraction and chromatography of cobalamins. Anal Biochem. 1978 Oct 1;90(1):119–125. doi: 10.1016/0003-2697(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Fink B. R., Shepard T. H., Blandau R. J. Teratogenic activity of nitrous oxide. Nature. 1967 Apr 8;214(5084):146–148. doi: 10.1038/214146a0. [DOI] [PubMed] [Google Scholar]
- Frenkel E. P. Abnormal fatty acid metabolism in peripheral nerves of patients with pernicious anemia. J Clin Invest. 1973 May;52(5):1237–1245. doi: 10.1172/JCI107291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel E. P., McCall M. S., White J. D. Recognition and resolution of errors in the radioisotopic assay of serum vitamin B12. Am J Clin Pathol. 1970 Jun;53(6):891–903. doi: 10.1093/ajcp/53.6.891. [DOI] [PubMed] [Google Scholar]
- GREEN C. D., EASTWOOD D. W. Effects of nitrous oxide inhalation on hemopoiesis in rats. Anesthesiology. 1963 May-Jun;24:341–345. doi: 10.1097/00000542-196305000-00015. [DOI] [PubMed] [Google Scholar]
- Green C. D. Strain sensitivity of rats to nitrous oxide. Anesth Analg. 1968 Sep-Oct;47(5):509–514. [PubMed] [Google Scholar]
- Green R., Van Tonder S. V., Oettle G. J., Cole G., Metz J. Neurological changes in fruit bats deficient in vitamin B12. Nature. 1975 Mar 13;254(5496):148–150. doi: 10.1038/254148a0. [DOI] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977 Dec;60(6):1381–1392. doi: 10.1172/JCI108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Isolation of cobalamin and cobalamin analogs by reverse affinity chromatography. Anal Biochem. 1978 Feb;84(2):486–490. doi: 10.1016/0003-2697(78)90067-2. [DOI] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Recognition of two intracellular cobalamin binding proteins and their identification as methylmalonyl-CoA mutase and methionine synthetase. Proc Natl Acad Sci U S A. 1977 Mar;74(3):921–925. doi: 10.1073/pnas.74.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Kondo H., Allen N. C., Podell E., Allen R. H. Cobalamin analogues are present in human plasma and can mask cobalamin deficiency because current radioisotope dilution assays are not specific for true cobalamin. N Engl J Med. 1978 Oct 12;299(15):785–792. doi: 10.1056/NEJM197810122991501. [DOI] [PubMed] [Google Scholar]
- Kondo H., Kolhouse J. F., Allen R. H. Presence of cobalamin analogues in animal tissues. Proc Natl Acad Sci U S A. 1980 Feb;77(2):817–821. doi: 10.1073/pnas.77.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke B. J., Talarico L., Shah N. K., Kelman A. D. Hematologic reaction to prolonged exposure to nitrous oxide. Anesthesiology. 1977 Oct;47(4):342–348. [PubMed] [Google Scholar]
- LASSEN H. C., HENRIKSEN E., NEUKIRCH F., KRISTENSEN H. S. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet. 1956 Apr 28;270(6922):527–530. doi: 10.1016/s0140-6736(56)90593-1. [DOI] [PubMed] [Google Scholar]
- LASSEN H. C., KRISTENSEN H. S. Remission in chronic myeloid leucaemia following prolonged nitrous oxide inhalation. Dan Med Bull. 1959 Nov;6:252–255. [PubMed] [Google Scholar]
- Layzer R. B., Fishman R. A., Schafer J. A. Neuropathy following abuse of nitrous oxide. Neurology. 1978 May;28(5):504–506. doi: 10.1212/wnl.28.5.504. [DOI] [PubMed] [Google Scholar]
- Layzer R. B. Myeloneuropathy after prolonged exposure to nitrous oxide. Lancet. 1978 Dec 9;2(8102):1227–1230. doi: 10.1016/s0140-6736(78)92101-3. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Youngdahl-Turner P., Willard H. F., Rosenberg L. E. Intracellular binding of radioactive hydroxocobalamin to cobalamin-dependent apoenzymes in rat liver. Proc Natl Acad Sci U S A. 1977 Mar;74(3):916–920. doi: 10.1073/pnas.74.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occupational disease among operating room personnel: a national study. Report of an Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room Personnel, American Society of Anesthesiologists. Anesthesiology. 1974 Oct;41(4):321–340. [PubMed] [Google Scholar]
- Parbrook G. D. Experimental studies into the effect of nitrous oxide on tumour cell growth. Br J Anaesth. 1967 Jul;39(7):549–553. doi: 10.1093/bja/39.7.549. [DOI] [PubMed] [Google Scholar]
- Parbrook G. D. Leucopenic effects of prolonged nitrous oxide treatment. Br J Anaesth. 1967 Feb;39(2):119–127. doi: 10.1093/bja/39.2.119. [DOI] [PubMed] [Google Scholar]
- Paulson G. W. "Recreational" misuse of nitrous oxide. J Am Dent Assoc. 1979 Mar;98(3):410–411. doi: 10.14219/jada.archive.1979.0055. [DOI] [PubMed] [Google Scholar]
- Poston J. M. Cobalamin-dependent formation of leucine and beta-leucine by rat and human tissue. Changes in pernicious anemia. J Biol Chem. 1980 Nov 10;255(21):10067–10072. [PubMed] [Google Scholar]
- Poston J. M. Leucine 2,3-aminomutase, an enzyme of leucine catabolism. J Biol Chem. 1976 Apr 10;251(7):1859–1863. [PubMed] [Google Scholar]
- Rao P. N. Mitotic synchrony in mammalian cells treated with nitrous oxide at high pressure. Science. 1968 May 17;160(3829):774–776. doi: 10.1126/science.160.3829.774. [DOI] [PubMed] [Google Scholar]
- Tortolani G., Bianchini P., Mantovani V. Separation and determination of cobalamins on an SP-Sephadex column. J Chromatogr. 1971 Dec 23;53(3):577–579. doi: 10.1016/s0021-9673(01)98517-6. [DOI] [PubMed] [Google Scholar]



