Abstract
Malaria sexual stage and mosquito transmission-blocking vaccines (SSM-TBV) have recently gained prominence as a necessary tool for malaria eradication. SSM-TBVs are unique in that, with the exception of parasite gametocyte antigens, they primarily target parasite or mosquito midgut surface antigens expressed only inside the mosquito. As such, the primary perceived limitation of SSM-TBVs is that the absence of natural boosting following immunization will limit its efficacy, since the antigens are never presented to the human immune system. An ideal, safe SSM-TBV formulation must overcome this limitation. We provide a focused evaluation of relevant nano-/microparticle technologies that can be applied toward the development of leading SSM-TBV candidates, and data from a proof-of-concept study demonstrating that a single inoculation and controlled release of antigen in mice, can elicit long-lasting protective antibody titers. We conclude by identifying the remaining critical gaps in knowledge and opportunities for moving SSM-TBVs to the field.
Keywords: Antigen, controlled release, immunity, malaria, midgut, mosquito, nanotechnology, natural boosting, sexual stages, transmission-blocking vaccine.
INTRODUCTION
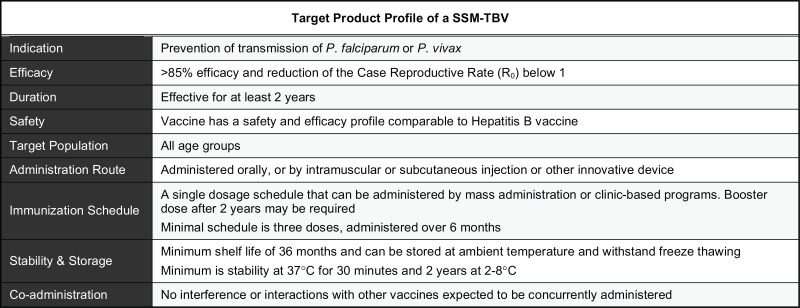
The malaria eradication research agenda has re-emphasized the need for effective sexual stage and mosquito transmission-blocking vaccines (SSM-TBV) [1], which prevents malaria parasite development in its mosquito vector and the subsequent cascade of secondary infections [2-5]. SSM-TBVs, in general, work through the action of inhibitory antibodies [5-7]. Thus, the minimum objective of immunization is to induce high titer antibodies sustainable for at least one transmission season (~3-6 months), but preferably for 2 years. Achieving this minimum goal would theoretically drive the case reproductive rate, (R0) <1. A summary of the target product profile (TPP) for SSM-TBVs is shown in Table 1. With the exception of Plasmodium falciparum or P. vivax gametocyte surface antigens that are expressed in the human, SSM-TBVs are considered unique in that they target parasite (gamete, zygote, or ookinete) or Anopheles mosquito midgut surface antigens that are only expressed in the mosquito. As such, one of the potential limitations of the TBV approach is that since the antigens are never naturally presented to the human immune system, the absence of natural boosting following immunization will limit their efficacy [8-13]. A complete P. knowlesi model in non-human primates (NHP) has been used to test the “natural boosting” hypothesis for Plasmodium gamete antigens [13]. It was found that following a two-dose immunization regimen using 105-107 P. knowlesi microgametes and macrogametes in a Freund’s complete adjuvant (FCA), the majority of the monkeys maintained a high level of functional transmission-blocking antibody titer for more than 1 year. Furthermore, annual challenge infections over a six year period were found to be sufficient for boosting and transmission-blocking immunity persisted in the majority of splenectomized NHPs. Importantly, as expected, they observed that transmission-blocking activity waned over time in the absence of boosting and that the challenge infection resulted in an increase in gamete-specific antibody levels. Although the likely gamete antigens had not yet been fully characterized at the time of this study, it was already known that gametocytes and gametes shared surface antigens [14, 15], thus it is possible that gametocyte exposure in the NHPs following challenge was responsible for boosting. This study further supported the notion that boosting would increase the efficacy and utility of SSM-TBVs but raised the question of the need for highly potent adjuvants such as FCA, which is considered a serious obstacle in human vaccine development.
Table 1.
The Proposed Target Product Profile (TPP) for a Malaria Sexual Stage and Mosquito Transmission-Blocking Vaccine (SSM-TBV) [61]
 |
The four leading SSM-TBVs (Table 2) include two gametocyte surface antigens, Pfs230 [16-20] and Pfs48/45 [21], the ookinete surface protein Pfs25 [22] and the Anopheles gambiae alanyl aminopeptidase N (APN1), which is an abundant, midgut-specific apical microvilli surface glycoprotein that has been shown to mediate ookinete invasion and oocyst development [7, 23]. Of these, only Pfs25 and APN1 are expressed explicitly inside the mosquito midgut. Note that the goal of this report is not to evaluate the complete repertoire of proven and possible SSM-TBV candidates, and the reader is directed to several excellent reviews for additional information [3, 4, 24-29]. Among the four leading candidates, only Pfs25 has completed Phase I clinical trials, albeit with equivocal results [29]. Efforts are underway to produce the full-length Pfs/Pvs230 [30-32] and Pfs48/45 antigens [33-35], which have proven to be a difficult undertaking using different expression platforms due to their size and/or conformation, as well as the high A+T content of plasmodial genes; and these issues have a direct impact on vaccine process development. The APN1 antigen, on the other hand, does not require the full-length antigen, is highly immunogenic [7] and is entering process development, with an optimistic initiation of Phase I clinical trials within the next 3-4 years. Since Pfs25 and APN1-based vaccines are the least likely to benefit from boosting following natural infection, we focused on these two antigens in this article to examine their current state of development, as well as similarities and differences in the context of several identified target product profiles and the “natural boosting” issue (Table 1). Furthermore, we have also used APN1 as a model antigen to directly address the above issue using nano- and microparticle technologies.
Table 2.
Update of the Current Status and Characteristics of the Leading SSM-TBV Candidates
| Target Antigen | Current Status | Attributes |
|---|---|---|
|
| ||
| P230 | Recombinant antigen expression through a variety of systems including plant, cell free wheat germ systems. | Present in the gametocyte and can confer natural boosting [10, 19] |
| Immunogenicity is poor and requires a strong adjuvant [19, 62, 63] | ||
| Molecule is large, resulting in difficulty in expression and maintenance of conformational epitopes [63] | ||
|
| ||
| P48/45 | Recombinant antigen expression using E. coli (codon harmonized) | Conformational epitopes necessitates an appropriate expression system [33] |
| Immunogenic protein in animals (alum) and is further enhanced by using a strong adjuvant [33] | ||
|
| ||
| P25 | Phase I clinical trials | Immunogenic varies depending on route [63] but is generally considered poorly immunogenic by itself and may require a strong adjuvant [29, 64-66] or conjugation to a molecular adjuvant or protein carrier [67] |
| + Conjugated to recombinant Pseudomonas aeruginosa ExoProtein A [62] | Reactogenic formulations prevented continuation of the first Phase I clinical trial [65] | |
| Phase I Clinical trial of ExoProtein A product is ongoing | Successfully produced the small immunogen in yeast and plants [68] | |
|
| ||
| APN1 | Entering Process Development | Immunogenic in mice [7] and non-human primates (Dinglasan, unpublished) using alum as adjuvant |
| Does not require an adjuvant for complete seroconversion in mice [7] | ||
An ideal SSM-TBV formulation with a highly immunogenic antigen must therefore have the following characteristics: (i) it should be safe; (ii) it should not require a cold-chain; (iii) it should easily be administered; and (iv) a single immunization should confer long-lasting protection. A biodegradable microparticle (BMP) system, which provides sustained release of antigen and adjuvant properties, is capable of meeting these challenges. Several recent studies have demonstrated the utility of this general vaccine approach in vertebrate models [36-40]. Microparticle size is an important determinant for cell uptake [41, 42] and may also influence the antigen release rate [43]. In line with this, recent studies have shown that smaller particle delivery systems are effective in eliciting a robust immune response to the target immunogen [44-47]. The bioabsorption rate of BMPs and antigen release rate can be engineered to provide boosting from weeks to several months. Particles carrying single or multiple antigens can arguably mimic viral antigen presentation thus rapidly inducing a potent and long-lasting cellular and humoral response either by direct immune stimulation of antigen presenting cells (APCs) or/and by delivering antigen to the lymph node [30, 37, 48]. In fact, virosomes follow this approach and have shown to be effective carriers for proteins and subunit vaccines against a variety of pathogens, including malaria [49], but to date, this approach has not been used to deliver SSM-TBV antigens. With these goals in mind, we conducted proof-of-concept studies to test the hypothesis that safe biodegradable microparticles can mimic natural boosting through sustained release of antigen and, in doing so, elicit significant transmission-blocking antibodies against Plasmodium.
MATERIALS AND METHODS
Preparation of Biodegradable Microparticles (BMPs) with Different Size Range and Different Antigen Loading Levels
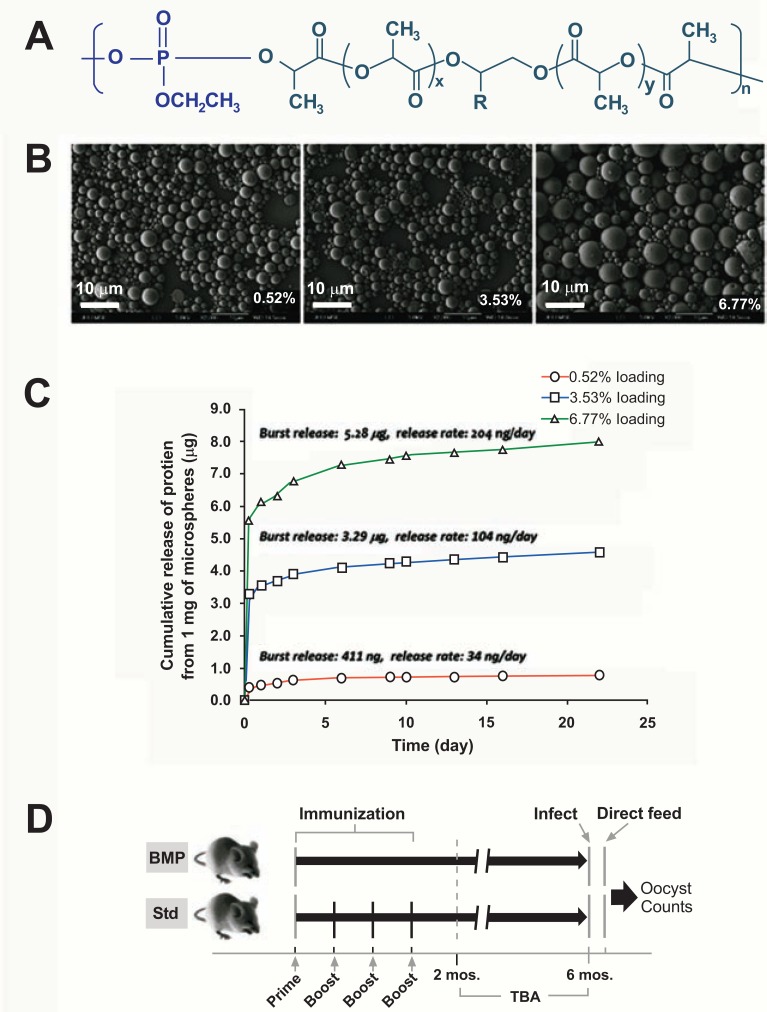
Recombinant APN1 was produced in E. coli as previously described [23]. Polylactofate (PLE) was used to prepare BMPs. PLE is a poly(lactide-co-glycolide) derivative with good biocompatibility and better control of biodegradation rate and physical properties [50, 51] (Fig. 1A). BMPs were prepared by a modified double emulsion method [50], and characterized by scanning electron microscopy. The release kinetics of APN1 from BMPs was characterized by monitoring the concentration of APN1 using ELISA. To modulate APN1 release, we used bovine serum albumin (BSA) as a filler protein.
Fig. (1).
Polylactofate biodegradable microparticles for single inoculation delivery of a malaria transmission-blocking vaccine antigen. (A) Structure of polylactofate (PLE). (B) Scanning electron micrographs of three batches of BMPs with 0.52%, 3.53% and 6.77% protein loading, respectively (Scale bars = 10 µm). (C) Effect of protein loading level on the cumulative release profile of encapsulated proteins from BMPs. (D) Immunization dosing regimens for BMP and control groups, and functional analysis by direct feeding assay (DFA).
Immunizations
BALB/c female mice were immunized with either (A) recombinant APN1 in PBS in suspension with alum, or (B) recombinant APN1 in PBS emulsified with incomplete Freund’s adjuvant (IFA), or (C) BMP-encapsulated recombinant APN1 delivered with alum, or (D) BMP encapsulated APN1 with IFA or (E) empty BMP with alum or (F) empty BMP with IFA. For all treatment groups, mice received 2 µg antigen/mouse/ dose. At day 0, mice received a subcutaneous (s.c.) injection of the appropriate inoculum in a volume of 100 μl per mouse. At 2, 4 and 6 weeks post priming, mice in the Control cohorts (treatments A and B, above) were boosted intraperitoneally (i.p.) with the same dose of the inoculum per mouse, whereas the BMP cohorts were boosted only with PBS. At these time points, each mouse was bled to collect sera for anti-APN1 antibody titer determination via ELISA (Fig. 1C).
ELISA and Cytokine Assay
ELISAs were performed as previously described, using recombinant APN1 as coating antigen [7]. For cytokine assays, the spleen was removed and homogenized at 10% wt/vol in 2% fetal bovine serum/minimal essential medium, and supernatants stored at -80°C until used. Cytokines were measured in tissue homogenates using bead-based multiplex cytokine kits (Bio-Plex, Bio-Rad), according to manufacturer’s instructions. The limits of detection were as follows: interleukin (IL)-1α, 1.32 pg/ml, IL-1β, 1.70 pg/ml; IL-2, 1.98 pg/ml; IL-3, 1.32 pg/ml; IL-4, 2.43 pg/ml; IL-5, 1.69 pg/ml; IL-5, 1.69 pg/ml; IL-6, 1.02 pg/ml; IL-9, 1.36 pg/ml; IL-10, 1.04 pg/ml; IL-12/23 p40, 1.15 pg/ml; IL-12 p70, 1.20 pg/ml; IL-13, 1.57 pg/ml; IL-17a, 1.44 pg/ml; interferon (IFN)-γ, 1.30 pg/ml; eotaxin, 1.70 pg/ml; granulocyte-colony stimulating factor, 1.69 pg/ml; granulocyte-macrophage-colony stimulating factor, 1.58 pg/ml; monocyte chemo-attractant protein, 1.71 pg/ml; macrophage inflammatory protein (MIP)-1α, 1.57 pg/ml; MIP-1β, 1.20 pg/ml; RANTES, 0.95 pg/ml; tumor necrosis factor (TNF)-α, 1.73 pg/ml. Cytokine measurements below the limit of detection as determined by the standard curve for each individual cytokine were assigned a value of the limit of detection/√2 for statistical analysis and plotting. Statistical significance was determined by One-way ANOVA with Bonferroni Post Test, α = 0.05.
Transmission-Blocking Assays
The Direct Feeding Assays (DFA) were conducted as previously described [7] at 2 months and at 6 months post-priming immunization (Fig. 1D). Since Plasmodium oocyst numbers are generally overdispersed in our system, statistical significance was assessed using the non-parametric Mann Whitney U Test, α = 0.05.
RESULTS
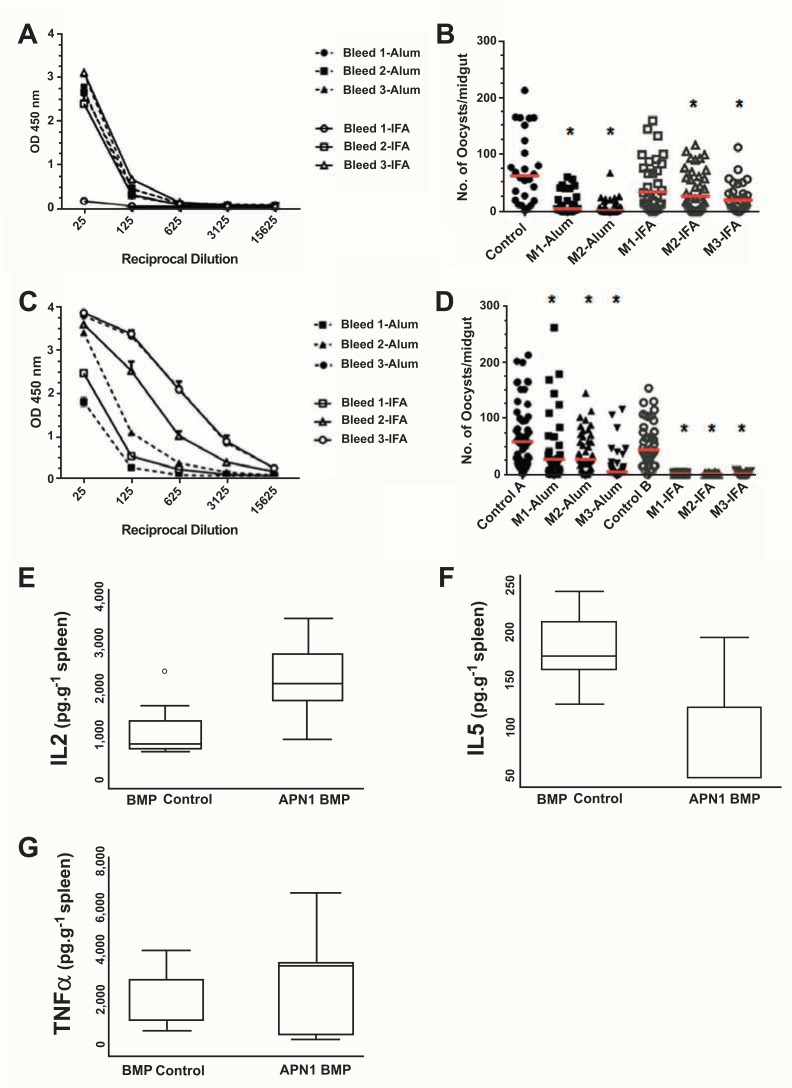
We generated PLE BMPs (Fig. 1B) and optimized the protocol for controlling the protein antigen loading levels. We then used loading level as a parameter to adjust the release rate of the antigen. Using bovine serum albumin (BSA) as a model antigen, we have shown that the amount of antigen released from the BMPs can be controlled by loading level as shown in Fig. (1C). For example, BMPs with 3.53% protein loading level released protein antigen at a rate of ~ 104 ng/day per mg of BMPs, after an initial burst release of 9.3% of the total protein loaded. These release rates amounted to a release of approximately 15% of total protein within the first 22 days. For this pilot study, we used BMPs with 3.53% of protein loading level. We compared the humoral response of mice using the schedule outlined in Fig. (1D). Mice immunized with a single inoculation of APN1-containing BMPs plus IFA or Alum alone (Fig. 2A) mounted a relatively poor antibody response in comparison to a prime and 3-boost regimen of APN1 plus IFA/Alum (Fig. 2C). Surprisingly, the immunoglobulin subtypes (IgG1, IgG2a, and IgG2b) generated in the group that received a single immunization of APN1-BMPs/alum were similar to that elicited by the APN1-alum (data not shown).
Fig. (2).
Characterization of the immune response and activity of antibodies elicited following immunization with APN1. (A) APN1-specific antibody titers (at bleeds 1-3) for mice that received only a single inoculation of BMP encapsulated APN1 with alum or IFA. (B) Direct Feeding Assay to assess short-term transmission-blocking potential of mouse APN1 antisera against Plasmodium berghei (ANKA 2.34) in Anopheles gambiae (KEELE) mosquitoes for groups in (A) at two months post-priming immunization (see Fig. 1D). (C) APN1-specific antibody titers (at bleeds 1-3, at two week intervals) for mice that received APN1 with either alum or IFA as adjuvant. (D) Direct Feeding Assay to assess short-term transmission-blocking potential of mouse APN1 antisera against P. berghei (ANKA 2.34) in An. gambiae (Keele) mosquitoes for groups in (C) at two months post-priming immunization. For A-D: Median oocyst numbers are represented by the horizontal line; control infections were from an agematched, unimmunized mouse; and the P-value was determined by Mann Whitney U Test and asterisks (*) indicate statistical significance at α = 0.05. (E-G) APN1-BMP induces pro-T-cell and B-cell cytokines. Twenty-three cytokines measured in homogenized spleen samples from mice that received either BMP (empty) or APN1-encapsulated BMPs. Data expressed on pg/g of tissue basis (corrected for spleen weight). The two significantly different cytokines (E) IL-2 and (F) IL-5 and one cytokine, TNF-α, which was not significantly different (G), are shown. Data presented as box and whiskers plots with outliers identified as dots. Median is the horizontal line within the box. Statistical significance was determined by one way ANOVA with Bonferroni Post Test, α = 0.05.
To determine the short-term and long-term efficacy of transmission-blocking serum antibodies against P. berghei we performed direct feeding assays (DFAs) two weeks following the final boost in the control group at 2 months (60 days) and at 6 months (180 days) (Figs. 1D, 2B, D). We compared parasite development in mosquitoes that were fed on four groups: (i) control cohort (primed with APN1/alum followed by three boosts); (ii) treatment group receiving a single inoculation of APN1-BMPplus alum, (iii) treatment group receiving a single inoculation of APN1-BMP plus IFA, and (iv) control (naïve/unimmunized or empty BMP immunized) infected mice. At 60 days, both the APN1-alum and APN1-IFA immunized controls elicited functional transmission-blocking antibodies against P. berghei (Fig. 2B). Despite the lower antibody titer observed previously, APN1-BMP-immunized mice generated a significant level of functional antibody titers that can effectively inhibit oocyst development in An. gambiae (Fig. 2C, D). We observed that at 6 months post-priming immunization, serum from mice immunized with APN1/alum, following a standard immunization regimen, no longer contained any transmission-blocking antibodies [refer to median oocyst number/prevalence for APN1-Alum Control (M3)]. In contrast, individual mice that received either APN1-BMPs/alum or APN1-BMPs/IFA still retained functional transmission-blocking antibody (Table 3). Cytokine levels analyzed by multiplex assay also demonstrated that APN1-BMPs significantly induced splenic pro-T-cell and B-cell cytokines such as IL-2 and IL-5. These data suggest a cell-specific immune effect rather than a general inflammatory process in response to BMP dosing, thereby validating the specificity of the immune response to the vaccine formulation (Fig. 2D-F).
Table 3.
Direct Feeding Assays (DFA) to Assess Long-Term Transmission-Blocking Potential of Mouse APN1 Antisera Against Plasmodium berghei (ANKA 2.34) in Anopheles gambiae (Keele) Mosquitoes. DFAs were Performed at 6 Months Post-Priming Immunization (see Fig. 1D)
| Group (Mouse #) | N | Median Oocyst # (Range) | % Inhibition | Prevalence | P-Value |
|---|---|---|---|---|---|
| Long-Term | |||||
| APN1-Alum Control (M3) | 23 | 82 (1-181) | — | 100% | — |
| APN1-BMP-Alum (M4) | 22 | 8.5 (0-84) | 90 | 82% | <0.0001 |
| APN1-BMP-Alum (M5) | n.d. | — | — | — | — |
| APN1-BMP-IFA (M4) | 32 | 16 (0-124) | 81 | 59% | <0.0001 |
| APN1-BMP-IFA (M5) | 22 | 0 (0-1) | 100 | 9% | <0.0001 |
Groups: APN1-Alum Control = recombinant APN1 + alum, using a prime + 3 boost immunization regimen (age-matched with BMP groups); APN1-BMP =APN1-BMP + alum (single inoculation); APN1-BMPIFA=APN1-BMP +Incomplete Freund's adjuvant (single inoculation). n.d., not determined since the mouse did not survive the mosquito feeding. P-value determined by Mann Whitney U Test, α = 0.05.
DISCUSSION
Although nano- and microparticle technology has been already shown to potentiate the immune response to pathogen-derived antigens [52, 53], including malaria [44, 45, 49, 54], its use in TBV delivery while previously postulated [9], remained relatively untested [44]. Our small scale study adds to the growing body of data, and moreover, successfully demonstrates that (1) APN1-BMPs with alum adjuvant elicit antigen-specific antibody titers after single dose immunization and induce the production of cell-activation rather than broad-spectrum pro-inflammatory cytokines; (2) the functional transmission-blocking activity of APN1 antisera against P. berghei from mice immunized with a single dose of APN1-BMP in An. gambiae mosquitoes; and (3) that with a potent adjuvant such as incomplete Freund’s adjuvant, immunization with BMPs elicits and maintains transmission-blocking titers in mice for 6 months. Furthermore, the protracted release kinetics of model antigen over 16 days in vitro by our PLE BMP demonstrates a more controlled profile as compared to gel core liposome or conventional liposome particles which have been shown to exhibit a 50% cumulative percentage release of antigen at 10-15 days and 5 days, respectively [44]. These data suggest that larger microparticles allow for enhanced control over the release profile. Recently, it was shown that incorporation of TLR9 agonists in 1-µm gel core liposomes can significantly enhance the immune response to the poorly immunogenic Pfs25 SSM-TBV antigen [44]. Thus, the use of molecular adjuvants as filler molecules may also be considered in future formulations. Taken together with our proof-of-concept data, we anticipate that co-encapsulation of adjuvant and administration of different BMPs with different release profiles (e.g. burst and fast release serve as priming and sustained/delayed release as boosting dose) will significantly enhance the overall immune responses.
CONCLUSION AND FUTURE PERSPECTIVES
Vaccines are traditionally developed with the prospect of eventual parenteral administration, and the TPP for SSM-TBVs suggests that this is the primary consideration for the development of the leading candidates (Table 1). Given the uniqueness of the SSM-TBV approach it is argued that non-classical concepts for vaccine delivery may be more suitable. In this section we highlight some concerns surrounding the use of NPs and BMPs when considering vaccine delivery not only through parenteral, oral or mucosal routes, but specifically via cutaneous immunization.
Does Size Matter?
It has been shown that 40 nm polystyrene nanoparticles (NP) that are surface-coated with antigen can be targeted to the lymph nodes to generate a robust immune response [46-48, 55]. NPs have also been shown to increase the breadth and avidity of the humoral response to a Plasmodium vivax blood stage antigen [37, 45] arising in part through a synergistic effect of surface displayed and encapsulated antigen in a single formulation. However, it is likely that the nature of the particle, the characteristics of the antigen, including intrinsic immunogenicity and molecular size, presence of conformational antibody epitopes, as well as the type of immune response that should be engendered will have a direct influence on the selection of biodegradable nanoparticles (BNP) vs BMP as carrier (reviewed in [56, 57]). It was found that larger particles engender a Type 2 response while smaller, virus-sized particles induced a largely cell-mediated Type 1 response [46]. An interesting approach would be to use different BNP and BMP carriers, leveraging the advantages of antigen targeting and antigen depot effect endowed by each type of particles to reach a specific immune response endpoint [36]. In the context of SSM-TBVs, it remains to be seen if different carrier modes can further potentiate the humoral response to confer long-term protection.
Does Route of Delivery Matter?
It has been shown that size also has a direct influence on the effectiveness of delivery when the route of administration is considered. Intradermal or subcutaneous inoculation of BNPs and BMPs bypasses the issue of tissue barriers and proteolytic environments, in the case of oral administration. However, the clear potential of this technology lies in the idea of needle-free vaccination. The use of BNP and BMPs as carriers for transcutaneous or cutaneous immunization has been extensively studied [57, 58] and it is well recognized that the main barrier for trans- or percutaneous delivery of antigen payload to the rich population of APCs in the epidermis and dermis is the stratum corneum lipid bilayer overlaying the epidermis [57]. Passive diffusion of antigen carried via nanocarriers through intercellular or follicular routes to access to the APCs in the epidermis and preferably the dermis has been demonstrated, strongly implying that presentation is size dependent [58].
While there are clear opportunities for the utility of BNPs and BMPs in the development of the next generation of SSM-TBVs, the current working model by many vaccine developers remains generally conservative. This is rightly so, since malaria vaccines must be low cost to allow for general distribution. The huge number of vaccine doses to cover the more than one third of the world’s population is likely to be borne by public-private partnerships and other novel funding models. However, there is hope for this approach since the prevailing strategy has been more recently revisited by the PATH Malaria Vaccine Initiative [59]. One of the biggest benefits of the BNP/BMP approach, namely the potential to mimic natural boosting, is quite attractive, especially in light of the prediction that titers of antibody (produced either naturally or following vaccination) against sexual stage and mosquito antigens will likely wane over time [60]. Furthermore, there is optimism that by leveraging the potential advantages conferred by particle-based approaches, the community will ultimately see the incorporation of vaccine antigens targeting different life stages of the parasite in a single particle formulation.
ACKNOWLEDGEMENTS
This work was supported by the Bloomberg Family Foundation and the Johns Hopkins Malaria Research Institute Pilot Grant Award Program. The authors thank Hilary Hurd and Paul Eggleston for the Anopheles gambiae KEELE strain.
ABBREVIATIONS
- BMP
= Biodegradable microparticle
- NP
= Nanoparticle
- TBV
= Transmission-blocking vaccine
- SSM-TBV
= Sexual stage and mosquito TBV
- APN1
= Alanyl aminopeptidase N 1
- PLGA
= Poly-lactic-co-glycolic acid
- FCA
= Freund’s complete adjuvant
- IFA
= Incomplete Freund’s adjuvant.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Alonso PL, Brown G, Arevalo-Herrera M, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8(1):e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19(17-19):2309–2314. doi: 10.1016/s0264-410x(00)00521-1. [DOI] [PubMed] [Google Scholar]
- 3.Dinglasan RR, Jacobs-Lorena M. Flipping the paradigm on malaria transmission-blocking vaccines. Trends Parasitol. 2008;24(8):364–370. doi: 10.1016/j.pt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavazec C, Bourgouin C. Mosquito-based transmission blocking vaccines for interrupting Plasmodium development. Microbes Infect. 2008;10(8):845–849. doi: 10.1016/j.micinf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Sinden RE. A biologist's perspective on malaria vaccine development. Hum Vaccin. 2010;6(1):3–11. doi: 10.4161/hv.6.1.9604. [DOI] [PubMed] [Google Scholar]
- 6.Ranawaka GR, Fleck SL, Blanco AR, Sinden RE. Characterization of the modes of action of anti-Pbs21 malaria transmission-blocking immunity: ookinete to oocyst differentiation in vivo. Parasitology. 1994;109(Pt 4):403–411. doi: 10.1017/s0031182000080653. [DOI] [PubMed] [Google Scholar]
- 7.Mathias DK, Plieskatt JL, Armistead JS, et al. Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine. Infect Immun. 2012;80(4):1606–1614. doi: 10.1128/IAI.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaslow DC. Immunogenicity of Plasmodium falciparum sexual stage antigens: implications for the design of a transmission blocking vaccine. Immunol Lett. 1990;25(1-3):83–86. doi: 10.1016/0165-2478(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 9.Kaslow DC. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr Opin Immunol. 1993;5(4):557–565. doi: 10.1016/0952-7915(93)90037-s. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow DC. Transmission-blocking vaccines: uses and current status of development. Int J Parasitol. 1997;27(2):183–189. doi: 10.1016/s0020-7519(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 11.Mendis KN, David PH, Carter R. Human immune responses against sexual stages of malaria parasites: considerations for malaria vaccines. Int J Parasitol. 1990;20(4):497–502. doi: 10.1016/0020-7519(90)90197-u. [DOI] [PubMed] [Google Scholar]
- 12.Halloran ME, Struchiner CJ, Spielman A. Modeling malaria vaccines. II: Population effects of stage-specific malaria vaccines dependent on natural boosting. Math Biosci. 1989;94(1):115–149. doi: 10.1016/0025-5564(89)90074-6. [DOI] [PubMed] [Google Scholar]
- 13.Gwadz RW, Koontz LC. Plasmodium knowlesi: persistence of transmission blocking immunity in monkeys immunized with gamete antigens. Infect Immun. 1984;44(1):137–140. doi: 10.1128/iai.44.1.137-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rener J, Carter R, Rosenberg Y, Miller LH. Anti-gamete monoclonal antibodies synergistically block transmission of malaria by preventing fertilization in the mosquito. Proc Natl Acad Sci USA. 1980;77(11):6797–6799. doi: 10.1073/pnas.77.11.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushal DC, Carter R, Rener J, Grotendorst CA, Miller LH, Howard RJ. Monoclonal antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitoes. J Immunol. 1983;131(5):2557–2562. [PubMed] [Google Scholar]
- 16.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139(12):4213–4217. [PubMed] [Google Scholar]
- 17.Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65(8):3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson KC, Keister DB, Muratova O, Kaslow DC. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol. 1995;75(1):33–42. doi: 10.1016/0166-6851(95)02507-3. [DOI] [PubMed] [Google Scholar]
- 19.Graves PM, Carter R, Burkot TR, Rener J, Kaushal DC, Williams JL. Effects of transmission-blocking monoclonal antibodies on different isolates of Plasmodium falciparum. Infect Immun. 1985;48(3):611–616. doi: 10.1128/iai.48.3.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson KC. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 2003;25(7):351–359. doi: 10.1046/j.1365-3024.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 21.Kocken CH, Jansen J, Kaan AM, et al. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol. 1993;61(1):59–68. doi: 10.1016/0166-6851(93)90158-t. [DOI] [PubMed] [Google Scholar]
- 22.Stowers AW, Keister DB, Muratova O, Kaslow DC. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect Immun. 2000;68(10):5530–5538. doi: 10.1128/iai.68.10.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinglasan RR, Kalume DE, Kanzok SM, et al. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci USA. 2007;104(33):13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaslow DC. Transmission-blocking vaccines. Chem Immunol. 2002;80:287–307. doi: 10.1159/000058850. [DOI] [PubMed] [Google Scholar]
- 25.Sinden RE, Carter R, Drakeley C, Leroy D. The biology of sexual development of Plasmodium: the design and implementation of transmission-blocking strategies. Malar J. 2012;11:70. doi: 10.1186/1475-2875-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coutinho-Abreu IV, Ramalho-Ortigao M. Transmission blocking vaccines to control insect-borne diseases: a review. Mem Inst Oswaldo Cruz. 2010;105(1):1–12. doi: 10.1590/s0074-02762010000100001. [DOI] [PubMed] [Google Scholar]
- 27.Arevalo-Herrera M, Solarte Y, Marin C, et al. Malaria transmission blocking immunity and sexual stage vaccines for interrupting malaria transmission in Latin America. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):202–211. doi: 10.1590/s0074-02762011000900025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24(2):377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134(Pt 14):1911–1929. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- 30.Tachibana M, Sato C, Otsuki H, et al. Plasmodium vivax gametocyte protein Pvs230 is a transmission-blocking vaccine candidate. Vaccine. 2012;30(10):1807–1812. doi: 10.1016/j.vaccine.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Tachibana M, Wu Y, Iriko H, et al. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin Vaccine Immunol. 2011;18(8):1343–1350. doi: 10.1128/CVI.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrance CE, Rhee A, Jones RM, et al. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol. 2011;18(8):1351–1357. doi: 10.1128/CVI.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury DR, Angov E, Kariuki T, Kumar N. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One. 2009;4(7):e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Outchkourov NS, Roeffen W, Kaan A, et al. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA. 2008;105(11):4301–4305. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones CS, Luong T, Hannon M, et al. Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl Microbiol Biotechnol. 2012 doi: 10.1007/s00253-012-4071-7. in press. [DOI] [PubMed] [Google Scholar]
- 36.Fredriksen BN, Grip J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.) Vaccine. 2012;30(3):656–667. doi: 10.1016/j.vaccine.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 37.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci USA. 2012;109(4):1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uppada SB, Bhat AA, Sah A, Donthamshetty RN. Enhanced humoral and mucosal immune responses after intranasal immunization with chimeric multiple antigen peptide of LcrV antigen epitopes of Yersinia pestis coupled to palmitate in mice. Vaccine. 2011;29(50):9352–9360. doi: 10.1016/j.vaccine.2011.09.129. [DOI] [PubMed] [Google Scholar]
- 39.Fredriksen BN, Saevareid K, McAuley L, Lane ME, Bogwald J, Dalmo RA. Early immune responses in Atlantic salmon (Salmo salar L.) after immunization with PLGA nanoparticles loaded with a model antigen and beta-glucan. Vaccine. 2011;29(46):8338–8349. doi: 10.1016/j.vaccine.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 40.dos Santos SA, Zarate-Blades CR, de Sa Galetti FC, et al. A subunit vaccine based on biodegradable microspheres carrying rHsp65 protein and KLK protects BALB/c mice against tuberculosis infection. Hum Vaccin. 2010;6(12):1047–1053. doi: 10.4161/hv.6.12.13350. [DOI] [PubMed] [Google Scholar]
- 41.Xiang SD, Scholzen A, Minigo G, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40(1):1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Cruz LJ, Tacken PJ, Fokkink R, et al. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J Control Release. 2010;144(2):118–126. doi: 10.1016/j.jconrel.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 43.De Koker S, Lambrecht BN, Willart MA, et al. Designing polymeric particles for antigen delivery. Chem Soc Rev. 2011;40(1):320–339. doi: 10.1039/b914943k. [DOI] [PubMed] [Google Scholar]
- 44.Tiwari S, Goyal AK, Mishra N, et al. Development and characterization of novel carrier gel core liposomes based transmission blocking malaria vaccine. J Control Release. 2009;140(2):157–165. doi: 10.1016/j.jconrel.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS One. 2012;7(2):e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fifis T, Gamvrellis A, Crimeen-Irwin B, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 47.Hardy CL, LeMasurier JS, Belz GT, et al. Inert 50-nm polystyrene nanoparticles that modify pulmonary dendritic cell function and inhibit allergic airway inflammation. J Immunol. 2012;188(3):1431–1441. doi: 10.4049/jimmunol.1100156. [DOI] [PubMed] [Google Scholar]
- 48.Fifis T, Mottram P, Bogdanoska V, Hanley J, Plebanski M. Short peptide sequences containing MHC class I and/or class II epitopes linked to nano-beads induce strong immunity and inhibition of growth of antigen-specific tumour challenge in mice. Vaccine. 2004;23(2):258–266. doi: 10.1016/j.vaccine.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 49.Moreno R, Jiang L, Moehle K, et al. Exploiting conformationally constrained peptidomimetics and an efficient human-compatible delivery system in synthetic vaccine design. Chembiochem. 2001;2(11):838–843. doi: 10.1002/1439-7633(20011105)2:11<838::AID-CBIC838>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 50.Mao HQ, Zhao Z, Dang W, et al. Biodegradable poly(phosphoester)s. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. New York, NY: Johns Wiley & Sons, Inc; 1999. pp. 45–60. [Google Scholar]
- 51.McNeela EA, Lavelle EC. Recent advances in microparticle and nanoparticle delivery vehicles for mucosal vaccination. Curr Top Microbiol Immunol. 2012;354:75–99. doi: 10.1007/82_2011_140. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z, Wang J, Mao HQ, Leong KW. Polyphosphoesters in drug and gene delivery. Adv Drug Deliv Rev. 2003;55(4):483–499. doi: 10.1016/s0169-409x(03)00040-1. [DOI] [PubMed] [Google Scholar]
- 53.Tyagi RK, Garg NK, Sahu T. Vaccination Strategies against Malaria: novel carrier(s) more than a tour de force. J Control Release. 2012;162(1):242–254. doi: 10.1016/j.jconrel.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 54.Kaba SA, Brando C, Guo Q, et al. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183(11):7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jilek S, Merkle HP, Walter E. DNA-loaded biodegradable microparticles as vaccine delivery systems and their interaction with dendritic cells. Adv Drug Deliv Rev. 2005;57(3):377–390. doi: 10.1016/j.addr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Jennings GT, Bachmann MF. Designing recombinant vaccines with viral properties: a rational approach to more effective vaccines. Curr Mol Med. 2007;7(2):143–155. doi: 10.2174/156652407780059140. [DOI] [PubMed] [Google Scholar]
- 57.Hansen S, Lehr CM. Nanoparticles for transcutaneous vaccination. Microb Biotechnol. 2012;5(2):156–167. doi: 10.1111/j.1751-7915.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Combadiere B, Mahe B. Particle-based vaccines for transcutaneous vaccination. Comp Immunol Microbiol Infect Dis. 2008;31(2-3):293–315. doi: 10.1016/j.cimid.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 59.PATH-Malaria Vaccine Initiative. http://www.malariavaccine.org/rd-collaborations.php . [Access November 2012].
- 60.Bousema JT, Drakeley CJ, Sauerwein RW. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med. 2006;6(2):223–229. doi: 10.2174/156652406776055140. [DOI] [PubMed] [Google Scholar]
- 61.The malERA Consultative Group on Vaccines. A Research Agenda for Malaria Eradication: Vaccines. PLoS Med. 2011;8(1):e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bustamante PJ, Woodruff DC, Oh J, Keister DB, Muratova O, Williamson KC. Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol. 2000;22(8):373–380. doi: 10.1046/j.1365-3024.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 63.Vincent AA, Fanning S, Caira FC, Williamson KC. Immunogenicity of malaria transmission-blocking vaccine candidate, y230.CA14 following crosslinking in the presence of tetanus toxoid. Parasite Immunol. 1999;21(11):573–581. doi: 10.1046/j.1365-3024.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 64.Qian F, Wu Y, Muratova O, et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25(20):3923–3933. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubler-Kielb J, Majadly F, Wu Y, et al. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci USA. 2007;104(1):293–298. doi: 10.1073/pnas.0609885104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Przysiecki C, Flanagan E, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci USA. 2006;103(48):18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrance CE, Chichester JA, Musiychuk K, et al. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccin. 2011;7(Suppl):191–198. doi: 10.4161/hv.7.0.14588. [DOI] [PubMed] [Google Scholar]