Abstract
Promoter methylation of the RAS-association domain family 1, isoform A gene (RASSF1A) is one of the most frequent events found in human tumours. In this study we set out to test the hypothesis that loss of Rassf1a can co-operate with inactivation of the adenomatous polyposis coli (Apc) gene to accelerate intestinal tumourigenesis using the Apc-Min (ApcMin/+) mouse model, as mutational or deletional inactivation of APC is a frequent early event in the genesis of intestinal cancer. Further, loss of RASSF1A has also been reported to occur in premalignant adenomas of the bowel. RASSF1A has been implicated in an array of pivotal cellular processes, including regulation of the cell cycle, apoptosis, microtubule stability and most recently in the β-catenin signalling pathway. By interbreeding isoform specific Rassf1a knockout mice with Apc+/Min mice we showed that loss of Rassf1a results in a significant increase in adenomas of the small intestine and accelerated intestinal tumourigenesis leading to the earlier death of adenocarcinoma-bearing mice and decreased overall survival. Comparative genomic hybridization of adenomas from Rassf1a−/−; Apc+/Min mice revealed no evidence of aneuploidy or gross chromosomal instability (no difference to adenomas from Rassf1a+/+; Apc+/Min mice). Immunohistochemical analysis of adenomas revealed increased nuclear β-catenin accumulation in adenomas from Rassf1a−/−; Apc+/Min mice, compared to those from Rassf1a+/+; Apc+/Min mice, but no differences in proliferation marker (Ki67) staining patterns. Collectively these data demonstrate co-operation between inactivation of Rassf1a and Apc resulting in accelerated intestinal tumourigenesis, with adenomas showing increased nuclear accumulation of β-catenin, supporting a mechanistic link via loss of the known interaction of Rassf1 with β-TrCP that usually mediates degradation of β-catenin.
Keywords: adenoma, adenocarcinoma, RASSF, APC, β-catenin
Introduction
The RASSF1A isoform of RASSF1 is frequently inactivated by epigenetic alterations in human cancers, and RASSF1A has been reported to play a role in stabilising microtubules, regulating cell cycle progression and activating pro-apoptotic pathways [reviewed in van der Weyden & Adams, 2007]. There have been several reports of RASSF1A promoter methylation with silencing of its expression in intestinal tumours with frequencies from around 2% for adenomas to as high as 60% for malignant colorectal cancers [van Engeland et al., 2002; Lee et al., 2004; Oliveira et al., 2005]. The most frequent early event in >80% of sporadic colorectal cancers (CRCs) is point mutation or deletion of the adenomatous polyposis coli (APC) gene, which is also mutated in the germline of patients with the familial CRC syndrome, familial adenomatous polyposis (FAP) [Nishisho et al., 1991]. APC inactivation leads directly to reduced β-catenin degradation with nuclear accumulation of β-catenin producing aberrant Wnt pathway signalling as a key factor in adenoma initiation [Su et al., 1992; Carothers et al., 2001]. Whether concomitant inactivation of RASSF1A and APC is of functional significance in intestinal cancer is yet to be established. Interestingly it has been proposed that the acquisition of activating mutations in K-ras, which has been reported to occur in ~30-40% of colorectal tumours, is mutually exclusive to epigenetic inactivation of RASSF1A [van Engeland et al., 2002]. Thus, tumours that have lost RASSF1A and APC but retain wild-type K-ras may represent a different subclass of this tumour.
Apc+/Min mice represent a valuable model of intestinal tumourigenesis, since sporadic loss of heterozygosity (LOH) of the wild-type allele of Apc recapitulates the initiation of adenomagenesis observed in humans. Similarly, Rassf1a null mice show an increased incidence of spontaneous and induced tumorigenesis [van der Weyden et al., 2005; Tommasi et al., 2005]. Importantly, isoform-specific Rassf1a null mice irradiated with 3.5 Gy of ionizing radiation to stimulate DNA damage, show an increased susceptibility to tumours associated with the intestinal tract (adenomas and adenocarcinomas) [van der Weyden et al., 2005]. Thus, using these models, we set out to test the hypothesis that loss of Rassf1a can co-operate with inactivation of Apc to accelerate intestinal tumourigenesis in vivo. Additionally, our aim was to use this model to address the possible mechanism of any such co-operation between these genes.
Rassf1a-deficient Apc+/Min mice show increased intestinal adenoma formation
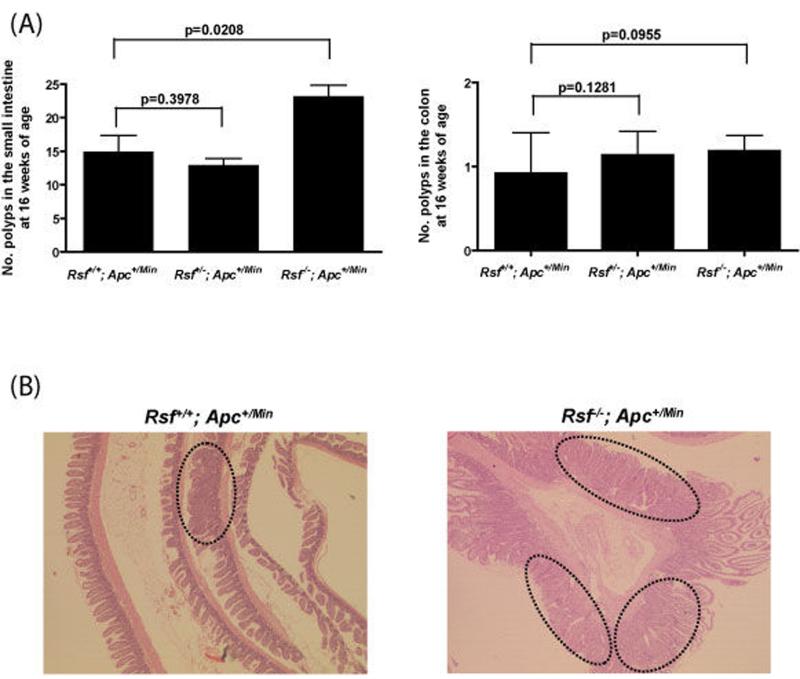
Isoform-specific Rassf1a null mice (on a mixed C57BL6/J–129S5 background) [van der Weyden et al., 2005] and Apc+/Min mice (on a C57BL6/J background) [Su et al., 2002] were interbred to generate Rassf1a+/+, Rassf1a+/− and Rassf1a−/− mice on an Apc+/Min background. Rassf1a mice were born at expected Mendelian frequencies on the Apc+/Min background (data not shown). Littermates of each genotype were used for all studies and mice were housed in a pathogen-free barrier environment. Rassf1a [van der Weyden et al., 2005] and Apc+/Min [Su et al., 1992] genotyping was carried out by PCR according to previously published procedures. Since Apc+/Min mice typically develop multiple intestinal adenomas by 16 weeks of age [Su et al., 1992; Luongo et al., 1994; Moser et al., 1990], we initially set out to determine the relative multiplicity of adenoma formation at the 16 week timepoint according to the three Rassf1a backgrounds. At 16 weeks, wild-type, Rassf1a heterozygotes and Rassf1a null homozygotes on a Min background were sacrificed and their intestines removed and examined under the dissecting microscope for the presence of visible adenomatous polyps. As shown in Figure 1a, Rassf1a−/−; Apc+/Min mice showed significantly more adenomas (24.3 ± 1.6, n=30) than Rassf1a+/−; Apc+/Min mice (14.0 ± 1.0, n=21) or Rassf1a+/+; Apc+/Min mice (15.6 ± 2.1, n=11; p=0.005 by unpaired Students t-test (two-tailed) for Rassf1a+/+ vs. Rassf1a−/−; data shown as mean ± SEM). This observation was confirmed when the intestines and colon were individually prepared as Swiss-rolls, histologically processed and examined by light microscopy for adenomas (Figure 1b). There was no significant difference in adenoma number between Rassf1a+/+; Apc+/Min and Rassf1A+/−; Apc+/Min mice (p=0.465). The vast majority of the adenomas observed were in the small intestine and were hemispherical in shape forming sessile polyps on the surface of the mucosa, although the occasional pedunculated colonic polyp was observed. Small intestinal polyps varied in size from 0.5 and 2 mm in diameter, whereas large intestinal polyps varied between 1 and 3 mm, and there was no significant difference in polyp size between Rassf1a+/+; Apc+/Min and Rassf1A−/−; Apc+/Min mice. Collectively these data show that inactivation of Rassf1a and Apc co-operate to increase the number of intestinal adenomas formed.
Figure 1.
Rassf1a-deficient Apc+/Min mice show increased intestinal adenoma formation. At 16 weeks of age intestines were collected from Rassf1a+/+ (n=11), Rassf1a+/− (n=21) and Rassf1a−/− (n=30) mice on an Apc+/Min background. (a) The intestines were opened and analysed for number and location of adenomas (polyps) with the help of a dissecting microscope. The bar indicates mean value for each genotype. (b) The intestines were then prepared as Swiss-rolls and histologically analysed following haematoxylin-eosin staining (circles outline the adenomas). All sections shown are representative. Magnification: x25.
Rassf1a-deficient Apc+/Min mice show decreased survival
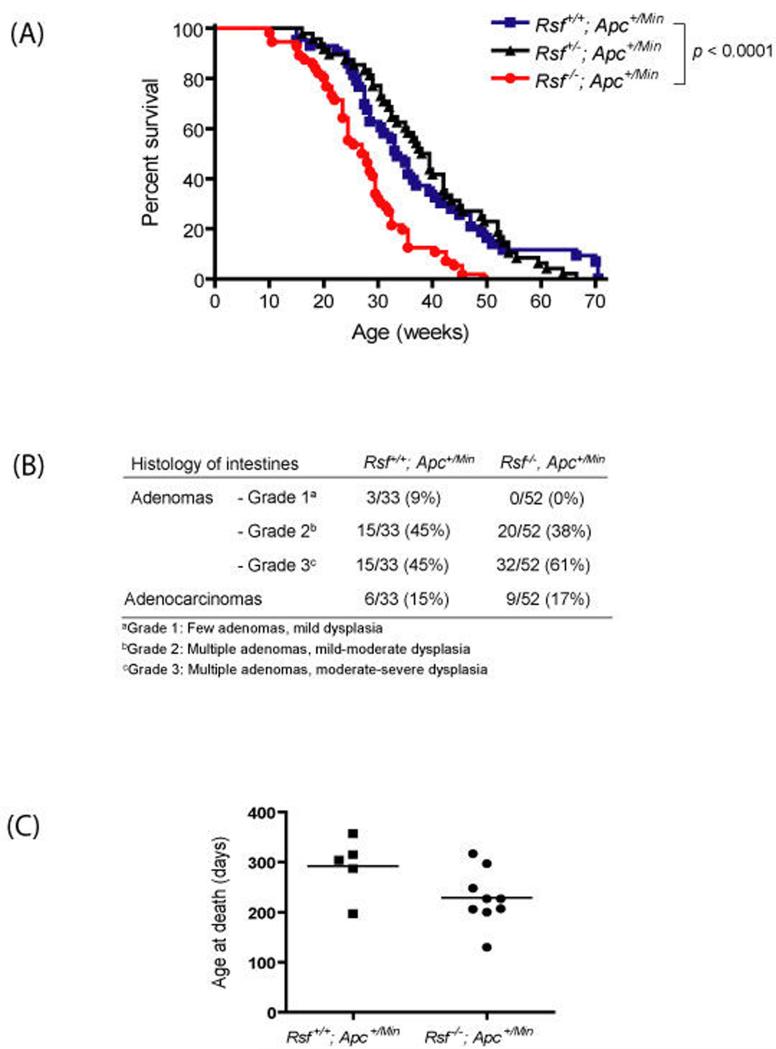
Given that Rassf1a−/−; Apc+/Min mice develop more adenomas than their littermates by 16 weeks of age, we next set out to determine whether there were any effects on progression to intestinal adenocarcinoma. We placed cohorts of Rassf1a+/+; ApcMin/+, Rassf1a+/−; Apc+/Min and Rassf1a−/−; Apc+/Min mice on tumour watch until they became moribund, at which point the mice were humanely sacrificed and a full necropsy performed, with all organs being processed for histopathological analysis. As shown in Figure 2a, Rassf1a−/−; Apc+/Min mice showed a decreased survival (27.5 weeks median survival), compared to Rassf1a+/−; Apc+/Min mice (38.5 weeks median survival) and Rassf1a+/+; Apc+/Min mice (33.5 weeks median survival; p<0.0001 by Logrank test for Rassf1a+/+ vs. Rassf1a−/−). Although there was no difference in the numbers of mice developing leukaemia or lymphoma (3/33 [9%] and 5/52 [9%] for Rassf1a+/+ and Rassf1a−/− mice, respectively), histopathological analysis was carried out on the small intestines and colons to look for a difference in the numbers of mice which developed (i) adenomas, (ii) adenomas of a specific pathological grade of dysplasia, or (iii) adenocarcinomas (Figure 2b). There was a greater trend towards moderate to high grade dysplasia (combined grades 2 & 3, relative to grade 1 mild dysplasia) for Rassf1a−/−; Apc+/Min adenomas compared to Rassf1a+/+; Apc+/Min adenomas (trend approaching significance with p=0.055 by Fisher’s Exact test). Interestingly, the age at death for intestinal adenocarcinoma-bearing mice was significantly lower in Rassf1a−/−; Apc+/Min mice (228.8 ± 18.4 days, n=9) compared with Rassf1a+/+; Apc+/Min mice (292.0 ± 26.4 days, n=5; p=0.034 by unpaired t-test, one-tailed), as shown in Figure 2c. Such earlier death may reflect greater numbers of adenomas in this cohort, increasing the likelihood of one of them progressing to malignancy at an earlier time. Thus, while loss of Rassf1a increases the formation and/or early growth of intestinal adenomas on the Apc+/Min background, it appears not to contribute significantly to the progression of these premalignant lesions to adenocarcinomas, consistent with RASSF1A inactivation being an early event in tumorigenesis [reviewed in van der Weyden & Adams, 2007].
Figure 2.
Rassf1a-deficient Apc+/Min mice show decreased survival. (a) Kaplan-Meier plots show the percentages of survival of Rassf1a+/+, Rassf1a+/− and Rassf1a−/− mice on an Apc+/Min background. (b) When the mice became moribund, their intestines were collected and prepared as Swiss-rolls for histological analysis following haematoxylin-eosin staining. The processed intestines from Rassf1a+/+; Apc+/Min (n=33) and Rassf1a−/−; Apc+/Min (n=52) were analysed for the presence of adenomas (grouped into grades 1-3 based on their number and degree of dysplasia) and adenocarcinomas. (c) The age at death for mice bearing intestinal adenocarcinomas was compared between Rassf1a+/+; Apc+/Min (n=6) and Rassf1a−/−; Apc+/Min (n=9) mice. The bar indicates mean value for each genotype.
Rassf1a-deficient Apc+/Min mice develop intestinal adenomas that display increased nuclear β-catenin accumulation
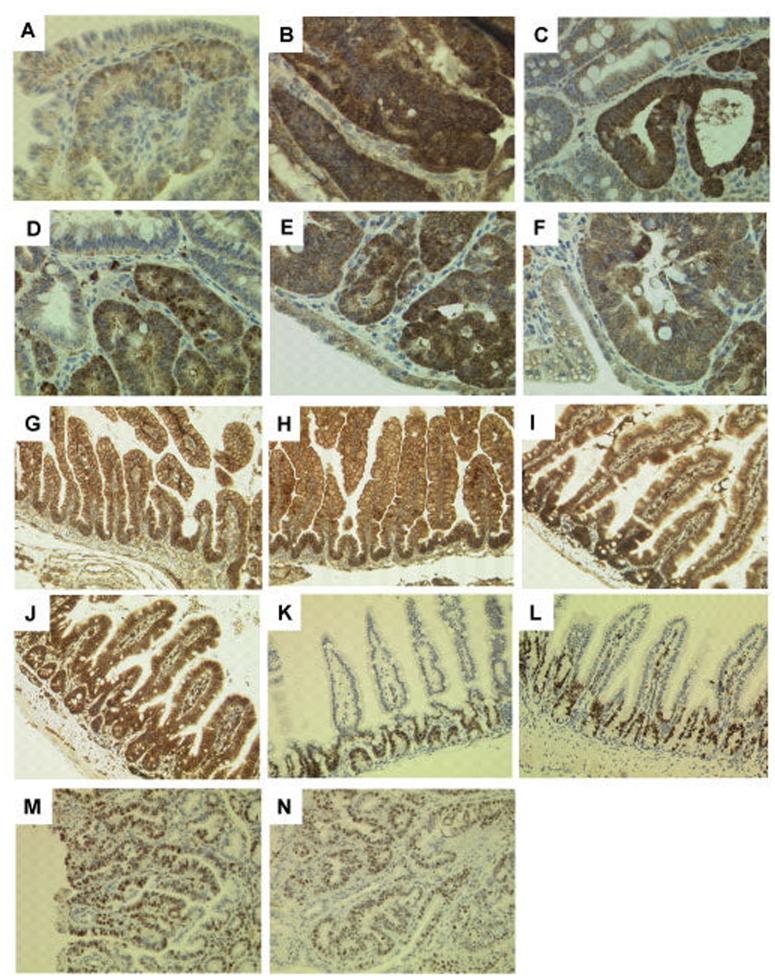
APC, in association with the serine-threonine glycogen synthase kinase, GSK-3β, down-regulates intracellular levels of β-catenin and the oncogenic effects of APC deficiency are in large part attributable to excess levels of β-catenin [Su et al., 1992]. Loss of the wild-type Apc allele in Apc+/Min murine adenomas results in inactivation of both Apc alleles with increased intracellular accumulation of β-catenin [Carothers et al., 2001], and it was recently found that over-expression of RASSF1C, or silencing of RASSF1A, also resulted in increased nuclear β-catenin accumulation due to an inhibition of βTrCP-mediated β-catenin degradation [Estrabaud et al., 2007]. Importantly, immunohistochemical analysis of β-catenin expression in Rassf1a−/−; Apc+/Min murine adenomas at 16 weeks of age showed increased nuclear accumulation of β-catenin in terms of both a greater number of cells showing this pattern and an increased intensity of nuclear β-catenin staining, compared to histologically matched Rassf1a+/+; Apc+/Min murine adenomas (Figure 3a, b). Adenomas collected at the time of death from these two groups of mice on tumour watch did not show such clear differences in the levels of β-catenin expression (Figure 3c, d). Interestingly, a gut tumour (large jejunal adenoma with severe dysplasia) arising from an irradiated Rassf1a−/− mouse [van der Weyden et al., 2005] also showed strong nuclear β-catenin staining (Figures 3e, f), suggesting that concomitant loss of Rassf1a and activation of β-catenin had occurred.
Figure 3.
β-catenin and Ki-67 staining in intestines. Intestines at 16 weeks showed β-catenin staining in the adenomas of Rassf1a+/+; Apc+/Min mice (a) with increased nuclear accumulation in adenomas of Rassf1a−/−; Apc+/Min mice (b). Adenomas collected at the time of death from Rassf1a+/+; Apc+/Min (c) and Rassf1a−/−; Apc+/Min (d) mice on tumour watch did not show such clear differences in nuclear β-catenin expression. (e, f) Strong nuclear β-catenin staining was observed in an intestinal adenoma arising from an irradiated Rassf1a−/− mouse. In contrast, no difference in the level of expression or subcellular localization of β-catenin was found in intestines from Rassf1a+/+ (g, i) or Rassf1A−/− (h, j) mice at 2 days or 12 weeks (respectively). Intestines at 16 weeks showed no difference in the number or distribution of Ki-67 positive cells in either the normal epithelium (k, l) or adenomas (m, n) of Rassf1a+/+; Apc+/Min and Rassf1a−/−; Apc+/Min mice, respectively. Immunohistochemistry was performed using anti-β-catenin (1:500 dilution; Sigma-Aldrich, Dorset, UK) or anti-Ki-67 (1:50 dilution; sp6 clone, DCS Diagnostics, Hamburg, Germany). Immunohistochemical signal was detected by secondary biotinylated donkey anti-rabbit antibody (1:500 dilution; Stratech Scientific, Suffolk, UK), followed by Vector ABC tertiary kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. All immunohistochemistry was performed on a BondMax machine (Vision Biosystems, Newcastle, UK) according to the manufacturer’s instructions and involved antigen retrieval by BondMax Epitope retrieval solution heated on the machine for 20 minutes. All sections shown are representative. Magnification: x400 (a-f) and x200 (g-n).
The physical interaction of RASSF1C with β-TrCP via the SS18GYXS19 motif found in RASSF1C has been proposed to result in the inhibition of β-TrCP-mediated degradation of β-catenin, and this may result in tumour promotion [Estrabaud et al., 2007]. Intriguingly, inhibition of RASSF1A or overexpression of RASSF1C was shown to inhibit β-TrCP in transiently transfected HeLa cells. We set out to investigate this mechanism further by examining expression of β-catenin in the normal intestines of our Rassf1a null mice which have isoform-specific deletion of Rassf1a but express wild-type levels of Rassf1c mRNA [van der Weyden et al., 2005]. We saw no difference in the levels of expression or subcellular localization of β-catenin in the normal epithelium of the small and large intestines of Rassf1a null mice when compared to wild-type controls, either at 2 days of age (Figures 3g, h), 12 weeks of age (Figures 3i, j) or 17 weeks of age (data not shown). As Rassf1a null mice become viable healthy young adults (prior to tumour susceptibility later on), this strongly suggests that loss of Rassf1a on its own does not result in a sufficiently elevated constitutive activation of β-catenin, since activated β-catenin signalling either by overexpression of β-catenin itself [van Amerongen and Berns, 2006] or by loss of Apc results in embryonic lethality [Su et al., 2002].
Thus, it seems likely that increased accumulation of β-catenin due to interference of the interaction between β-TrCP and RASSF1C, resulting from loss of RASSF1A, may only become relevant in the setting of tumour formation where β-catenin is already partly activated, such as via inactivation by mutation and/or loss of Apc. A similar mechanism of co-operative activation of β-catenin has been demonstrated by inactivation of the secreted frizzled related protein (SFRP) gene together with APC inactivation in human colon cancer [Taketo, 2004]. Thus, Rassf1a inactivation during intestinal tumour formation may further deregulate Wnt pathway signalling leading to increased accumulation of β-catenin, which can further contribute to the oncogenic effects of aberrant Wnt/β-catenin signalling [Reya & Clevers, 2005].
Rassf1a-status does not appear to affect the pattern of proliferation marker staining in adenomas or normal intestinal epithelium from Apc+/Min mice
Given that Rassf1a−/−; Apc+/Min mice show increased adenoma formation at 16 weeks (Figure 1), and RASSF1A has been reported to play an important role in regulating cell cycle/mitotic progression [reviewed in van der Weyden & Adams, 2007], we looked to see whether there was an increase in the compartment of cells within the intestine that was cycling/proliferating, as assessed by immunohistochemistry with Ki-67 antibody (the Ki-67 nuclear antigen is present throughout most of the cell cycle, and Ki-67 immunohistochemistry provides a reliable means of rapidly evaluating the growth fraction of normal and neoplastic cell populations [Gerdes et al., 1984]). However, we observed no difference in the number or distribution of Ki-67 positive cells in either the normal intestinal epithelium or the adenomas of Rassf1a−/−; Apc+/Min mice compared to Rassf1a+/+; Apc+/Min mice controls (Figure 3k-n), suggesting that deregulation of the cell cycle detectable by this method is unlikely to be the mechanism of action by which Rassf1a inactivation enhances adenoma formation in Min mice. Similarly, no differences were found in the prevalence of apoptotic figures identified in haematoxylin-eosin stained sections of either normal intestinal epithelium or adenomas from these two groups of mice (data not shown).
No evidence of aneuploidy or gross genomic instability in intestinal adenomas from Rassf1a−/−; Apc+/Min mice
The RASSF1A gene has been shown to interact with the pro-apoptotic kinase MST1 and to complex with MST2 and WW45, homologues of Sav and LATS1 respectively [Guo et al., 2007]. Dysregulation of this complex by inactivation of Rassf1a has been proposed to result in cytokinesis failure and delayed mitosis [Guo et al., 2007], and failed or stalled exits from mitosis may promote genomic instability. Furthermore, transient knockdown of RASSF1A in foreskin fibroblasts has been shown to cause mitotic abnormalities including multiple centrosomes and lagging chromosomes that were postulated to contribute to chromosomal instability [Song et al., 2004]. However, although tumour-prone, Rassf1a null mice do not show any evidence of gross genomic instability [van der Weyden et al., 2005]. Thus, to determine if co-operation between inactivated RASSF1A and APC could promote genomic instability within tumours, we subjected adenomas from Rassf1a−/−; Apc+/Min and Rassf1a+/+; Apc+/Min mice to comparative genomic hybridization to gain a genome-wide profile of genomic gains and losses. The statistically significant chromosomal alterations found in these Rassf1A, Apc+/Min adenomas are listed in Supplemental Table 1. Two commonly recurring chromosomal alterations, namely a ~3.5 Mb amplification on chromosome 14 (present in 5/12 samples) and a 282 kb deletion on chromosome 17 (present in 6/12 samples) represent previously documented copy number variants (CNV) between the C57BL/6J and 129Sv mouse strains [Adams et al., 2005; Graubert et al., 2007]. Interestingly only one Rassf1A, Apc+/Min adenoma (number 11) showed a ~4 Mb deletion on chromosome 18 encompassing the murine Apc gene. However, there was no evidence of aneuploidy (i.e., loss or gains of whole chromosomes), suggesting that a defect in microtubule stability is not a major determinant of the accelerated intestinal tumourigenesis seen in the Rassf1a−/−; Apc+/Min mice. Furthermore, there was no difference in average copy number alterations in adenomas from these two groups (9.3 ± 1.5 gains or losses per adenoma for Rassf1a+/+; Apc+/Min mice and 7.1 ± 5.3 gains or losses per adenoma for Rassf1a−/−; Apc+/Min mice). These levels of copy number alterations are consistent with the frequency observed for adenomas from the Apc+/1638N mouse model [Alberici et al., 2007].
In this study we set out to test the hypothesis that inactivation of both Rassf1a and Apc could co-operate in the formation of intestinal tumours in vivo. We observed increased adenoma formation in Rassf1a−/−; Apc+/Min mice at 16 weeks of age, decreased survival in tumour watch animals and a decreased latency of adenocarcinoma formation, suggesting that inactivation of both Rassf1a and Apc, which is frequently observed in tumours of the human intestinal tract, is an important mechanism of tumour initiation and early growth of intestinal adenomas. By immunohistochemical analysis of the adenomas from Rassf1a−/−; Apc+/Min compared to those from Rassf1a+/+; Apc+/Min mice, we were able to demonstrate enhanced nuclear accumulation of β-catenin, indicative of increased β-catenin signalling. This may represent an important mechanistic link by which inactivation of Apc and Rassf1a co-operate in tumour formation, as both Apc (via GSK3β) and Rassf1 (via β-TrCP) usually bring about β-catenin degradation. Thus, abnormality of both of these β-catenin degradation pathways may combine to produce higher levels of nuclear β-catenin in tumour cells than occur with either alone, conferring a co-operative oncogenic effect of inactivation of both Rassf1a and Apc.
Supplementary Material
Acknowledgements
Work in the D.J. Adams Laboratory is funded by Cancer Research UK (CR-UK) and the Wellcome Trust, work in the A. Bradley Laboratory is funded by the Wellcome Trust and work in the M.J. Arends Laboratory is supported by CR-UK. We thank John Brown for performing the immunohistochemistry and Beverley Haynes for processing of the tissues. Open access to this article is funded by the Wellcome Trust.
References
- Adams DJ, Dermitzakis ET, Cox T, Smith J, Davies R, Banerjee R, et al. Complex haplotypes, copy number polymorphisms and coding variation in two recently divergent mouse strains. Nat Genet. 2005;37:532–536. doi: 10.1038/ng1551. [DOI] [PubMed] [Google Scholar]
- Alberici P, de Pater E, Cardoso J, Bevelander M, Molenaar L, Jonkers J, et al. Aneuploidy arises at early stages of Apc-driven intestinal tumorigenesis and pinpoints conserved chromosomal loci of allelic imbalance between mouse and human. Am J Pathol. 2007;170:377–387. doi: 10.2353/ajpath.2007.060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers AM, Melstrom KA, Jr, Mueller JD, Weyant MJ, Bertagnolli MM. Progressive changes in adherens junction structure during intestinal adenoma formation in Apc mutant mice. J Biol Chem. 2001;276:39094–39102. doi: 10.1074/jbc.M103450200. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Jonkers J, Kitson H, Fiegler H, Humphray S, Scott C, et al. A whole-genome mouse BAC microarray with 1-Mb resolution for analysis of DNA copy number changes by array comparative genomic hybridization. Genome Res. 2004;14:188–196. doi: 10.1101/gr.1878804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F, Rios D, Griffiths M, Smith J, Ning Z, Cox T, et al. TranscriptSNPView: a genome-wide catalog of mouse coding variation. Nat Genet. 2006;38:853. doi: 10.1038/ng0806-853a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrabaud E, Lassot I, Blot G, Le Rouzic E, Tanchou V, Quemeneur E, et al. RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of beta-catenin by interacting with βTrCP. Cancer Res. 2007;67:1054–1061. doi: 10.1158/0008-5472.CAN-06-2530. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Graubert TA, Cahan P, Edwin D, Selzer RR, Richmond TA, Eis PS, et al. A High-Resolution Map of Segmental DNA Copy Number Variation in the Mouse Genome. PLoS Genetics. 2007;3:e3. doi: 10.1371/journal.pgen.0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol. 2007;17:700–705. doi: 10.1016/j.cub.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Hupé P, Stransky N, Thiery JP, Radvanyi F, Barillot E. Analysis of array CGH data: from signal ratio to gain and loss of DNA regions. Bioinformatics. 2004;20:3413–3422. doi: 10.1093/bioinformatics/bth418. [DOI] [PubMed] [Google Scholar]
- Lee S, Hwang KS, Lee HJ, Kim JS, Kang GH. Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Lab Invest. 2004;84:884–893. doi: 10.1038/labinvest.3700108. [DOI] [PubMed] [Google Scholar]
- Livingston DM. Cancer: Chromosome defects in the colon. Nature. 2001;410:536–537. doi: 10.1038/35069185. [DOI] [PubMed] [Google Scholar]
- Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Velho S, Domingo E, Preto A, Hofstra RM, Hamelin R, et al. Concomitant RASSF1A hypermethylation and KRAS/BRAF mutations occur preferentially in MSI sporadic colorectal cancer. Oncogene. 2005;24:7630–7634. doi: 10.1038/sj.onc.1208906. [DOI] [PubMed] [Google Scholar]
- Taketo MM. Shutting down Wnt signal-activated cancer. Nature Genetics. 2004;36:320–322. doi: 10.1038/ng0404-320. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer; New York: 2005. pp. 397–420. [Google Scholar]
- Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol. 2004;6:129–137. doi: 10.1038/ncb1091. [DOI] [PubMed] [Google Scholar]
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Tommasi S, Dammann R, Zhang Z, Wang Y, Liu L, Tsark WM, et al. Tumor susceptibility of Rassf1a knockout mice. Cancer Res. 2005;65:92–98. [PubMed] [Google Scholar]
- van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- van der Weyden L, Tachibana KK, Gonzalez MA, Adams DJ, Ng BL, Petty R, et al. The RASSF1A isoform of RASSF1 promotes microtubule stability and suppresses tumorigenesis. Mol Cell Biol. 2005;25:8356–8367. doi: 10.1128/MCB.25.18.8356-8367.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L, Adams DJ. The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta. 2007;1776:58–85. doi: 10.1016/j.bbcan.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland M, Roemen G, Brink M, Pachen MM, Weijenberg MP, de Bruine AP, et al. K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene. 2002;21:3792–3795. doi: 10.1038/sj.onc.1205466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.