Abstract
Recombineering is a technology that utilizes the efficient homologous recombination functions encoded by λ phage to manipulate DNA in E.coli. Construction of knockout vectors has been greatly facilitated by recombineering as it allows one to choose any genomic region to manipulate. We describe here an efficient recombineering-based protocol for making mouse conditional knockout targeting vectors.
Keywords: conditional knockout, recombineering, vector, gene targeting, mouse, E.coli
1. Introduction
The development of mouse knockout (gene targeting) technology is based on two scientific discoveries in the 1980s. First, mouse embryonic stem cells (ES) derived from wild type mouse embryos were found to be able to propagate in Petri dishes (1, 2), and were subsequently demonstrated to contribute to the germline in chimera mice produced using ES cells (3). Second, homologous recombination in mammalian cells enabled precise manipulation of a locus whereby a selection marker flanked by two homology arms could insert into a pre-determined locus (4-6). Today, with the improved protocols for establishing and culturing germline-robust mouse ES cells and the advances in DNA manipulation, one can generate essentially all types of genetic mutations in ES cells and in the mouse (7). To decode gene functions genome-wide, large mutagenesis programmes in the mouse have recently taken off aiming to mutate all coding genes in the mouse genome primarily through gene targeting (8). The success of these large projects will undoubtedly generate enormous genetic resources for the research communities for decades to come.
The first generation of mouse knockout (KO) alleles were either deletion of an exon or simply insertion of a selection marker into a locus to disrupt the expression of the gene (9). For many genes, owing to their essential roles in embryonic development, the knockout mutants die in uteri, thereby precluding studying their functions in late development or in specific tissues of adult mice. In some extreme cases, even loss of one copy of a gene is not tolerated in development (VEGF) (10). The embryonic lethality problem is circumvented by using conditional knockout (cko) approaches that two loxP sites flank the critical exon (s) of a gene so that upon spatial and temporal Cre recombinase expression, this critical exon is deleted (11). Operationally, construction of cko targeting vectors is laborious since one has to find perfect restriction digestion-ligation strategies to put together usually six to seven pieces of DNA fragments. Indeed, it had been a bottleneck for producing cko mice until recombineering technology became available.
Recombineering is a technology developed in the late 1990s that utilizes the efficient homologous recombination functions encoded by λ phage or cryptic phage Rac to perform DNA manipulation in E.coli (12-14). Recombining linear double-strand (ds) DNA requires three λ phage proteins, Gam, Beta and Exo, which are collectively called λ Red proteins. The λ Gam protein inhibits RecBCD and SbcCD exonuclease activities of E.coli, thus preserving linear dsDNA and allowing it to be used as a substrate for recombination (15). Beta protein is an ssDNA-binding protein that promotes annealing of complementary DNA strands (14, 16). Beta can bind stably to ssDNA longer than 35 nucleotides (16), and can protect ssDNA overhangs from single-strand nuclease degradation. This property of Beta protein makes it possible to use only about 50 nucleotides of homology for efficient recombination. The short homology can be conveniently supplied by incorporating the 50 nt genomic DNA sequence into PCR primer oligonucleotides (12, 17). Exo is a dsDNA-dependent 5′-3′ exonuclease that processes linear dsDNA and generates a 3′ ssDNA overhang at each end, the substrate that beta protein binds (18).
Several efficient recombineering systems have been developed that utilize the λ Red proteins (12, 17, 19-21). The system developed in the laboratories of Don Court and Neal Copeland appears to be commonly used for making conditional targeting vector (22, 23). In this system, initially, recombineering was performed in E.coli strains that harbour a defective λ prophage (17), which retains pL operon, where the Red genes are located, and the critical features of transcriptional control of this operon. Transcription of pL is under control of CI857 repressor which shuts down pL transcription at 32°C. At 42°C, CI857 repressor becomes inactive, so Red genes are transcribed and Red proteins are accumulated. Owing to the strong pL promoter, growth of E.coli at 42°C for 10-15 minutes is enough for efficient and clean homologous recombination in these cells. The Court/Copeland recombineering system is very efficient possibly due to the fact that the three Red genes are expressed from their natural operon with the strong but tightly regulated pL promoter, and therefore, the three proteins are in appropriate molar ratio as they form a complex in vivo. The original recombineering strains were further modified to express Cre (EL350) or Flpe recombinases (EL250) upon arabinose induction. These new features make the Court/Copeland strains popular for making complicated DNA constructs that have loxP or FRT sites (22, 23).
Using recombineering, we and other colleagues have previously described methods for constructing targeting vectors (19, 23-27). However, due to some unknown reasons, some BAC DNAs are difficult to transform into EL350 or EL250 recombineering competent strains, or have rearrangements after transformation. We have recently described new reagents that deliver recombineering functions directly into the cells hosting BACs or PACs (27).
In this chapter, we describe a protocol for making conditional knockout targeting vectors using pSim18 plasmid, which carries the three Red genes under the control of pL promoter that is in turn regulated by the temperature sensitive CI857 repressor (27). By simple plasmid transformation, heat-inducible recombineering functions are delivered to BACs. The conditional targeting vectors constructed in this protocol have a lacZ reporter incorporated into the targeted allele.
2. Materials
2.1. Mobile recombineering reagents
pSim18 has the pSC101 replication origin which is low copy and temperature sensitive. This plasmid contains the three Red genes, exo, bet and gam, together with CI857 repressor and pL promoter. Additionally, pSim18 plasmid has the coding sequence of a Hygromycin resistant cassette inserted between CI857 and pL promoter (27). Consequently, transformants of pSim18 are selected with Hygromycin (see Note 1).
The retrieval vector, PL611, is derived from pBR322, which can host relatively large piece of mammalian genomic DNA. PL613 is the lacZ reporter cassette plasmid. I-SceI-Bsd-I-CeuI and loxP-F3-Neo-F3 are the selection cassettes for introducing loxP sites into the BACs. Neo is PGK-EM7-Neo so it is selectable in both E.coli and in ES cells. Plasmid Cm-MC1TK has a Chloramphenicol resistance cassette (Cm) and the negative selection marker in ES cells, MC1TK. Cm-MC1TK cassette is flanked by two 600bp homology regions to PL611 therefore Cm-MC1TK is added to the targeting vector backbone by recombineering. Details of these plasmids can be found in a recent publication (27).
2.2. Primers for PCR amplification of selection cassettes and the retrieval vector backbone
Design rules for the long oligos can be found in a recent publication (27) (see Note 2). The underlined sequences are mouse genomic DNA used as homology in recombineering.
- Primers for amplifying the Bsd cassette:
- For-5′-TCTAGCCTCACATAGGGGAGAAAGTGTATTTCTCAGTTATACTTTAAGCCCTGGCATTTTTTTAAAGTGTCTGGGACATTCTAGGGATAACAGGGTAATG;
- Rev-5′-TAACCAACAGTTTACCAGCCAACTGCAACATTTAAGGATGTAGAAGAGACAATGGCCTAGGGACAAGGATGAGTCTAGCTTCGCTACCTTAGGACCGTTA.
- Primers for amplifying the Neo cassette:
- For-5′-AAAGTTTTATTTTATTTTATTTTTAAATGGTTATCAAATTGAATGTGAAATGTGCAAAGGCCCTGGAATGTGATGAAATATGTAAAACGACGGCCAGTGA;
- Rev-5′-TCTACTTCTTTTGGCGCCAGAATTTCATTAAATGCATCATTTTAAACAAGTATTGTCACAAGATGAACTTCTTGCTAATGAGGAAACAGCTATGACCATG.
- Primers for amplifying retrieval plasmid backbone:
- For-5′-GAGACTTGGTTCAAGAAACAAATATGTGTCCCTTTTGTTGTTGTGCTAAATTGGGAGTGAGGTTTAAAAAAAAAATCAGATACGACTCACTATAGGGAG;
- Rev-5′-TAATGCCTTTTATCCAAAGCCAGGAGACTTTTATCTTTTTAAGCATCGGCAAAGTAAGGTGTTTGGCTCTTACTTTTATTTTAGTGAGGGTTAATTATCG.
2.3. PCR amplification
Plasmids PL611 (retrieval vector), I-SceI-Bsd-I-CeuI cassette and loxP-F3-Neo-F3 cassette are digested with EcoRI/BamHI, and the digestion products, 3.1kb (PL611), 0.6kb (Bsd), and 2.1kb (Neo) are purified from the gel using Qiagen gel extraction kit (see Note 3).
1.0 ng of each the purified DNA fragments is used as PCR templates. PCR amplification is carried out using Extensor Hi-Fidelity PCR Master Mix 2 (2X, ABgene). 25 μl of the master mix was added to 1 μl of the template (1.0 ng), 2 μl of each primer (10 μM) and 20 μl of PCR-grade water.
PCR was performed using PTC-225 PCR machine (Peltier Thermal Cycler) with the following settings: 94°C for 4 min, this is followed by 35 cycles of 94°C for 30s, 60°C for 30s, 68°C for 1 min (Bsd cassette) or 2-3 min (Neo and retrieval cassette). This is then followed by 68°C for 5 min.
After PCR reactions, 1.0 μl of DpnI and 0.5 μl of exonuclease I (New England Biolabs) are added per 50 μl PCR products and incubated at 37°C for 1 h followed by heat inactivation at 80°C for 20 min. The PCR products are then purified using Qiagen mini-preparation columns and eluted in 50 μl of PCR-grade water (see Note 4).
2.4. Antibiotics and LB
Antibiotics are used at the following concentrations: Ampicillin (Amp), 50μg/ml; Chloramphenical (Cm), 12.5μg/ml; Kanamycin (Kan), 20μg/ml; and Hygromycin (Hygro), 75μg/ml.
Fast-Media and agar with Blasticidin (Bsd) (fas-bl-s), Puromycin (Puro) (fas-pr-s) are purchased from InvivoGen. Prepare agar and TB as per instructions on packets, taking care not to overheat the mixture. Cool media rapidly in ice slurry for a few minutes after heating in a microwave. Rapid cooling of the heated mixture reduces degradation of the antibiotics. Puro selection in E.coli cells is not as stringent as other commonly used antibiotics, we do not recommend to use Puromycin selection in 96-well liquid media culture.
LB contains 10 g of tryptone, 5 g of yeast extract and 5g NaCl per litre.
2.5. Restriction enzymes
I-SceI and I-CeuI are available from New England Biolabs. I-PpoI is from Promega.
2.6. Electroporation of E.coli
Electroporation is performed under the following condition: 1.75 kV, 25 μF with the pulse controller set at 200 Ω. After electroporation, 1.0 ml LB is added to the cuvette which is subsequently incubated at 32°C for at least an hour prior to plating.
3. Methods
3.1. Construction of a lacZ reporter conditional null targeting vector
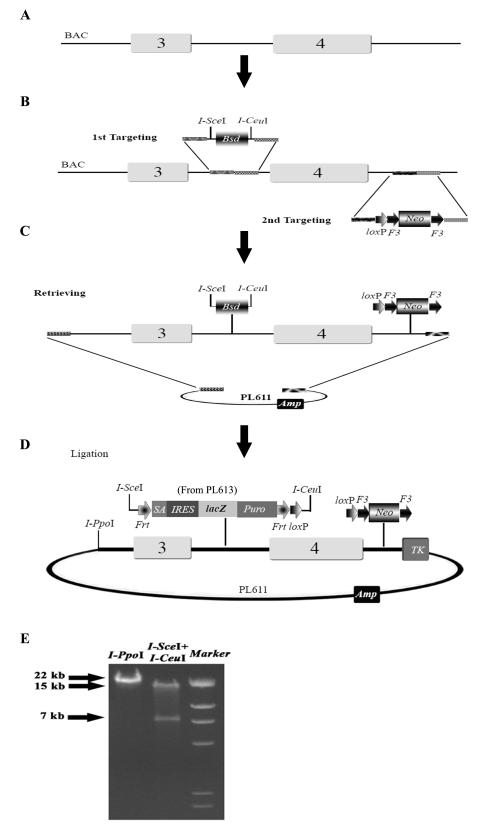
To generate a cko allele, two loxP sites flanking exon 4 of Bcl11a gene, are introduced into the genome. Upon expression of tissue-specific or inducible Cre recombinase, recombination occurs between the two loxP sites, resulting in deletion of the intervening genomic DNA sequence, thus creating a null allele. We describe here steps to make a cko targeting vector for a multi-purpose allele that can serve as a conventional KO, a conditional KO and a reporter allele. For high throughput operation in 96-well plates, please refer to our recent publication (27). The overall strategy is illustrated in Fig. 1A-D.
Fig. 1.
The workflow of generating a reporter conditional null targeting vector. (A) The BAC clone containing the region of interest is made recombineering competent by transformed with pSim18 plasmid. Exon 4, which encodes the main functional domains of Bcl11a protein, is the intended deletion region and would be flanked by loxP sites in the conditional allele. (B) The Bsd cassette flanked by two rare cutter sites, I-SceI and I-CeuI, is targeted to intron 3. Subsequently, the loxP-F3-PGK-EM7-Neo-F3 (Neo) cassette is targeted into intron 4. Shaded bars represent the short homology arms (50-80 nts) used for recombineering. In a typical cko vector, we select between 4-5 kb genomic DNA as the left homology arm (5′), and 2-3kb as the right homology arm (3′). The genomic DNA region to be deleted is generally between 1-7 kb. (C) The doubly targeted genomic DNA on the BAC is retrieved to PL611 (AmpR). (D) The Bsd cassette is replaced by the lacZ reporter cassette (from PL613) in a simple restriction digestion and ligation process. The final targeting vector has the lacZ reporter flanked by two FRT sites with one loxP site, and has the F3-flanked Neo cassette with another loxP site. Finally, the negative selection marker MC1TK is added to the vector backbone by recombineering. The final targeting vector is linearized with I-PpoI for ES cell transfection. The true conditional knockout allele is obtained by excising the lacZ and the Neo cassettes with Flpe in the targeted ES cells or in the mouse germline. We have shown that FRT and F3 sites do not recombine in the mouse germline with the constitutive presence of Flpe. (E) Restriction digestion patterns of the final targeting vector. Restriction digestion with I-PpoI linearizes the targeting vector (22 kb). Digestion with both I-SceI and I-CeuI excises the 7 kb lacZ reporter cassette.
3.1.1. Conferring recombineering competence to BAC cells by transformation with pSim18
Inoculate BAC cells (containing region of interest) into 1.0 ml of LB with Chloramphenical (in a 15 ml polypropylene tube) for overnight growth at 37°C with shaking at 200 rpm.
Transfer cells into a 1.5 ml eppendorf tube and pellet cells by spinning the tube at max speed using a bench top centrifuge for 25 seconds.
Decant supernatant and wash three times with ice-cold water. Cells are collected by spinning at max speed using a bench top centrifuge for 25 seconds at each wash step.
Resuspend cells in 50 μl of ice-cold water with 1 ng of pSim18 and perform electroporation.
Add 1.0 ml of LB to the cuvette and incubate transformation mixture at 32°C for 1 hour.
Plate out cells onto a LB-Hygro plate and incubate the plate at 32°C overnight.
3.1.2. Targeting selection cassettes to BACs (Fig. 1A-B)
Pick one HygroR BAC colony and inoculate it into 1.0 ml of LB with Chloramphenical and Hygromycin (in a 15 ml polypropylene tube) and incubate overnight at 32°C with shaking at 200 rpm.
Inoculate 25, 35, 45 and 55 μl of the overnight culture into four 15 ml polypropylene tubes, each containing 1.0 ml of fresh LB and incubate at 32°C with shaking at 200 rpm for 2 hours.
Without measuring OD, transfer the cultures to separate wells in a 42°C heat block (Grant Instrument, Cambridge, UK) and incubate for 15 min.
Put the heat block on ice and incubate for 5 min.
Transfer cells into four 1.5 ml eppendorf tubes and centrifuge at maximum speed using a bench top centrifuge for 25 sec. Decant supernatant and wash three times with ice-cold water. Cells are collected by spinning at max speed using a bench top centrifuge for 25 seconds at each wash step.
Combine cells from the four tubes and resuspend them in 50 μl of ice-cold water with about 300 ng-1.0μg PCR product (Bsd cassette) and perform electroporation.
Add 1.0 ml of LB to the cuvette and incubate the transformation mixture at 32°C for 1 hour.
Plate out cells onto a LB-Bsd plate and incubate the plate at 32°C overnight.
Pick 10 BsdR colonies. Streak the colonies onto two plates, LB-Amp and LB-Hygro, and incubate the plates at 32°C for overnight. The desired colonies should be AmpS and HygroR. Sensitivity to Amp indicates that these BsdR colonies are true targeted ones and not the contamination from the original Bsd plasmid. BsdR-HygroR colonies still retain pSim18 and can be directly used for the next round of recombineering.
The AmpS-BsdR-HygroR cells are used for targeting the PCR amplified Neo cassette to the BAC (repeating steps 1-9).
The AmpS-BsdR-KanR-HygroR cells are now ready for the retrieval step.
3.1.3. Retrieving genomic DNA to a plasmid backbone (Fig. 1C)
Inoculate one BsdR-KanR-HygroR BAC colony into 1.0 ml of LB with Kan in a 15 ml polypropylene tube and incubate the tube overnight at 32°C with shaking at 200 rpm.
Inoculate 25, 35, 45 and 55 μl of overnight culture into four 15 ml polypropylene tubes, each containing 1.0 ml of LB and incubate at 32°C with shaking at 200 rpm for 2 hours.
Without measuring OD, transfer the cultures to individual wells in a 42°C heat block and incubate for 15 min.
Put the heat block on ice and incubate for 5 min.
Transfer cells into 1.5 ml eppendorf tubes and centrifuge at maximum speed using a bench top centrifuge for 25 sec. Decant supernatant and wash three times with ice-cold water. Cells are collected by spinning at max speed using a bench top centrifuge for 25 seconds at each wash step.
Combine cells from the four tubes and resuspend them in 50 μl of ice-cold water with about 300ng-1.0μg PCR product (PL611 retrieval cassette) and perform electroporation.
Add 1.0 ml of LB to the cuvette and incubate transformation mixture at 32°C for one hour.
Plate out cells onto a LB-Amp plate and incubate the plate at 32°C overnight.
There are usually hundreds or even thousands of AmpR colonies on the plate. In some cases, most of the colonies are background from either self-ligation or intra-molecular recombination of the retrieval plasmid vector. The true retrieval plasmid, however, can be easily identified through re-transformation from the background AmpR cells because only the true retrieved plasmid carries the Bsd and Neo Cassettes.
Add 2 ml of LB to the plate, swirl the plate to collect cells.
Isolate plasmids from the cell mixture using Qiaprep Spin Miniprep kit (Qiagen) and electroporate into DH10B electro-competent cells with 1.0 μl of the plasmid preparation. After one hour incubation at 37°C, plate the transformants onto a LB-Kan plate.
Inoculate three KanR colonies for plasmid preparation and restriction digestion. These KanR colonies are the correctly retrieved plasmid.
3.1.4. Replacement of Bsd cassette with the lacZ reporter (Fig. 1D)
Set up restriction digestion reactions of the retrieved plasmid (10 μl, about 1.5 μg) or PL613 (10 μl, about 1.5 μg): 2 μl of I-SceI, 1 μl of I-CeuI (New England Biolabs, NEB), 3 μl of NEB buffer 4, 0.3 μl of BSA and 13.7 μl of water and incubate at 37°C for 2 hours (See Note 4).
Purify the restriction digested retrieved plasmid using Qiaprep Mini-preparation columns (Qiagen) and elute DNA in 30 μl of PCR-grade water (See Note 5).
Run the restriction digestion reaction of PL613 through a 1.0% agarose gel, purify the lacZ reporter cassette (the 7 kb band) using QIAquick Gel Extraction kit (Qiagen).
Set up ligation reaction of purified digestion products using 12 μl of lacZ reporter cassette (600 ng), 10 μl of purified retrieved plasmid (50 ng), 2.5 μl of T4 DNA ligase buffer with 1.0 μl of T4 DNA ligase (New England Biolabs) and incubate at room temperature for 2 hours (see Note 6).
Add 5 μl of ligation products to chemical competent TOP10 cells (Invitrogen) and incubate on ice for 30 min (can use electroporation for the transformation).
Heat shock at 42°C without shaking for 30 sec.
Add 250 μl of SOC and incubate transformation mixture at 32°C for 1 hour.
Plate out cells onto a LB-Puro-Kan plate and incubate the plate at 37°C overnight. Colonies are typically observed after 16-24 hours.
Inoculate four PuroR-KanR colonies into 3 ml of LB (in 15 ml polypropylene tubes) with Kanamycin, and culture at 37°C overnight with shaking at 200 rpm.
Isolate plasmids using Qiaprep Spin Miniprep kit (Qiagen) and set up restriction digestion to confirm the identity of the plasmid.
3.1.5. Targeting the negative selection cassette to the vector plasmid backbone
This step is to add the Cm-MC1TK cassette to the plasmid backbone.
Inoculate 25, 35, 45 and 55 μl of overnight culture of EL350 cells (recombineering competent) into four 15 ml polypropylene tubes, each containing 1.0 ml of fresh LB and incubate at 32°C with shaking at 200 rpm for 2 hours.
Without measuring OD, transfer the cultures to separate wells in a 42°C heat block and incubate for 15 min.
Transfer the heat block on ice and incubate for 5 min.
Transfer cells into individual 1.5 ml eppendorf tubes and centrifuge at maximum speed using a bench top centrifuge for 25 sec. Decant supernatant and wash three times with ice-cold water. Cells are collected by spinning at max speed using a bench top centrifuge for 25 seconds at each wash step.
Combine cells from the four tubes and resuspend them in 50 μl of ice-cold water with about 10-100ng purified Cm-MC1TK cassette and perform electroporation.
Add 1.0 ml of LB to the cuvette and incubate transformation mixture at 32°C for 1 hour.
Plate out cells onto a LB-Kan-Cm plate and incubate the plate at 32°C overnight.
Inoculate a few PuroR-KanR-CmR colonies into 3 ml of LB with Kanamycin in 15 ml polypropylene tubes and grow at 37°C overnight with shaking at 200 rpm.
Isolate plasmid DNA using Qiaprep Spin Miniprep kit (Qiagen). Dilute the plasmid DNA 1:100. Use 1.0 μl of the diluted DNA to electroporate into DH10B cells and plate onto a LB-Kan plate. This re-transformation step eliminates plasmid multimers formed during recombineering.
Isolate plasmid DNA using Qiaprep Spin Miniprep kit (Qiagen) to obtain the final targeting vector.
3.1.6. Verification of the final targeting construct
Set up restriction digestions using 1.0 μl of the final targeting vectors (150 ng) with either 2 μl of I-PpoI or 2 μl of I-SceI plus 1.0 μl of I-CeuI in a total reaction volume of 30 μl and incubate at 37°C for 2 hours.
Run the restriction digestion products through a 1% gel to check for expected digestion patterns (Fig. 1E).
Sequence the final targeting vectors to verify the key DNA junctions, including the two junctions between the plasmid backbone and the two ends of the retrieved genomic DNA fragment, and the loxP, FRT and F3 sites.
Acknowledgement
This work is supported by The Wellcome Trust.
Footnotes
pSim18 is prepared in the conventional plasmid preparation way. Since it is a low copy plasmid, the yields are usually lower than regular high copy plasmids used in the laboratories such as pBluescript.
The 50-80 nucleotides homology in the long oligos should avoid genomic regions that have known repeats and stretches of Gs (over three). The two homology arms flanking a selection cassette should not have more than 5 nucleotides identical between them. We find that HPLC purified long oligos perform better than desalted-only oligos. HPLC presumably eliminates most of the incorrect oligos that are present in significantly amounts in the crude desalted oligos.
The selection cassettes need to be gel-purified prior to PCR amplification. We usually use 500ng plasmid DNA for digestion in a 30μl volume with 20 units of each restriction enzyme. The reaction is usually for 2 hours at 37°C. In case small amount of background is still present after gel purification, PCR products are digested with DpnI restriction enzyme. DpnI only cleaves DNA from dam+ strains. Plasmid DNA prepared from commonly used E.coli strains, such as DH5α and DH10B, are sensitive to DpnI digestion. We test the PCR amplified cassette DNA to make sure that no background colony is obtained after electroporating the DNA into commercially purchased electro-competent DH10B cells.
The activity of I-SceI in buffer 4 is 50%, therefore we used twice as much of I-SceI in the double digestion reaction with I-CeuI.
Briefly, add water to the digestion mixture or PCR tube to make volume to 100 μl. Add 500 μl PB from Qiaprep kit and mix well. Load the mixture to a Qiaprep Mini-preparation column. Wash the column and elute DNA according to the plasmid mini-preparation protocol.
We found that increasing the molar ratio of the insert (lacZ reporter cassette) vs. the vector (retrieved backbone) to 10:1 results in a drastic increase the number of PuroR/KanR colonies. This is probably due to the fact that the lacZ reporter (7kb) has to compete with the smaller Bsd cassette (0.6kb) in the retrieved plasmid (Fig. 1D). Gel purification of the targeting vector after I-SceI/I-CeuI digestion to eliminate the Bsd fragment further improves the ligation efficiency.
Reference
- 1.Evans MJ, Kaufman MH. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley A, Evans M, Kaufman MH, Robertson E. Nature. 1984;309:255–6. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas KR, Capecchi MR. Cell. 1987;51:503–12. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 5.Smithies O. Nat Med. 2001;7:1083–6. doi: 10.1038/nm1001-1083. [DOI] [PubMed] [Google Scholar]
- 6.Mansour SL, Thomas KR, Capecchi MR. Nature. 1988;336:348–52. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 7.van der Weyden L, Adams DJ, Bradley A. Physiol Genomics. 2002;11:133–64. doi: 10.1152/physiolgenomics.00074.2002. [DOI] [PubMed] [Google Scholar]
- 8.Collins FS, Rossant J, Wurst W. Cell. 2007;128:9–13. doi: 10.1016/j.cell.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Bradley A, Hasty P, Davis A, Ramirez-Solis R. Biotechnology (N Y) 1992;10:534–9. doi: 10.1038/nbt0592-534. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 11.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Science. 1994;265:103–6. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Buchholz F, Muyrers JP, Stewart AF. Nat Genet. 1998;20:123–8. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 13.Copeland NG, Jenkins NA, Court DL. Nat Rev Genet. 2001;2:769–79. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 14.Court DL, Sawitzke JA, Thomason LC. Annu Rev Genet. 2002;36:361–88. doi: 10.1146/annurev.genet.36.061102.093104. [DOI] [PubMed] [Google Scholar]
- 15.Sawitzke JA, Thomason LC, Costantino N, Bubunenko M, Datta S, Court DL. Methods Enzymol. 2007;421:171–99. doi: 10.1016/S0076-6879(06)21015-2. [DOI] [PubMed] [Google Scholar]
- 16.Mythili E, Kumar KA, Muniyappa K. Gene. 1996;182:81–7. doi: 10.1016/s0378-1119(96)00518-5. [DOI] [PubMed] [Google Scholar]
- 17.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. Proc Natl Acad Sci U S A. 2000;97:5978–83. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassuto E, Lash T, Sriprakash KS, Radding CM. Proc Natl Acad Sci U S A. 1971;68:1639–43. doi: 10.1073/pnas.68.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Li MZ, Elledge SJ. Nat Genet. 2002;30:31–39. doi: 10.1038/ng797. [DOI] [PubMed] [Google Scholar]
- 20.Datsenko KA, Wanner BL. Proc Natl Acad Sci U S A. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy KC. J Bacteriol. 1998;180:2063–71. doi: 10.1128/jb.180.8.2063-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Jenkins NA, Copeland NG. Genome Res. 2003;13:476–84. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angrand PO, Daigle N, van der Hoeven F, Scholer HR, Stewart AF. Nucleic Acids Res. 1999;27:e16. doi: 10.1093/nar/27.17.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD. Nat Biotechnol. 2003;21:652–9. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 26.Cotta-de-Almeida V, Schonhoff S, Shibata T, Leiter A, Snapper SB. Genome Res. 2003;13:2190–4. doi: 10.1101/gr.1356503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan W, Costantino N, Li R, Lee SC, Su Q, Melvin D, Court DL, Liu P. Nucleic Acids Res. 2007;35:e64. doi: 10.1093/nar/gkm163. [DOI] [PMC free article] [PubMed] [Google Scholar]